Abstract
Vasodilatation is a vital mechanism of systemic blood flow regulation that occurs during periods of increased energy demand. The AMP-dependent protein kinase (AMPK) is a serine/threonine kinase that is activated by conditions that increase the AMP-to-ATP ratio, such as exercise and metabolic stress. We hypothesized that AMPK could trigger vasodilatation and participate in blood flow regulation. Rings of thoracic aorta were isolated from C57Bl6 mice and mice deficient in the AMPK catalytic α1 (AMPKα1−/−) or α2 (AMPKα2−/−) subunit and their littermate controls, and mounted in an organ bath. Aortas were preconstricted with phenylephrine (1 μm) and activation of AMPK was induced by addition of increasing concentrations of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR). AICAR (0.1–3 mm) dose-dependently induced relaxation of precontracted C57BL6, AMPKα1+/+ and α2+/+ aorta (P < 0.001, n = 5–7 per group). This AICAR induced vasorelaxation was not inhibited by the addition of adenosine receptor antagonists. Moreover, when aortic rings were freed of endothelium by gentle rubbing, AICAR still induced aortic ring relaxation, suggesting a direct effect of AICAR on smooth muscle cells. When aortic rings were pretreated with l-NMMA (30 μm) to inhibit nitric oxide synthase activity, AICAR still induced relaxation. Western blot analysis of C57Bl6 mice denuded aorta showed that AMPK was phosphorylated after incubation with AICAR and that AMPKα1 was the main catalytic subunit expressed. Finally, AICAR-induced relaxation of aortic rings was completely abolished in AMPKα1−/− but not AMPKα2−/− mice. Taken together, the results show that activation of AMPKα1 but not AMPKα2 is able to induce aortic relaxation in mice, in an endothelium- and eNOS-independent manner.
AMPK is a ubiquitous serine/threonine protein kinase activated by pathological stimuli, such as oxidative damage, osmotic shock, hypoxia and glucose deprivation, as well as by physiological stimuli such as exercise and muscle contraction, and by hormones including leptin and adiponectin (Hardie et al. 2003). AMPK is activated in response to decreased cellular energy charge (high AMP/ATP ratio) and is involved in regulating carbohydrate and fat metabolism (Hardie et al. 2003; Sambandam & Lopaschuk, 2003).
AMPK exists in cells as a heterotrimeric complex composed of a catalytic subunit (α) and two regulatory subunits (α and γ). Two α subunit isoforms exist, α1 and α2, which are unevenly distributed in the tissues. AMPK is expressed both in endothelial cells and in smooth muscle cells. The predominant isoform expressed in vascular endothelial cells is α1 (Zou et al. 2004; Davis et al. 2006). AMPK expression in vascular smooth muscles is different from its expression in striated muscles (Rubin et al. 2005). Both α1 and α2 catalytic subunits are expressed in arterial smooth muscle cells, although their relative proportion differs between different arteries (Rubin et al. 2005; Evans et al. 2006).
AMPK can be artificially activated by treatment with the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR). This nucleoside is taken up by cells, resulting in accumulation of the monophosphorylated derivative 5-aminoimidazole-4-carboxamide ribonucleotide (ZMP) and activation of AMPK (Corton et al. 1995). Treatment of human aortic smooth muscle cells or isolated rabbit aortas with AICAR induces phosphorylation of AMPK and of acetyl-CoA carboxylase, a key target of AMPK, resulting in inhibition of growth factor-induced cell proliferation (Igata et al. 2005). A target of AMPK is endothelial nitric oxide synthase (eNOS), an important modulator of angiogenesis and vascular tone. It has been clearly established that AMPK may associate with and phosphorylate eNOS in cardiomyocytes and endothelial cells (Chen et al. 1999), in association with the heat shock protein 90 (Davis et al. 2006), thus increasing eNOS activity and NO production. Direct activation of AMPK with AICAR stimulates NO synthesis in human aortic endothelial cells (Morrow et al. 2003). Furthermore AMPK can be activated independently in endothelial cells by extracellular nucleotides and adenosine through P2 receptors and adenosine transporters (da Silva et al. 2006). Finally, metabolically challenged endothelium-denuded porcine carotid artery segments exhibit a rapid increase in AMPK activity after metabolic stress associated with the recruitment of signalling pathways that may regulate smooth-muscle contraction (Rubin et al. 2005). However, AICAR failed to relax endothelin-1 precontracted carotid artery rings in this species (Rubin et al. 2005). These data suggest that AMPK may play a complex role in vascular function and remodelling. However, the possible involvement of AMPK in vasorelaxation has not at present been directly shown.
In this study we investigated whether pharmacological activation of AMPK by AICAR could induce relaxation of preconstricted mouse aorta and whether this effect was mediated by endothelium and/or NOS activation. Furthermore, AMPK isoform specificity of AICAR-induced relaxation was investigated in AMPKα1 and α2 knock-out mice and their littermate controls (Viollet et al. 2003; Jorgensen et al. 2004). The results show that activation of AMPKα1 induces vasorelaxation of mouse aorta in an endothelium- and NOS-independent manner.
Methods
Chemicals and materials for mechanical experiments
The following drugs and chemicals were supplied by Sigma-Aldrich Chimie (Saint-Quentin Fallavier, France): acetylcholine chloride, phenylephrine, KCl, 8-(p-sulfophenyl)theophylline (SPT) and 1,3-dipropyl-8-(p-sulfophenyl)xanthine (DPSX). NG-Monomethyl l-arginine (l-NMMA) was purchased from Calbiochem (EMD Biosciences, Inc, an Affiliate of Merck, Darmstadt, Germany) and AICAR from Toronto Research Chemicals Inc. (Toronto, Canada). Stock solutions of these drugs were all prepared in distilled water. Further dilutions were prepared in Krebs–Henseleit buffer.
Animal models
The generation of mouse knock-outs for AMPKα1 and AMPKα2 catalytic subunits has been described elsewhere (Viollet et al. 2003; Jorgensen et al. 2004). AMPKα1 background mice were C57Bl6/129Sv/FVB-N and AMPKα2 mice were C57Bl6 background (6 backcrosses). All genetically modified mice were compared to their appropriate controls. Because the genetic backgrounds of the two models were different, adult male AMPKα1−/− or AMPKα2−/− and their control littermates or wild-type C57Bl6 mice (WT) were used in this study. Genotypes of the knock-out mice were determined by PCR with primer couples for AMPKα1 deleted allele (forward: GGG CTG CAG GAA TTC GAT ATC AAG C and reverse: CCT TCC TGA AAT GAC TTC TGG TGC); for AMPKα1 wild-type allele (forward: AGCCGACTTTGGTAAGGATG and reverse: CCCACTTTCCATTTTCTCCA); for AMPKα2 deleted allele (forward: GCT TAG CAC GTT ACC CTG GAT GG and reverse: GCA TTG AAC CAC AGT CCT TCC TC); and for AMPKα2 wild-type allele (forward: GCT TAG CAC GTT ACC CTG GAT GG and reverse: GTT ATC AGC CCA ACT AAT TAC AC). Animals were housed under temperature-controlled conditions (21°C) and had free access to water and to a standard mouse chow. The investigation was conducted in accordance with our institutional guidelines defined by the European Community guiding principles in the care and use of animals and French decree no. 87/848.
Functional measurements of aortic reactivity
Mice (n = 4–7 per group) were anaesthetized by intraperitoneal injection of sodium pentobarbital (60 mg kg−1). Thoracic aortas were dissected free and placed in ice-cold Krebs–Henseleit buffer continuously gassed with a mixture of 95% oxygen and 5% carbon dioxide and containing the following (mm): NaCl 116.3, KCl 5.4, CaCl2 1.8, NaH2PO4 1.04, MgSO4 0.83, NaHCO3 19, glucose 5.5; pH 7.4. The blood vessels were cleaned of connective tissue and cut into 2 mm rings. The endothelial lining was removed from one or two segments by gentle rubbing. The rings were suspended isometrically between two stainless steel hooks in organ chambers containing Krebs solution at 37°C, and the top hook was connected to a force-displacement transducer connected to an amplifier (Bionic instrument; Phymep, France) and a data acquisition system (PowerLab; ADInstruments Ltd, Australia), for continuous recording of isometric tension. The rings were stretched in a stepwise manner and allowed to equilibrate at 500 mg load over a period of 1 h. Functional integrity of the arterial rings was then challenged with KCl (60 mm), and the presence or absence of functional endothelium was assessed by acetylcholine (ACh, 1 μm)-induced relaxation of aortic rings preconstricted with phenylephrine (1 μm).
In each group of animals, the influence of AICAR on vascular reactivity was measured in aortic rings precontracted with a submaximal concentration of phenylephrine (1 μm). After reaching a tension plateau value, increasing concentrations of AICAR (or adenosine in some cases) were administered. Each increase in AICAR concentration was made in the bath once a plateau of relaxation was reached (after about 30 min). In some experiments, the inhibitor of NO synthase l-NMMA (30 μm) or the non-selective adenosine receptor antagonist SPT (10 μm) and the A1 selective adenosine receptor antagonist DPSX (30 nm) were added to the bath before phenylephrine administration. Relaxation values were expressed as percentage decreases in phenylephrine-induced vasoconstriction.
Western blot analysis
Aortas from WT, AMPKα2−/− and AMPKα1−/− mice were isolated in Krebs buffer and cleaned of connective tissue. They were then opened longitudinally and endothelium was removed by gentle rubbing. Tissues were homogenized in a lysate buffer containing (mm): Hepes 50, KCl 50, EDTA 1, EGTA 1, α-glycerol-phosphate 5, orthovanadate 1, DTT 1, NaPPi 5, phenylmethylsulphonyl fluoride (PMFS) 2 (all from Sigma-Aldrich Chimie), a cocktail of protease inhibitors (Calbiochem Set V EDTA free) and 0.1% Triton X-100. Twenty-five micrograms of proteins was used for Western blot detection of AMPK isoforms. Immunoblot protein levels of AMPK subunits were determined by using anti-AMPKα1 and anti-AMPKα2 (a generous gift from G. Hardie), and anti-pan-AMPK-α (Cell Signalling Technology Inc, Danvers, MA, USA) antibodies. For the control of phosphorylation, C57Bl6 mice denuded aortas were incubated for 30 min in 0, 0.1 or 3 mm AICAR. Tissues were homogenized as above. Immunoblot protein levels of total and phosphorylated AMPK were determined by using the anti-pan-antibody and anti phosphorylated antibody (Cell Signalling). The signals were detected by an ECL-based detection system. Band intensities were quantified by scanning and processing with the program Image J (v1.37 for Mac OSX).
Statistical analysis
The results are expressed as means ± s.e.m. The differences between groups were determined by two-way analysis of variance (ANOVA) with repeated measures followed by the Bonferroni-corrected t test or by Student's t test for paired or unpaired data as appropriate. All differences were considered significant when P < 0.05.
Results
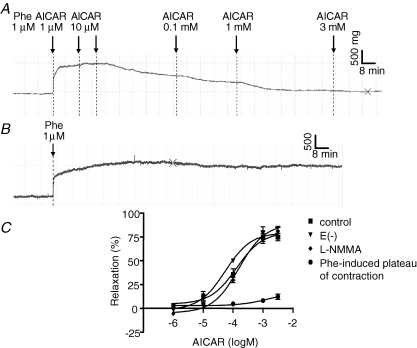
In preliminary experiments conducted using C57Bl6 mice, a large range of AICAR concentrations (1 μm to 3 mm) was tested on phenylephrine-preconstricted mouse aortic rings (Fig. 1A–C). AICAR dose-dependently induced aortic ring vasorelaxation at a concentration of 0.1 mm and above (ANOVA, P < 0.001, n = 7). The maximal effect obtained with AICAR (3 mm) was a 74.8%± 5.4% relaxation of the phenylephrine-induced contraction. These effects developed slowly and stabilized within 30 min after each addition of AICAR. Relaxing effects did not result from the decrease in phenylephrine-induced contraction amplitude, as shown in Fig. 1B–C. Because AMPK is known to phosphorylate eNOS, we investigated the effects of l-NMMA, a known inhibitor of NOS. Surprisingly, AICAR-induced vasorelaxation was not affected by treatment with 30 μm l-NMMA or by the absence of endothelium (Fig. 1C). This suggests that AICAR-induced vasorelaxation is eNOS and endothelium independent. The effects of AICAR on the phosphorylation of AMPK were verified by Western blotting in denuded aortas (Fig. 2). AMPK was partially phosphorylated after incubating the aortas with 0.1 mm AICAR and clearly phosphorylated with 3 mm AICAR. AICAR concentrations of 0.1 mm, 1 mm and 3 mm were used for subsequent experiments.
Figure 1.
AICAR-induced relaxation of C57B16 mouse aorta A, representative tension trace showing the effects of AICAR (1 μm to 3 mm) on isolated C57Bl6 mouse aortic ring after phenylephrine-induced contraction. B, representative tension trace showing the stability of phenylephrine-induced contraction. C, mean values of AICAR (1 μm to 3 mm)-induced relaxation of phenylephrine preconstricted C57Bl6 aortic rings (control conditions), and the same experiment in the presence of l-NMMA (30 μm), or after removal of the endothelium. Data were analysed by nonlinear regression sigmoidal dose–response curve using the Prism 4.01 software (GraphPad Software, San Diego, CA, USA). Results are expressed as means ± s.e.m. of 4–7 mice aorta.
Figure 2.
Phosphorylation of AMPK by AICAR in denuded C57Bl6 mouse aorta Immunoblots showing expression level of total AMPKα (anti-pan-AMPKα antibody) and phosphorylated AMPK (anti-phospho-AMPK antibody), after 30 min incubation in AICAR 0, 0.1 or 3 mm. Twenty-five micrograms of protein from C57Bl6 mice aortic rings were loaded in each lane. N = 3–4 different animals in each group.
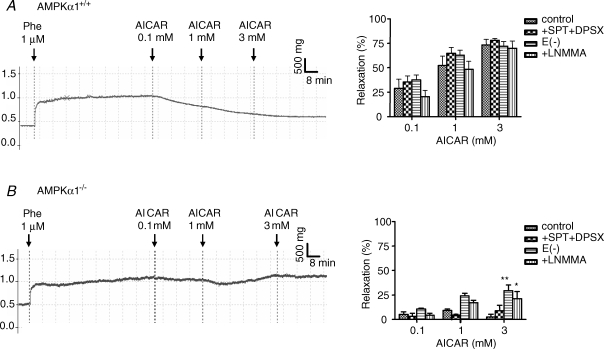
The contraction amplitude of aortic rings preconstricted with 1 μm phenylephrine did not differ between AMPKα1−/−, AMPKα2−/− mice and their littermate controls (in g: 0.49 ± 0.02 for C57Bl6, 0.52 ± 0.06 for WT AMPKα1, 0.41 ± 0.05 for AMPKα1−/−, 0.45 ± 0.05 for WT AMPKα2 and 0.42 ± 0.03 for AMPKα2−/− mice). As shown in Fig. 3A, AICAR dose-dependently relaxed preconstricted AMPKα1+/+ aortic rings (ANOVA, P < 0.001, n = 5). In order to ensure that the relaxation effect of AICAR was not due to an adenosine like effect, we tested the effects of two adenosine receptor antagonists, SPT (10 μm) plus DPSX (30 nm), on the AICAR-dependent relaxation. The relaxation induced by AICAR (0.1, 1, 3 mm) was not altered by preincubation of the rings with SPT + DPSX (Fig. 3A). We then tested the ability of increasing concentrations of adenosine to relax aortic rings. The addition of 100 μm adenosine had no significant effect on the contraction induced by phenylephrine (tension: 0.896 ± 0.059 g without versus 0.914 ± 0.107 g with 100 μm adenosine, n = 5). Accordingly, the phenylephrine-induced contraction was not altered by 100 μm adenosine in the presence of SPT + DPSX (tension: 0.918 ± 0.085 g without versus 0.918 ± 0.118 g with adenosine + SPT + DPSX). This shows that the AICAR effects were not due to a non-specific adenosine receptor activation. As previously observed in C57Bl6 mice, AICAR-induced vasorelaxation was also not affected by the absence of endothelium or by treatment with 30 μm l-NMMA. Taken together, these results show that the aortic vasorelaxation induced by AICAR was neither endothelium nor NO dependent in AMPKα1+/+ mice (Fig. 3A).
Figure 3.
Representative tension traces (left) and mean values (right) showing the effects of AICAR (0.1, 1, 3 mM) on isolated AMPKα1+/+ (A) and AMPKα1−/− (B) mouse aortic rings after phenylephrine-induced contraction, in the absence or in the presence of SPT (10 μM) plus DPSX (30 nM), L-NMMA (30 μM), or after removal of the endothelium Results are expressed as means ± s.e.m. of 4–6 mice aorta. *P < 0.05, **P < 0.01 versus control conditions.
When tested on AMPKα1−/− mouse aorta, AICAR did not relax preconstricted aortic rings, suggesting that AICAR relaxes mouse aortic rings via AMPKα1 subunit activation (Fig. 3B). These effects were not associated with a difference in phenylephrine-induced aortic preconstriction, as stated above. Moreover, the absence of any AICAR effect was also observed after treatment with 10 μm SPT plus 30 nm DPSX. In the same tissues, removal of endothelium and l-NMMA ring incubation revealed a small vasorelaxing effect for 3 mm AICAR (29.3%± 6.0%. and 21.2%± 7.3%, respectively, P < 0.05 and P < 0.01 versus controls; Fig. 3B).
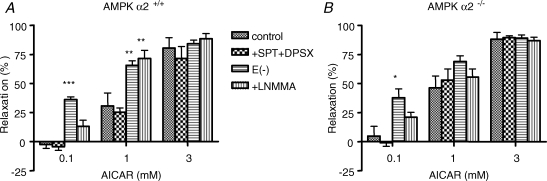
To further explore the involvement of each isoform of the AMPK catalytic subunits on AICAR-induced aortic relaxation, the same experiments were conducted on AMPKα2−/− and littermate AMPKα2+/+ mice. As shown in Fig. 4A, AICAR induced concentration-dependent vasorelaxation of preconstricted AMPKα2+/+ aortic rings (ANOVA, P < 0.001, n = 5), although the sensitivity to AICAR was reduced in these mice since there was almost no relaxation with 0.1 mm AICAR. These relaxing effects were similar to those in AMPKα1+/+ mice, in terms of the delay of action and time of stabilization. Maximal relaxation was obtained with 3 mm AICAR (80.6%± 8.9% in AMPK2+/+ aortas; Fig. 4A). The two adenosine receptor antagonists, 10 μm SPT plus 30 nm DPSX, did not modify these effects. As shown in Fig. 4A, AICAR-induced aortic vasorelaxation was significantly increased in the presence of 30 μm l-NMMA for 1 mm AICAR (P < 0.01), and in the absence of endothelium for 0.1 mm and 1 mm AICAR (P < 0.001 and P < 0.01, respectively). In AMPKα2−/− mice, AICAR also induced a concentration-dependent vasorelaxation (ANOVA, P < 0.001, Emax = 88.2%± 5.7%, Fig. 4B) that was not different from the one observed in their littermate controls, again indicating that the α2 subunit is not implicated in the vasorelaxation induced by AICAR. In this group, the relaxing effects of AICAR were not altered by preincubation of the rings with the two adenosine receptor antagonists, 10 μm SPT plus 30 nm DPSX, showing that this relaxation was not associated with adenosine receptor activation (Fig. 4B). Furthermore, neither the absence of endothelium nor 30 μm l-NMMA treatment reduced the AICAR-induced relaxation.
Figure 4.
Mean values of AICAR (0.1, 1, 3 mM)-induced relaxation of preconstricted AMPK α2+/+ (A) and AMPKα2−/− mouse (B) aortic rings under control conditions, in the presence of SPT (10 μM) plus DPSX (30 nM), L-NMMA (30 μM), or after removal of the endothelium Results are expressed as means ± s.e.m. of 4–6 mice aorta. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control conditions.
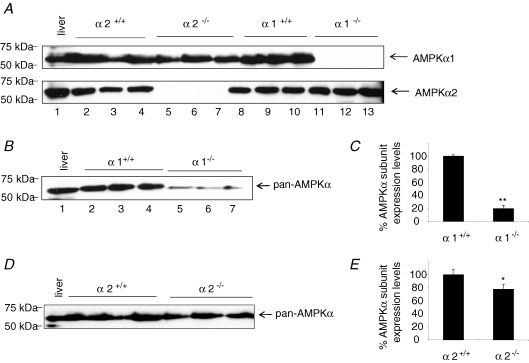
In an attempt to identify the reason for the AMPK isoform specificity of the AICAR response, we determined the expression of the AMPK catalytic subunits in denuded aorta (Fig. 5). As expected, AMPKα1 was present in WT and AMPKα2−/− aorta and absent in AMPKα1−/− aorta, thus showing that endothelium-free mouse aorta expresses the α1 subunit (Fig. 5A). Similarly, AMPKα2 was observed in WT and AMPKα1−/− aorta but not in AMPKα2−/− aorta, showing that α2 subunit is also expressed in vascular smooth muscle. To determine the relative expression of AMPKα2 and AMPKα1 subunits, we performed immunoblots on denuded aorta from AMPKα1+/+ and AMPKα1−/− animals on the one hand, from AMPKα2+/+ and AMPKα2−/− animals on the other hand, using anti-pan-AMPKα antibody, which recognizes both α1 and α2 subunits of AMPK (Fig. 5B and D). In Fig. 5B, the 63 kDa labelled protein band corresponds either to both α1 and α2 subunits in AMPKα1+/+ aorta protein samples, or to remaining α2 subunit in AMPKα1−/− aorta protein samples. Quantification of signal intensity showed that α2 subunit expression levels in AMPKα1−/− denuded aorta accounted only for 20% of the total (α1 and α2) AMPK catalytic subunit expression (Fig. 5C). In Fig. 5D, the 63 kDa labelled protein band corresponds either to both α1 and α2 subunits in AMPKα2+/+ aorta, or to remaining α1 subunit in AMPKα2−/− aorta. Quantification of signal intensity showed that α1 subunit expression levels in AMPKα2−/− denuded aorta accounted for 80% of the total (α1 and α2) AMPK catalytic subunit expression (Fig. 5E). Taken together, these data clearly show that α1 subunit of AMPK is the predominant form expressed in denuded mouse aorta, confirming the data obtained previously with porcine carotid arteries (Rubin et al. 2005).
Figure 5.
AMPK catalytic subunit isoforms in mouse-denuded aorta A, immunoblots showing expression pattern for AMPKα1 (upper blot) and AMPKα2 (lower blot) subunit levels. Lane 1, mouse liver (used as control); lanes 2–4, mouse AMPKα2+/+ aorta; lanes 5–7, mouse AMPKα2−/− aorta; lanes 8–10, mouse AMPKα1+/+ aorta; lanes 11–13, mouse AMPKα1−/− aorta. Twenty-five micrograms of protein from WT, AMPKα1−/− and AMPKα2−/− mouse denuded aortic rings, were loaded in each lane. B, AMPKα subunit expression in AMPKα1+/+ and AMPKα1−/− denuded aorta detected by anti-pan-AMPKα antibody. Lane 1, mouse liver (used as control); lanes 2–4, mouse AMPKα1+/+ aorta; lanes 5–7, mouse AMPKα1−/− aorta. C, quantification of AMPKα subunit expression levels in AMPKα1+/+ and AMPKα1−/− aorta (n = 5 different animals in each group). AMPKα expression has been normalized to the value observed in AMPKα1+/+ aorta. D, AMPKα subunit expression in AMPKα2+/+ and AMPKα2−/− denuded aorta detected by anti-pan-AMPKα antibody. Lane 1, mouse liver (used as control); lanes 2–4, mouse AMPKα2+/+ aorta; lanes 5–7, mouse AMPKα2−/− aorta. E, quantification of AMPKα subunit expression levels in AMPKα2+/+ and AMPKα2−/− aorta (n = 5 different animals in each group). AMPKα expression has been normalized to the value observed in AMPKα2+/+ aorta. *P < 0.05, **P < 0.01.
Discussion
We investigated the potential vasorelaxing capacity of AMPK in mouse aortic rings. In this study we have shown that (1) AICAR, a pharmacological activator of AMPK, induces a dose-dependent relaxation of mouse aortic rings. (2) This effect is associated with AMPK phosphorylation. (3) It is independent of adenosine receptors. (4) It is also independent of endothelium and NOS activation. (5) The vasorelaxing effects of AICAR are completely abolished in AMPKα1 deficient mice but not in AMPKα2 deficient mice, showing that AICAR-induced aortic vasorelaxation depends on the AMPKα1 catalytic subunit.
In aorta preconstricted with phenylephrine, AICAR dose-dependently induced vasorelaxation. This relaxation was slow but near-complete with 3 mm AICAR, and it was obtained in the four strains of mice that expressed the α1 AMPK subunit. The AICAR concentration needed for maximal relaxation was able to cause AMPK phosphorylation and was in a range already known to activate AMPK in different tissues (Morrow et al. 2003; Nagata et al. 2003). These results are in contrast to a study on porcine carotid rings prestimulated with endothelin-1, which exhibited only minor (≈12%) relaxation in the presence of 2 mm AICAR, and without AMPK activation (Rubin et al. 2005). The same study found that metabolic challenge induced 100% relaxation associated with AMPK activation. The reason why AICAR failed to activate AMPK in porcine carotid arteries in contrast to mouse aorta could be due to species and/or vascular bed differences. Normally, AICAR enters cells and is metabolized to ZMP, an intermediate in the pathway of de novo purine synthesis. ZMP can be further metabolized, suggesting that its accumulation will depend on the balance between synthesis and degradation rates, which may differ between tissues.
Because AICAR is a nucleoside, we investigated whether AICAR or its metabolites exhibit adenosine like effects. It was recently shown in HUVEC that adenosine can by itself activate AMPK phosphorylation via an adenosine receptor-independent pathway involving transport of adenosine across the cell membrane and its further conversion to AMP (da Silva et al. 2006). We found that, adenosine did not exhibit significant vasorelaxing effects in mice aortas. Moreover, we used a combination of adenosine receptor inhibitors: 10 μm SPT, which is a non-selective adenosine receptor antagonist known to inhibit adenosine-induced vasorelaxation (Lewis et al. 1994), and 30 nm DPSX, which is a more selective A1 receptor antagonist (Daly et al. 1985). In the presence of these adenosine receptor antagonists, AICAR was still able to relax precontracted aorta. This demonstrates that the effects of AICAR are independent of adenosine signalling, strongly supporting the idea that AMPK mediates AICAR-induced vasorelaxation in mouse aorta.
The endothelium regulates vascular tone through the synthesis and release of vasoactive compounds that mediate relaxation as well as contraction of vessels. The main relaxing factor is NO derived from endothelial NO synthase. It has now been established that AMPK is able to phosphorylate and thereby activate eNOS, increasing NO production.
Surprisingly, when aortic rings were freed from endothelium by gentle rubbing, AICAR still induced vasorelaxation, suggesting that its effects were not mediated by the endothelium. Moreover, preincubation of aorta with l-NMMA, an inhibitor of NO synthase, failed to block AICAR-induced vasorelaxation, suggesting that neither endothelial nor smooth muscle NOS were involved in the vasorelaxing effects of AICAR. Interestingly, the agent metformin, which acts at least in part through activation of AMPK (Zou et al. 2004; Davis et al. 2006), also relaxed endothelium-denuded or l-NAME-treated rat aortic rings precontracted with phenylephrine (Majithiya & Balaraman, 2006). Taken together these results suggest that AMPK can induce vasorelaxation in an endothelium- and NOS-independent manner, by a direct action on smooth muscle cells.
To confirm the involvement of AMPK in the vasorelaxing effects of AICAR, and to investigate isoform specificity, experiments were repeated in AMPKα1 or α2 knock-out mice. The vasorelaxing effects of AICAR were completely abolished in α1 but not in α2 deficient mice. Others have shown that both metformin and AICAR significantly increase eNOS Ser1179 phosphorylation in wild-type but not in AMPKα1−/− treated mice, indicating that AMPKα1 is the main isoform in mouse aorta (Davis et al. 2006). By contrast, another study showed that AICAR increased both α1 and α2 associated AMPK activity in pulmonary artery smooth muscle cells, although it increased AMPKα1 activity to a greater extent than AMPKα2 activity (Evans et al. 2005). In our study, immunoblots of endothelium-freed aortas revealed that the AMPKα1 subunit is present in WT and AMPKα2−/− mice, while the AMPKα2 isoform is expressed at a lower level. Consistent with this, it was previously shown that there was little if any AMPKα2 subunit in carotid smooth muscle (Rubin et al. 2005). Thus, the relaxing effects of AICAR on aortic rings of AMPKα2−/− mice seem to be due to the presence of AMPKα1 subunit in this tissue. The small potentiation of AICAR-induced relaxation observed in denuded aorta or in the presence of l-NMMA seems to depend on the mouse strain, as it was absent in α1+/+, slight in C57Bl6 and more important in α2+/+ mice. Moreover, the effect disappeared in α2−/− mice while it was revealed in α1−/− mice only for high AICAR concentrations. This would suggest a slight AMPKα2-dependent endothelium-mediated vasoconstricting effect. Taken together, these results show that AMPK is able to induce aortic ring relaxation through direct activation of AMPKα1 in smooth muscle cells. Smooth muscle contraction and relaxation depend on the phosphorylation state of the smooth muscle myosin regulatory light chain (MLC). Contraction is initiated by Ca2+-dependent activation of the MLC kinase, and by Rho kinase- and PKC-dependent inhibition of MLC phosphatase (MLCP). Relaxation of smooth muscle cell depends on calcium withdrawal, calcium desensitization, MLCP activation and/or MLCK inhibition (Somlyo & Somlyo, 2003). For example, hypoxia, which was shown to involve AMPK phosphorylation and activation (Rubin et al. 2005), can induce vasorelaxation by calcium-dependent mechanisms involving regulation of potassium channels (Thorne et al. 2004), or by calcium-independent mechanism involving activation of MLCP, and dephosphorylation of the Rho-kinase phosphorylated myosin binding subunit of MLCP (MYPT1) (Wardle et al. 2006). More work is needed to establish whether AICAR-induced activation of AMPK can interfere with calcium homeostasis, or the phosphorylation/dephosphorylation cascade of MLC, how and at which step.
In summary, the present results show that activation of smooth muscle cell AMPKα1 subunit but not α2 induces a dose-dependent vasorelaxation of mouse aorta that is independent of NO and the presence of endothelium. As a metabolic sensor, vascular AMPK could be involved in the metabolic regulation of blood flow. Hypoxia increases the AMP/ATP ratio in pulmonary artery smooth muscle cells and induces a twofold increase in AMPK activity (Evans et al. 2005). Metabolic challenge also rapidly and reversibly activates AMPKα1 in porcine carotid arteries (Rubin et al. 2005), suggesting that AMPK activation may participate in the regulation of blood pressure. Indeed, AICAR treatment decreased blood pressure in rats displaying features of the insulin resistance syndrome (Buhl et al. 2002). AMPK activation may be an additional mechanism by which hypoxia or metabolic challenge can induce vasorelaxation of large vessels, thereby increasing oxygen availability in peripheral tissues. AMPK thus appears as a new player in the complex signalling pathways that regulate vascular tone.
Acknowledgments
We thank Dr Rodolphe Fischmeister, Prof Bernard Lacour and Dr Sophie Vaulont for continuous support, James Wilding for carefully reading the manuscript and for revising the English, Anthony Lucas for technical assistance and Valérie Domegrgve-Dupont for animal care. The authors are grateful to Grahame Hardie (University of Dundee, Scotland, UK) for providing anti-AMPKα1 and -AMPKα2 antibodies. R.V.-C. is supported by the Centre National de la Recherche Scientifique and Y.A. by Fondation pour la Recherche Médicale. This work was supported by the Association Française contre les Myopathies, Fondation de France and the European Union Contracts nos LSHM-CT-2005-018833/EUGeneHeart and LSHM-CT-2004-005272/exgenesis. We thank Prof Vincent Richard for helpful discussion.
References
- Buhl ES, Jessen N, Pold R, Ledet T, Flyvbjerg A, Pedersen SB, Pedersen O, Schmitz O, Lund S. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett W, Shamim MT, Butts-Lamb P, Waters J. 1,3-Dialkyl-8-(p-sulfophenyl)xanthines: potent water-soluble antagonists for A1- and A2-adenosine receptors. J Med Chem. 1985;28:487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- da Silva CG, Jarzyna R, Specht A, Kaczmarek E. Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters. Circ Res. 2006;98:e39–47. doi: 10.1161/01.RES.0000215436.92414.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- Evans AM, Hardie DG, Galione A, Peers C, Kumar P, Wyatt CN. AMP-activated protein kinase couples mitochondrial inhibition by hypoxia to cell-specific Ca2+ signalling mechanisms in oxygen-sensing cells. Novartis Found Symp. 2006;272:234–252. [PubMed] [Google Scholar]
- Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the α2 but not α1 5′AMP-activated protein kinase isoform abolishes AICAR- but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- Lewis CD, Hourani SM, Long CJ, Collis MG. Characterization of adenosine receptors in the rat isolated aorta. Gen Pharmacol. 1994;25:1381–1387. doi: 10.1016/0306-3623(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Majithiya JB, Balaraman R. Metformin reduces blood pressure and restores endothelial function in aorta of streptozotocin-induced diabetic rats. Life Sci. 2006;78:2615–2624. doi: 10.1016/j.lfs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- Sambandam N, Lopaschuk GD. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Prog Lipid Res. 2003;42:238–256. doi: 10.1016/s0163-7827(02)00065-6. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Thorne GD, Ishida Y, Paul RJ. Hypoxic vasorelaxation: Ca2+-dependent and Ca2+-independent mechanisms. Cell Calcium. 2004;36:201–208. doi: 10.1016/j.ceca.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle RL, Gu M, Ishida Y, Paul RJ. Ca2+-desensitizing hypoxic vasorelaxation: pivotal role for the myosin binding subunit of myosin phosphatase (MYPT1) in porcine coronary artery. J Physiol. 2006;572:259–267. doi: 10.1113/jphysiol.2005.104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, 4th, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]