Abstract
The onset of motor learning in rats coincides with exclusive expression of GABAA receptors containing α6 and δ subunits in the granule neurons of the cerebellum. This development temporally correlates with the presence of a spontaneously active chloride current through α6-containing GABAA receptors, known as tonic inhibition. Here we report that the coexpression of α6, β2, and δ subunits produced receptor–channels which possessed two distinct and separable states of agonist affinity, one exhibiting micromolar and the other nanomolar affinities for GABA. The high-affinity state was associated with a significant level of spontaneous channel activity. Increasing the level of expression or the ratio of β2 to α6 and δ subunits increased the prevalence of the high-affinity state. Comparative studies of α6β2δ, α1β2δ, α6β2γ2, α1β2γ2 and α4β2δ receptors under equivalent levels of expression demonstrated that the significant level of spontaneous channel activity is uniquely attributable to α6β2δ receptors. The pharmacology of spontaneous channel activity arising from α6β2δ receptor expression corresponded to that of tonic inhibition. For example, GABAA receptor antagonists, including furosemide, blocked the spontaneous current. Further, the neuroactive steroid 5α-THDOC and classical glycine receptor agonists β-alanine and taurine directly activated α6β2δ receptors with high potency. Specific mutation within the GABA-dependent activation domain (βY157F) impaired both low- and high-affinity components of GABA agonist activity in α6βY157Fδ receptors, but did not attenuate the spontaneous current. In comparison, a mutation located between the second and third transmembrane segments of the δ subunit (δR287M) significantly diminished the nanomolar component and the spontaneous activity. The possibility that the high affinity state of the α6β2δ receptor modulates the granule neuron activity as well as potential mechanisms affecting its expression are discussed.
Ligand-gated ion channels comprise a large family of membrane-embedded receptors which play a central role in neuronal transmission. The binding of a neurotransmitter to the receptor domain of this class of membrane proteins opens an integrated ion channel, allowing selected ions to permeate. Such receptor–channels are either excitatory or inhibitory in nature, modulating the frequency of action potential initiation. The most widely expressed inhibitory class of ligand-gated ion channels are γ-aminobutyric acid type A (GABAA) receptors. Assembled from a diverse range of subunits termed α(1-6),β(1-3), γ(1-3), δ, π and ɛ, each subtype of GABAA receptor exhibits unique kinetics, agonist affinity and pharmacology (Hevers & Luddens, 1998; Sieghart & Sperk, 2002; Wallner et al. 2003; Hanchar et al. 2005).
Cerebellar granule neurons are central to the control of information flow through the cerebellar cortex and are postulated to play a fundamental role in motor learning activity (Marr, 1969; Tyrrell & Willshaw, 1992; Thompson & Stephenson, 1994; Mellor et al. 1998). These neurons evince unique anatomical characteristics and GABAA subunit expression that temporally coincide with the learning and development of motor skills. During the first postnatal week in rats, granule cells start migrating towards the inner granule layer, where they progressively begin to express both α6 and δ GABAA subunits (Laurie et al. 1992; Persohn et al. 1992; Wisden et al. 1996). The α6 subunit mRNA becomes detectable approximately 1 week after birth and is followed by the α6-dependent expression of the δ subunit (Laurie et al. 1992; Jones et al. 1997; Nusser et al. 1999). The expression of α6 and δ subunits gradually increases throughout postnatal development, reaching their highest levels in adulthood (Laurie et al. 1992; Persohn et al. 1992; Jechlinger et al. 1998). This exclusive expression paradigm makes receptors containing α6 and δ subunits the predominant GABAA receptor subtype expressed within cerebellar granule neurons in adulthood (Quirk et al. 1994; Nusser et al. 1999; Tretter et al. 2001). The temporal expression of α6 and δ subunits within the granule neurons correlates with the development of a spontaneous chloride current known as tonic inhibition (Kaneda et al. 1995; Brickley et al. 1996; Wall & Usowicz, 1997; Hamann et al. 2002). The spillover or diffusion of GABA from synaptic events is thought to activate the α6-containing GABAA receptors resulting in a tonic inhibition (Isaacson et al. 1993; Rossi & Hamann, 1998; Hamann et al. 2002; Mody & Pearce, 2004; Semyanov et al. 2004; Farrant & Nusser, 2005). Studies in animal models are gradually establishing the importance of tonic inhibition in the regulation of motor activity (Thompson et al. 1998; Chiu et al. 2005). For example, GABA transporter type 1 (GAT1) knockout mice display various neuronal deficits, including tremor and ataxia, that may arise due to a significant increase in the level of tonic chloride conductance within the cerebellar granule neurons (Chiu et al. 2005).
To simulate the temporal coexpression of α6 and δ subunits within granule neurons, we investigated the characteristics of α6βδ receptors under different levels and conditions of expression. The structure–function relationship of α6β2δ receptors was further examined using mutations of conserved residues within the β2 or δ subunit.
Methods
Oocyte preparation
The Xenopus laevis frogs were anaesthetized by bathing in a solution containing 0.1% MS222 (Tricaine methane sulphonate, Sigma-Alderich, St Louis, MO, USA). Before ovariectomy, the state of anaesthesia was assessed by pinching the toe of the frog. After surgery, the frog was killed by decapitation according to a protocol approved by the Institutional Animal Care and Use Committee. Oocytes were placed in a calcium-free oocyte Ringer solution (calcium-free OR2; 83.5 mm NaCl, 2.5 mm KCl, 1 mm MgCl2, 1 mm Na2HPO4 and 5 mm Hepes, pH 7.5) plus 0.3% collagenase A (Roche Applied Science, Indianapolis, IN, USA) for approximately 1 h. Stage V and VI oocytes were isolated and maintained by incubating in OR2 (82.5 mm NaCl, 1 mm CaCl2, 2.5 mm KCl, 1 mm MgCl2, 2 mm sodium pyruvate, 1 mm Na2HPO4, 50 U ml−1 penicillin, 50 U ml−1 streptomycin and 5 mm Hepes, pH 7.5) with 2% horse serum at 18°C.
Quantification of complementary RNAs (cRNA) and oocyte injections
The procedure for in vitro transcription of cRNA have been previously described (Walters et al. 2000). The quality of cRNA was determined by electrophoresis on a 1% formaldehyde-containing agarose gel. cRNA concentrations were measured spectrophotometrically. For most experiments, we tested two preparations of cRNAs for each subunit.
Micropipettes for injecting cRNA were fabricated using a Sutter P87 horizontal puller (Sutter Instruments Co., Novato, CA, USA) and, to ensure uniformity of size, the tip of each micropipette was cut with microscissors under 45× magnification next to a control-cut needle. Using a Picospritzer II (General Valve Corporation, Fairfield, NJ, USA), cRNA subunits reconstituted in diethylpyrocarbonate-treated water were injected into Xenopus laevis oocytes at a ratio of 1α : 1β2 : 1.8(γ2 or δ). The cRNA combinations were injected in amounts of 1.5–3 ng, 5–7 ng, and 8–12 ng per oocyte to produce, respectively, low, intermediate and high levels of expression. For comparison of the different GABAA subtypes, we coinjected 5–7 ng of cRNA (intermediate expression level) for each combination (α6β2δ, α1β2δ, α6β2γ2, α1β2γ2 and α4β2δ), using two sets of cRNA-mixture preparations and two batches of oocytes.
Drug preparations
Forusemide, bicuculline and picrotoxinin were purchased from Sigma-Alderich Corp. (St Louis, MO, USA). Allotetrahydrodeoxycorticosterone (5α-THODC) was obtained from Steraloids, Inc (Newport, RI, USA). Forusemide, bicuculline, picrotoxinin and 5α-THODC were dissolved in dimethylsulfoxide at their respective stock solution concentrations of 100, 40, 100 and 20 mm. The test solutions were made by diluting the stock solutions in the recording OR2 solution (mm: NaCl, 82.5; KCl, 2.5; Hepes, 5; CaCl2, 1; MgCl2, 1; pH 7.5). The highest concentration of the vehicle solution (0.5% of DMSO) did not significantly alter the level of α6β2δ recptors activity.
Electrophysiology
Three to four days after injection, oocytes were placed on a mesh within a small perfusing volume chamber (∼75 μl), with t1/2 and clearance times of approximately 3 and 10 s, respectively. For complete description of the drug application system see Walters et al. (2000).
We used a two-electrode voltage-clamp amplifier (Turbo TEC-05 npi, Adams and List, Westbury, NY, USA) to record currents in response to the application of drugs. Recording microelectrodes were fabricated with a Narishige PP-83 puller (Narishige, Japan) and filled with 3 m KCl. We used electrodes with input resistances of 0.7–1.6 MΩ. Membrane potential was clamped to −70 mV. Data were visualized on a TA-240 chart recorder (Gould Instrument System, Valley View, CA, USA) during the experiments and stored online using Pulse Fit.
Measurement of the spontaneous current and statistical analysis
High concentrations of GABA (mm) do not evoke a current in mock-injected oocytes indicating an absence of endogenous GABAA receptors. Upon impaling an oocyte with a pair of electrodes, the oocyte initially displayed a leak current (holding potential = −70 mV). This leak current did not reverse at −30 mV (the predicted reversal potential for chloride in oocytes under these conditions) and within 4–5 min reduced to < 30 nA. If waiting time following the impalement was longer (∼10–15 min), the leak current would have gradually decreased to a value of a few nanoamps, suggesting that the initial random leak may result from an incomplete sealing of the membrane around the electrode. In experiments where the time allocated for recording from each oocyte were short (∼4–5 min, due to large number of oocytes tested in one day), the averaged control leak current measured from the mock-injected oocytes was subtracted from the data and such corrections are noted in Results. However, in most experiments, the wait-time was more than 10 min and thus the mock-injected oocytes did not show any significant leak current. It is also important to note that spontaneous currents arising from α6β2δ receptors (in most experiments) are at least an order of magnitude higher than any control leak current recorded (the range of averages of leak current in control cells in different experiments was 14–23 nA where the wait time before measurement was < 5 min).
The analysis of variance (ANOVA one-way) and Fisher's LSD multiple comparison test were used for statistical investigation. All statistical calculations are presented as means ± standard error of the mean.
Data analysis
The EC50 and Hill coefficients for the agonists were estimated by fitting the data from concentration–response relationships to the Hill equation according to the following formula (Sigma plot 2000 or Origin 6.0):
Alternatively, the data were fitted with a sum of two Hill equations (Origin, 6.0)
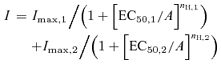 |
where I is the peak current at a given concentration of agonist (A), Imax is the maximum current, EC50 is the concentration of agonist yielding a half-maximal current, and nH is the Hill coefficient.
In the high or low expression condition for the wild-type α6β2δ receptors, where one component dominates, the fit of the data points to the sum of two Hill equations using Origin software does not give a satisfactory result (confidence > 0.95). Even in cases when the fitting was successful, the obtained double fit for most low and high expression experiments did not closely follow the experimental data point obtained at high or low concentration ranges, respectively. We postulate that under low or high expression conditions, the apparent desensitization/inactivation of the high affinity component may hinder reliable fitting with the sum of two Hill equations.
To quantify the inhibitory effect of antagonists, the data were fitted to the following equation
where I is the peak current at a given concentration of antagonist (An), Imax is the level of spontaneous current in the absence of antagonist, IC50 is the concentration of antagonist inhibiting half of the spontaneous current, and nH is the slope.
Results
α6β2δ receptors exhibit distinct agonist affinity states
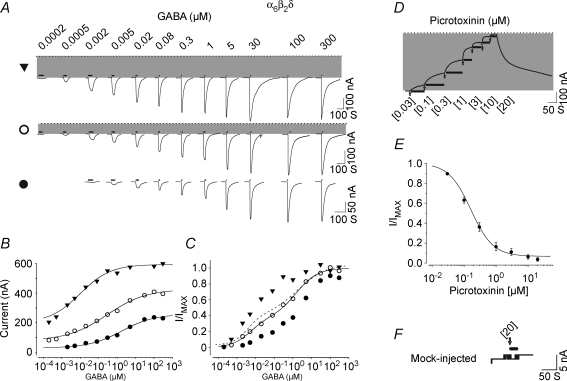
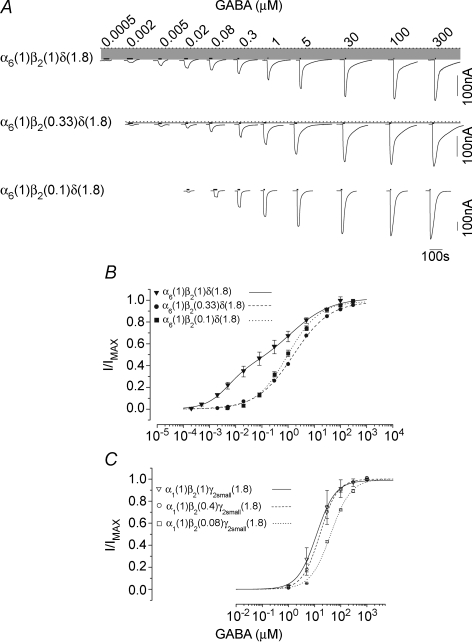
cRNA of rat wild-type α6, β2 and δ subunits (Bernard et al. 1998) was injected into Xenopus laevis oocytes in increments of 1.5–3, 5–7 and 8–12 ng per oocyte to produce low, intermediate and high levels of expression, respectively (at a ratio of 1α6 : 1β2 : 1.8δ). Three to four days after injection, GABA-activated currents were recorded using a GABA concentration range from 0.0002 to 300 μm. Figure 1 shows the current traces and GABA concentration–response relationships for three representative oocytes with low, intermediate and high levels of expression of α6β2δ receptors. At low expression levels (Fig. 1, filled circles; holding potential −70 mV), the GABA concentration–response relationship yielded an EC50 (a concentration eliciting half-maximal current) of 1.77 μm and a Hill coefficient (nH) of 0.49 (GABA maximal current; GABA Imax = 206 nA). At intermediate expression levels (Fig. 1, open circles), a spontaneous current became apparent (shaded area; 80 nA; GABA Imax = 328 nA) which reversed at −33 mV (reversal potential = −28.70 ± 1.64 mV, range −22 to −35 mV; n = 15) in accordance with the predicted chloride reversal potential (Taleb & Betz, 1994). Fitting of the data points from this oocyte with a single Hill equation yielded an EC50 of 0.28 μm and an nH of 0.41, representing a more than 5-fold increase in GABA sensitivity as compared to the low expression data set. At high expression levels, the spontaneous chloride current increased to over 200 nA (Fig. 1, filled triangles; 269 ± 61 nA, n = 15). The presence of a larger spontaneous current (200 nA) was concomitant with a further increase in overall GABA sensitivity (EC50 = 0.01 μm, nH = 0.53) and desensitization at GABA concentrations greater than the EC50 value (the group data for GABA EC50, nH and maximum values for the low, intermediate and high expression are given in Table 1). Collectively, the range of GABA EC50 values derived from different levels of expression ranged from 0.01 to 4.86 μm, with an nH of ∼0.5–0.7 (n = 24).
Figure 1.
α6β2δ receptors exhibit spontaneous activity and two separable state of agonist affinities A, representative GABA current traces for high (top), intermediate (middle) and low (bottom) expression conditions with oocytes clamped at −70 mV. The dotted line indicates the zero-current level; the shaded area represents the spontaneous activity. The thick lines above the current traces represent the duration of GABA application. The duration of agonist application decreased with increasing concentration of agonist since the currents reached steady state (peaked) more rapidly at higher concentrations of agonist. B, plot of GABA concentration–response relationships including the spontaneous currents. All data were fitted with a single Hill equation. C, concentration–response relationship of the normalized GABA currents. The EC50, nH and maxima parameters for the group data for high, intermediate and low expression conditions are shown in Table 1. The continuous line shows the plot of the fit of a sum of two Hill equations to the data points from an oocyte with intermediate expression level demonstrating the presence of two components with different sensitivities to GABA. The dashed line shows the overall plot of the fit (group data) of sum of two Hill equations to the GABA concentration–response data points for the α6β2δ receptor at median expression. D, a representative current trace for consecutive application of 0.03–20 μm picrotoxinin on an oocyte with high expression of α6β2δ recptor. The thick lines below the current traces represent the duration of antagonist application. The arrow indicates the start of the picrotoxinin application at the concentration shown below it. E, the concentration–response relationship for picrotoxinin block of the spontaneous current form α6β2δ receptor. Picrotoxinin inhibited greater than 90% of the spontaneous current with an IC50 of approximately 0.2 μm.F, the effect of 20 μm picrotoxinin application on the leak current (20 nA) from a mock-injected oocyte (see methods).
Table 1.
Parameters obtained from fitting the Hill equation to GABA, β-alanine, taurine, I4AA, and 5α-THDOC data
| GABAAR subtype | EC50 (μm) | nH | Imax (nA) | n |
|---|---|---|---|---|
| GABA-dependent activation | ||||
| α6β2(1)δ | 7 | |||
| High-affinity component | 0.005 ± 0.001 | 1.16 ± 0.11 | 259.50 ± 73.96 | |
| Low-affinity component | 2.29 ± 0.85 | 0.56 ± 0.05 | 479.10 ± 123.94 | |
| Low expression | 2.62 ± 0.39 | 0.63 ± 0.02 | 129.06 ± 10.72 | 9 |
| Intermediate expression | 0.46 ± 0.12 | 0.54 ± 0.02 | 317.39 ± 56.43 | 7 |
| High expression | 0.04 ± 0.009 | 0.65 ± 0.04 | 690.60 ± 80.23 | 11 |
| α6β2(0.3)δ | 1.52 ± 0.38 | 0.63 ± 0.03 | 362.80 ± 97.95 | 6 |
| α6β2(0.1)δ | 1.25 ± 0.21 | 0.74 ± 0.02 | 321.67 ± 50.62 | 10 |
| α1β2(1)γ2S | 2.87 ± 1.20 | 1.33 ± 0.10 | 2287.43 ± 237.76 | 7 |
| α1β2(0.4)γ2S | 10.61 ± 3.27 | 1.22 ± 0.10 | 2270.33 ± 354.79 | 6 |
| α1β2(0.08)γ2S | 37.20 ± 4.58 | 1.26 ± 0.14 | 974.33 ± 497.32 | 3 |
| α1β2δ | 6.45 ± 0.85 | 1.14 ± 0.05 | 510.02 ± 80.43 | 9 |
| α6β2γ2S | 22.30 ± 1.98 | 0.88 ± 0.14 | 484.00 ± 122.45 | 4 |
| α4β2δ | 1.53 ± 0.14 | 0.85 ± 0.03 | 338.09 ± 52.61 | 11 |
| α6β2δR287M | 3.71 ± 0.94 | 0.69 ± 0.18 | 44.67 ± 2.33 | 3 |
| α6βY157Fδ | 13 | |||
| High-affinity component | 0.37 ± 0.05 | 0.97 ± 0.04 | 355.54 ± 80.52 | |
| High-affinity component | 104.65 ± 28.04 | 0.85 ± 0.14 | 389.23 ± 63.43 | |
| α6βY157F (0.1)δ | 53.27 ± 13.99 | 0.94 ± 0.14 | 161.33 ± 36.99 | 3 |
| I4AA-dependent activation | ||||
| α6β2δ | 4 | |||
| High-affinity component | 0.06 ± 0.01 | 0.82 ± 0.04 | 254.68 ± 52.02 | |
| High-affinity component | 202.25 ± 17.71 | 2.61 ± 0.44 | 183.41 ± 16.77 | |
| β-Alanine-dependent activation | ||||
| α6β2δ | 5 | |||
| High-affinity component | 1.16 ± 0.72 | 0.89 ± 0.12 | 160.61 ± 69.75 | |
| High-affinity component | 594.66 ± 142.86 | 0.67 ± 0.05 | 277.94 ± 64.02 | |
| Low expression | 412.14 ± 68.76 | 0.71 ± 0.03 | 302.74 ± 58.79 | 10 |
| Intermediate expression | 111.86 ± 13.72 | 0.55 ± 0.15 | 426.33 ± 166.71 | 3 |
| High expression | 2.56 ± 1.52 | 0.59 ± 0.16 | 945.00 ± 76.51 | 3 |
| Taurine-dependent activation | ||||
| α6β2δ | 3 | |||
| High-affinity component | 5.80 ± 0.84 | 1.03 ± 0.05 | 225.33 ± 43.42 | |
| High-affinity component | 806.67 ± 193.50 | 0.91 ± 0.29 | 205.00 ± 40.31 | |
| Low expression | 1817.58 ± 865.99 | 0.69 ± 0.06 | 145.40 ± 13.31 | 4 |
| Intermediate expression | 45.16 ± 25.57 | 0.55 ± 0.05 | 398.30 ± 63.92 | 3 |
| High expression | 6.03 ± 1.62 | 0.90 ± 0.10 | 607.63 ± 160.86 | 4 |
| 5α-THDOC-dependent activation | ||||
| α6β2δ | 0.89 ± 0.49 | 0.97 ± 0.06 | 550.67 ± 67.25 | 3 |
The shallow slope of these concentration–response relationships suggests the presence of a mixture of receptor–channels with differing agonist sensitivities (Kuhse et al. 1993; Amin & Weiss, 1996). Refitting the data from the oocyte from the intermediate expression group (open circles) with the sum of two Hill equations suggested two distinct affinity components within the nanomolar and micromolar ranges (EC50 = 0.0052 μm, nH = 0.87, and GABA Imax = 124 nA for the high-affinity component, and EC50 = 1.54 μm, nH = 0.76, GABA Imax = 202 nA for the low-affinity component; Fig. 1C). The mean EC50 and nH parameters derived for the two components at the intermediate expression level are presented in Table 1 (see also dashed line in Fig. 1C). The high-affinity component displayed a 400-fold greater apparent affinity for GABA than the low-affinity component (EC50 values of 0.005 versus 2.29 μm). The relative magnitude of the two components (high to low affinity) was 0.54 (259.50 to 479.10 nA, see Table 1).
Reversal potential measurements indicate that a chloride conductance underlies the spontaneous current but do not demonstrate that the current is mediated by α6β2δ receptors (since there are also endogenous chloride channels present within oocytes). Picrotoxinin, a specific pore blocker of GABAA receptors, was used to determine whether the spontaneous chloride current originates from α6β2δ receptor expression. Figure 1D and E shows the picrotoxinin-induced current traces and concentration–response relationship from an oocyte expressing high levels of α6β2δ receptor. Seven incremental concentrations of picrotoxinin (from 0.03 to 20 μm) were applied to oocytes expressing α6β2δ subunits to construct a concentration–response relationship. Concentrations were applied incrementally because picrotoxinin possesses a high-affinity binding component for the α6β2δ receptor that is resilient to complete wash out. Picrotoxinin blocked the spontaneous current arising from α6β2δ receptors with both high efficacy and potency. The IC50 value for the picrotoxinin action was approximately 0.2 μm, with picrotoxinin, at 20 μm, blocking more than 90% of the spontaneous current (for all parameters, see Table 2). Figure 1F shows a control in which picrotoxinin is added to a mock-injected oocyte displaying 20 nA of leak current. Picrotoxinin (20 μm) did not attenuate the control leak current. These experiments demonstrate that the spontaneous current observed following expression of α6, β2 and δ cRNAs originates from GABAA receptors.
Table 2.
Parameters obtained from fitting the Hill equation to data from Picrotoxinin-, Furosemide- and Zn2+-dependent block of the spontaneous current
| GABAAR subtype | IC50 (μm) | nH | Maximal inhibition | n |
|---|---|---|---|---|
| α6β2δ | ||||
| Picrotoxinin | 0.16 ± 0.02 | 1.26 ± 0.06 | 93.68 ± 2.29% | 6 |
| Furosemide | 12.31 ± 0.51 | 1.03 ± 0.05 | 75.82 ± 4.59% | 9 |
| Zn2+ | 1.42 ± 0.21 | 0.99 ± 0.08 | 56.77 ± 4.18% | 5 |
| α6β1δ | ||||
| Picrotoxinin | 0.28 ± 0.05 | 0.95 ± 0.06 | 86.06 ± 2.68% | 5 |
| α6β3δ | ||||
| Picrotoxinin | 0.28 ± 0.05 | 1.18 ± 0.09 | 93.04 ± 2.04% | 6 |
GABAA antagonists block the spontaneous activity arising from α6β2δ receptors
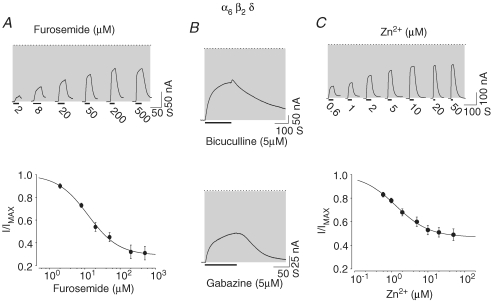
We examined the effects of several specific GABAA antagonists on the spontaneous current arising from α6β2δ receptors. Among the antagonists tested, furosemide proved to be a specific antagonist for GABAA receptors containing the α6 subunit (Korpi et al. 1995; Korpi & Luddens, 1997). Furosemide was added at 2, 8, 20, 50, 200 and 500 μm concentrations to oocytes expressing α6β2δ receptors. Figure 2A shows the corresponding current traces and the concentration–response relationship for inhibition of the spontaneous current. Furosemide inhibited 76% of the spontaneous current with an IC50 of 12.3 μm (see Table 2).
Figure 2.
Furosemide, bicuculline, gabazine and Zn2+ inhibit the spontaneous current arising from α6β2δ receptors A, furosemide blocked the spontaneous activity of α6β2δ receptors. Current traces and the concentration–response relationship for furosemide-dependent inhibition of the spontaneous current arising from an oocyte with a high level of expression of α6β2δ receptors. The dotted line indicates the zero-current level; the shaded area represents the spontaneous activity. The thick lines below the current traces represent the duration of antagonist application. B, bicuculline and gabazine inhibited the spontaneous activity of α6β2δ receptors. Current traces representing bicuculline (5 μm) and gabazine (5 μm) inhibitory action on the spontaneous activity. C, the representative current traces and concentration–response relationship for Zn2+ block of the spontaneous current arising from α6β2δ receptors.
Bicuculline and gabazine (SR95531) are specific competitive antagonists for GABAA receptors and 5 μm bicuculline or 5 μm gabazine blocked the GABA-independent component of the α6β2δ receptors current by 48 ± 5% and 29 ± 5%, respectively (n = 4; Fig. 2B), demonstrating that competitive antagonists of GABAA receptors attenuate the spontaneous activity arising within α6β2δ receptors.
Zinc inhibits GABAergic responses within neurons and is postulated to function as an endogenous modulator of ion channels in the CNS (Legendre & Westbrook, 1991; Smart, 1992; Dunne et al. 2002; Smart et al. 2004). We tested the effect of Zn2+ on the spontaneous current arising from α6β2δ receptor expression. Figure 2C shows representative current traces and the concentration–response relationship of the Zn2+-mediated inhibition of the spontaneous current (IC50 of ∼1.42 μm; Table 2).
Thus a range of established GABAA antagonists, including furosemide, bicuculline, gabazine, picrotoxinin and Zn2+ all inhibit spontaneous activity arising from the expression of α6β2δ receptors.
Expression of a functional receptor–channel requires α6, β2, and δ subunits
The presence of two components in the α6β2δ GABA concentration–response relationship suggests the coexistence of at least two distinct and separable populations of ion channels. We tested the capacity of α6 and β2, β2 and δ, and α6 and δ, as well as that of β2 alone to express ligand-gated ion channels by injecting these cRNA combinations into oocytes at quantities that yield a high level of expression for the α6β2δ receptor (8–12 ng per oocyte). Four days post-injection, oocytes were tested for the presence of spontaneous activity and GABA-dependent activity using GABA concentrations of up to 500 μm. These subunit combinations yielded neither functional receptor–channels, nor a spontaneous current (n = 46). At significantly greater quantities of cRNA (20–30 ng per oocyte), β2 or β2 and α6 yielded receptor–channels which displayed spontaneous channel activity. However, the resulting β2 or α6β2 receptors exhibited a markedly reduced GABA maximal current (< 30 nA for β2n = 15 and < 150 nA for α6β2n = 30). Moreover, even at expression levels of 20–30 ng of cRNA per oocyte (∼4-fold the quantities used for intermediate expression of α6β2δ), neither β2 nor α6β2 receptors produced the magnitude of spontaneous current activity observed in α6β2δ receptors (data not shown). Together these results suggest that neither the β2 nor the α6β2 receptors contribute significantly to the observed channel activity arising from the expression of α6β2δ receptors.
Spontaneous channel activity is a unique property of the α6β2δ receptor
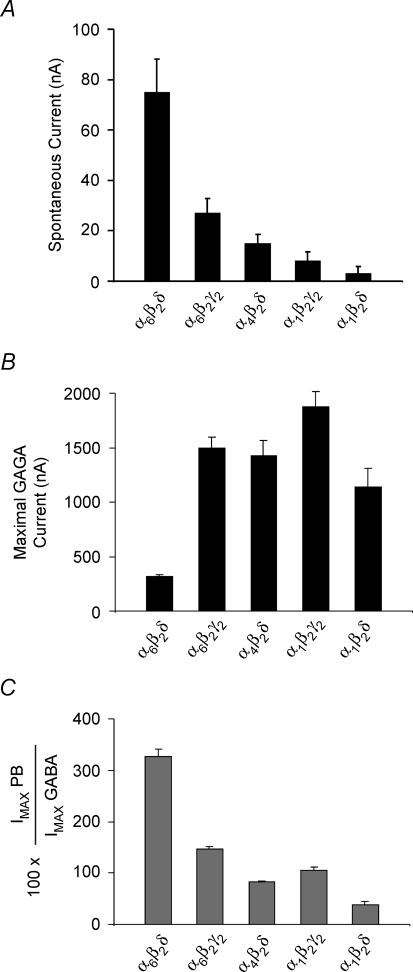
Using the intermediate expression protocol (5–7 ng of cRNA), we tested expression of α1β2γ2S(Short), α1β2δ, α4β2δ, α6β2γ2S and α6β2δ subunit combinations to determine if these receptors produced spontaneous channel activity. For each oocyte expressing a given subtype of GABAA receptor, we measured the magnitude of the spontaneous current as well as the GABA- and pentobarbital-induced maximal current 4 days post-injection. Figure 3A shows the background leak-subtracted magnitudes of spontaneous current for the aforementioned GABAA subunit combinations (see Methods and Table 3). The spontaneous current activity recorded from α6β2δ receptors was significantly higher than that for any other GABAA receptors (ANOVA one-way analysis F ratio = 15.76; P < 0.001). Fisher's LSD multiple comparison test also showed that the magnitudes of spontaneous currents arising from α6β2δ receptors were different from those of other GABAA receptors (P < 0.05). These experiments demonstrated that the significant level of spontaneous channel activity is a property unique to α6β2δ receptors.
Figure 3.
Comparison of the spontaneous currents, GABA-induced maxima and pentobarbital relative maxima to GABA for α6β2δ, α6β2γ, α4β2δ, α1β2γ, and α1β2δ receptors under equivalent expression conditions A, comparison of the spontaneous currents for α6β2δ, α6β2γ2, α4β2δ, α1β2γ2 and α1β2δ receptors under equivalent expression conditions. The α6β2δ receptor exhibited a significantly higher spontaneous current than did the other GABAA receptors. B, comparison of the GABA-induced maximal current for α6β2δ, α6β2γ2, α4β2δ, α1β2γ2 and α1β2δ receptors under equivalent expression conditions. GABA maximal current was determined from experiments in A using GABA concentrations 20–50 times the respective EC50 value. GABA had the lowest efficacy (maximal) for α6β2δ receptors. C, comparison of the relative maximal current of pentobarbital to GABA for the GABAA receptor subtypes. Pentobarbital maximal current was determined from experiments in A using 1 mm concentration. Pentobarbital exhibited a significantly higher efficacy than did GABA for α6β2δ receptors.
Table 3.
Spontaneous, GABA-induced (20–50× their relative EC50 values) and pentobarbital-evoked (1 mm) maximum currents (nA) for different GABAA receptor subtypes
| GABAAR subtype | Spontaneous | GABA | Pentobarbital | ImaxPB/ImaxGABA | n |
|---|---|---|---|---|---|
| α6β2δ | 98 ± 13.2 | 318 ± 14 | 1027 ± 53 | 326 ± 15% | 30 |
| α6β2γ2S | 50 ± 5.9 | 1499 ± 98 | 2156 ± 122 | 147 ± 4% | 25 |
| α4β2δ | 38 ± 4 | 1440 ± 110 | 1185 ± 127 | 83 ± 2% | 24 |
| α1β2γ2S | 31 ± 3.6 | 1874 ± 138 | 1974 ± 210 | 106 ± 6% | 25 |
| α1β2δ | 26 ± 2.9 | 1136 ± 173 | 570 ± 144 | 39 ± 6% | 27 |
| Control | Leak | ||||
| Mock-injected | 23 ± 2.5 | — | — | — | 16 |
GABA exhibits a low efficacy for α6β2δ receptors
The maximal GABA-induced current for each subunit combination tested (α1β2γ2S, α1β2δ, α4β2δ, α6β2γ2S and α6β2δ) was determined in the preceding experiments (where the spontaneous currents at intermediate expression levels were compared). GABA concentrations were used at 20–50 times the respective EC50 values (for EC50 values, see Table 1). A comparison of the maximal GABA-evoked currents for the five GABAA receptor subtypes is shown in Fig. 3B (for current values see Table 3). The maximal GABA-evoked current for α6β2δ receptors was only 16–28% of that of the other subunit combinations tested. These data reveal that the GABA-sensitive component of α6β2δ receptors is significantly smaller than that seen for other GABAA receptor subunit combinations. A comparison of the spontaneous current relative to the total current (maximal GABA-induced plus the spontaneous current) demonstrates that for α6β2δ receptors the spontaneous activity represented approximately 23% of the total attainable current.
Pentobarbital, an intravenous anaesthetic, is a potent modulator of GABAA receptors and at high concentrations can directly activate them. Previous studies have shown that the GABA-dependent and pentobarbital-dependent activation domains are distinct, given that pentobarbital can activate a mutated GABAA receptor whose GABA-dependent activation domain is impaired (Amin & Weiss, 1993; Amin, 1999). We also determined the maximal pentobarbital-induced current for each GABAA receptor subtype within the preceding experiments at intermediate expression levels (on the same oocytes where the spontaneous activity and the GABA maxima were determined). Figure 3C shows the maximal current induced by 1 mm pentobarbital relative to that induced by GABA (pentobarbital Imax/GABA Imax× 100) for the GABAA receptor subtypes tested (see also Table 3). For all GABAA receptors tested, excepting α6β2δ receptors, pentobarbital produced similar or lower maximal current than GABA. The pentobarbital-evoked maximal current was more than three times greater than that induced by GABA for α6β2δ receptors and was similar in magnitude to the GABA maximal current for other GABAA receptors. Thus, pentobarbital is markedly more efficacious than GABA and acts as a full agonist for the α6β2δ receptor when compared to GABA.
α6β1δ and α6β3δ receptors also exhibit the high-affinity state
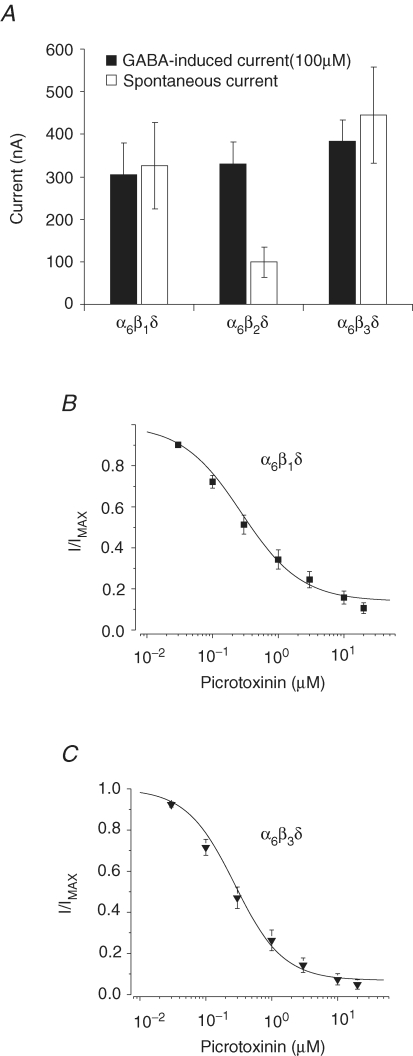
Cerebellar granule neurons express high levels of α1, γ2, α6, δ, β2 and β3 subunits in the adult rats (Laurie et al. 1992; Persohn et al. 1992; Wisden et al. 1996; Jechlinger et al. 1998). The α6 and δ subunits in combination with either β1, β2 or β3 cRNAs (at intermediate expression levels) were coinjected into oocytes and the maximal GABA-induced current (100 μm) and the extent of the spontaneous activity for each receptor subtype was measured to determine whether the high-affinity state and the spontaneous activity of the α6β2δ receptor depend upon the subtype of β subunit (Fig. 4A). GABA induced a similar maximal current for α6β1δ, α6β2δ and α6β3δ receptors. Further, all three α6β1-3δ receptors displayed high levels of spontaneous activity, indicating the presence of the high-affinity state. The level of spontaneous activity was greater for α6β1δ and α6β3δ than for α6β2δ receptors, suggesting that the α6β1δ and α6β3δ receptors may exhibit a higher propensity to assemble into the high-affinity state. Next, we determined a picrotoxinin concentration–response relationship (0.03–20 μm) to establish whether the spontaneous activity indeed arises from α6β1δ and α6β3δ receptors (Fig. 4B and C). Similar to the α6β2δ receptor, picrotoxinin blocked the spontaneous current arising from α6β1δ and α6β3δ receptors with high potency (IC50 of ∼0.3, see Table 2) and efficacy (∼90% block at 20 μm). These experiments demonstrated that either the β1, β2 or β3 subunit may coassemble with α6 and δ subunits to express spontaneously active receptor–channels in the high-affinity state.
Figure 4.
Expression of the high-affinity state of α6β2δ receptors is independent of the isoform of the β subunit GABA induced similar maximal current for α6β1δ, α6β2δ, and α6β3δ receptors (filled bars) with high levels of spontaneous activity indicating the presence of the high-affinity state (open bars). B and C, the concentration–response relationship for picrotoxinin block of the spontaneous current from α6β1δ and α6β3δ receptors.
Potency and efficacy of different GABA and glycine agonists upon α6β2δ receptors
We compared the maximal induced current evoked by GABA with that of two other established GABA agonists, trans-4-aminocrotonic acid (TACA) and imidazole-4-acetic acid (I4AA), in the α6β2δ receptor (at intermediate expression levels). Previous studies have established that TACA is a full agonist and I4AA as a partial agonist in GABAA receptors (Woodward et al. 1993; Chebib & Johnston, 1999; Mortensen et al. 2004). Figure 5A shows the maximal current induced by I4AA (1100 μm∼200 × EC50) and TACA (500 μm∼200 × EC50) relative to that of saturating concentrations of GABA (300 μm). For I4AA, the relative maximal current to that of evoked by GABA was 0.59 ± 0.06 (n = 9). In comparison, maximal currents evoked by TACA were consistently larger than that those evoked by GABA (1.22 ± 0.03, n = 5) demonstrating that for α6β2δ receptors both GABA and I4AA behave as partial agonists relative to TACA.
Figure 5.
Comparison of the I4AA and TACA maximal-induced currents as well as β-alanine and taurine concentration-response relationship for α6β2δ receptors A, comparison of the I4AA and TACA maximal-induced currents relative to GABA for α6β2δ receptors. B, concentration–response relationship for I4AA and the fit of a sum of two Hill equations to these data points. The α6β2δ receptors response to I4AA concentrations exhibited two components with marked difference in apparent affinity. C, the β-alanine concentration–response relationships for oocytes with high (▾), intermediate (○) and low (•) α6β2δ expression. The dashed line shows the plot of the fit (average from group data) of sum of two Hill equations to the β-alanine concentration–response data point for wild-type α6β2δ receptor at intermediate expression condition. D, taurine concentration–response relationships for α6β2δ receptors at low (•), intermediate (○), or high (▾) expression condition. The dashed line shows the plot of the fit (average from group data) using a sum of two Hill equations to the taurine data points for the α6β2δ receptor at intermediate expression condition.
A concentration–response relationship was constructed with 10 concentrations of I4AA, ranging from 0.001 to 4000 μm. Figure 5B depicts the fit of a sum of two Hill equations to these data points (under intermediate expression conditions). The response of α6β2δ receptors to I4AA concentrations exhibited two components with a marked difference in their apparent affinity. For I4AA, the high- and the low-affinity components had EC50 values of 0.06 and 202.25 μm, respectively, translating into a 3400-fold difference in apparent affinity between the two components (Table 1). The relative maxima of the high- to the low-affinity components of the responses to I4AA was 1.39, as compared to 0.54 for GABA, suggesting that I4AA may have a significantly greater efficacy for the high-affinity state than for the low affinity state.
Both β-alanine and taurine are classical glycine receptor agonists (Kuhse et al. 1993) and may act as neurotransmitters within the CNS. Using a range of β-alanine concentrations from 0.05 to 10 000 μm, we determined the efficacy and potency of this agonist in oocytes expressing low, intermediate and high levels of α6β2δ receptors (displaying 0, 70 and 190 nA of spontaneous current, respectively; Fig. 5C). For an oocyte with a low level of expression (filled circles), the EC50 for β-alanine agonist was ∼0.5 mm (the group data for β-alanine are shown in Table 1). With increasing expression, the sensitivity of the α6β2δ receptor to β-alanine increased concomitantly with the high spontaneous activity (see open circles and filled triangles), reducing the EC50 value to approximately 1 μm (single fit to the Hill equation). The fit of the β-alanine data from the α6β2δ intermediate expression to the sum of two Hill equations yielded EC50 values of 1.16 and 594.66 μm, respectively, for the high and the low affinity components (see Table 1). A comparison of the overall GABA and β-alanine maximal currents demonstrated that β-alanine was as efficacious as GABA for α6β2δ receptors (Imaxβ-alanine at 10 000 μm/Imax GABA at 300 μm = 1.08 ± 0.03, n = 7).
Figure 5D shows taurine concentration–response relationships for three sets of oocytes with low (filled circles), intermediate (open circles) and high (filled triangles) levels of α6β2δ expression (dispalying 40, 80 and 140 nA of spontaneous current, respectively; the group data for taurine are shown in Table 1). Fitting of the data points to the sum of two Hill equations yielded EC50 values of 5 and 807 μm for the high- and low-affinity components, respectively (Table 1). Taurine behaved as a partial agonist for α6β2δ receptors as compared to GABA or β-alanine. The relative efficacy of taurine (50 000 μm) to β-alanine (10 000 μm) at near saturating concentrations was 0.69 ± 0.04 (n = 7).
The level of β2 subunit expression determines the apparent affinity of α6β2δ receptors
We injected the α6, β2 and δ cRNA into oocytes in the ratios of 1α6 : 0.1 β2 : 1.8δ, 1α6 : 0.3 β2 : 1.8δ, or in the control 1α6 : 1 β2 : 1.8δ. The amount of injected cRNA was 5–7 ng of cRNA (intermediate expression) except for 1α6 : 0.1 β2 : 1.8δ where 8–12 ng (high expression) of cRNA was injected. A GABA concentration–response relationship was constructed as shown in Fig. 6A and B. At the 0.1 β2 ratio (1α6 : 0.1 β2 : 1.8δ; filled squares), the α6β2δ receptors were predominantly present in the low-affinity state and displayed three notable properties: (1) the resulting α6β2δ receptors were insensitive to GABA concentrations below 0.02 μm (EC50 of 1.3 μm; Table 1); (2) after removal of GABA, the current's return to baseline was satisfied by a fit to a single exponential; and (3) these receptor–channels showed no discernible spontaneous activity. With an increase in the β2 ratio (0.3 β2, filled circles), GABA-induced currents were detected at concentrations as low as 0.002 μm (EC50 of ∼1.5 μm; single fit, see Table 1). Moreover, the higher sensitivity to GABA was concomitant with an appearance of spontaneous channel activity (shaded area) and a shallower Hill coefficient than for the 0.1 β2 ratio. Further, current decay, following agonist washout at higher concentrations followed a multiexponential decay (analysis not shown). At the control ratio of 1α6 : 1 β2 : 1.8δ (filled triangles), both high and low affinity components were readily discernible. The appearance of the high-affinity component thus coincided with a significant level of spontaneous channel activity with the current decay following removal of the agonist exhibiting a multiexponential paradigm. In experiments with the β2 cRNA at a ratio 2–4 times higher (e.g. 1α6 : 2 or 4 β2 : 1.8δ; data not shown), the resulting α6β2δ receptors were predominantly present in the high-affinity state, similar to that observed under condition of high expression (see also Fig. 1).
Figure 6.
The effect of change in β2 subunit ratio on the expression of α6β2δ and α1β2γ2 receptors A, the current traces of receptor–channels from the expression of 1α6 : 0.1 β2 : 1.8δ, 1α6 : 0.3 β2 : 1.8δ, or control 1α6 : 1 β2 : 1.8δ ratios. The dotted line indicates the zero-current level; the shaded area represents the spontaneous activity. The thick lines above the current traces represent the duration of GABA application. B, the GABA concentration–response relationship for the three tested ratios of α6, β2, and δ subunits. The data derived from 1α6 : 0.1 β2 : 1.8δ and 1α6 : 0.3 β2 : 1.8δ were fitted with a single Hill equation, while the data for 1α6 : 1 β2 : 1.8δ were fitted with a sum of two Hill equations. C, the concentration–response relationship for 1α1 : 0.08 β2 : 1.8γ2S, 1α1 : 0.4 β2 : 1.8γ2S and control1α1 : 1 β2 : 1.8γ2S subunit combination.
The α1β2γ2S receptor is one of the most abundantly expressed GABAA receptor subtypes present within the CNS. To assess whether the marked change in GABA sensitivity brought on by altering the β2 ratio is unique to α6β2δ receptors, we repeated the preceding experiments, but varying the ratio of β2 to α1 and γ2S cRNA. We injected these cRNAs, in ratios of either 1α1 : 0.08 β2 : 1.8γ2S, 1α1 : 0.4 β2 : 1.8γ2S or the control ratio of 1α1 : 1 β2 : 1.8γ2S into oocytes (intermediate expression condition) and determined GABA concentration–response relationships (Fig. 6C). For the 0.08 β2(1α1 : 0.08 β2 : 1.8γ2S; open squares), the EC50 and Hill coefficient parameters from a fit to a single Hill equation were 37.2 μm and 1.26, respectively (Table 1). These values were similar to those obtained from previous experiments with α1β2γ2S receptors in which the maximal GABA current was limited to ∼1000 nA (for the range of maximal currents and EC50 values, see Table 1). At 0.4 β2 (open circles) and at the control ratio (open triangles), the sensitivity of the resulting α1β2γ2S receptors increased by approximately 4- and 12-fold, respectively, in comparison to 0.08 β2 with GABA maxima greater than 2 μA in magnitude (Table 1). Previous studies have shown that coinjection of cRNA for α1, β2, and γ2S also yields α1β2 receptors which show an approximately 10-fold higher sensitivity to GABA than the α1β2γ2S receptor (Walters et al. 2000). The presence of α1β2 receptors may contribute, in part, to the observed increase in GABA sensitivity of α1β2γ2S receptors. Nevertheless neither expression conditions for α1β2γ2S receptors resulted in the appearance of spontaneous channel activity.
With the increase in the ratio of β2 cRNA, both α6β2δ and α1β2γ2S receptors showed increases in their apparent affinity for GABA. However, the magnitude of the shift in GABA sensitivity to lower concentrations was 12-fold for the α1β2γ2S receptor in comparison to more than 400-fold for the α6β2δ receptor. The increase in GABA sensitivity was concomitant with the appearance of spontaneous channel activity within the α6β2δ receptor, but not for the α1β2γ2S receptor.
5α-THDOC directly activates α6β2δ receptors
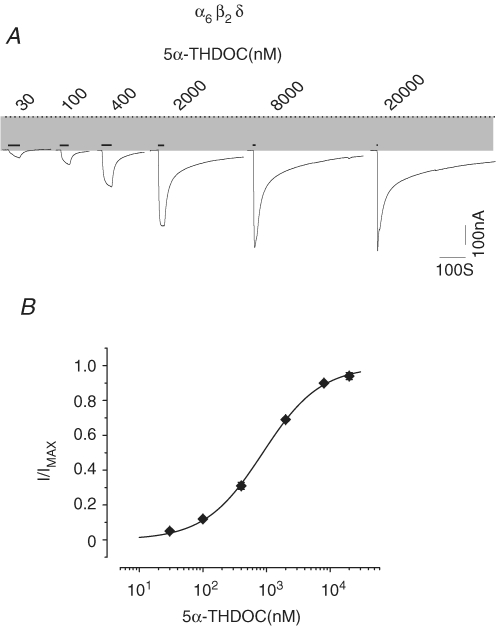
Neuroactive steroids are metabolites of the principal sex and stress steroid hormones and represent a large class of endogenous compounds active within the CNS (Belelli & Lambert, 2005). One such metabolite, 5α-THDOC, is a potent modulator of GABAA receptors (Puia et al. 1994), and is a highly hydrophobic compound that mediates its actions through a mechanism that is distinct from that of GABA binding (Morris & Amin, 2004). We examined the direct action of 5α-THDOC upon α6β2δ receptors at a range of concentrations. Figure 7 shows 5α-THDOC-induced current traces and the concentration–response relationship for α6β2δ receptors (at intermediate- and high-expression conditions). Concentrations as low as 30 nm of 5α-THDOC augmented the spontaneous current. For direct activation by 5α-THDOC, EC50 and nH values of 0.89 μm and 0.97 were derived, respectively (see Table 1). The 5α-THDOC-induced maximal current at 20 μm was similar in magnitude to that of GABA. Thus, 5α-THDOC, previously known for its modulatory action on GABAA receptors, can directly activate α6β2δ receptors with high potency.
Figure 7.
The 5α-THDOC directly activates α6β2δ receptors A, 5α-THDOC-induced current traces. The dotted line indicates the zero-current level; the shaded area represents the spontaneous activity. The thick lines above the current traces represent the duration of 5α-THDOC applications. B, the 5α-THDOC concentration–response relationship and fit of data point to a Hill equation.
Mutation of the βY157F impairs both the high and the low affinity states of α6β2δ receptors
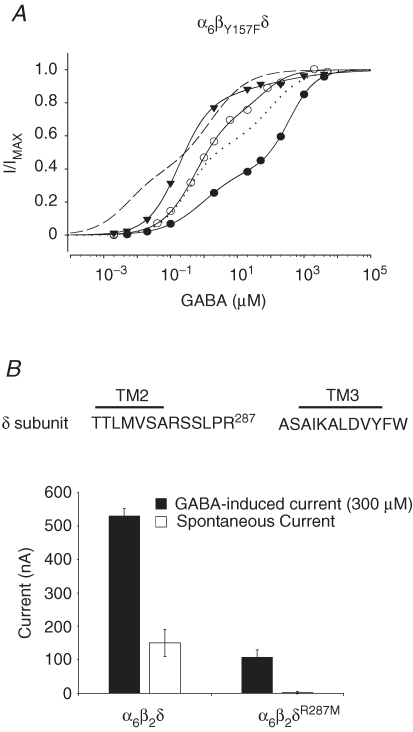
Previous studies have demonstrated that the mutation of Tyr157 to Phe within the β2 subunit (βY157F) impairs the GABA-dependent activation of α1βY157Fγ2S receptors (∼50-fold increase in EC50, Amin & Weiss 1993). To examine the effect of this mutation on the spontaneous activity and the two GABA components of the α6β2δ receptor, we coinjected cRNA of α6, βY157F and δ subunits into oocytes at low, intermediate, and high levels of expression and determined the respective GABA concentration–response relationship (Fig. 8A). These oocytes exhibited spontaneous current activity of 30 nA, 70 nA and 180 nA at low, intermediate and high expression levels, respectively. Thus, α6βY157Fδ receptors exhibited a spontaneous current similar in magnitude to that of the wild-type receptors. Fitting these data points to the sum of two Hill equations yielded EC50 values of 0.37 and 105 μm GABA (see Table 1). Comparison of the EC50 values for the wild-type and mutant receptors showed that the βY157F mutation had produced an approximately 50-fold reduction in the apparent affinity for GABA for both components.
Figure 8.
The effect of β2 or δ subunit mutation on the expression of α6β2δ receptors A, the GABA concentration–response relationship for α6βY157Fδ receptors for three oocytes with 30 nA (•), 70 nA (○) and 180 nA (▾) of spontaneous current. The mutation within the GABA-dependent activation domain (α6βY157Fδ) did not abolish the spontaneous activity. The continuous lines represent the fit of the GABA data points with a sum of two Hill equations for α6βY157Fδ receptors at different expression levels. The dashed line shows the overall plot of the fit of the GABA concentration–response relationship data with a sum of two Hill equations for the wild-type α6β2δ receptor (intermediate expression). The dotted line shows a plot of the fit (group data) of the GABA concentration–response relationship data with a sum of two Hill equations for the α6βY157Fδ receptor. B, the δR287M mutation attenuated the spontaneous activity and the GABA maximal current. Top shows the amino acid sequence between the TM2 and TM3 domains of the δ subunit, indicating the position of Arg287. At equivalent expression conditions, the wild-type receptor showed high levels in comparison to no spontaneous activity for α6β2δR287M receptors (corrected for control leak, see methods). The maximal-induced GABA current for mutated receptor was significantly reduced as compared to that for wild-type receptor.
We then examined α6βY157Fδ receptors at the injected cRNA ratio of 1α6 : 0.1 βY157F1.8δ. Under these expression conditions, α6βY157Fδ receptors persisted in the low-affinity state (no spontaneous channel activity), with an EC50 of 53 μm representing a 50-fold lower sensitivity to GABA in comparison to the α6β2δ at 0.1 β2 (see Table 1).
In summary, mutation of the crucial Tyr within the β2 subunit produced similar impairment of both the low- and high-affinity components, but the appearance of spontaneous activity was unaffected by the βY157F mutation. These findings suggest that the domains important in establishment of the spontaneous current may be distinct from those crucial for GABA-dependent activation.
δR287M mutation attenuates both spontaneous activity and the GABA maximal current
Studies of families with a history of generalized epilepsy associated with febrile seizures have identified a single mutation at Lys289 to Met, a residue which is located within the extracellular loop between the second and third transmembrane spanning domains (TM2 and TM3) of the γ2S subunit. This mutation significantly decreases the magnitude of the maximal GABA-induced current in α1β2γK289M receptors (Baulac et al. 2001). To examine the role of δ subunits in the expressional characteristics of α6β2δ receptors, we mutated the equivalent residue of the δ subunit, Arg287, to Met. GABA concentration–response relationship experiments for α6β2δR287M receptors yielded an EC50 and nH values of 3.71 μm and 0.69, respectively (Table 1), values which are comparable to the EC50 and nH parameters found for the low-affinity components of the wild-type receptor. Analogous to the effect of the γK289M mutation within α1β2γK289M receptors, the δR287M mutation appeared to induce a diminished maximal GABA-evoked current (Table 1). Accordingly we compared both the spontaneous current and the maxima of GABA-evoked (300 μm) currents of wild-type and α6β2δR287M receptors under equivalent expression conditions (Fig. 8B). Under these conditions, the wild-type receptors showed significant spontaneous activity (150 ± 41 nA, n = 7; ANOVA one-way analysis F ratio = 14.31, P < 0.001) as compared to the absence of spontaneous activity (2 ± 4 nA, n = 8, corrected for control leak current of 14 ± 3 nA, n = 7; see Methods) in α6β2δR287M receptors. Further, we observed that the maximal-induced GABA current in the mutant receptors was also significantly diminished. The GABA maximal current exhibited by α6β2δR287M receptors was 107 ± 23 nA (n = 7) as compared to 530 ± 23 nA (n = 8) for the wild-type receptors. These experiments demonstrated that the δ subunit contributes to the appearance of the high-affinity state in α6β2δ receptors.
Discussion
The α6β2δ receptors show two distinct states of agonist affinity
We have demonstrated that coexpression of α6, β2 and δ subunits produces receptor–channels which display two distinct and separable states of agonist affinity with nanomolar and micromolar sensitivity to GABA. In the high-affinity state, α6β2δ receptors showed a significant level of spontaneous activity. Increasing the expression level, or the ratio of β2 subunit, shifted the equilibrium of the receptor subtypes from the low- to the high-affinity state.
Previous studies of granule neurons in both culture and slice recordings from the cerebellum have demonstrated a significant increase in GABA sensitivity during the maturation of granule neurons (Mathews et al. 1994; Puia et al. 1994; Zempel & Steinbach, 1995; Tia et al. 1996b, Tia 1996a; Ueno et al. 1996; Hevers & Luddens, 2002). However, the degree of shift in GABA sensitivity and spontaneous current shown here has not been reported in studies within granule neuron or with the transient expression of α6β3δ receptors in tissue-culture systems (Saxena & Macdonald, 1996). First, the detection of such a current may be hindered by the presence of a mixture of GABAA receptor subtypes within granule cells, most of which exhibit a significantly lower sensitivity of GABA than the α6β2δ receptors. Second, the predominant expression of α6β2-3δ receptors in the high-affinity state may occur less frequently and only in fully developed granule cells. Such neurons may be more difficult to culture or to study electrophysiologically in slice recordings. Third, in transient transfection studies the level of expression is difficult to control and electrophysiological experiments are often carried out only 24 hours after transfection, thereby limiting the expression level. Finally, in slice recordings, the persistence of low concentrations of GABA may mask the presence of spontaneous channel activity of α6βδ receptors.
The presense of two state of agonist affinities or a spontaneous current shown for α6β2δ receptors, has precedent within the ligand-gated ion channel family (Khrestchatisky et al. 1989; Sigel et al. 1989; Krishek et al. 1996; Zwart & Vijverberg, 1998; Neelands et al. 1999; Buisson & Bertrand, 2001; Mortensen et al. 2003; Taleb & Betz, 1994). For example, among GABAA receptors, α1β2ɛ exhibit a high sensitivity to GABA as well as spontaneous activity (Zwart & Vijverberg, 1998; Neelands et al. 1999; Mortensen et al. 2003).
Mutation of β2versusδ subunits
Earlier reports have shown that the βY157F mutation impairs the GABA-dependent activation of α1βY157Fγ2S receptors yet produces little effect on pentobarbital-dependent activation, indicating that the pentobarbital- and GABA-dependent activation domains are distinct (Amin & Weiss, 1993; Amin, 1999). Within the α6β2δ receptors, mutation of βY157F impaired the low- and the high-affinity GABA components to similar extents, without attenuating the spontaneous activity of the α6β2δ receptors, suggesting that the domain important for spontaneous activity may also be different from that of the agonist-binding domain.
The mutation of Lys298 to Met within the γ2 subunit and of the comparable residue in the δ subunit significantly decreased the magnitude of the GABA-induced maximal current within both α1β2γ2 receptor (Baulac et al. 2001) and α6β2δ receptors. The mutation within the δ subunit also abolished the spontaneous channel activity within α6β2δ receptors, indicating the contribution of the δ subunits to the unique properties of α6β2δ receptors.
Alternative scenarios for expression of α6β2δ receptors within two states of agonist affinities
Earlier reports have shown that GABAA receptors composed of α1, β2 and γ subunits have a fixed stoichiometry in which 2α1, 2β2 and 1γ2 subunits assemble in a pentameric configuration (Chang et al. 1996; Kellenberger et al. 1996; Baumann et al. 2001; Tretter et al. 2001). Comparison of the amino acid sequence among the subunits of GABAA receptors shows that the δ subunit has a greater homology to the β subunit than the γ subunit. At very high expression levels, the β2 subunit can form a homo-oligmeric receptor–channels with very low maximal current. The β subunit is also essential for the expression of GABAA receptors since the cRNA combinations lacking β do not express ligand-gated ion channels within oocytes. This may explain the apparent correlation between the increase in the β2 levels (by changing the ratio or increasing the amount of cRNA injected) and the level of α6β2δ expression. Given the significance of the β2 subunit in expression of GABAA receptors, a greater homology of the δ to the β subunit may be an important clue undelying the unique kinetic signature of α6β2δ receptors.
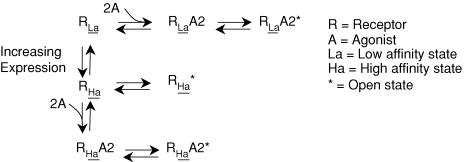
Recent studies have shown that the kinetics of GABAA receptors within hippocampal neurons changes upon clustering of these receptor–channels (Petrini et al. 2003; Petrini et al. 2004). Additional studies with recombinant GABAA receptors demonstrate that coexpression of GABAA receptor-associated proteins markedly alters the kinetics of the GABAA receptors increasing their conductance (Everitt et al. 2004). We propose two alternative scenarios that could account for expression of α6β2δ receptors into two distinct states of affinity. First, the α6β2δ receptors could assemble with two different stoichiometries (e.g. 2α6, 2β2 and 1δ2versus 1α6, 3β2 and 1δ2). Second, the α6β2δ receptors may have a high propensity to cluster where close interaction with the neighbouring receptor-channels may energetically favour the channel configuration in the open state (Fig. 9).
Figure 9.
A model showing different states of α6β2δ receptors upon clustering
Pharmacological parallel between the spontaneous activity and the tonic inhibition
The spontaneous activity of α6β2δ receptors exhibited a pharmacology corresponding to that of tonic inhibition in adult rat granule cells. The following support this notion. First, similar to the block of spontaneous current of α6β2δ receptors shown here, bicuculline, gabazine (SR95531) and picrotoxinin all inhibit the tonic chloride current within the granule neurons (Kaneda et al. 1995; Brickley et al. 1996; Wall & Usowicz, 1997; Rossi & Hamann, 1998; Hamann et al. 2002). Second, earlier reports have demonstrated that the ability of bicuculline to inhibit the tonic current diminishes by adulthood. For example, Wall & Usowicz (1997) showed that bicuculline does not completely block the tonic current within a subpopulation of granule neurons at the adult stage. In addition, gabazine does not completely block the tonic current in every neuron tested (Hamann et al. 2002). In our studies, bicuculline and gabazine did not completely block the spontaneous current of α6β2δ receptor. Third, furosemide, a specific antagonist for GABAA receptors containing the α6 subunit (Korpi et al. 1995; Korpi & Luddens, 1997), blocks the tonic inhibition (Hamann et al. 2002) to a similar extent to the block of spontaneous activity of the α6β2δ receptors. Fourth, the kinetics of washouts were significantly slower for bicuculline than for furosemide block of the spontaneous activity of α6β2δ receptors (Fig. 2). This is analogous to the markedly slower return of current to the baseline after bicuculline wash than after furosemide wash in studies of tonic inhibition from adult animals (Hamann et al. 2002). The very slow wash of the competitive antagonist bicuculline in these studies may indicate the presence of GABAA receptor within the high-affinity state. Fifth, β-alanine in the micromolar concentration range can activate α6β2δ receptors. Earlier reports have demonstrated that β-alanine, in micromolar concentrations, can also induce a bicuculline-sensitive current in adult cerebellar granule cells exhibiting a tonic current (Wall & Usowicz, 1997). Finally, nanomolar concentrations of 5α-THDOC can enhance tonic conductance through α6-containing receptors (Stell et al. 2003). In parallel, 5α-THDOC at nanomolar concentrations can directly activate α6β2δ receptors in the high-affinity state.
Potential contribution of the high-affinity state of α6β2-3δ receptors to granule neuron
The developmentally controlled expression of α6β2δ receptors may be an important component in the formation of motor memory in the CNS. We hypothesize that the intrinsic spontaneous activity from α6β2δ receptors contributes to some extent to the formation of motor memory at later stages of rat development. Conceivably, the level of α6βδ expression alone could have a key function in the formation of single-cell memory, producing a graded but ligand-independent tonic activity in cerebellar granule neurons, where spillover of GABA can further modulate its strength. A few findings support this hypothesis. (1) The expression of α6, β and δ subunits in the cerebellum increases during postnatal development, reaching a markedly high level by adulthood (Laurie et al. 1992; Jones et al. 1997). The high expression of α6, β and δ subunits increases the likelihood of the transition of α6β2-3δ receptors to the high-affinity state. (2) There is an extensive pharmacological similarity between spontaneous activity of the α6β2δ receptor and the tonic inhibition in rat granule neurons. (3) In an α6-knockout animal model in which motor functions remain normal, a spontaneously open K+ channel appears to subsume the role of the α6-containing GABAA receptors (Brickley et al. 2001). (4) The α6β2-3δ receptors are extrasynaptically located (Nusser et al. 1998). This is consistent with the view that these receptor–channels may, to some extent, be independent from neurotransmitters for conveying their entire functional spectrum. (5) Electrophysiological studies have demonstrated the existence of GABAA receptor exhibiting spontaneous current in cultured rat neurons (Birnir et al. 2000). (6) Recent binding studies have shown a bimodal distribution of GABA affinity with components in the nanomolar and micromolar range within membrane preparations of cerebellum (Maksay & Biro, 2005). We found that the derived GABA-binding values were similar to the EC50 values for the two GABA components of α6β2δ receptors (Table 1). Two distinct affinity states were also shown for taurine with binding values matching closely with the EC50 of the two taurine components of α6β2δ receptors (Table 1). In comparison, the high-affinity component was altogether absent in membrane preparations from the hippocampus, where α6 and δ subunits are absent.
We have demonstrated that α6β2δ receptors can exist in low- and high-affinity states in which the expression level can affect the equilibrium between the two states. The high-affinity state is always accompanied by a spontaneous current that exhibited an overall pharmacology similar to that of tonic inhibition. Additional studies are needed to determine the relative contributions of the neurotransmitter-dependent and spontaneous currents arising from α6β2-3δ receptors in mediating tonic inhibition in the adult cerebellum.
Acknowledgments
We are indebted to Dr W. Sieghart for his kind gift of the α4 and to Dr H. Lüdden for β1 and β3 cDNAs. We thank S. Gionet for help in preparation, J. Cole for professional editing and Dr R. J. Walters for his critical reading of the manuscript. This work was supported by The Established Investigator Award from the American Heart Association to J.A.
References
- Amin J. A single hydrophobic residue confers barbiturate sensitivity to γ-aminobutyric acid type C receptor. Mol Pharmacol. 1999;55:411–423. [PubMed] [Google Scholar]
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital [see comment] Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss DS. Insights into the activation mechanism of rho1 GABA receptors obtained by coexpression of wild type and activation-impaired subunits. Proc Biol Sci. 1996;263:273–282. doi: 10.1098/rspb.1996.0042. [DOI] [PubMed] [Google Scholar]
- Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat Genet. 2001;28:46–48. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bernard E, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology XV. Subtypes of the γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Birnir B, Eghbali M, Everitt AB, Gage PW. Bicuculline, pentobarbital and diazepam modulate spontaneous GABAA channels in rat hippocampal neurons. Br J Pharmacol. 2000;131:695–704. doi: 10.1038/sj.bjp.0703621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance [see comment] Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebib M, Johnston GA. The ‘ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- Chiu CS, Brickley S, Jensen K, Southwell A, McKinney S, Cull-Candy S, Mody I, Lester HA. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne EL, Hosie AM, Wooltorton JR, Duguid IC, Harvey K, Moss SJ, Harvey RJ, Smart TG. An N-terminal histidine regulates Zn2+ inhibition on the murine GABAA receptor β3 subunit. Br J Pharmacol. 2002;137:29–38. doi: 10.1038/sj.bjp.0704835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt AB, Luu T, Cromer B, Tierney ML, Birnir B, Olsen RW, Gage PW. Conductance of recombinant GABAA channels is increased in cells co-expressing GABAA receptor-associated protein. J Biol Chem. 2004;279:21701–21706. doi: 10.1074/jbc.M312806200. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. Pharmacological heterogeneity of γ-aminobutyric acid receptors during development suggests distinct classes of rat cerebellar granule cells in situ. Neuropharmacology. 2002;42:34–47. doi: 10.1016/s0028-3908(01)00158-7. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. Subunit composition and quantitative importance of hetero-oligomeric receptors: GABAA receptors containing α6 subunits. J Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol. 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Eckenstein S, Baur R, Malherbe P, Buhr A, Sigel E. Subunit stoichiometry of oligomeric membrane proteins: GABAA receptors isolated by selective immunoprecipitation from the cell surface. Neuropharmacology. 1996;35:1403–1411. doi: 10.1016/s0028-3908(96)00034-2. [DOI] [PubMed] [Google Scholar]
- Khrestchatisky M, MacLennan AJ, Chiang MY, Xu WT, Jackson MB, Brecha N, Sternini C, Olsen RW, Tobin AJ. A novel α subunit in rat brain GABAA receptors. Neuron. 1989;3:745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Luddens H. Selective antagonist for the cerebellar granule cell-specific γ-aminobutyric acid type A receptor. Mol Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- Korpi ER, Luddens H. Furosemide interactions with brain GABAA receptors. Br J Pharmacol. 1997;120:741–748. doi: 10.1038/sj.bjp.0700922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. Homomeric β1 γ-aminobutyric acid A receptor-ion channels: evaluation of pharmacological and physiological properties. Mol Pharmacol. 1996;49:494–504. [PubMed] [Google Scholar]
- Kuhse J, Laube B, Magalei D, Betz H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993;11:1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Westbrook GL. Noncompetitive inhibition of γ-aminobutyric acidA channels by Zn. Mol Pharmacol. 1991;39:267–274. [PubMed] [Google Scholar]
- Maksay G, Biro T. High affinity, heterogeneous displacement of [3H]EBOB binding to cerebellar GABAA receptors by neurosteroids and GABA agonists. Neuropharmacology. 2005;49:431–438. doi: 10.1016/j.neuropharm.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews GC, Bolos-Sy AM, Holland KD, Isenberg KE, Covey DF, Ferrendelli JA, Rothman SM. Developmental alteration in GABAA receptor structure and physiological properties in cultured cerebellar granule neurons. Neuron. 1994;13:149–158. doi: 10.1016/0896-6273(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: electrical activity regulates the GABAA receptor α6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Morris KD, Amin J. Insight into the mechanism of action of neuroactive steroids. Mol Pharmacol. 2004;66:56–69. doi: 10.1124/mol.66.1.56. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Kristiansen U, Ebert B, Frolund B, Krogsgaard-Larsen P, Smart TG. Activation of single heteromeric GABAA receptor ion channels by full and partial agonists. J Physiol. 2004;557:389–413. doi: 10.1113/jphysiol.2003.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Wafford KA, Wingrove P, Ebert B. Pharmacology of GABAA receptors exhibiting different levels of spontaneous activity. Eur J Pharmacol. 2003;476:17–24. doi: 10.1016/s0014-2999(03)02125-3. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Fisher JL, Bianchi M, Macdonald RL. Spontaneous and γ-aminobutyric acid (GABA)-activated GABAA receptor channels formed by ε subunit-containing isoforms. Mol Pharmacol. 1999;55:168–178. doi: 10.1124/mol.55.1.168. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, Somogyi P. Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the α6 subunit gene. Eur J Neurosci. 1999;11:1685–1697. doi: 10.1046/j.1460-9568.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Marchionni I, Zacchi P, Sieghart W, Cherubini E. Clustering of extrasynaptic GABAA receptors modulates tonic inhibition in cultured hippocampal neurons. J Biol Chem. 2004;279:45833–45843. doi: 10.1074/jbc.M407229200. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Zacchi P, Barberis A, Mozrzymas JW, Cherubini E. Declusterization of GABAA receptors affects the kinetic properties of GABAergic currents in cultured hippocampal neurons. J Biol Chem. 2003;278:16271–16279. doi: 10.1074/jbc.M213081200. [DOI] [PubMed] [Google Scholar]
- Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl− currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Quirk K, Gillard NP, Ragan CI, Whiting PJ, McKernan RM. Model of subunit composition of γ-aminobutyric acid A receptor subtypes expressed in rat cerebellum with respect to their α and γ/δ subunits. J Biol Chem. 1994;269:16020–16028. [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry [erratum appears in Neuron (1998) 21, 527] Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Malherbe P, Mohler H. The rat β1-subunit of the GABAA receptor forms a picrotoxin-sensitive anion channel open in the absence of GABA. FEBS Lett. 1989;257:377–379. doi: 10.1016/0014-5793(89)81576-5. [DOI] [PubMed] [Google Scholar]
- Smart TG. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol. 1992;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Hosie AM, Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb O, Betz H. Expression of the human glycine receptor α1 subunit in Xenopus oocytes: apparent affinities of agonists increase at high receptor density. EMBO J. 1994;13:1318–1324. doi: 10.1002/j.1460-2075.1994.tb06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Stephenson FA. GABAA receptor subtypes expressed in cerebellar granule cells: a developmental study. J Neurochem. 1994;62:2037–2044. doi: 10.1046/j.1471-4159.1994.62052037.x. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Tehrani MH, Barnes EM, Jr, Stephenson FA. Decreased expression of GABAA receptor α6 and β3 subunits in stargazer mutant mice: a possible role for brain-derived neurotrophic factor in the regulation of cerebellar GABAA receptor expression? Brain Res Mol Brain Res. 1998;60:282–290. doi: 10.1016/s0169-328x(98)00205-8. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J Neurosci. 1996a;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology. 1996b;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Tretter V, Hauer B, Nusser Z, Mihalek RM, Hoger H, Homanics GE, Somogyi P, Sieghart W. Targeted disruption of the GABAA receptor δ subunit gene leads to an up-regulation of γ2 subunit-containing receptors in cerebellar granule cells. J Biol Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- Tyrrell T, Willshaw D. Cerebellar cortex: its simulation and the relevance of Marr's theory. Philos Trans R Soc Lond B Biol Sci. 1992;336:239–257. doi: 10.1098/rstb.1992.0059. [DOI] [PubMed] [Google Scholar]
- Ueno S, Zempel JM, Steinbach JH. Differences in the expression of GABAA receptors between functionally innervated and non-innervated granule neurons in neonatal rat cerebellar cultures. Brain Res. 1996;714:49–56. doi: 10.1016/0006-8993(95)01457-8. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3 delta and α6β3δγ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RJ, Hadley SH, Morris KD, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci. 2000;3:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- Wisden W, Korpi ER, Bahn S. The cerebellum: a model system for studying GABAA receptor diversity. Neuropharmacology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- Woodward RM, Polenzani L, Miledi R. Characterization of bicuculline/baclofen-insensitive (rho-like) γ-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of γ-aminobutyric acidA and γ-aminobutyric acidB receptor agonists and antagonists. Mol Pharmacol. 1993;43:609–625. [PubMed] [Google Scholar]
- Zempel JM, Steinbach JH. Neonatal rat cerebellar granule and Purkinje neurons in culture express different GABAA receptors. Eur J Neurosci. 1995;7:1895–1905. doi: 10.1111/j.1460-9568.1995.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–1131. [PubMed] [Google Scholar]