Abstract
Synthetic glucocorticoids (sGCs) are routinely used to treat women at risk of preterm labour to promote fetal lung maturation. There is now strong evidence that exposure to excess glucocorticoid during periods of rapid brain development has permanent consequences for endocrine function and behaviour in the offspring. Prenatal exposure to sGC alters the expression of N-methyl-d-aspartate receptor (NMDA-R) subunits in the fetal and neonatal hippocampus. Given the integral role of the NMDA-R in synaptic plasticity, we hypothesized that prenatal sGC exposure will have effects on hippocampal long-term potentiation (LTP) after birth. Further, this may occur in either the presence or absence of elevated cortisol concentrations, in vitro. Pregnant guinea-pigs were injected with betamethasone (Beta, 1 mg kg−1) or vehicle on gestational days (gd) 40, 41, 50, 51, 60 and 61 (term ∼70 days), a regimen comparable to that given to pregnant women. On postnatal day 21, LTP was examined at Schaffer collateral synapses in the CA1 region of hippocampal slices prepared from juvenile animals exposed to betamethasone or vehicle, in utero. Subsequently, the acute glucocorticoid receptor (GR)- and mineralocorticoid receptor (MR)-dependent effects of cortisol (0.1–10 μm; bath applied 30 min before LTP induction) were examined. There was no effect of prenatal sGC treatment on LTP under basal conditions. The application of 10 μm cortisol depressed excitatory synaptic transmission in all treatment groups regardless of sex. Similarly, LTP was depressed by 10 μm cortisol in all groups, with the exception of Beta-exposed females, in which LTP was unaltered. Hippocampal MR and GR protein levels were increased in Beta-exposed females, but not in any other prenatal treatment group. This study reveals sex-specific effects of prenatal exposure to sGC on LTP in the presence of elevated cortisol, a situation that would occur in vivo during stress.
Preterm delivery occurs in 7–10% of births in North America and results in ∼75% of neonatal deaths (NIH, 1995). Prenatal maternal treatment with synthetic glucocorticoids (sGC) is highly effective in reducing the frequency of respiratory complications (Block et al. 1977). However, until recently many women received repeated courses of sGC (Quinlivan et al. 1998) owing to the difficulties in diagnosing preterm labour. Now, animal studies and, more recently, those in humans, have shown that prenatal exposure to sGC can have long-term consequences for metabolic, endocrine and neurological function (Owen et al. 2005). In this respect, a recent study has shown that children exposed to repeated prenatal sGC exhibit hyperactivity and attention disorders (French et al. 2004).
The pervasive effects of prenatal sGC on neurological function after birth are probably attributable to their similarity to endogenous glucocorticoids (cortisol and corticosterone). Exposure of the fetal brain to normal concentrations of endogenous glucocorticoid is essential for neuronal survival (Sloviter et al. 1993), differentiation (Rua et al. 1995) and both structural and functional development of synapses (Huang et al. 2001; Antonow-Schlorke et al. 2003), but the consequences of excess exposure are still being elucidated. Glucocorticoids act via glucocorticoid receptors (GR) and mineralocorticoid receptors (MR; Beato et al. 1996). Areas of the brain such as the hippocampus, which expresses very high levels of GR and MR in adult life (Liu et al. 2001), but also during fetal development (Bohn et al. 1994; Owen & Matthews, 2003), are particularly susceptible to excess sGC exposure. Additionally, hippocampal corticosteroid receptor expression is critical in regulating glucocorticoid negative-feedback actions on hypothalamic–pituitary axis (HPA) function and endogenous glucocorticoid release (Sapolsky et al. 1984; Bohn et al. 1994).
The hippocampus is also a key structure in the formation of spatial memory (Morris et al. 1982), and long-term potentiation (LTP) has become a primary candidate underlying the cellular basis for memory (Bliss & Collingridge, 1993). Glucocorticoids have contrasting effects on LTP, where MR stimulation favours potentiation while GR activation enhances depression (Joels & de Kloet, 1991). Mineralocorticoid receptors mediate glucocorticoid actions at low, basal levels because their binding affinity is approximately 10-fold higher than that of GR, which are activated when glucocorticoid concentrations are high, such as those that occur during stress (Reul & de Kloet, 1985). Therefore, there are differential effects of endogenous glucocorticoids on regulation of LTP, depending on their local concentrations.
The guinea-pig has been used extensively to investigate the effects of prenatal sGC and stress on endocrine function and behaviour in juvenile and adult offspring (Owen & Matthews, 2003, 2007; Kapoor & Matthews, 2005; Banjanin et al. 2004; Owen et al. 2004). The period of rapid growth in both human and guinea-pig brain occurs in utero, whereas in rats and mice this period occurs postnatally. This important stage of brain development is particularly susceptible to insult (Dobbing & Sands, 1971; Owen et al. 2005).
We have shown that prenatal exposure to sGC significantly changes hippocampal expression of N-methyl-d-aspartate receptor (NMDA-R) subunits, an integral receptor in LTP induction, in the fetal and neonatal brain (Owen et al. 2005; Owen & Matthews, 2007). We have also identified permanent changes in the expression of hippocampal GR and MR in offspring exposed to sGCs, in utero (Liu et al. 2001; Dean et al. 2001; Owen & Matthews, 2003). Additionally, downstream effectors of LTP, such as calcium calmodulin kinase II (CaMKII), can be modulated by glucocorticoids (Gerges et al. 2004). There is currently no information concerning the impact of prenatal sGC exposure on LTP in offspring. In the present study, we hypothesized that prenatal exposure to sGC modifies LTP after birth, but also affects the modulatory role of glucocorticoids on hippocampal LTP through changes in hippocampal GR and MR expression.
Methods
Animals and experimental treatments
These studies were performed using protocols approved by the Animal Care Committee at the University of Toronto and in accordance with the Canadian Council for Animal Care. Female guinea-pigs were mated in our animal facility as previously described (McCabe et al. 2001). This method produces accurately time-dated pregnant guinea-pigs. Pregnant animals received subcutaneous (s.c.) injections of betamethasone (Beta, n = 12, 1 mg kg−1; Betaject, Sabex, Boucherville, QC, Canada; 6 mg ml−1) or saline (Control, n = 10), on gestational day (gd) 40, 41, 50, 51, 60 and 61, as previously described (McCabe et al. 2001). Animals were weighed on gd 30 and every 5 days thereafter until gd 65 but otherwise left undisturbed until they underwent treatment. Animals delivered normally (term ∼70 days), and offspring remained with their mothers until experimentation on postnatal day (pnd) 21. Guinea-pigs give birth to approximately three offspring. Pup weight was assessed at birth and every 5 days thereafter. The dose of betamethasone used was fourfold higher than that administered to pregnant women at risk of preterm labour because the binding affinity of the guinea-pig GR is approximately fourfold lower than in humans (Keightley & Fuller, 1995).
Electrophysiological recordings
Control conditions
On pnd 21 (± 2), offspring were removed between 08.30 and 09.30 h, anaesthetized with halothane and killed by decapitation. Hippocampal slices (400 μm) were collected as we have previously described (Bai et al. 2001) in artificial cerebrospinal fluid (ACSF; mm: 124 NaCl, 3 KCl, 1 MgSO4·7H2O, 2 CaCl2, 12.5 NaH2PO4·H2O, 2.6 NaHCO3 and 10 d-glucose; 300 mosmol l−1). A single slice was transferred to a submerged recording chamber and superfused with ACSF (2 ml min−1) saturated with 95% O2–5% CO2 at 30 ± 2°C (Pharmacia Fine Chemicals, Uppsala, Sweden). Hippocampal slices were equilibrated (10 min) prior to recording. Field potentials were elicited using constant-current stimuli delivered to the Schaffer collaterals through a bipolar stimulating electrode. Field excitatory postsynaptic potential (fEPSP) recordings were made using glass micropipettes with a resistance of 3–5 MΩ (Electrode Puller Type PP-83, no. 8904, Narishige Scientific Instruments, Tokyo, Japan; Glass Thinwall 3′ filaments, World Precision Instruments, Sarasota, FL, USA) filled with ACSF and placed in the stratum radiatum (dendritic layer of CA1). The fEPSPs were recorded using Clampex 9 (Axon Instruments, Union City, CA, USA). Test stimuli were evoked at 0.05 Hz with the stimulus intensity set to produce 30–40% of the maximum synaptic responses. Tetanic stimulation consisted of two trains of 100 Hz stimuli lasting 500 ms, at an intertrain interval of 20 s. In control conditions, this stimulation caused LTP that attained a stable level by 10 min after tetanus and which persisted for at least 60 min. For clarity, records from the first 30 min after tetanus are shown. Raw data were amplified using Clampex 9 (Axon Instruments) and analysed with Clampfit software (Axon Instruments). The slope of fEPSPs was calculated off-line by performing a linear regression through the data points corresponding to the rising phase (10–50%) of the synaptic response. The value of fEPSP slope from the 10 min period immediately before tetanus was defined as baseline (100%). For analysis, raw slopes before and after tetanus were normalized to the average of the baseline responses resulting in positive values. Two or three viable slices were obtained from each guinea-pig and these were allocated to different electrophysiological experimental groups or for Western blot analysis (see below). Recordings were discarded if responses varied by more than 15% during the baseline recording period.
Cortisol modulation of LTP
In order to determine cortisol-dependent modulation of LTP, hydrocortisone (cortisol; Sigma-Aldrich, St Louis, MO, USA), the primary endogenous glucocorticoid in guinea-pigs, was added to the ACSF perfusion after establishing 10 min of stable baseline recordings. Cortisol (0.1, 1.0 or 10 μm) was added to the ACSF 30 min prior to the induction of LTP (2 trains, 500 ms, 100 Hz), whereupon perfusion was switched back to ACSF containing no cortisol. Recordings continued for 1 h following LTP induction but for clarity only the first 30 min are shown. Hippocampal slices that had not been used for recording were rapidly frozen and stored at −80°C for later protein analysis.
Western blot analysis
Western blot analysis was undertaken as previously described (Owen & Matthews, 2003). Briefly, control hippocampal slices that had not been used for recordings were homogenized in radioimmunoprecipitation (RIPA) lysis buffer and centrifuged. Protein concentration of the supernatant was determined by the Bradford method (Bradford, 1976). 2X Laemmli sample buffer (Sigma, Oakville, ON, Canada) was added to each sample (25 μg protein), which was then denatured. Samples were separated by SDS-PAGE (8% resolving polyacrylamide gel) and transferred to a nitrocellulose membrane (Bio-Rad, Mississauga, ON, Canada). Membranes were blocked overnight (4°C) in skimmed milk (5% w/v) phosphate-buffered saline with Tween 20 (PBS-T) and then incubated with the primary antibody of interest at optimized dilutions [MR, 1:200 (Santa Cruz, CA, USA, sc-11412); GR, 1:2000 (Santa Cruz, sc-8995); NMDA-receptor subunit 1 (NR1), 1:500 (Chemicon International, CA, USA, AB1516); NMDA-receptor subunit 2A (NR2A), 1:10 000 (Chemicon International, AB1555); NMDA-receptor subunit 2B (NR2B), 1:5000 (Chemicon International, AB1548); CaMKII, 1:5000 (Santa Cruz, sc-13082); and P-CaMKII, 1:5000 (Santa Cruz, Thr286, sc-12886). Membranes were then washed and incubated with HRP-conjugated goat antirabbit IgG (1:5000; 1 h, 23°C; Perkin Elmer, Woodbridge, ON, Canada), exposed to Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer) and bands visualized using Kodak Blue X-OMAT film (Perkin Elmer). Membranes were stripped in Restore Western Blot Stripping buffer (20 ml, 30 min, 23°C; Pierce, MJS Bioynx, Mississauga, ON, Canada). The absolute optical density of each protein was analysed with computerized imaging software. All signals were standardized to α-tubulin (1:10 000, Santa Cruz, sc-5546). Western blots were performed a minimum of two times for each animal. Data were averaged for each animal.
Endocrine analysis
Total plasma cortisol concentrations were measured in duplicate in plasma collected at the time of hippocampal collection using a coated tube radioimmunoassay kit (ICN Biomedicals, Inc., Costa Mesa, CA, USA), which has been used previously in the guinea-pig (McCabe et al. 2001). All samples were run in the same assays to negate interassay bias.
Statistical analysis
Group data are presented as means ± s.e.m. and were statistically analysed using ANOVA followed by Duncan's method of post hoc comparison (Statistica, StatSoft Inc., Tulsa, OK, USA). Statistical significance was set at P < 0.05. If two-way ANOVA revealed a significant effect of sex or a significant interaction between sex and prenatal treatment, males and females were analysed separately. For electrophysiological recordings, the normalized slope values were analysed by repeated measures ANOVA. Recordings were separated according to baseline, the short-term potentiation (STP) defined as the EPSP immediately following tetanus, and the early LTP defined as the 30 min following tetanus. Protein expression by Western blot was analysed by ANOVA.
Results
Pregnancy and weight of offspring
There was no difference in maternal weight (control 1170 ± 34.2 g; Beta 1105 ± 33.8 g), gestation length (control 69.1 ± 0.4 days; Beta 69.6 ± 0.5 days) or litter size (control 3.6 ± 0.32; Beta 3.2 ± 0.25) between prenatal treatment groups. There was a significant effect of sex (P < 0.0001) and an interaction between prenatal treatment and sex (P < 0.0001) with respect to weights. There was a sex-specific treatment difference with respect to birthweight (Table 1), where Beta females had reduced total body weight at birth compared to control females (P < 0.02) while Beta males were significantly heavier than control males (P < 0.04). In contrast to females, Beta males remained significantly heavier through pnd 10 (pnd 5 P < 0.036 and pnd 10 P < 0.035), but prenatal treatment differences resolved by pnd 15 (Table 1).
Table 1.
Offspring weights (g) on postnatal day (pnd) 0, 5, 10, 15 and 21 in control female (F; n = 20), Beta F (n = 18), control male (M; n = 16) and Beta M (n = 15)
| Treatment | Sex | pnd 0 | pnd 5 | pnd 10 | pnd 15 | pnd 21 |
|---|---|---|---|---|---|---|
| Control | F | 101.6 ± 2.6 | 121.2 ± 3.9 | 160.8 ± 5.3 | 208.3 ± 6.4 | 256.8 ± 7.4 |
| Control | M | 96.6 ± 3.0 | 120.9 ± 4.4 | 165.0 ± 7.3 | 220.8 ± 9.8 | 289.1 ± 10.7† |
| Beta | F | 92.2 ± 2.9* | 116.1 ± 4.5 | 160.8 ± 5.0 | 206.8 ± 5.4 | 258.2 ± 7.0 |
| Beta | M | 106.0 ± 2.9*† | 135.1 ± 5.1*† | 185.5 ± 6.0*† | 238.5 ± 5.6† | 305.8 ± 9.3† |
Data are expressed as means ± s.e.m.
Significant effect (P < 0.05) of prenatal betamethasone treatment within same sex and age group.
Significant (P < 0.05) sex difference within prenatal treatment group at same age.
Offspring HPA activity
There was no effect of prenatal sGC treatment on total plasma cortisol at pnd 21. However, there was a significant sex effect (P < 0.0001), where males (control 74.2 ± 12.8 ng ml−1, n = 15; and Beta 75.3 ± 12.6 ng ml−1, n = 15) exhibited significantly lower total plasma cortisol concentrations than females (control 194.9 ± 26.3 ng ml−1, n = 18; and Beta 201.6 ± 30.6 ng ml−1, n = 17; P < 0.001) within each prenatal treatment group.
Electrophysiology
Control baseline and long-term potentiation
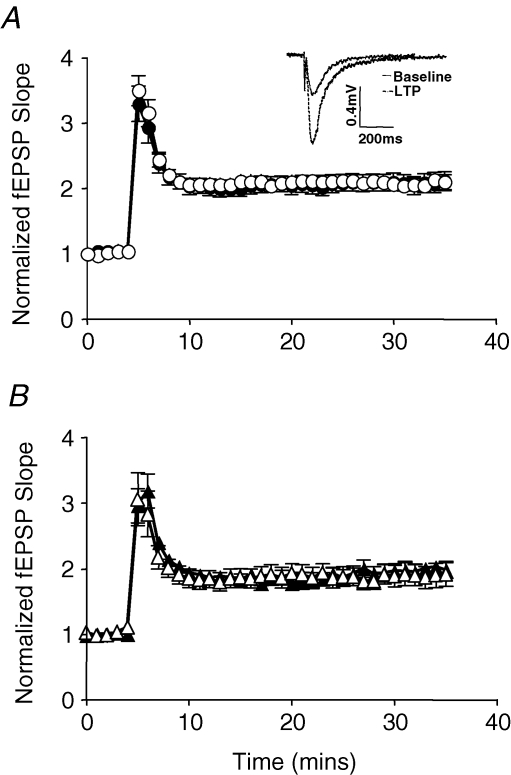
Two-way ANOVA revealed that there were no prenatal treatment effects or sex effects on normalized baseline fEPSPs, STP or LTP under control (no cortisol) conditions (Fig. 1). No differences were found in the fEPSP amplitude during baseline recording before tetanus, STP or LTP at 30 min following tetanus.
Figure 1.
Control (no cortisol) LTP in (A) female control (○; n = 16) and female Beta (•; n = 13) and (B) male control (▵; n = 10) and male Beta (▴; n = 7) Data are expressed as means ± s.e.m. Inset shows representative traces.
Modulation by cortisol
Application of cortisol (0.1 μm), 30 min prior to the induction of LTP, caused significant potentiation of baseline fEPSPs in female and male hippocampi from both prenatal treatment groups over the entire 30 min application (Fig. 2A and B; P < 0.001). However, there was no overall effect of prenatal sGC treatment or sex on STP or LTP following exposure to 0.1 μm cortisol.
Figure 2.
Cortisol effects on baseline fEPSPs and LTP during and after 30 min perfusion with cortisol A and B, 0.1 μm cortisol. A, female control (○; n = 9) and female Beta (•; n = 8). B, male control (▵; n = 8) and male Beta (▴; n = 10). C and D, 1.0 μm cortisol. C, female control (○; n = 10) and female Beta (•; n = 10). D, male control (▵; n = 7) and male Beta (▴; n = 11). E and F, 10 μm cortisol. E, female control (○; n = 9) and female Beta (•; n = 9) offspring. F, male control (▵; n = 8) and male Beta (▴; n = 11). Data are expressed as means ± s.e.m.* Significant effect (P < 0.05) of prenatal betamethasone treatment on STP and LTP after cortisol pretreatment. † Significant effect (P < 0.05) of cortisol over entire 30 min perfusion in control offspring. ‡ Significant effect (P < 0.05) of cortisol over entire 30 min perfusion in Beta offspring.
There was a significant overall effect of sex (P < 0.05) on fEPSPs during 1.0 μm cortisol application. Incubation with 1.0 μm cortisol had a significant potentiating effect on baseline fEPSPs over the 30 min application period in hippocampal slices derived from Beta females (P < 0.001), Beta males (P < 0.001) and control males (P < 0.001) but not in control females (Fig. 2C and D). There were no significant effects of prenatal sGC treatment or sex on STP or LTP.
Application of cortisol (10 μm) had a significant inhibitory effect on baseline fEPSPs in male and female offspring of both prenatal treatment groups (Figs 2E and F; P < 0.00001). There was an overall interaction between sex and prenatal treatment on STP and LTP (P < 0.05) after 10 μm cortisol application. This concentration of cortisol had a significant inhibitory effect on LTP in hippocampi derived from control males, control females and Beta males when compared with LTP in the cortisol-free condition (Fig. 2A, B, E and F; P < 0.05). However, there was no effect of cortisol (10 μm) on LTP in Beta females (Fig. 2E). This resulted in a significant effect of prenatal treatment in the females, with Beta females showing an enhanced STP (P < 0.05) and LTP (P < 0.05) when compared with control females, i.e. a prevention of the cortisol-induced inhibition. In contrast, in males, there was no significant effect of prenatal sGC treatment on LTP following exposure to 10 μm cortisol (Fig. 2F).
Hippocampal GR and MR protein
Two-way ANOVA revealed an interaction between prenatal treatment and sex on both GR (P < 0.05) and MR (P < 0.01) and a significant effect of sex on GR (P < 0.001). In females, prenatal sGC treatment increased hippocampal GR (P < 0.02) and MR (P < 0.003) compared with control animals (Fig. 3A and B). There was no effect of prenatal sGC treatment on GR or MR protein in male hippocampi. There was a significant sex difference in hippocampal GR and MR protein, with control males exhibiting higher expression than control females (GR, P < 0.0005; and MR, P < 0.006; Fig. 3). Prenatal sGC treatment abolished these sex differences.
Figure 3.
Hippocampal protein expression of GR (A) and MR (B) expressed as a ratio to α-tubulin Data are means ± s.e.m. relative optical density (ROD) for female and male offspring treated prenatally with either betamethasone (filled bars) or saline (open bars). * Significant effect (P < 0.05) of prenatal betamethasone treatment within sex. † Significant effect (P < 0.05) of sex in control offspring. Representative blots are bands from two different subjects per treatment group from one experiment.
Hippocampal NMDA-R subunit protein
There was a significant overall effect of prenatal treatment on NR1, NR2A and NR2B expression and an almost significant interaction between prenatal treatment and sex on NR2A and NR2B (P < 0.07 and P < 0.08, respectively). Prenatal sGC treatment resulted in a significant reduction in hippocampal NR1 (P < 0.05), NR2A (P < 0.01) and NR2B protein expression (P < 0.02) in male offspring (Table 2). There were no differences between control and Beta female offspring. While there were no sex differences in NR2A expression, males exhibited significantly lower hippocampal NR2B expression than females (P < 0.01) following prenatal sGC treatment.
Table 2.
Hippocampal protein expression of NMDA receptor subunits (NR1, NR2A and NR2B) in control female (F; n = 12), Beta F (n = 10) control male (M; n = 13) and Beta M (n = 9)
| Treatment | Sex | NR1 | NR2A | NR2B |
|---|---|---|---|---|
| Control | F | 0.62 ± 0.03 | 0.56 ± 0.02 | 0.69 ± 0.03 |
| Control | M | 0.65 ± 0.03 | 0.60 ± 0.02 | 0.73 ± 0.04 |
| Beta | F | 0.58 ± 0.03 | 0.55 ± 0.02 | 0.67 ± 0.02 |
| Beta | M | 0.56 ± 0.02* | 0.51 ± 0.01* | 0.60 ± 0.02*† |
Data are expressed as relative optical density (ROD) of protein of interest/α-tubulin ratio, means ± s.e.m.
Significant effect (P < 0.05) of prenatal treatment within sex.
Significant (P < 0.05) sex difference within prenatal treatment group.
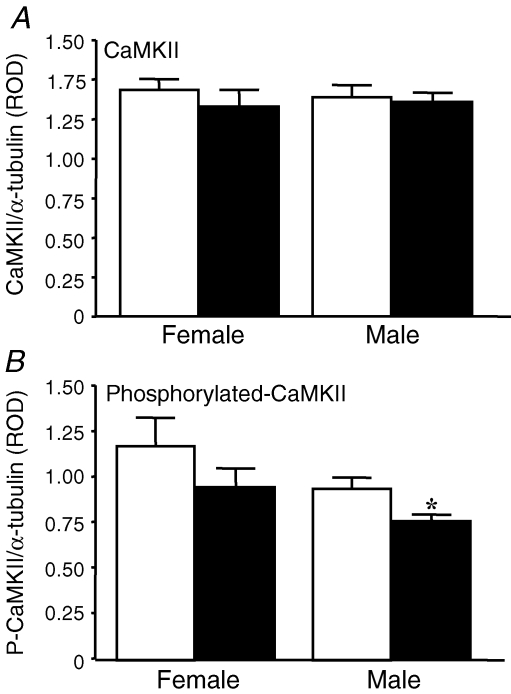
Hippocampal CaMKII and phosphorylated CaMKII protein
At CA1 synapses, CaMKII is a kinase downstream of the NMDA receptor, which is required for induction of LTP (Blitzer et al. 2005). There were no effects of prenatal sGC treatment or sex on hippocampal CaMKII protein (Fig. 4). There was an overall effect of prenatal sGC treatment and sex (P < 0.05) on phosphorylated CaMKII (P-CaMKII) expression. Post hoc analysis revealed that hippocampal levels of P-CaMKII expression were lower in Beta males compared with control males (P < 0.03). However, there were no significant effects of prenatal sGC treatment on hippocampal P-CaMKII expression in female offspring.
Figure 4.
Hippocampal expression of CaMKII (A) and P-CaMKII (B) expressed as a ratio to α-tubulin Data are means ± s.e.m. relative optical density (ROD) for female and male offspring treated prenatally with either betamethasone (filled bars) or saline (open bars). * Significant effect (P < 0.05) of prenatal betamethasone treatment within sex.
Discussion
This mechanistic study is the first to examine the effects of prenatal exposure to sGC on hippocampal LTP in offspring. Prenatal exposure to sGC did not produce differences in fEPSPs, STP or LTP under control or lower dose cortisol (0.1 and 1.0 μm) conditions. However, in female offspring, prenatal sGC treatment prevented the inhibition of STP and LTP induced by cortisol (10 μm). Further, we have identified changes in hippocampal GR and MR levels that may account, in part, for the differences in cortisol modulation of STP and LTP after prenatal exposure to sGC in female offspring. Differences in synaptic plasticity occurred in the absence of effects on bodyweight or endogenous cortisol levels at the time of experimentation.
Application of 0.1 μm cortisol, in vitro, resulted in a potentiation while 10 μm significantly depressed fEPSPs during baseline recordings. These results are consistent with the MR-dependent decrease and the GR-mediated increase in the after-hyperpolarization (AHP), a determinant of neuronal excitability previously reported in the rat hippocampus (Joels & de Kloet, 1990, 1991). Neuronal excitability may also be altered by modulation of voltage-dependent Ca2+ currents (VDCC) and/or NMDA-mediated Ca2+ currents, where GR prolongs and MR attenuates the transient Ca2+ current (Karst et al. 1994; Takahashi et al. 2002). Although MR effects appear when concentrations of cortisol in the perfusate exceed those that would normally be associated with maximal occupation of GRs (Kd∼5 nm; Reul & de Kloet, 1985), in vivo, the levels of cortisol penetrating the hippocampal slice are likely to be very significantly reduced compared with the in vivo situation, given the artificial perfusion (van Haarst et al. 1996).
Glucocorticoid modulation of STP and LTP was observed at the highest concentration of cortisol (10 μm). Both STP and LTP were suppressed in hippocampal slices from the Beta males and control animals but not in the Beta females, in which the potentiation was similar to that observed in the absence of cortisol. Glucocorticoid receptor-mediated inhibition and MR-mediated facilitation of LTP have been reported previously in the rat (Pavlides et al. 1996). A lack of inhibition resulting from exposure to cortisol would suggest a decreased GR and/or increased MR contribution. Results from this study indicate that hippocampal GR and MR expression are both significantly increased in Beta females compared with control females. However, the greater increase in MR may override any effects of the modestly elevated GR, favouring MR-mediated potentiation. In the present study, analysis was confined to GR and MR protein in the entire hippocampus and so we can only speculate as to the relative amount of cortisol binding to GR and MR in specific fields of the hippocampal formation.
In a study by Pavlides et al. (1996), the authors caution that handling of the animals immediately before slice preparation might affect subsequent in vitro results owing to activation of the HPA axis immediately prior to tissue collection. In the present study, measurement of plasma cortisol concentrations immediately prior to hippocampal slice collection established no significant difference between control and Beta females. Thus, we are confident in attributing the glucocorticoid effect identified to the in vitro cortisol application.
Studies supporting the classical view of genomic effects of glucocorticoids have confirmed that changes in excitability depend on DNA binding and protein synthesis (Karst & Joels, 1991; Karst et al. 2000). Others argue that the time to observe effects (within 10 min) and the use of membrane-impermeable ligands favours the existence of non-genomic, membrane recognition sites (Hua & Chen, 1989; Takahashi et al. 2002). In the present study, effects of cortisol on baseline fEPSPs were seen within 10 min of steroid application and are likely to reflect membrane-associated actions. In contrast, the actions of cortisol on LTP may involve early genomic effects, or a combination of membrane and genomic interactions. Further time course and pharmacological studies are required to resolve the relative involvement of the different pathways.
In the present study, we have identified significant sex differences in cortisol modulation of LTP following prenatal betamethasone treatment. The age at which experiments were performed was selected so as to avoid puberty and thus differences resulting from high levels of circulating sex steroid hormones. Oestrogen and testosterone are known to be potent modulators of LTP in the adult rat; oestrogen enhances while testosterone suppresses LTP (Foy et al. 1999; Harley et al. 2000). We have shown oestrogen levels to be very low in prepubertal female guinea-pigs and that prenatal sGC exposure does not influence these levels (S. G. Matthews & E. Setiawan, unpublished observations). However, the sex differences observed in plasma cortisol at pnd 21 could potentially contribute to the sex-specific differences observed in the modulation of LTP by cortisol. Further studies are clearly warranted to investigate the mechanism that underlies the sex differences identified.
Differential expression of NR2A and NR2B subunits confers biophysical and pharmacological properties to the NMDA-R (Cull-Candy & Leszkiewicz, 2004), and there has been some debate as to which is the critical subunit in induction of LTP (Liu et al. 2004; Berberich et al. 2005). In the present study, we identified a significant reduction in hippocampal NR1, NR2A and NR2B protein expression in male offspring that had been prenatally exposed to betamethasone. However, there were no significant differences in NR2A protein expression relative to NR2B (both decreased to a similar level). Further, there was no difference in the expression of any of the NR subunits in female offspring that had been prenatally exposed to betamethasone, and that had displayed altered LTP following exposure to 10 μm cortisol. It should also be noted that protein analysis was undertaken in a slice containing all fields of the hippocampus, and it is possible that there were significant changes in the expression of NMDA-R subunits in specific subregions of the hippocampus. In this regard, we have shown reduced NR1 mRNA in the CA1 region of the hippocampus on pnd 10 in female guinea-pigs treated with an identical prenatal betamethasone regimen to that described in the present study (Owen & Matthews, 2007). This region-specific difference in NR1 mRNA expression might contribute to the differences in LTP seen in Beta females compared with control animals, independent of GR- or MR-mediated actions. Unfortunately, the nature of the present study did not allow us to undertake analysis of regional expression within the hippocampus.
Calcium calmodulin kinase II is another key molecule in LTP formation, and its phosphorylated form (P-CaMKII) is a key downstream effector of LTP maintenance (Lisman et al. 2002). Surprisingly, a decrease in basal P-CaMKII in Beta males did not translate into differences in LTP. Other studies have found that stress-induced inhibition of LTP corresponds with reduced P-CaMKII and CaMKII in the adult rat (Gerges et al. 2004). Future studies are required to investigate P-CaMKII and CaMKII levels after LTP induction in tissue slices that have been exposed to cortisol in vitro.
Stress, both physical and psychological, has detrimental effects on LTP in the hippocampus (Foy et al. 1987; Shors et al. 1989) that are largely due to increased glucocorticoid release (Korz & Frey, 2003). In the present study, increasing concentrations of cortisol, in vitro, were used to mimic stress-induced increases in cortisol. Our results indicate that prenatal exposure to sGC may attenuate the stress-induced inhibition of LTP in females. This might indicate a potential protective effect of prenatal sGC exposure on LTP during periods of acute stress in female offspring. However, glucocorticoids do not act independently, since other HPA axis markers, such as arginine vasopressin and catecholamines, correlate with learning and memory and, in turn, can modulate LTP (Izumi et al. 1992; Sakurai et al. 1998). Further, other structures, such as the amygdala, are key components in stress-induced effects on LTP (Kim et al. 2005). Clearly, a direct extrapolation to an in vivo stress situation and the resultant effects on synaptic plasticity cannot be made owing to the neuroanatomical and neuroendocrine isolation of the in vitro preparation, and results must be interpreted with care.
Differences in synaptic plasticity and their correlation to differences in behavioural aspects of learning and memory are still highly debated (Holscher, 1999). Even so, synaptic plasticity is likely to play a role in memory formation, given that inhibition of LTP inhibits memory and that structures that express LTP are also integral in memory formation (Okada et al. 2003). In vivo studies, such as those in hippocampal place cells using implanted electrodes (Dragoi et al. 2003), are strengthening the connection between electrophysiological and behavioural changes and will answer these questions more definitively. The results of the present study suggest that prenatal exposure to sGC may result in sex-specific mechanistic changes in memory function in situations of high cortisol, such as stress.
The long-term consequences of single or repeated course prenatal sGC exposure on behaviour in humans are largely unknown. Evidence is beginning to emerge to indicate that children who were exposed to either prenatal sGC or increased endogenous glucocorticoid resulting from maternal stress may be at higher risk of emotional and behavioural abnormalities. A recent study conducted in school-aged children who received three or more courses of antenatal betamethasone showed that such treatment was associated with an increased risk of postnatal aggressive/destructive behaviour, increased distractibility and hyperactivity (French et al. 2004). Animal studies have identified effects of prenatal exposure to sGC on neuron degeneration and function (Slotkin et al. 1992; Uno et al. 1994), brain volume and hippocampal volume (Moss et al. 2005), and longitudinal changes in central control of blood pressure (O'Connor et al. 2002). In the present study, to our surprise, there was no effect of prenatal sGC exposure on LTP in the absence of exogenous cortisol application. This suggests that the mechanisms regulating LTP, under resting conditions, in prepubertal animals, may be resistant to the influences of prenatal exposure to sGC. However, this study does show that multiple courses of prenatal sGC can produce a sex-specific difference in LTP in the presence of cortisol, which may lead to changes in memory performance during periods of stress.
Acknowledgments
We would like to acknowledge Linda Liu for her technical assistance in the protein analysis. This study was funded by the Canadian Institutes of Health Research with grants to J.F.M. and S.G.M. (MOP49511). E.S. received a CIHR Genes, Environment and Health Trainee award and Ontario Graduate Scholarship in Science and Technology.
References
- Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol. 2003;547:117–123. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Banjanin S, Kapoor A, Matthews S. Prenatal glucocorticoid exposure alters hypothalamic–pituitary–adrenal function and blood pressure in mature male guinea pigs. J Physiol. 2004;558:305–318. doi: 10.1113/jphysiol.2004.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Chavez S, Truss M. Transcriptional regulation by steroid hormones. Steroids. 1996;61:240–251. doi: 10.1016/0039-128x(96)00030-x. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry. 2005;57:113–119. doi: 10.1016/j.biopsych.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Block MF, Kling OR, Crosby WM. Antenatal glucocorticoid therapy for the prevention of respiratory distress syndrome in the premature infant. Obstet Gynecol. 1977;50:186–190. [PubMed] [Google Scholar]
- Bohn MC, Dean D, Hussain S, Giuliano R. Development of mRNAs for glucocorticoid and mineralocorticoid receptors in rat hippocampus. Dev Brain Res. 1994;77:157–162. doi: 10.1016/0165-3806(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004(255):re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dean F, Yu C, Lingas RI, Matthews SG. Prenatal glucocorticoid modifies hypothalamo-pituitary-adrenal regulation in prepubertal guinea pigs. Neuroendocrinology. 2001;73:194–202. doi: 10.1159/000054636. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Vulnerability of the developing brain. Neonate. 1971;19:363–378. doi: 10.1159/000240430. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, Buzsaki G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17β-Estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190:588–595. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Aleisa AM, Schwarz LA, Alkadhi KA. Reduced basal CaMKII levels in hippocampal CA1 region: possible cause of stress-induced impairment of LTP in chronically stressed rats. Hippocampus. 2004;14:402–410. doi: 10.1002/hipo.10193. [DOI] [PubMed] [Google Scholar]
- Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–697. doi: 10.1002/1098-1063(2000)10:6<693::AID-HIPO1007>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Holscher C. Synaptic plasticity and learning and memory: LTP and beyond. J Neurosci Res. 1999;58:62–75. [PubMed] [Google Scholar]
- Hua SY, Chen YZ. Membrane receptor-mediated electrophysiological effects of glucocorticoid on mammalian neurons. Endocrinology. 1989;124:687–691. doi: 10.1210/endo-124-2-687. [DOI] [PubMed] [Google Scholar]
- Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. Int J Dev Neurosci. 2001;19:415–425. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Norepinephrine reverses N-methyl-d-aspartate-mediated inhibition of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1992;142:163–166. doi: 10.1016/0304-3940(92)90364-d. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Mineralocorticoid receptor-mediated changes in membrane properties of rat CA1 pyramidal neurons in vitro. Proc Natl Acad Sci U S A. 1990;87:4495–4498. doi: 10.1073/pnas.87.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Effect of corticosteroid hormones on electrical activity in rat hippocampus. J Steroid Biochem Mol Biol. 1991;40:83–86. doi: 10.1016/0960-0760(91)90170-a. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Joels M. The induction of corticosteroid actions on membrane properties of hippocampal CA1 neurons requires protein synthesis. Neurosci Lett. 1991;130:27–31. doi: 10.1016/0304-3940(91)90219-j. [DOI] [PubMed] [Google Scholar]
- Karst H, Karten YJ, Reichardt HM, de Kloet ER, Schutz G, Joels M. Corticosteroid actions in hippocampus require DNA binding of glucocorticoid receptor homodimers. Nat Neurosci. 2000;3:977–978. doi: 10.1038/79910. [DOI] [PubMed] [Google Scholar]
- Karst H, Wadman WJ, Joels M. Corticosteroid receptor-dependent modulation of calcium currents in rat hippocampal CA1 neurons. Brain Res. 1994;649:234–242. doi: 10.1016/0006-8993(94)91069-3. [DOI] [PubMed] [Google Scholar]
- Keightley MC, Fuller PJ. Cortisol resistance and the guinea pig glucocorticoid receptor. Steroids. 1995;60:87–92. doi: 10.1016/0039-128x(94)00014-4. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, Han JS. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: involvement of adrenal steroid receptors. J Neurosci. 2003;23:7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–E739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;30:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moss TJ, Doherty DA, Nitsos I, Sloboda DM, Harding R, Newnham JP. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am J Obstet Gynecol. 2005;192:146–152. doi: 10.1016/j.ajog.2004.06.065. [DOI] [PubMed] [Google Scholar]
- NIH Consensus development conference. Effect of corticosteroids for fetal maturation and perinatal outcomes. Am J Obstet Gynecol. 1995;173:253–344. [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- Okada T, Yamada N, Tsuzuki K, Horikawa HP, Tanaka K, Ozawa S. Long-term potentiation in the hippocampal CA1 area and dentate gyrus plays different roles in spatial learning. Eur J Neurosci. 2003;17:341–349. doi: 10.1046/j.1460-9568.2003.02458.x. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Repeated maternal glucocorticoid treatment affects activity and hippocampal NMDA receptor expression in juvenile guinea pigs. J Physiol. 2007;578:249–257. doi: 10.1113/jphysiol.2006.122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D, Setiawan E, Li A, McCabe L, Matthews SG. Regulation of N-methyl-d-aspartate receptor subunit expression in the fetal guinea pig brain. Biol Reprod. 2004;71:676–683. doi: 10.1095/biolreprod.104.027946. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Ogawa S, Kimura A, McEwen BS. Role of adrenal steroid mineralocorticoid and glucocorticoid receptors in long-term potentiation in the CA1 field of hippocampal slices. Brain Res. 1996;738:229–235. doi: 10.1016/s0006-8993(96)00776-7. [DOI] [PubMed] [Google Scholar]
- Quinlivan JA, Evans SF, Dunlop SA, Beazley LD, Newnham JP. Use of corticosteroids by Australian obstetricians – a survey of clinical practice. Aust N Z J Obst Gynaecol. 1998;38:1–7. doi: 10.1111/j.1479-828x.1998.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rua C, Trejo JL, Machin C, Arahuetes RM. Effects of maternal adrenalectomy and glucocorticoid administration on the development of rat hippocampus. J Hirnforsch. 1995;36:473–483. [PubMed] [Google Scholar]
- Sakurai E, Maeda T, Kaneko S, Akaike A, Satoh M. Inhibition by [Arg8]-vasopressin of long term potentiation in guinea pig hippocampal slice. Jpn J Pharmacol. 1998;77:103–105. doi: 10.1254/jjp.77.103. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, McCook EC, Tayyeb MI, Eylers JP, Seidler FJ. Glucocorticoids and the development of neuronal function: effects of prenatal dexamethasone exposure on central noradrenergic activity. Biol Neonate. 1992;61:326–336. doi: 10.1159/000243761. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dean E, Neubort S. Electron microscopic analysis of adrenalectomy-induced hippocampal granule cell degeneration in the rat: apoptosis in the adult central nervous system. J Comp Neurol. 1993;330:337–351. doi: 10.1002/cne.903300305. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kimoto T, Tanabe N, Hattori TA, Yasumatsu N, Kawato S. Corticosterone acutely prolonged N-methyl-d-aspartate receptor-mediated Ca2+ elevation in cultured rat hippocampal neurons. J Neurochem. 2002;83:1441–1451. doi: 10.1046/j.1471-4159.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- van Haarst AD, Welberg LA, Sutanto W, Oitzl MS, de Kloet ER. 11β-Hydroxysteroid dehydrogenase activity in the hippocampus: implications for in vivo corticosterone receptor binding and cell nuclear retention. J Neuroendocrinol. 1996;8:595–600. [PubMed] [Google Scholar]