Abstract
For many decades, eye-tracking has been used to investigate gaze behaviour in the normal population. Recent studies have extended its use to individuals with disorders on the autism spectrum. Such studies typically focus on the processing of socially salient stimuli. In this review, we discuss the potential for this technique to reveal the strategies adopted by individuals with high-functioning autism when processing social information. Studies suggest that eye-tracking techniques have the potential to offer insight into the downstream difficulties in everyday social interaction which such individuals experience.
Autism is a pervasive developmental disorder, characterized by a triad of impairments: social communication problems, difficulties with reciprocal social interactions, and unusual patterns of repetitive behaviour (Wing & Gould, 1979). It was first described in 1943 by Leo Kanner, a child psychiatrist (Kanner, 1943). The prevalence of autism is estimated at 1–2 per thousand individuals (Fombonne, 1999). In the past decade it has been suggested that autism is not a categorical disorder, but instead lies on a continuum, along with Asperger syndrome and pervasive developmental disorder not otherwise specified (PDD-NOS). These conditions are collectively referred to as the autism spectrum disorders (ASDs, DSM-IV; American Psychiatric Association, 2000). Classical autism of the type first described by Kanner is typically associated with mental retardation (Kanner, 1943). However, approximately 20% of people with autism have average or above-average IQ. Individuals with Asperger syndrome (AS) do not suffer from language delay or mental retardation, but have the impairments characteristic of autism in reciprocal social interaction, abnormal patterns of repetitive behaviour and social communication difficulties. A recent study estimated the prevalence of all childhood ASDs at just over 1% (Baird et al. 2006).
An ongoing line of research has centred on finding a core cognitive deficit – that is, a fundamental difference in the way information is processed in the brain that underlies these conditions. However, as yet no single deficit has been found that can explain the range of symptoms found in the ASDs (Schultz, 2005). One problem is the gulf, particularly in individuals with ‘high functioning’ autism in which IQ is unimpaired, between formally assessed cognitive ability and everyday social interaction ability (Klin et al. 2002a). For example, it has been postulated that the social difficulties in autism arise from a deficit in theory of mind (ToM) – the ability to understand the mental states of others (Baron-Cohen et al. 1985). However, individuals with high-functioning autism can often pass formal tests of ToM while having real difficulties in everyday social situations (Klin et al. 2000). This gulf could arise because individuals with autism do not perform these tasks in the same way as do other people, but adopt some kind of unusual strategy to complete the task (Grossman et al. 2000; Klin et al. 2000; Volkmar et al. 2004). In this review we will address the potential use of eye-tracking in investigating the strategies used by individuals with autism, with a focus on tasks involving social information processing.
Social deficits in autism
Kanner's original description of autism highlighted the social and emotional aspects of this disorder (Kanner, 1943). It is these aspects that appear to be unique to autism, whereas communication deficits and repetitive behaviour patterns also occur in other disorders (Schultz, 2005). It is possible that deficits in social interaction might in fact play a causative role in the emergence of other characteristics manifest in autism, such as language delay (Klin et al. 2003). A number of studies have sought to probe the nature of these social deficits by investigating the processing of social information in ASD. The most commonly used stimuli are pictures of human faces, but videotapes of social interactions, human voices, and abstract animations have also been employed. It has been suggested that the perception of social information of this type is closely related to the reciprocal social interaction deficits observed in autism (Joseph & Tager-Flusberg, 2004).
Simply filming subjects whilst they look at a picture or watch a video clip can give a crude indication of their gaze direction. More specialised equipment allows the direction of gaze to be determined more accurately. One technique is to use a ‘scleral search coil’– a contact lens incorporating wire coils – in conjunction with a magnetic field around the subject's head. A less invasive approach is to illuminate the eye with an infra-red beam, and then capture the reflected image with a video camera. The position of the pupil provides sufficient information to determine where on a screen a subject is looking. It is also possible to capture the reflection from the eye, in addition to the pupil. The relative position of these two points is more independent of head position. In addition, novel techniques are under development that detect the position of the fovea directly, potentially allowing improvements in accuracy (Gramatikov et al. 2007).
It should be noted that evidence for actual impaired performance in face-processing tasks in autism is not unequivocal, though differences in processing style, such as a bias towards use of local features, are more consistent across studies (for a recent review of this issue, see Jemel et al. 2006). There have been calls for a shift of emphasis in autism research away from overall performance on tasks and towards the study of the processes and strategies used to perform these tasks (Klin et al. 2002a; Volkmar et al. 2004). In the second part of this review we consider how eye-tracking technology can be used for this purpose.
Eye-tracking as a tool for investigating behaviour
The study of gaze behaviour has long been used to investigate how stimuli are processed. The premise behind this is that when a person looks directly at (‘fixates’) an object, its image falls on the fovea, the part of the retina specialized for detailed visual processing. The eyes therefore need to move in order to inspect the whole of a visual scene in detail (Norton & Stark, 1971). Recordings of gaze behaviour thus indicate where in a visual scene a person was seeking detailed information. This is useful when combined with a test of cognitive performance, such as a test of ToM, or emotion recognition, because it provides information in addition to a person's overall score on the test. Information about which parts of the test stimulus the subject fixated can provide insight into the strategies he or she might have been using to complete the test.
Early studies of looking behaviour involved simply filming subjects whilst they looked at a picture or watched a video clip, and scoring the videotape to obtain a crude indication of their gaze direction. Modern eye-tracking techniques allow the direction of gaze to be determined more accurately. There are a variety of ways of accomplishing this, but the most popular approach is to illuminate the eye with an infra-red beam, and then capture the reflected image with a video camera. Two points are identified from the captured image: the reflection from the cornea of the eye, normally the brightest point on the image, and the pupil, normally the second brightest. The relative position of these two points provides sufficient information to determine where on a screen a subject is looking. Eye-tracking using only the position of the pupil, without the corneal reflection, is also possible. In addition, novel techniques are under development which detect the position of the fovea directly, potentially allowing improvements in accuracy (Gramatikov et al. 2007).
The temporal resolution available in the collection of eye-tracking data varies according to the type and model of eye-tracker used. Pupil-only and pupil-CR eyetrackers typically operate at sampling rates of between 50 Hz (e.g. ASL R6 model; http://www.a-s-l.com) and 2 kHz (e.g. SR Research Eyelink 2k; http://www.sr-research.com). Direct tracking of the fovea can be accomplished at speeds of up to 200 Hz (Gramatikov et al. 2007). Spatial resolution varies from 0.005 degrees of visual angle (Clarke et al., 2002) to 0.5 degrees (ASL Model 310; http://www.a-s-l.com), or approximately 0.1 degrees for methods that involve direct detection of the fovea (Gramatikov et al. 2007).
The studies described in this review have largely made use of two approaches: either calculating the total percentage of time that the subject's gaze was on a particular region of the stimulus, or processing the data to find the regions of the stimulus on which the subject ‘fixated’. A fixation occurs when the observer looks at the same point for long enough to allow the processing of visual information from that point. Typically, if the point of gaze remains within 1 degree of visual angle for at least 100 milliseconds this is classified as a fixation, though some eye-tracking studies have used different criteria (e.g. Pelphrey et al. 2002).
Studying gaze patterns in autism
Eye-tracking has long been used to investigate the gaze patterns of normal adults. Recently it has been employed to study individuals with autism. As described earlier, studies typically involve images or video clips of people, or simply human faces. Normal adults show a very specific pattern of gaze when viewing faces, fixating mainly on the eyes, but also on the nose and mouth, the so-called ‘core features’ (Walker-Smith et al. 1977; Luria & Strauss, 1978).
One of the first studies using eye-tracking in autism (Pelphrey et al. 2002) monitored the eye movements of five adult males with autism and five controls whilst they performed a test of emotion recognition from photographs of facial expressions. The subjects with autism spent a smaller percentage of time examining the core features of the face (eyes, nose and mouth; subsequent analysis showed this effect to be driven by less gaze time to the eyes and nose). Also, when gaze data were analysed in terms of fixations, fewer of the autism group's fixations were to these core facial features, though these differences were not significant at the level of individual features.
Similar results were found in a neuroimaging study by Dalton et al. (2005). Subjects completed two tasks whilst their eye movements were monitored and brain activity was recorded using fMRI. Task 1 involved discriminating between emotional and neutral facial expressions. Task 2 involved deciding whether a face was familiar or unfamiliar. In both tasks, the autism group showed fewer fixations to the eye region. However, there were no differences between the autism and control groups in the number of fixations in general, or in the number of fixations to the mouth. The functional imaging findings of this study are discussed in the next section of this review.
Eye-tracking can also be used with video clips, as exemplified in a study by Klin et al. (2002b), who studied the fixation patterns of 15 young males with autism, and 15 controls, while they watched film clips featuring characters engaged in social interaction. The subjects were not given any specific task, but were simply instructed to watch the video clips. Video clips are more ecologically valid than static photographs of single individuals in that they simulate a real-life social situation in which there are multiple distracting people and objects in the scene. As with still photographs (Pelphrey et al. 2002; Dalton et al. 2005), the autism group looked less frequently at the eyes of the characters. In contrast, they looked more frequently at the mouths and bodies, and at other objects in the scene. The other main finding was that fixation of the mouth was a strong predictor of an autistic individual's social competence. Studies of cognitive function in autism have rarely been able to demonstrate a link between scores on formal tests and measures of social ability or disability. This finding suggests that eye-tracking could be a way of closing the gulf between performance on cognitive tests and everyday social ability of individuals with autism.
Another fruitful approach is to look at parallels between gaze behaviour in autism and other clinical groups. Reduced fixation of the eyes is also found in SM, a patient with lesions to the amygdala (Adolphs et al. 2005), raising the possibility that abnormal fixation patterns in autism are due to abnormal functioning of the amygdala. It has also been found that individuals with autism share another feature with SM – a reduced ability to recognize facial expressions of fear (Pelphrey et al. 2002), which in SM was shown to be due to reduced fixation of the eyes (Adolphs et al. 2005). Congruent with these findings, a recent study found that individuals with autism who made fewer fixations to the eyes were worse at recognizing fear (Corden & Skuse, personal communication).
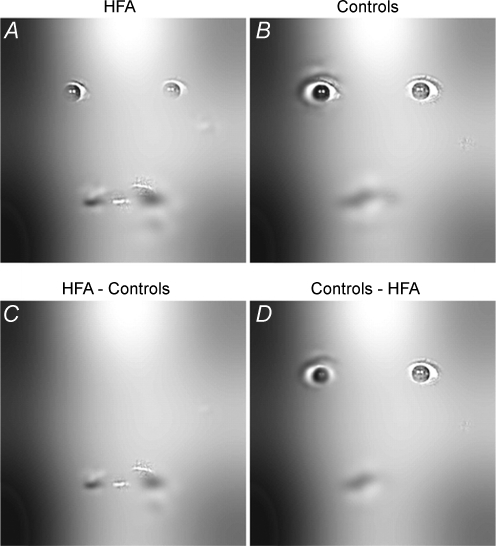
A limitation to studying fixation patterns is that they cannot indicate how the brain uses the visual information it receives. For example, even if an individual shows normal fixation of the eyes, they may not make use of the information available in the eyes. Spezio et al. (2007) used eye-tracking, together with a novel method of presenting stimuli, to investigate which parts of the face subjects were using to recognize emotional expressions. Using the so-called ‘bubbles’ method (Gosselin & Schyns, 2001), they created images in which only certain parts of the face were visible. They found, like Klin et al. (2002b), that subjects with autism made more fixations to the mouth, and that subjects with autism had a greater reliance on information from the mouth in order to identify the emotion (see Fig. 1).
Figure 1.
The areas of the face from which subjects used information when performing an emotion recognition task Subjects with autism had a greater reliance on information from the mouth region, rather than the eyes. From Spezio et al. (2007) reproduced with permission of Springer Science and Business Media.
Using eye-tracking in conjunction with fMRI
Using eye-tracking in concert with functional imaging raises the possibility of correlating differences in gaze patterns with brain activations. This approach was adopted by Dalton et al. (2005). In addition to finding diminished eye region fixation in subjects with autism, this study demonstrated differences in brain activity – the response of the fusiform gyrus (FG) to faces was diminished in subjects with autism. They found that activity in the FG was positively correlated with the number of fixations an individual made to the eyes, and concluded that the underactivation of the FG in individuals with autism was due to a general failure to look at the eyes. Dalton et al. also found a positive correlation between eye fixation and amygdala activation, which they interpreted as evidence of eye avoidance. They propose that in autism this originates from a hyperactive amygdala, which produces unpleasant levels of arousal if the eye region is fixated. However, this is only one of many possible interpretations of these data, and a problem with studies of this type is the difficulty in inferring a causal relationship between gaze patterns and brain activation.
Another limitation to be considered is the differences that are introduced by transferring a task to the scanning environment. In an fMRI scanner, a subject is in a highly unnatural environment – lying horizontal, unable to move, and exposed to loud, unusual noise. Perhaps the most critical difference is the lack of any visual or social distractions – a situation markedly different from everyday social encounters. This should be considered when extrapolating from eye-tracking findings obtained in a scanning paradigm.
Gaze patterns and the broad autism phenotype
A recent study extended the use of eye-tracking to the relatives of individuals with autism. Dalton et al. (2006) measured fixation patterns in 12 individuals with autism, 10 of their siblings and 12 controls, whilst they looked at photographs of faces. They found that both the subjects with autism and their siblings made fewer fixations to the eyes. Family studies are a common approach to investigating the genetic basis of autism. There is evidence for a strong genetic component to this disorder (Pickles et al. 1995), but it is thought that what is inherited in families is not severe autism, but a ‘broad autism phenotype’ (BAP) – a mild predisposition towards autistic traits, which when combined with environmental influences might develop into autism in some cases (for a review see Piven, 2001). Because of the strong heritability of the BAP, the concept is likely to be useful in the search for genes linked to the development of autism. The findings of Dalton et al. (2006) suggest that abnormal fixation patterns might form part of this BAP.
Related to this is the idea that diminished eye fixation could be an early behavioural marker, useful for identifying infants at risk of developing autism later in life. Merin et al. (2006) studied the gaze behaviour of 31 younger siblings of children with autism. This differs from the study of Dalton et al. (2006), which specifically looked at siblings who were unaffected. The infants in the study of Merin et al. (2006) were too young (at 6 months) for their diagnostic status to be ascertained. However, given the raised incidence of autism in siblings compared to the general population (Sumi et al. 2006), these infants can be considered a high-risk cohort.
Merin et al. (2006) recorded the infants' point of regard during infant–mother interaction, and found that a subgroup of the high-risk infants showed reduced gaze to the mothers' eyes, and increased gaze to the mouth. These results suggest a potential application of eye-tracking in the early identification of autism and related disorders.
Conflicting findings in eye-tracking research
It is important to note that some studies of individuals with autism found no difference in gaze patterns (van der Geest et al. 2002a,van der Geest 2002b). There are a number of possible reasons for these differences between results. Firstly, they could be due to differences in the participant groups – the studies that found no difference involved children, whereas the majority of studies (with the exception of Dalton et al. 2006) that have found a difference in gaze behaviour have involved adult participants. Second, specialist training on emotion recognition, as occurs in some educational units for individuals with autism, could have an impact on how an individual performs in testing.
Alternatively, the critical difference could be in the nature of the stimuli used. It has been suggested (Kemner & van Engeland, 2003) that gaze differences in autism only exist in response to dynamic stimuli (e.g. Klin et al. 2002b) and are due to impaired dorsal stream function. Studies that failed to find a difference have used static stimuli (van der Geest et al. 2002a,van der Geest 2002b). However, other studies using static stimuli have found gaze abnormalities in autism (Pelphrey et al. 2002; Dalton et al. 2005, Dalton 2006; Spezio et al. 2007).
Finally, the importance of the testing paradigm itself should be highlighted. The specific instructions given to the participant could be a crucial factor as they impact on what precisely is being measured – differences in the gaze strategies used when a subject is completing a specific task versus differences in spontaneous behaviour. Individuals with autism might conceivably look at the face in a normal way when required to do so by a task, yet fail to explore a face visually without specific reason to do so. On the other hand, they might show normal spontaneous gaze behaviour, but an inability to examine the appropriate parts of a face when performing the task. It should be noted that one study included both a free-viewing and a task-directed condition in their study, and found the same pattern of results in both (Pelphrey et al. 2002).
The ideal task for investigating differences in gaze behaviour will vary according to the particular theoretical question under investigation. However, it might be worth considering that unstructured, naturalistic testing paradigms are reported to be more likely to reveal deficits in social processing in autism (Klin et al. 2002a; Ponnet et al. 2004).
Summary and future directions
Despite some negative findings, most studies have shown that individuals with autism look at social stimuli differently, in particular looking less at the eye region of the face (Pelphrey et al. 2002; Dalton et al. 2005; Klin et al. 2002b). With regard to the mouth region, some studies have found increased fixation (Spezio et al. 2007; Klin et al. 2002b) but others have found no clear difference (Pelphrey et al. 2002; Dalton et al. 2005).
Eye-tracking allows the direct, objective and quantitative observation of behaviour, and through the analysis of fixation patterns can indicate which information from a scene is available to the brain. The potential for its use in behavioural and neuroimaging studies has not yet been fully realized. As shown in the studies reviewed in this article, it can be used to investigate the mechanisms underlying abnormal brain activity (Dalton et al. 2005), and reduced task performance (Corden et al. 2007) in individuals with autism. Because of its relative low cost eye-tracking can also be used in larger studies. Most studies so far have been based on relatively small subject groups. Larger studies would add weight to earlier findings of diminished eye fixation, and clarify whether individuals with autism also show abnormal fixation of the mouth.
It would be beneficial if eye-tracking studies were broadened in scope to investigate how far abnormal fixation patterns extend to other stimuli. Social perceptual deficits in autism are not limited to familiar stimuli such as faces, but extend to highly abstract stimuli such as animated shapes (Abell et al. 2000; Castelli et al. 2002; Boraston et al. 2007), which could also be used in conjunction with eye-tracking. Similarly, it is unknown whether fixation abnormalities in autism are restricted to social stimuli at all, and this issue needs to be resolved before the root cause of such abnormalities can be addressed.
One issue for further discussion is that of ecological validity, that is the extent to which the stimuli and protocol approximate the real-life situation that is under study. Although the non-intrusive nature of the eye-tracking technique and the use of videos of realistic social interaction (e.g. Klin et al. 2002b) can enhance ecological validity, some limitations remain. With some exceptions (Merin et al. 2006), experimental studies do not generally involve real people, but pictures and videotapes of people. When investigating traits such as fixations to the eye region this should be borne in mind since a photograph does not ‘look back’ at the subject, whereas a real person would. The implications of this are a topic for further exploration.
References
- Abell F, Happe F, Frith U. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. Cognitive Dev. 2000;15:1–16. [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition. American Psychiatric Publishing.
- Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D, Charman T. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP) Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a ‘theory of mind’? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Boraston Z, Blakemore SJ, Chilvers R, Skuse D. Impaired sadness recognition is linked to social interaction deficit in autism. Neuropsychologia. 2007;45:1501–1510. doi: 10.1016/j.neuropsychologia.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Clarke AH, Ditterich J, Druen K, Schonfeld U, Steineke C. Using high frame rate CMOS sensors for three-dimensional eye tracking. Behav Res Methods Instrum Comput. 2002;34:549–560. doi: 10.3758/bf03195484. [DOI] [PubMed] [Google Scholar]
- Corden B, Chilvers R, Skuse D. Avoidance of emotionally arousing stimuli predicts social-perceptual impairment in Aspergers syndrome. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.08.005. in press. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2006;61:512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nature Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. The epidemiology of autism: a review. Psychol Med. 1999;29:769–786. doi: 10.1017/s0033291799008508. [DOI] [PubMed] [Google Scholar]
- Gosselin F, Schyns PG. Bubbles: a technique to reveal the use of information in recognition tasks. Vision Res. 2001;41:2261–2271. doi: 10.1016/s0042-6989(01)00097-9. [DOI] [PubMed] [Google Scholar]
- Gramatikov BI, Zalloutm OH, Wu YK, Hunter DG, Guyton DL. Directional eye fixation sensor using birefringence-based foveal detection. Appl Optics. 2007;46:1809–1818. doi: 10.1364/ao.46.001809. [DOI] [PubMed] [Google Scholar]
- Grossman JB, Klin A, Carter AS, Volkmar FR. Verbal bias in recognition of facial emotions in children with Asperger syndrome. J Child Psychol Psychiatry. 2000;41:369–379. [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: fact or artefact? J Autism Dev Disord. 2006;36:91–106. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H. The relationship of theory of mind and executive functions to symptom type and severity in children with autism. Dev Psychopathol. 2004;16:137–155. doi: 10.1017/S095457940404444X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kemner C, van Engeland H. Autism and visual fixation. Am J Psychiatry. 2003;160:1358–1359. doi: 10.1176/appi.ajp.160.7.1358-a. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. Am J Psychiatry. 2002a;159:895–908. doi: 10.1176/appi.ajp.159.6.895. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002b;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Schultz R, Cohen D. Theory of mind in action: developmental perspectives on social neuroscience. In: Baron-Cohen S, Tager-Flusberg H, Cohen D, editors. Understanding Other Minds: Perspectives from Developmental Neuroscience. 2. Oxford: Oxford University Press; 2000. pp. 357–388. [Google Scholar]
- Langdell T. Recognition of faces: an approach to the study of autism. J Child Psychol Psychiatry. 1978;19:255–268. doi: 10.1111/j.1469-7610.1978.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Luria SM, Strauss MS. Comparison of eye movements over faces in photographic positives and negatives. Perception. 1978;7:349–358. doi: 10.1068/p070349. [DOI] [PubMed] [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interaction distinguish a subgroup of 6-month-old infants at risk for autism from comparison infants. J Autism Dev Disord. 2006;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Norton D, Stark L. Eye movements and visual perception. Sci Am. 1971;224:35–43. [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pickles A, Bolton P, Macdonald H, Bailey A, Le Couteur A, Sim CH, Rutter M. Latent-class analysis of recurrence risks for complex phenotypes with selection and measurement error: a twin and family history study of autism. Am J Human Genet. 1995;57:717–726. [PMC free article] [PubMed] [Google Scholar]
- Piven J. The broad autism phenotype: a complementary strategy for molecular genetic studies of autism. Am J Med Genet. 2001;105:34–35. [PubMed] [Google Scholar]
- Ponnet KS, Roeyers H, Buysse A, De Clercq A, Van der Heyden E. Advanced mind-reading in adults with Asperger syndrome. Autism. 2004;8:249–266. doi: 10.1177/1362361304045214. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:415–427. doi: 10.1098/rstb.2002.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal use of facial information in high-functioning autism. J Autism Dev Disord. 2007 doi: 10.1007/s10803-006-0232-9. in press. [DOI] [PubMed] [Google Scholar]
- Sumi S, Taniai H, Miyachi T, Tanemura M. Sibling risk of pervasive developmental disorder estimated by means of an epidemiologic survey in Nagoya, Japan. J Human Genet. 2006;51:518–522. doi: 10.1007/s10038-006-0392-7. [DOI] [PubMed] [Google Scholar]
- van der Geest JN, Kemner C, Camfferman G, Verbaten MN, van Engeland H. Looking at images with human figures: comparison between autistic and normal children. J Autism Dev Disord. 2002a;32:69–75. doi: 10.1023/a:1014832420206. [DOI] [PubMed] [Google Scholar]
- van der Geest JN, Kemner C, Verbaten MN, van Engeland H. Gaze behavior of children with pervasive developmental disorder toward human faces: a fixation time study. J Child Psychol Psychiatry. 2002b;43:669–678. doi: 10.1111/1469-7610.00055. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- Walker-Smith GJ, Gale AG, Findlay JM. Eye movement strategies involved in face perception. Perception. 1977;6:313–326. doi: 10.1068/p060313. [DOI] [PubMed] [Google Scholar]
- Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J Autism Dev Disord. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Yin RK. Looking at upside-down faces. J Exp Psychol. 1969;81:141–145. [Google Scholar]