Abstract
Dopamine (DA) effects on prefrontal cortex (PFC) neurons are essential for the cognitive functions mediated by this cortical area. However, the cellular mechanisms of DA neuromodulation in neocortex are not well understood. We characterized the effects of D1-type DA receptor (D1R) activation on the amplification (increase in duration and area) of excitatory postsynaptic potentials (EPSPs) at depolarized potentials, in layer 5 pyramidal neurons from rat PFC. Simulated EPSPs (sEPSPs) were elicited by current injection, to determine the effects of D1R activation independent of modulation of transmitter release or glutamate receptor currents. Application of the D1R agonist SKF81297 attenuated sEPSP amplification at depolarized potentials in a concentration-dependent manner. The SKF81297 effects were inhibited by the D1R antagonist SCH23390. The voltage-gated Na+ channel blocker tetrodotoxin (TTX) abolished the effects of SKF81297 on sEPSP amplification, suggesting that Na+ currents are necessary for the D1R effect. Furthermore, blockade of 4-AP- and TEA-sensitive K+ channels in the presence of TTX significantly increased EPSP amplification, arguing against the possibility that SKF81297 up-regulates currents that attenuate sEPSP amplification. SKF81297 application attenuated the subthreshold response to injection of depolarizing current ramps, in a manner consistent with a decrease in the persistent Na+ current. In addition, D1R activation decreased the effectiveness of temporal EPSP summation during 20 Hz sEPSP trains, selectively at depolarized membrane potentials. Therefore, the effects of D1R activation on Na+ channel-dependent EPSP amplification may regulate the impact of coincidence detection versus temporal integration mechanisms in PFC pyramidal neurons.
Input from midbrain dopamine (DA) cell nuclei onto prefrontal cortex (PFC) neurons is essential for the cognitive functions mediated by this cortical area. Consequently, disturbances of DA signalling in the PFC are central to the pathophysiology of cognitive dysfunction in mental disorders such as schizophrenia (Lewis & Gonzalez-Burgos, 2006). Furthermore, behavioural alterations in drug addiction are thought to involve altered DA regulation of the interactions between the PFC and other brain regions, including the basal ganglia (Kalivas et al. 2005).
In the mammalian brain, DA appears to act exclusively via high affinity G-protein coupled receptors (Neve & Neve, 1997; Missale et al. 1998). Therefore, DA may influence neural activity by modulating the effects of ligand- and voltage-dependent channels but not via fast synaptic communication. Although the neuromodulatory effects of DA in the PFC have been intensively studied (Seamans & Yang, 2004), many aspects of its actions are still not clearly understood. One way in which DA neuromodulation may influence PFC-dependent cognitive functions is by regulating the flow of neural activity in PFC circuits.
Transmission of activity in local circuits depends in part on the mode in which PFC pyramidal cells integrate synaptic inputs to generate spikes. EPSP–spike coupling may occur via coincidence detection or temporal integration mechanisms (Koch et al. 1996; Konig et al. 1996). Whether single pyramidal cells behave as coincidence detectors or temporal integrators depends, in part, on the EPSP decay time course: the faster the EPSP decay, the more coincident the inputs must be to summate and trigger spikes (Koch et al. 1996; Konig et al. 1996). Accordingly, the EPSP decay time course is critical for the ability of neocortical pyramidal neurons to generate outputs based on detection of input timing (Grande et al. 2004).
EPSP–spike coupling under most circumstances occurs in the axon initial segment (Stuart et al. 1997; Palmer & Stuart, 2006; Shu et al. 2006), a compartment of the pyramidal cell membrane that seems to contain a relatively high density of Na+ channels. Recordings from pyramidal neurons in slices of neocortex revealed that, near spike threshold, voltage-dependent Na+ channels produce a significant prolongation of the EPSP decay time (Sutor & Hablitz, 1989; Deisz et al. 1991; Stuart & Sakmann, 1995; Fricker & Miles, 2000; Gonzalez-Burgos & Barrionuevo, 2001; Zsiros & Hestrin, 2005). Such increase in EPSP decay and integral at depolarized potentials, called EPSP amplification, enhances the EPSP-induced depolarization without changing the synaptic conductance (Stuart & Sakmann, 1995; Schwindt & Crill, 1995; Andreasen & Lambert, 1999; Fricker & Miles, 2000).
Because it is apparent during sustained membrane depolarization, amplification of small EPSPs is thought to be mediated by activation of the persistent Na+ current (INaP) (Stafstrom et al. 1985; Sutor & Hablitz, 1989; Deisz et al. 1991; Stuart & Sakmann, 1995). The INaP displays little or no inactivation during prolonged membrane depolarization that strongly inactivates the fast Na+ current (Crill, 1996). Although the molecular basis of INaP generation is still a matter of active investigation, recent data suggest that there are no specific Na+ channel protein subtypes that exclusively mediate the INaP. Apparently INaP generation is the consequence of special gating features of channels containing either Nav1.1, Nav1.2 or Nav1.6 α subunits, with all of these channel subtypes giving rise to both INaP and fast Na+ current (Goldin, 2001; Maurice et al. 2001; Astman et al. 2006). Acting via G-protein coupled receptors, DA controls Na+ currents via multiple intracellular signalling cascades (Cantrell & Catterall, 2001). Therefore, regulation of Na+ current-dependent EPSP amplification is an important mechanism by which DA could affect the EPSP decay time course and therefore the integrative properties of PFC pyramidal neurons.
Here, we characterized the effects of DA receptor activation on EPSP amplification in layer 5 pyramidal neurons from the rat medial PFC. Shortly after activation of D1-type DA receptors (D1Rs) the magnitude of EPSP amplification at depolarized membrane potentials decreased. D1R activation also decreased the subthreshold depolarization elicited by slow depolarizing current ramps. The D1R-mediated effects on sEPSPs and on the response to current ramps were consistent with down-regulation of the INaP and, furthermore, were abolished by prior blockade of Na+ channels. Our findings also suggest that an important functional consequence of this D1R-mediated effect is to decrease the effectiveness of temporal integration, thus favouring coincidence detection, specifically when PFC pyramidal neurons are in a depolarized membrane potential state.
Methods
Slice preparation
Male Wistar rats (19–28 days old) were deeply anaesthetized with halothane and decapitated following procedures in accordance with NIH guidelines and approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. The brain was quickly removed and immersed in ice-cold, artificial cerebrospinal fluid (ACSF) containing (mm): sucrose 210.0, NaCl 10.0, KCl 1.9, Na2HPO4 1.2, NaHCO3 33.0, MgCl2 6.0, CaCl2 1.0, glucose 10.0 and kynurenic acid 2.0; pH 7.3–7.4, and continuously bubbled with 95% O2–5% CO2. The frontal cortex was next sectioned into 350 μm-thick slices in the coronal plane, using a vibrating microtome (VT1000S, Leica Microsystems, Nussloch, Germany). Slices were immediately placed in an incubation chamber filled with a solution maintained at 36°C and containing (mm): NaCl 126.0, KCl 2.5, Na2HPO4 1.2, glucose 10.0, NaHCO3 25.0, MgCl2 1.0 and CaCl2 2.0, pH 7.3–7.4 when bubbled with 95% O2–5% CO2. After 60 min of incubation, brain slices were stabilized at room temperature in the same solution for at least 50 min before they were transferred to the recording chamber.
Electrophysiological recordings
For recording, slices were transferred to a submersion recording chamber and superfused at a rate of 1–2 ml min−1 with an oxygenated solution of the following composition (mm): NaCl 126.0, KCl 2.5, Na2HPO4 1.2, glucose 10.0, NaHCO3 25.0, MgCl2 1.0 and CaCl2 2.0, pH 7.3–7.4 when bubbled with 95% O2–5% CO2, at a temperature of 30–32°C. The superfusion solution contained bicuculline or gabazine (10 μm) and 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX; 10 μm) to block GABA- and glutamate-mediated fast synaptic transmission, respectively. In addition, the recording solution contained 1.5 mm of the antioxidant ascorbic acid.
Voltage recordings were obtained, using patch pipettes, from layer 5 pyramidal cells visually identified in the infralimbic or prelimbic regions of the medial frontal cortex, under a microscope equipped with infrared illumination and differential interference contrast video-microscopy. Pipettes pulled from borosilicate glass had a resistance of 6–11 MΩ when filled with the following pipette solution (mm): K-gluconate 120, NaCl 10, Hepes 10, EGTA 0.2, MgATP 4.5, NaGTP 0.3, and Na-phosphocreatine 14, pH 7.2–7.4. Voltage recordings were obtained from the soma using Axoclamp 200A amplifiers (Axon Instruments, Union City, CA, USA) and signals were low-pass filtered at 3 kHz, digitized at 10 kHz, and stored on disk for off-line analysis. Data acquisition and analysis were performed using Signal 2.0 (Cambridge Electronic Design, Cambridge, UK). Membrane potential was not corrected for liquid junction potential. Series resistance and pipette capacitance were monitored and cancelled using built-in bridge circuit. Only recordings with a stable series resistance of less than 20 MΩ were used for analysis. Only neurons that had a resting membrane potential of at least −65 mV were included in this study.
In order to determine the effects of changes in membrane potential on the EPSP shape, the cells' membrane potential was varied in 5 mV increments, from hyperpolarized values (approximately −80 mV) to depolarized values near action potential threshold (approximately −50 mV, Fig. 1Aa), by injecting current via the recording electrode. To study the effects of DA receptor activation on EPSP amplification after fast synaptic transmission was blocked, we generated EPSP-like depolarizations (Fig. 1Aa), referred to as simulated EPSPs (sEPSPs). For this purpose, EPSC-like depolarizing current pulses of constant peak amplitude (50 pA) were injected into the soma of the recorded neuron. Similar to a previous study of PFC neurons (Gonzalez-Burgos & Barrionuevo, 2001), these current pulses elicited membrane depolarizations that closely resembled actual EPSPs (Fig. 1Aa). The analysis and the measurements of sEPSP area, amplitude, rise time and decay time were done on average traces obtained from at least 10 sequential sweeps. At depolarized potentials near spike threshold, the sweeps in which the cell fired action potentials after the injection of EPSC-like current were excluded from the average. At the resting membrane potential, the sEPSPs had an average amplitude of 1.09 ± 0.09 mV and a 10–90% rise time of 7.06 ± 0.94 ms. The 10–90% decay phase was well fitted by a single exponential decay function with a mean time constant of 25.57 ± 2.12 ms. The area of the sEPSPs was estimated by calculating the integral under the sEPSP waveform up to 280 ms after the EPSP onset. Typically, the membrane potential was held near −80 mV and SKF81297 was applied to the recording chamber. After 2–3 min of SKF81297 application, the membrane potential was varied between −80 and ∼−50 mV, and sEPSP amplification was measured within 7 min of SKF81297 application. In the absence of current injection, SKF81297 (5 μm) produced a significant membrane depolarization within 30–60 s of its application (the membrane potential was −70.1 ± 1.3 mV in control conditions and −64.3 ± 1.4 mV in the presence of SKF81297, n = 9, P < 0.00001, Student's t test). Here, the effects of D1R activation were determined independently of D1R-induced changes in the membrane potential, which was maintained near controlled values using injection of steady-state current. The D2 receptor agonist quinpirole was applied in an identical manner than SK81297. When the effects of the D1R antagonist SCH23390 were tested, the compound was bath applied 5–7 min prior to SKF81297, which was then applied similarly to what described above, in the continuous presence of SCH23390.
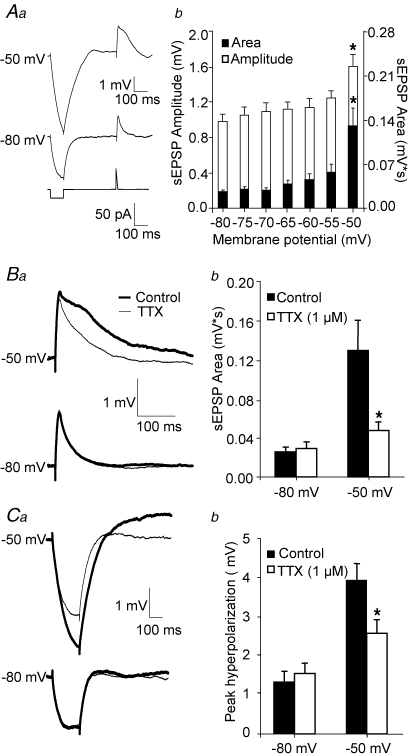
Figure 1.
Amplification of sEPSPs elicited by somatic current injection is abolished by bath-applied TTX Aa, EPSP-like depolarizations elicited by somatic current injection (lower traces, see methods) at −80 mV (middle traces), and at a depolarized potential near firing threshold (upper traces). The duration and area under the EPSP waveform were significantly increased by depolarization. The response to small hyperpolarizing steps was similarly enhanced. b, bar graph summarizing the effects of membrane potential on sEPSP amplitude and area. Single factor ANOVA (n = 10 cells) demonstrated significant effects of membrane potential on sEPSP amplitude (F6,63 = 4.92; P < 0.001) and sEPSP area (F6,63 = 7.44, P < 0.001). Amplitude and area at −50 mV were significantly different compared to those at −80 mV (*P < 0.05). Ba, example sweeps illustrating the attenuation of sEPSP amplification at depolarized potentials by TTX (1 μm). b, bar graph summarizing the effects of TTX (1 μm) on sEPSPs. Two factor ANOVA revealed significant effects of membrane potential (F1,36 = 14.65 P < 0.001), of TTX application (F1,36 = 5.93 *P < 0.02) and a significant interaction (F1,36 = 7.09, P < 0.02). Ca, example sweeps illustrating the effects of TTX (1 μm) on the response to hyperpolarizing steps elicited at hyperpolarized and depolarized potentials. b, bar graphs summarizing the effects of TTX (1 μm) on the response to hyperpolarizing steps (n = 10 cells). Two factor ANOVA revealed significant effects of membrane potential (F1,36 = 32.83 P < 0.001), and a significant interaction (F1,36 = 6.15 *P = 0.02), suggesting that the effects of TTX were conditional to membrane potential value.
To monitor changes in the input resistance of the cells, 500 ms prior to the sEPSP, a small hyperpolarizing current pulse (−20 pA, for 100 ms) was injected into the recorded neuron. The response to these brief and small hyperpolarization current steps was also used to determine the deactivation of tonically activated currents at depolarized potentials. The amplitude and area of sEPSPs recorded at different steady-state potentials with or without the preceding hyperpolarizing steps did not differ significantly. This result showed that the effects of steady-state membrane potential completely predominated over the possible effects of the brief and small hyperpolarization produced by the steps injected 500 ms prior to the sEPSP. To further assess the effects of D1R activation, a slow depolarizing current ramp of 600 ms duration was injected into the soma 500 ms after the sEPSP. The amplitude of the current at the end of the ramp (usually between 200 and 600 pA) was adjusted for each cell so that 2–3 spikes were fired near the end of the ramp. Once the proper level of current at the end of the ramp was determined, it was kept constant throughout the recordings from each individual neuron. At least 10 sweeps were acquired at each potential and averaged for subsequent measurements.
In order to determine the changes in temporal summation of EPSPs in the presence of SKF81297, we injected into the soma trains of five EPSC-like current pulses of identical amplitude (50 pA) and time course, at frequencies of 20, 50, and 100 Hz, while the membrane potential was varied from −80 mV to −50 mV. The magnitude of temporal summation was estimated via the peak depolarization attained during the sEPSP trains, as compared to the steady state potential prior to the onset of the train.
Statistical analysis
Data are expressed as the mean ± s.e.m. Statistical analysis was performed using Statistica (StatSoft, Tulsa, OK, USA). The significance of the differences between group means was tested employing Student's t test or ANOVA followed by planned comparisons, as indicated in each case. The differences between group means were considered as statistically significant if P < 0.05.
Drugs
The following drugs were purchased from Sigma Chemical Co. (St Louis, MO, USA): 2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl)pyridazinium bromide (SR-95531 or gabazine); (±)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrobromide (SKF81297), trans-(–)-4aR-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline hydrochloride (Quinpirole), 4-aminopyridine (4-AP) and tetraethylammonium chloride (TEA), R(±)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-be-nzazepine hydrochloride (SCH23390). 4-Ethylphenylamino-1,2-dimethyl-6-methylamino-pyrimidinium chloride (ZD7288), was obtained from Tocris Bioscience (Ellisville, MO, USA). Concentrated stock solutions were prepared daily in water and diluted in external recording solution shortly before application.
Results
Activation of D1 receptors modulates Na+ channel-dependent amplification of sEPSPs at depolarized potentials
Medial PFC layer 5 pyramidal neurons included in this study (n = 101) displayed significant spike frequency adaptation (n = 92) or, in a minority of cases (n = 9), a weakly bursting firing pattern. Because the effects of D1R activation did not differ between these two electrophysiological cell classes, recordings from regular spiking and weakly bursting neurons were pooled for statistical analysis.
We determined the effects of DA receptor activation on EPSP amplification independent of its potential effects on fast synaptic conductances or transmitter release. To this end, synaptic transmission was blocked, and EPSP-like depolarizations, or simulated EPSPs (sEPSPs), were elicited injecting EPSC-like currents into the recorded neurons. Previous studies showed that amplification of EPSPs elicited in the apical dendrite is markedly reduced by local application of the Na+ channel blocker TTX to the soma, but not to the apical dendrite of neocortical layer 5 pyramidal cells (Stuart & Sakmann, 1995; Gonzalez-Burgos & Barrionuevo, 2001). These data suggest that independent of the dendritic location of the active inputs, EPSP amplification is mediated by Na+ channels confined to perisomatic membrane compartments. Thus, previous data suggest that EPSP amplification could be properly studied generating sEPSPs by somatic current injection. Membrane depolarization near firing threshold significantly increased the amplitude (1.3-fold increase) and area (3–4-fold increase) of sEPSPs evoked by somatic current injection (Fig. 1Aa and b). At these potentials, the sEPSPs elicited spikes in some of the sweeps (data not shown). Furthermore, bath application of TTX (1 μm) revealed that amplification of sEPSPs elicited by somatic current injection is strongly dependent on voltage-activated Na+ channels (Fig. 1Ba and b). Membrane depolarization also produced an increase in the response to hyperpolarizing current steps (Fig. 1Ca), which was markedly reduced by TTX application (Fig. 1Ca and b). These results are consistent with previous findings showing that brief hyperpolarizing current steps injected at depolarized steady potentials deactivate a tonic Na+ current that effectively amplifies the hyperpolarizing effect of the step in control conditions (Stuart, 1999). Because TTX was applied at a concentration (1 μm) that should produce maximal Na+ current inhibition, additional active currents must be involved in the increase in the response to the hyperpolarizing step observed here, because such increase was not totally abolished by Na+ current blockade (Fig. 1Ca and b). Because TTX is a highly selective Na+ channel toxin, the most likely mechanism by which it attenuates EPSP amplification is the direct inhibition of Na+ channels localized in the recorded pyramidal neuron. An alternative possibility is that TTX had indirect effects, for instance by reducing release of some neuromodulator from other pyramidal cells or from interneurons in the slice network. However, intracellular application of the Na+ channel blocker QX314 abolished EPSP amplification at depolarized potentials (Deisz et al. 1991; Gonzalez-Burgos & Barrionuevo, 2001), strongly suggesting that blockade of Na+ channels in the recorded neuron, and not in other components of the cortical circuit, mediates the TTX effect on EPSP amplification.
In the rat medial frontal cortex, mRNA and binding sites are more abundant for D1Rs than for receptors of the D2 family (Seamans & Yang, 2004). Thus, to determine the effects of DA receptor activation on layer 5 PFC neurons, we first tested the effects of the selective D1R agonist SKF81297. As shown in Fig. 2Aa, SKF81297 (5 μm) significantly down-regulated the increase in sEPSP area at depolarized potentials near spike threshold. Figure 2Ab illustrates the changes in sEPSP area with membrane potential in control conditions and during SKF81297 application. The effect of SKF81297 on sEPSP amplification developed rapidly and in most neurons was only partially reversed by washout, independent of the fact that the neurons were maintained hyperpolarized at −80 mV for 5–7 min before the effects of washout on sEPSP amplification were tested (Fig. 2Ba). The reversal of the effect of SKF81297 on sEPSPs recorded at −50 mV was not statistically significant after 15 min of washout (Fig. 2Bb). In the same neurons, sEPSPs recorded at −80 mV were not significantly affected throughout the same time period (Fig. 2Bb). This suggests that the decrease in sEPSP amplification observed after application of SKF81297 did not result from time-dependent changes in recording conditions or in membrane properties. This was further demonstrated by the absence of significant changes in sEPSP amplification during application and washout of low concentrations of SKF81297, during application of SKF81297 in the presence of the D1R antagonist SCH23390, or following application of the D2 receptor agonist quinpirole (see below).
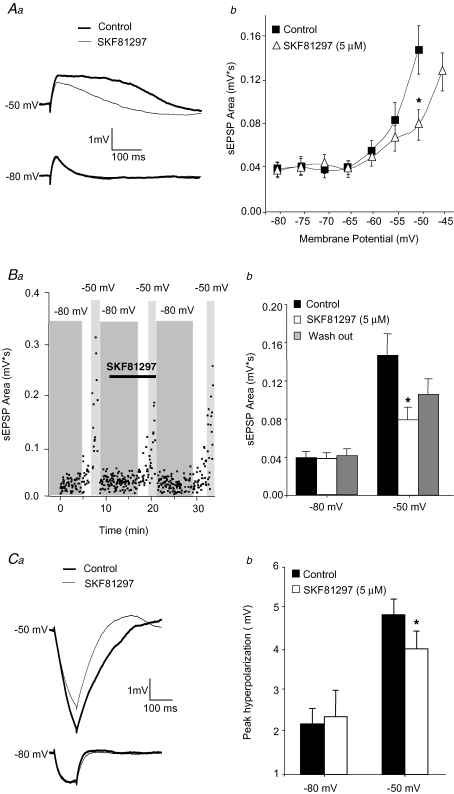
Figure 2.
Activation of D1 receptors attenuates sEPSP amplification at depolarized membrane potentials Aa, example traces showing that SKF81297 (5 μm) attenuates the increase in sEPSP area at depolarized potentials but has no effects on sEPSPs recorded at −80 mV. b, summary graph showing the changes in sEPSP area with membrane potential in control conditions and after application of SKF81297 (5 μm). Two factor ANOVA (n = 9 cells) demonstrated a significant effect of membrane potential (F6,112 = 13.1, P < 0.001) and an absence of significant global effect of SKF81297 application (F1,112 = 3.74, P = 0.056). However, a significant interaction between membrane potential and SKF91297 (F6,112 = 2.29, P < 0.05) indicated an effect of SKF81297 that was conditional to membrane potential value. Planned comparisons demonstrated a significant effect of SKF81297 at −50 mV (*P < 0.01) but not at other membrane potentials. Ba, sEPSP area was plotted as a function of time in an example experiment illustrating the relatively rapid time course of the effect of SKF81297, and its partial reversal during washout. The horizontal bar indicates the time during which SKF81297 was applied to the slices. The vertical grey bars indicate the time at which the cells were held near −50 mV or −80 mV. b, bar graphs summarizing the data on the effects of SKF81297 and their partial reversal after 15 min of drug washout. During recordings at −50 mV, single factor ANOVA (n = 9 cells) followed by planned comparisons demonstrated a significant effect of SKF81297 application (*P < 0.02) but absence of significant effect of washout. At −80 mV, no significant differences between group means were found. Ca, example recordings showing that the attenuation of the response to hyperpolarizing steps by SKF81297 was significant only at depolarized membrane potentials. b, bar graph summarizing the effects of D1R activation on the pyramidal cell response to hyperpolarizing current steps at hyperpolarized and depolarized membrane potentials. Two factor ANOVA (n = 9 cells) demonstrated a significant effect of membrane potential (F1,16 = 11.3, P < 0.005) but no overall effect of SKF81297 application (F1,16 = 2.84, P = 0.11). However, the significant interaction between factors (F1,16 = 7.44, P < 0.02) suggested an effect of SKF81297 that was conditional to membrane potential. Planned comparisons showed that SKF81297 had a significant effect at −50 mV (*P < 0.02) but not at −80 mV.
Application of SKF81297 (5 μm) did not significantly affect the peak amplitude of the sEPSPs at any of the tested potentials (sEPSP amplitude at −80 mV, in control conditions: 1.39 ± 0.18 mV, in the presence of SKF81297: 1.48 ± 0.18 mV; sEPSP amplitude at −50 mV, in control conditions: 1.73 ± 0.19 mV, in the presence of SKF81297: 1.65 ± 0.20 mV; two factor ANOVA indicated an absence of SKF81297 effect, P = 0.82, n = 9 cells), in contrast with its effects on the sEPSP area. Similar to the effects of TTX (Fig. 1), SKF81297 decreased the response to hyperpolarizing steps at depolarized, but not at hyperpolarized, membrane potentials (Fig. 2Ca and b). The absence of SKF81297 effects on hyperpolarizing steps at hyperpolarized potentials suggests that D1R activation did not significantly change the cells' input resistance.
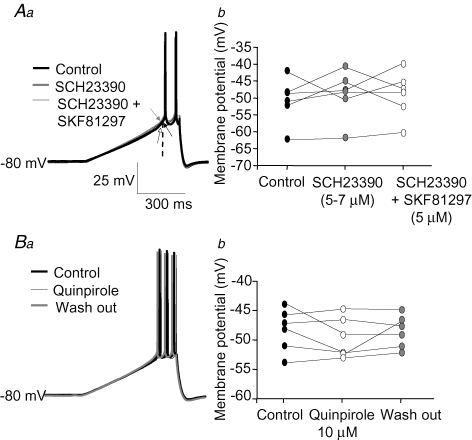
Pharmacological compounds that activate D1Rs may also directly affect the activity of voltage-gated channels in a D1R-independent manner (Nisenbaum et al. 1998; Zackheim & Abercrombie, 2001). Thus, in order to test if the modulation of EPSP amplification by SKF81297 was mediated via specific activation of D1Rs, we first determined the effects of different concentrations of SKF81297 on sEPSP amplification at depolarized potentials. Consistent with a specific receptor-mediated effect, we found that the reduction of sEPSP area at depolarized potentials by SKF81297 was concentration dependent (Fig. 3A and B). The SKF81297 concentration that produced 50% of the maximum effect, estimated by interpolation of a sigmoidal curve fitted to the data (Fig. 3B), was ∼1.3 μm. In the same neurons, in contrast, SKF81297 had no significant effects on sEPSP area at hyperpolarized membrane potentials at any of the drug concentrations tested (Figs 2Bb and 3A). Mediation of the effects of SKF81297 by specific activation of D1Rs was also indicated by experiments testing the effects of the D1R antagonist SCH23390 (Fig. 3C). Bath application of SCH23390 (5–7 μm) for 5–10 min had no significant effects per se, but significantly antagonized the effects of SKF81297 (5 μm) on sEPSP area at depolarized membrane potentials (Fig. 3C). Similarly, in the presence of SCH23390 (5–7 μm), SKF81297 (5 μm) had no significant effects on the response to hyperpolarizing steps injected at hyperpolarized or depolarized potentials (the peak hyperpolarization induced by the steps was, at −80 mV, control: 1.48 ± 0.18 mV, SKF81297 + SCH23390: 1.53 ± 0.20 mV; at −50 mV, control: 3.89 ± 0.40 mV, SKF81297 + SCH23390: 3.93 ± 0.44 mV; two factor ANOVA indicated a significant effect of membrane potential, P < 0.0001; an absence of effect of SKF81297 applied in the presence of SCH23390, P = 0.54; and no significant interaction, P = 0.69; n = 6 cells).
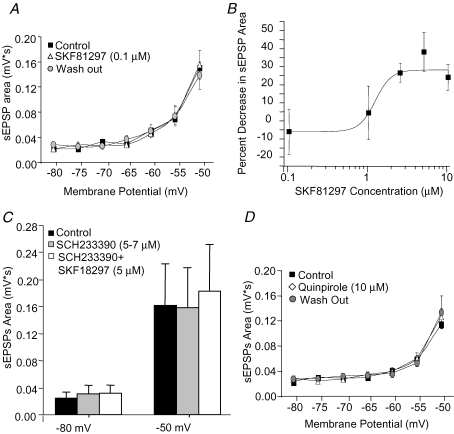
Figure 3.
The effect of SKF81297 on sEPSP amplification is mediated by selective activation of D1Rs A, summary graph showing the absence of significant effects of SKF81297 bath-applied at a concentration of 0.1 μm. Two factor ANOVA indicated a significant effect of membrane potential (F6,83 = 25.6, P < 0.001) and absence of effect of SKF81297 application (F1,83 = 0.05, P = 0.82). B, the effects of different concentrations of SKF81297 on sEPSP area could be well fitted by a sigmoideal function. The number of cells for each SKF81297 concentration was: 0.1 μm, n = 13; 1 μmn = 9; 2.5 μmn = 9; 5 μmn = 9; 10 μmn = 10. C, bath application of SCH23390 (5 or 7 μm) alone did not alter the sEPSP area at hyperpolarized potentials nor its increase by depolarization. However, SCH23390 antagonized the effects of a subsequent application of SKF81297 (5 μm) on sEPSP area at depolarized potentials. Two factor ANOVA (n = 6 cells) indicated a significant effect of membrane potential (F1,10 = 74.2, P < 0.001) and no significant effects of SCH23390 or SKF81297 bath applied in the presence of SCH23390 (F2,20 = 1.25, P = 0.31). D, bath application of the D2 receptor agonist quinpirole (10 μm) did not produce significant effects on sEPSP area, independently of the membrane potential. Single factor ANOVA (n = 7 cells) indicated a significant effect of membrane potential (F6,35 = 26.6, P < 0.001) and no significant effects of quinpirole application or washout (F2,70 = 0.41, P = 0.66) and no significant interaction between factors (F12,70 = 0.61, P = 0.83).
Although less abundant than the D1Rs (Gaspar et al. 1995; Seamans & Yang, 2004), activation of D2 family receptors in the PFC elicits significant physiological and behavioural effects (Seamans et al. 2001; Seamans & Yang, 2004; Trantham-Davidson et al. 2004). In addition, in striatal cholinergic interneurons, D2 receptor activation decreases Na+ currents via an increase in slow inactivation (Maurice et al. 2004). In PFC cells, however, application of the selective D2 receptor agonist, quinpirole (10 μm), to the PFC slices did not significantly alter the effects of membrane potential on sEPSP area (Fig. 3D). Similarly, quinpirole produced no statistically significant effects on the response to small hyperpolarizing steps injected at hyperpolarized or depolarized potentials (the peak hyperpolarization induced by the steps was, at −80 mV, control: 1.25 ± 0.13 mV, quinpirole: 1.17 ± 0.13 mV; at −50 mV, control: 3.74 ± 0.49 mV, quinpirole: 3.87 ± 0.24 mV; two factor ANOVA indicated a significant effect of membrane potential, P < 0.0001; absence of effect of quinpirole, P = 0.33; and no significant interaction, P = 0.68; n = 7 cells). These findings suggest that in layer 5 pyramidal neurons, activation of D2 receptors is not coupled to regulation of EPSP amplification.
Blockade of Na+ channels occludes the D1R-mediated effects
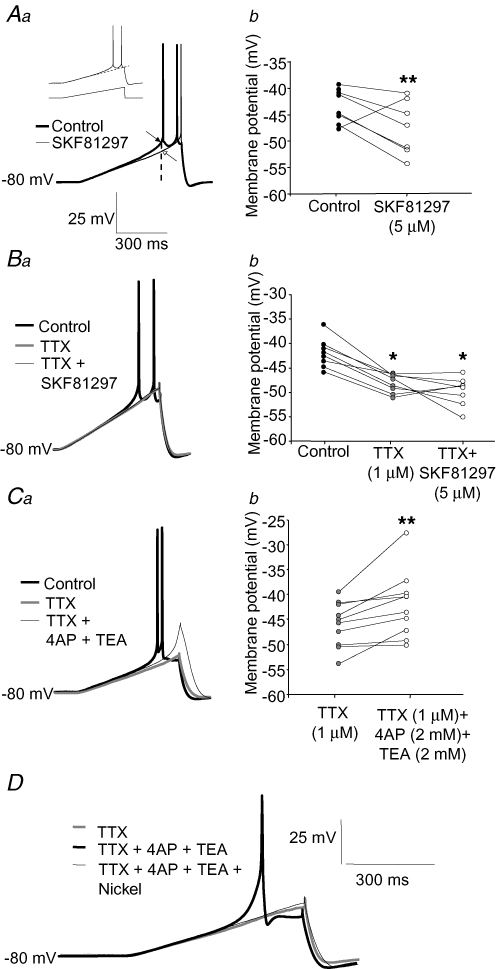
In layer 5 pyramidal neurons from rat PFC, blockade of outward K+ currents markedly increases EPSP amplification (Gonzalez-Burgos & Barrionuevo, 2001), suggesting that K+ currents counteract the EPSP amplification generated by Na+ channels. Therefore, SKF81297 may regulate EPSP amplification by decreasing the Na+ currents that promote EPSP amplification or by enhancing the K+ currents that counteract the Na+ channel effect. To determine if Na+ channel activation is necessary for the modulation of EPSP amplification by D1R activation, we first examined the effects of SKF81297 on sEPSPs after Na+ channels were blocked by TTX. As shown in Fig. 4Aa and b, bath application of SKF81297 (5 μm) in the presence of TTX (1 μm) had no significant effects on the sEPSP area at depolarized potentials, indicating that modulation of sEPSP area by SKF81297 is dependent on Na+ channel activation.
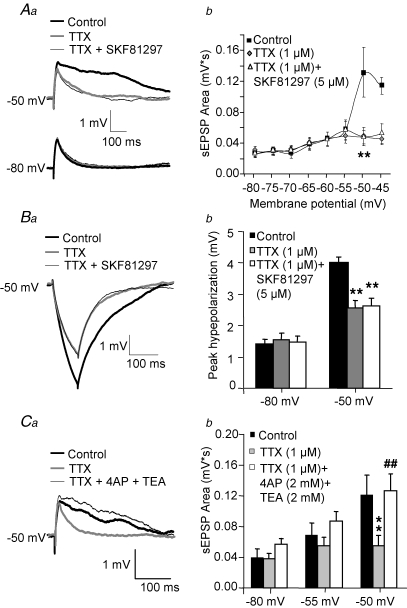
Figure 4.
The D1R-mediated attenuation of sEPSP amplification at depolarized potentials was prevented by blockade of Na+ channels Aa, example traces illustrating that during TTX application (1 μm), SKF81297 (5 μm) produced no effects on sEPSP amplification at depolarized potentials. b, graph of sEPSP area versus membrane potential summarizing the effects of SKF81297, applied subsequently to TTX application. Two factor ANOVA (n = 10 cells) indicated significant effects of membrane potential (F6,63 = 4.0, P < 0.002) and drug application (F2,126 = 7.2, P < 0.002). A significant interaction between these (F12,126 = 8.2, P < 0.001) showed that the effects of drug application were conditional to the value of membrane potential. Planned comparisons showed that at −50 mV TTX significantly reduced sEPSP area (**P < 0.001) but SKF81297 applied after TTX did not have statistically significant effects. None of the drugs had significant effects at potentials more negative than −50 mV. Ba, example traces showing that TTX (1 μm) application prevented the attenuation by SKF81297 (5 μm) of the response to hyperpolarizing steps at depolarized potentials. b, bar graphs summarizing the effects of TTX application on the action of subsequent application of SKF81297 on the increase in response to hyperpolarizing current steps at depolarized membrane potentials. Two factor ANOVA (n = 10 cells) showed significant effects of membrane potential (F1,54 = 40.6, P < 0.001) but no global effect of drug application (F2,54 = 2.4278, P = 0.09). However, a significant interaction between factors (F2,54 = 3.99, P < 0.05) indicated that the effects of drug application were conditional to membrane potential value. Planned comparisons demonstrated a significant decrease in the amplitude of the hyperpolarizing response at −50 mV (**P < 0.001) but not at −80 mV. SKF81297 applied following TTX application had no statistically significant effects when compared to SKF81297 conditions. Ca, example recordings of sEPSPs at a depolarized potential in control conditions, after addition of TTX (1 μm) and after subsequent addition of the K+ channel blockers 4-AP and TEA (2 mm each) together with TTX. b, bar graph summarizing the effects of Na+ and K+ channel blockers on sEPSP area at hyperpolarized and depolarized membrane potentials. Two factor ANOVA (n = 14 cells) indicated significant effects of membrane potential (F2,39 = 6.3, P < 0.005) and drug application (F2,78 = 17.1, P < 0.001) and a significant interaction between factors (F4,78 = 4.3, P < 0.005). Planned comparisons showed that at −50 mV TTX decreased sEPSP area compared to control (**P < 0.001) and that subsequent addition of 4-AP and TEA reversed this effect (##P < 0.001). At −80 mV, the effects of drug application were not significant.
As shown in Fig. 1, TTX application attenuates the increased response to hyperpolarizing current steps at depolarized potentials, consistent with the idea that the short hyperpolarizing steps deactivate a persistent Na+ current (Stuart, 1999). If the SKF81297-induced attenuation of the response to hyperpolarizing steps at depolarized potentials (Fig. 2Ca and b) is similarly mediated by modulation of a persistent inward current, more specifically the INaP, then the SKF81297 effect should be prevented by TTX application. As illustrated in Fig. 4Ba and b, SKF81297 (5 μm) applied subsequently to TTX (1 μm) did not significantly alter the response to hyperpolarizing current steps at depolarized potentials.
The dependence of SKF81297 effects on Na+ channels is consistent with a D1R-mediated Na+ current down-regulation. However, TTX application may indirectly preclude the activation of other channels which could be the actual targets of D1R neuromodulation. For example, D1R activation may increase the effect of the 4-AP- and TEA-sensitive K+ channels that attenuate EPSP amplification (Gonzalez-Burgos & Barrionuevo, 2001). If such K+ currents generally affect EPSP amplification in a manner that is strictly secondary to activation of Na+ currents, then TTX would indirectly abolish the D1R effect. To address this possibility, we determined the effects of K+ channel blockers on sEPSPs after Na+ currents were inhibited with TTX. As shown in Fig. 4Ca and b, after Na+ currents were blocked by TTX application (1 μm), subsequent application of submaximal concentrations of the K+ channel blockers TEA and 4-AP (2 mm each) produced a significant increase in the sEPSP area at depolarized potentials. These results suggest that K+ currents actively counteract sEPSP amplification independent of Na+ channel activation. Most likely, the sEPSP amplification enabled by inhibition of K+ channels after Na+ channels were blocked by TTX is due to activation of voltage-dependent Ca2+ channels (Seamans et al. 1997; Gonzalez-Burgos & Barrionuevo, 2001). Together, our results suggest that the effects of D1R activation are independent of the K+ currents that counteract EPSP amplification by Na+ channels.
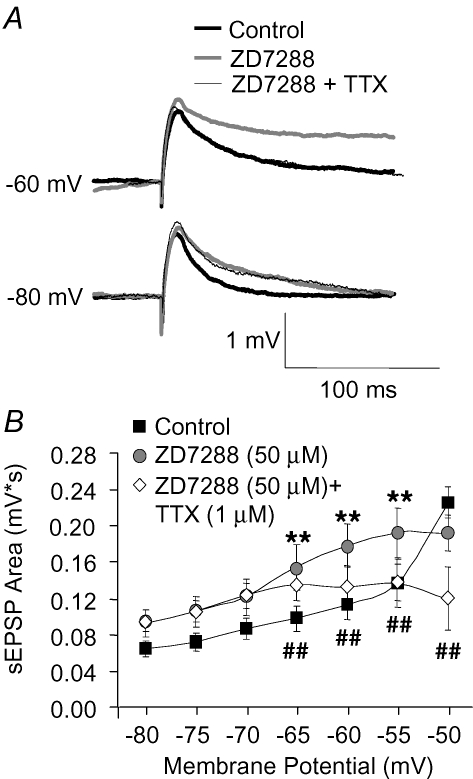
In cortical pyramidal neurons, EPSP duration is increased after blockade of the hyperpolarization-activated current (Ih), suggesting that Ih accelerates the EPSP decay phase (Magee, 1998; Stuart & Spruston, 1998). Ih potentiation could contribute to the decrease in sEPSP amplification by SKF81297 because this current is prominent in layer 5 PFC pyramidal neurons (Day et al. 2005) and is enhanced by D1R activation in cortical pyramidal cells (Rosenkranz & Johnston, 2006). However, at the depolarized potentials at which EPSP amplification is modulated by D1R activation, Ih activation may be limited, and its driving force significantly reduced. To determine the role of Ih in sEPSP amplification at depolarized potentials, we tested the effects of the Ih blocker ZD7288. Bath application of ZD7288 (50 μm) increased the sEPSP area at hyperpolarized potentials, but not at depolarized potentials near spike threshold (Fig. 5A and B). The increase in sEPSP area by ZD7288 relative to control conditions was more pronounced at intermediate potentials, ranging from −65 to −55 mV (Fig. 5A and B). These results suggest that the effects of ZD7288 on the EPSP area are more complex than those expected from an increase in the membrane resistance and time constant after Ih blockade. One possibility is that at intermediate potentials, blockade of the Ih enhances the sEPSP amplification by Na+ channels. In agreement with this interpretation, application of TTX (1 μm) reduced the increase in sEPSP area induced by ZD7288 (50 μm) application (Fig. 5A and B). These results suggest that, to shape the EPSP area at the soma, Ih and Na+ currents interact in a membrane potential-dependent manner. In addition, Ih blockade by ZD7288 had weak, non-significant effects on sEPSP amplification at the depolarized potentials at which EPSP amplification and the effects of D1R activation are most significant (Fig. 5B). On the other hand, ZD7288 blockade consistently increased the area of sEPSPs recorded at negative potentials (Fig. 5B). At these potentials (for instance, −80, −75 and −70 mV), neither SKF81297 nor TTX had noticeable effects on the sEPSP area. Our results thus suggest that Ih is unlikely to significantly contribute to the effects of SKF81297. We cannot rule out, however, that D1R-mediated modulation of Ih in the apical dendrite, where the density of Ih channels is highest (Magee, 1998; Stuart & Spruston, 1998; Kole et al. 2006), has significant effects that are not readily revealed when studying sEPSPs generated by somatic current injection.
Figure 5.
Blockade of the hyperpolarization-activated current (Ih) prolongs sEPSP duration in a voltage-dependent manner A, example traces showing sEPSPs recorded at hyperpolarized and depolarized potentials in control conditions, following application of the Ih blocker ZD7288 (50 μm) and after addition of TTX (1 μm) in the presence of ZD7288. Note that the increase in sEPSP duration by ZD7288 was unaffected by TTX at −80 mV but was reduced by TTX at −60 mV. B, graph of sEPSP area as a function of membrane potential, summarizing the effects of ZD7288 and TTX on the sEPSP at different membrane potentials. Two factor ANOVA (n = 12 cells) indicated significant effects of membrane potential (F6,56 = 3.02, P < 0.02) and drug application (F2,112 = 24.3, P < 0.001) and a significant interaction between these factors (F12,112 = 7.1, P < 0.001). Planned comparisons showed that: (1) at the most hyperpolarized potentials (−80, −75 and −70 mV, pooled) ZD7288 significantly increased sEPSP area compared to control (*P < 0.05) but TTX had no significant effects; (2) at intermediate potentials (−65, −60 and −55 mV, pooled) ZD7288 significantly increased sEPSP area compared to control (**P < 0.001), and TTX application significantly reversed this increase (##P < 0.001); (3) at the near threshold depolarized potential (−50 mV) ZD7288 had no significant effects and TTX addition significantly reduced sEPSP area compared to control (##P < 0.001).
Persistent Na+ current modulation may underlie the effects of D1 receptor activation
Our results show that D1R activation decreases the EPSP area at depolarized potentials in a manner consistent with down-regulation of the TTX-sensitive persistent sodium current INaP, which is thought to mediate EPSP amplification (Stafstrom et al. 1985; Deisz et al. 1991). To determine whether activation of D1Rs in layer 5 PFC neurons has effects consistent with INaP modulation, we tested the effects of SKF81297 on the response of the membrane potential to injection of slow depolarizing current ramps. Slow depolarizing voltage changes favour activation of the INaP, as opposed to the transient Na+ current (Geijo-Barrientos & Pastore, 1995; Gorelova & Yang, 2000; Maurice et al. 2001; Vervaeke et al. 2006). In control conditions, when the steady-state membrane potential was held close to −80 mV, the response to the initial portion of the depolarizing ramps was approximately linear (Fig. 6Aa, inset). Subsequently, the membrane potential deviated in the depolarizing direction until, at the ramp speeds employed (see methods), 2–3 spikes were fired near the end of the ramp (Fig. 6Aa, inset). Bath application of SKF81297 (5 μm) did not significantly change the response to the initial part of the ramps, but significantly attenuated the depolarization elicited closer to the ramp end (Fig. 6Aa). At the time point when the ramps reached spike threshold in control conditions, the ramp-induced depolarization was significantly decreased during SKF81297 application (Fig. 6Aa and b). These effects of SKF81297 on the depolarizing ramps are similar to those observed after selective reduction of the INaP activated by current ramps in hippocampal pyramidal neurons (Vervaeke et al. 2006).
Figure 6.
D1R activation attenuates the response of the pyramidal cell membrane potential to injection of slow depolarizing current ramps Aa, the inset shows the response of the pyramidal cell membrane potential (top trace) to injection of a slow depolarizing current ramp (bottom trace). The current was adjusted to elicit 2–3 spikes near the ramp end. Below the inset are shown example traces illustrating the effects of SKF81297 (5 μm) on the response to current ramps. The black arrow indicates the time of spike threshold in control conditions. The white arrow indicates the depolarization observed in the presence of SKF81297 (5 μm) at the time of spike threshold in control conditions (vertical dotted line). Calibration bars next to the traces in Aa apply also to the traces in Ba and Ca. b, graph summarizing the attenuation of the ramp-induced depolarization by SKF81297 (**P < 0.01 Student's t test, n = 7 cells). Ba, example traces illustrating that the attenuation of ramp-induced depolarization by application of TTX (1 μm) prevented the effects of subsequent application of SKF81297 (5 μm). b, graph summarizing the significant attenuation of ramp-induced depolarization by TTX and the absence of further effects observed after subsequent addition of SKF81297 (single factor ANOVA, F2,24 = 15.5, *P < 0.001, n = 7 cells). Planned comparisons indicated that the mean of both experimental groups differed from the control (*P < 0.05), but did not differ significantly between each other. Ca, example recordings showing that after application of TTX (1 μm) attenuated the response to current ramps, subsequent application of the K+ channel blockers 4AP and TEA rapidly enhanced the ramp-induced depolarization. b, graph summarizing the significant enhancement of the ramp-induced depolarization by K+ channel blockers (**P < 0.01, Student's paired t test, n = 9 cells). D, example recordings illustrating that in all cells tested, application of 4-AP and TEA (2 mm each) in the presence of TTX (1 μm) eventually caused the ramps to elicit slow regenerative potentials that resembled Ca2+ spikes. In this cell, such spikes were blocked by application of nickel (2 mm), an inorganic Ca2+ channel blocker.
The effects of SKF81297 on the response to depolarizing ramps are consistent with modulation of the INaP. However, SKF81297 may decrease the ramp depolarization in a manner independent of Na+ channels, for instance by enhancing K+ currents activated by the ramps. To test this possibility, we determined the effects of SKF81297 on the response to depolarizing ramps after Na+ channels were blocked with TTX. Application of TTX (1 μm) did not affect the initial phase of the response to the current ramp, but significantly attenuated the subthreshold depolarizing deflection that preceded the first spike in control conditions (Fig. 6Ba). Application of SKF81297 (5 μm) in the presence of TTX (1 μm) had no further effect on the response to depolarizing ramps (Fig. 6Ba). At the time of spike threshold in control conditions, the depolarization was significantly reduced in the presence of TTX (Fig. 6Bb), but was not further changed by the addition of SKF81297 (Fig. 6Bb).
The absence of effects of SKF81297 on the response to current ramps after TTX application is consistent with down-regulation of the INaP by D1R activation. However, D1R activation may attenuate the subthreshold ramps by modulating K+ currents activated secondary to Na+ channel activation. Experiments performed with K+ channel blockers argue against this possibility. In 12 out of 12 neurons, within 1 min after applying a mixture of the K+ channel blockers 4-AP and TEA (2 mm each) in the presence of TTX (1 μm), a significant deflection of the membrane potential in the depolarizing direction was observed (Fig. 6Ca and b). In the presence of TTX and K+ channel blockers, the ramps eventually triggered slow regenerative potentials (9 of 12 cells) which resembled Ca2+ spikes and were abolished by blockade of Ca2+ channels (Fig. 6D), as previously reported for layer 5 PFC pyramidal neurons (Gonzalez-Burgos & Barrionuevo, 2001; Nasif et al. 2005). These results show that after Na+ channel block by TTX, depolarizing ramps caused substantial activation of K+ channels. However, the absence of SKF81297 effects after TTX application suggests that D1R activation does not significantly alter the K+ currents activated by the depolarizing ramps below spike threshold.
Similar to its effects on EPSP amplification, the effects of SKF81297 on the response to depolarizing current ramps appeared to be mediated by selective activation of D1Rs. In addition, D2 receptor activation had no significant effects on the response to ramps. For example, application of SCH23390 did not have significant effects per se, but abolished the effects of SKF81297 on the subthreshold depolarization induced by the ramps (Fig. 7Aa and b). In addition, application of the specific D2 receptor agonist quinpirole (10 μm) did not significantly alter the subthreshold response to depolarizing current ramps (Fig. 7Ba and b).
Figure 7.
Pharmacology of the modulation by DA receptor activation of the response to depolarizing current ramps Aa, example traces illustrating the antagonism of the effects of SKF81297 (5 μm) on the response to current ramps by prior application of SCH23390 (5 μm). The arrows point to spike threshold in control conditions (black) and to the value of membrane depolarization measured, at the time of control spike threshold, in the presence of SCH23390 (grey) and in the presence of both SCH23390 and SKF81297 added subsequently (white). Calibration bars in Aa also apply to the traces in Ba. b, graph summarizing the effects, on the membrane potential at the time of spike threshold in control conditions, of SCH23390 (5 or 7 μm) application followed by subsequent application of SKF81297 (5 μm) to the same neurons. Single factor ANOVA indicated no significant difference between group means (F2,12 = 0.53, P = 0.60, n = 6 cells). Ba, example traces illustrating the response to depolarizing current ramps in control conditions, during application of the D2 agonist quinpirole and after 15 min of drug washout. b, graph summarizing the data on the membrane potential measured at the time of spike threshold in control conditions, during application of quinpirole (10 μm) and after 15 min of drug washout. Single factor ANOVA indicated no significant difference between group means (F2,10 = 0.85, P = 0.45, n = 7 cells).
The experiments with depolarizing current ramps revealed that the modulation of subthreshold depolarization by D1R activation is consistent with an inhibition of the INaP. In addition, D1R activation had significant effects on the firing of PFC neurons during injection of current ramps. For instance, in the presence of SKF81297, the latency to the first spike increased by ∼10% (the latency between onset of the ramp and the time to reach spike threshold was 0.49 ± 0.02 s in control conditions, and 0.54 ± 0.04 s in the presence of 5 μm SKF81297, n = 9, P < 0.02, Student's t test). Such delay in spike firing induced by SKF81297 could be explained by the decrease in the slope of the subthreshold depolarization (Fig. 6Aa) or by the elevation, observed during SKF81297 application, of the potential at which the ramps elicited spikes (spike threshold for ramps injected at −80 mV, in control conditions: −40.5 ± 0.8 mV; during SKF81297 application: −36.9 ± 0.9 mV, P < 0.001, n = 9 cells). When current ramps were combined with steady state membrane depolarization, in control conditions the spike threshold significantly shifted to more depolarized potentials (P < 0.05). Bath application of SKF81297 (5 μm) produced a further depolarizing shift in the threshold of ramp-elicited spikes, when the ramps were injected at depolarized steady-state potentials (spike threshold for ramps injected at −50 mV, in control conditions: −38.3 ± 0.8 mV; during SKF81297 application: −35.6 ± 0.6 mV, P < 0.01, n = 9 cells). These findings suggest the possibility that sustained depolarization enhances spike threshold by inducing, among other changes, slow inactivation of Na+ channels which is further enhanced by D1R stimulation (Carr et al. 2002; Carr et al. 2003). Activation of D1Rs also elevated the spike threshold when spikes were generated by sEPSPs elicited at depolarized potentials (spike threshold in control conditions: −37.4 ± 0.9 mV; spike threshold in the presence of SKF81297: −33.1 ± 0.9 mV). These results show that SKF81297 modulation of conductances that regulate action potential threshold in the depolarized state, possibly including the INaP, leads to a significant decrease in the excitability of layer 5 PFC neurons.
In previous studies, D1R activation was actually reported to produce an increase in PFC cell excitability (Yang & Seamans, 1996; Ceci et al. 1999; Henze et al. 2000; Gorelova & Yang, 2000; Lavin & Grace, 2001; Seamans & Yang, 2004; Chen et al. 2007). Such an increase in excitability, however, was somewhat delayed relative to D1R agonist application and persisted after drug washout (Henze et al. 2000; Gorelova & Yang, 2000; Lavin & Grace, 2001; Chen et al. 2007). To determine whether a similar delayed increase in spiking could be observed in response to the depolarizing ramps used in this study, in a subgroup of experiments action potential firing elicited by the ramps was monitored throughout a period of 45 min, before, during and after application of SKF81297. Consistent with the data shown in Fig. 6A, shortly after applying SKF81297 ramp-induced spiking was depressed, as reflected by a significant increase in first spike latency (the latency between onset of the ramp and the time to reach spike threshold was 0.52 ± 0.01 s in control conditions, 0.55 ± 0.01 s in the presence of 5–10 μm SKF81297, P < 0.05, n = 10 cells). In the same cells, after 35 min of SKF81297 washout, the speed of the subthreshold depolarization induced by the ramp was accelerated and the latency to the first spike significantly decreased compared to control conditions (0.46 ± 0.01 s, P < 0.01, n = 10 cells). A similar long-lasting decrease in first spike latency during injection of current ramps into PFC neurons was attributed previously to a delayed and persistent D1R-mediated increase in the activation of the INaP at subthreshold ramp potentials (Gorelova & Yang, 2000). The ionic mechanisms mediating the D1R-mediated delayed enhancement of PFC cell excitability observed here were not investigated.
Modulation of EPSP summation by D1 receptor activation
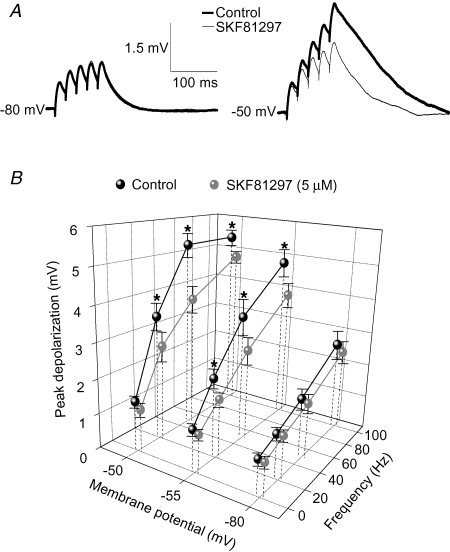
The decrease in EPSP area produced by D1R activation may narrow the effective time window for temporal summation of EPSPs at depolarized potentials. If so, D1R activation would restrict temporal integration of EPSPs at potentials near spike threshold, whereas detection of coincident inputs would be less significantly altered (Konig et al. 1996). To estimate the functional impact of D1R activation on the magnitude of temporal summation of EPSPs, we determined the effects of SKF81297 on sEPSP trains of different frequencies. Temporal summation was measured as the peak depolarization attained during the trains at hyperpolarized and depolarized potentials. When SKF81297 (5 μm) was bath-applied, the magnitude of EPSP summation was not affected at hyperpolarized potentials but decreased significantly at depolarized potentials (Fig. 8A). In control conditions, the peak depolarization attained during the sEPSP trains reached its maximum at a potential of ∼−50 mV and was similar for 50 and 100 Hz sEPSP trains (Fig. 8B). Overall, SKF81297 application produced no significant effects on sEPSP trains recorded at −80 mV (Fig. 8B). In contrast, D1R activation significantly affected sEPSP trains at more depolarized potentials in a frequency-dependent manner. The effects of SKF81297 at −50 mV were more pronounced for the 20 and 50 Hz trains than for 100 Hz trains (the peak depolarization during trains was decreased by 22% for 20 Hz trains, by 29% for 50 Hz trains and by 11% for 100 Hz trains; Fig. 8B). These findings show that the effects of D1R activation on the strength of EPSP trains at depolarized potentials are stronger when the dependence of temporal summation on EPSP duration is more critical.
Figure 8.
Effects of D1R activation on temporal summation of sEPSPs A, example traces of sEPSP trains generated by injection of five EPSC-like currents at a frequency of 50 Hz, at hyperpolarized and depolarized potentials in control conditions and in the presence of SKF81297 (5 μm). B, graph summarizing the effects of SKF81297 application on sEPSP trains of different frequencies (0.3, 20, 50 and 100 Hz) at hyperpolarized and depolarized membrane potentials. The magnitude of sEPSP summation was estimated by measuring the peak depolarization attained during the sEPSP train, relative to the membrane potential measured just prior to the sEPSP train. Factorial ANOVA (n = 8 cells) indicated significant effects of membrane potential (F2,79 = 66.5, P < 0.001), sEPSP train frequency (F3,79 = 30.8, P < 0.001 and of SKF81297 application (F1,79 = 52.9, P < 0.001). The significant interactions between the effects of SKF81297 and either membrane potential (F2,79 = 15.1, P < 0.001) or frequency (F3,79 = 3.52, P < 0.02) indicated that the SKF81297 effects were conditional to membrane potential and sEPSP train frequency. Planned comparisons were employed to test the significance of the difference between means of the control and SKF81297 groups (*P < 0.01).
Discussion
Ionic mechanisms involved in modulation of sEPSP amplification by D1R activation
In layer 5 PFC pyramidal neurons, the INaP is activated at potentials significantly more negative (∼10 mV) than those required for activation of the transient Na+ current (Carr et al. 2002). Moreover, the contribution of the transient Na+ current to the total subthreshold Na+ current is relatively small (Maurice et al. 2001). Thus, the INaP in PFC layer 5 pyramidal neurons has properties suitable to mediate EPSP amplification, as suggested in previous studies (Stafstrom et al. 1985; Deisz et al. 1991). In addition to INaP down-regulation, the decrease in EPSP amplification by SKF81297 could be mediated at least in part by potentiation of the K+ currents that counteract EPSP amplification (Gonzalez-Burgos & Barrionuevo, 2001). Our present results, however, suggest that modulation of K+ currents is not strongly involved in the effects of SKF81297, because Na+ channel block occluded the effects of D1R stimulation but not the effects of K+ channels on sEPSP amplification. Furthermore, D1R activation modulates K+ currents in PFC pyramidal cells, by predominantly causing K+ current suppression (Dong & White, 2003; Dong et al. 2004; Dong et al. 2005), an effect that would lead to enhanced sEPSP amplification.
A number of recent observations support the hypothesis that EPSP amplification, and therefore its modulation by D1Rs, is mediated by INaP. For example, Na+ channel-mediated EPSP amplification takes place in the perisomatic compartment of the pyramidal cell membrane (Stuart & Sakmann, 1995; Andreasen & Lambert, 1999; Gonzalez-Burgos & Barrionuevo, 2001; however, see Schwindt & Crill, 1995), more specifically in the axon initial segment (Stuart & Sakmann, 1995). INaP appears to be primarily generated in the axon initial segment, as suggested by the finding that axonal but not somatic or dendritic TTX applications abolish the INaP (Astman et al. 2006; however, see Mittmann et al. 1997). INaP generation in the axon initial segment may be due to a low K+ channel density, to a high Na+ channel density or to Na+ channel properties that facilitate INaP initiation (Colbert & Pan, 2002; Astman et al. 2006). For example, Nav1.6 α subunit-containing Na+ channels enter into a non-inactivating mode more frequently and produce more INaP than other channel isoforms (Maurice et al. 2001; Rush et al. 2005; Astman et al. 2006). Layer 5 PFC pyramidal neurons contain mRNA for the Nav1.6 Na+ channel subunit, and the INaP is significantly reduced in PFC neurons from Nav1.6 null mice (Maurice et al. 2001), suggesting a link between Nav1.6 expression and INaP in layer 5 PFC neurons. In certain cell types, Nav1.6 subunit-containing channels are predominant at the axon initial segment (Van Wart & Matthews, 2006). Whether Nav1.6 channels are similarly localized in the axon initial segment in cortical pyramidal cells remains to be directly investigated. In PFC, D1Rs are found in axons (Bergson et al. 1995; Paspalas & Goldman-Rakic, 2005), and specifically in the pyramidal cell axon initial segment (Bergson et al. 1995), where they may be strategically placed for INaP modulation.
Our finding that SKF81297 attenuates the subthreshold response to slow depolarizing current ramps is consistent with INaP modulation by D1Rs. That slow current ramps predominantly activate the persistent, as opposed to the transient Na+ current (Crill, 1996), was verified recently using dynamic clamp to reduce the INaP without altering the transient Na+ current (Vervaeke et al. 2006). Also consistent with INaP modulation by D1Rs is the observation that TTX application occluded the effects of SKF81297 on the response of PFC cells to current ramps. Such findings do not completely rule out the possibility that D1Rs attenuate the subthreshold ramps by increasing some K+ currents. For example, it is possible that blockade of the depolarization generated by the INaP largely prevents the activation of K+ currents. This possibility was ruled out by the finding that blockade of voltage-gated K+ currents in the presence of TTX revealed a significant, Na+ channel-independent K+ channel activation by the ramps. In addition, DA receptor activation seems to suppress, and not to enhance, voltage-gated K+ currents in PFC neurons (Yang & Seamans, 1996; Dong & White, 2003; Dong et al. 2004; Dong et al. 2005). An alternative possibility that cannot be ruled out is that D1R activation enhances Na+-dependent K+ currents, which are expressed in high levels in the frontal cortex (Bhattacharjee & Kaczmarek, 2005) and are significantly regulated by neuromodulators (Santi et al. 2006).
DA D1R activation was shown to down-regulate Na+ currents in acutely dissociated cortical pyramidal cells (Cantrell & Catterall, 2001) including rat PFC layer 5 pyramidal neurons (Maurice et al. 2001; Peterson et al. 2006). D1R activation reduces the Na+ currents by decreasing Na+ channel availability, but without changing the voltage dependence and kinetics of activation or of fast inactivation (Cantrell & Catterall, 2001). In addition to fast inactivation, Na+ channels exhibit a slow inactivation process that has kinetics in the order of seconds and that apparently affects both the transient Na+ current and the INaP (Carr et al. 2002; Carr et al. 2003; Chen et al. 2006). Enhancement of the slow inactivation process seems to be a central mechanism by which protein kinase A or protein kinase C pathways regulate Na+ channel availability (Carr et al. 2002; Carr et al. 2003; Chen et al. 2006). Resembling D1R-mediated modulation of Na+ currents (Cantrell et al. 1999; Carr et al. 2003), the suppression of EPSP amplification by D1Rs reported here is consistent with an enhanced slow inactivation: it is more significant at potentials near spike threshold and less significant at hyperpolarized potentials that remove slow inactivation. Thus, we suggest that the effects of D1R activation on EPSP amplification may be mediated by an enhancement of the Na+ channel slow inactivation. If this interpretation is correct, then sustained depolarization may have at least two important effects, first, to depolarize the cell into the voltage range of INaP activation, producing EPSP amplification, and second, to produce slow inactivation of Na+ channels which is enhanced by D1R activation, thus decreasing the amount of INaP available for EPSP amplification.
In the present study the attenuation of sEPSP amplification by D1R activation developed rapidly and was thus measured during the application of SKF81297; however, the effects were not completely reversed after 10–15 min of wash-out. In acutely dissociated neurons, D1R activation decreases Na+ currents in a similarly rapid, but also quickly reversible, manner (Maurice et al. 2001; Cantrell & Catterall, 2001). Previous studies have shown that the complete reversal of the enhanced slow inactivation of Na+ channels by neuromodulators requires hyperpolarization of PFC cells (Carr et al. 2003) or of striatal cholinergic interneurons (Maurice et al. 2004) for ∼30 s. In addition, in dissociated neurons reversal of the neuromodulator effect upon drug washout is accelerated by membrane hyperpolarization (Maurice et al. 2004). This suggests that even if channel phosphorylation or some other intracellular messenger effects reverse rapidly, the exit of the Na+ channels from the slow inactivated state still requires membrane hyperpolarization (Maurice et al. 2004). In our experiments, after the effect of SKF81297 was tested, the cells were maintained at −80 mV for a prolonged period (5–7 min) during drug washout (Fig. 2Ba). However, such prolonged hyperpolarization was not efficient in reversing the decrease in EPSP amplification induced by prior SKF81297 application (Fig. 2Ba and b). The reason for the discrepancies in the time course of D1R effects in our brain slice study versus acutely dissociated cells is not clear. One possibility is that in brain slice conditions, Na+ channels remain phosphorylated after agonist washout for a longer period, and thus depolarization after drug washout still induces more slow inactivation than in control conditions. In previous studies certain D1R-mediated effects were found to be delayed and persistent, relative to drug application (Yang & Seamans, 1996; Henze et al. 2000; Gorelova & Yang, 2000; Seamans & Yang, 2004; Chen et al. 2007). Specifically, a delayed and persistent increase in excitability has been attributed at least in part to an enhanced activation of the INaP (Yang & Seamans, 1996; Gorelova & Yang, 2000; Chen et al. 2007). In the present study, we found that the spiking elicited in PFC pyramidal neurons by current ramps was depressed during SKF81297 application and shortly after, but was enhanced in the same neurons during agonist washout (see Results). Whereas enhanced slow inactivation of Na+ channels probably contributed to the decreased excitability observed in this study shortly after SKF81297 application, the ionic mechanisms underlying the delayed increase in spiking observed subsequently in the same cells were not investigated.
Collectively, the results of this and previous studies suggest that D1R activation produces time-dependent effects that in some cases appear to be biphasic, i.e. short-term down-regulation versus long-term enhancement of Na+ currents. One possibility is that the time dependence of the D1R effects is somehow related to the intracellular signal transduction pathways mediating the effect. Some studies suggest that protein kinase A may mediate the transient effects of D1R activation, whereas the delayed and persistent effects of D1R activation in PFC neurons may be mediated by protein kinase C (Gorelova & Yang, 2000; Young & Yang, 2004; Chen et al. 2007). Direct stimulation of protein kinase C indeed increases the excitability of pyramidal cells with a delayed and persistent time course (Astman et al. 1998; Franceschetti et al. 2000). In dissociated cells, however, activation of D1Rs, serotonin receptors, muscarinic receptors, protein kinase A or protein kinase C rapidly and reversibly depresses both transient and persistent Na+ currents (Mittmann & Alzheimer, 1998; Maurice et al. 2001; Cantrell & Catterall, 2001; Carr et al. 2002; Carr et al. 2003; Chen et al. 2006). Thus, the time dependence and nature (i.e. suppression versus enhancement) of D1R effects cannot be explained, alone, by the signal transduction pathway involved. The mechanisms underlying short-term and long-lasting effects of D1R activation, as well as their relative importance in vivo, remain to be determined.
Similar to our results, a previous study reported a DA-mediated attenuation of the response to current ramps consistent with a rapid decrease of the INaP in PFC pyramidal neurons (Geijo-Barrientos & Pastore, 1995). In contrast, in acutely dissociated PFC cells D1R activation did not modulate the INaP efficiently, although it suppressed the transient Na+ current via a cAMP and protein kinase A signal transduction pathway (Maurice et al. 2001). In the same cells, cAMP analogue application modulates both transient and persistent Na+ currents (Maurice et al. 2001). Such differential effects of D1R activation on transient and persistent Na+ currents may indicate that the molecular mechanisms underlying macroscopic slow inactivation, or its modulation, may be different between these two currents. An alternative possibility is that in acutely isolated neurons protein kinase-mediated modulation of the INaP, which usually is initiated in the axon initial segment but not in the pyramidal cell body (Astman et al. 2006), is somehow uncoupled from D1R activation. Because in dissociated neurons the INaP is readily modulated by protein kinase C (Mittmann & Alzheimer, 1998; Carr et al. 2002) or serotonin receptor stimulation (Carr et al. 2002), such an uncoupling would have to be specific to D1R-mediated effects. Nav1.6 channels actually lack a phosphorylation site that seems to be important for protein kinase A-mediated effects (Maurice et al. 2001; Cantrell & Catterall, 2001). However, cAMP analogue application reduced the INaP in PFC neurons (Maurice et al. 2001), suggesting that Na+ channels of other subtypes, in addition to Nav1.6, mediate INaP in PFC cells. Consistent with this idea, in layer 5 PFC pyramidal neurons of Nav1.6 null mice, the INaP is reduced but not completely eliminated (Maurice et al. 2001).
Functional consequences of modulation of sEPSP amplification by D1R activation
Our results show that the modulatory effects of D1R activation on EPSPs are markedly dependent on the state of the PFC cell membrane potential. Specifically, SKF81297 had significant effects at potentials near spike threshold but no effects at hyperpolarized membrane potential states. In vivo, the PFC cell membrane potential alternates between hyperpolarized and depolarized states, displaying a form of cellular bistability that depends on network activity (Lewis & O'Donnell, 2000). Our data suggest that D1R activation may decrease the effectiveness of temporal summation thus favouring coincidence detection, as opposed to temporal integration, in depolarized PFC pyramidal neurons. Elevation of the spike threshold by D1R stimulation in the depolarized state, as that shown here, may further contribute to the bias for detection of coincident inputs. Similar to other D1R-mediated effects, regulation of excitability seems to be time dependent: at short times after onset of drug application, a decrease in excitability was observed in this and previous studies (Geijo-Barrientos & Pastore, 1995; Gulledge & Jaffe, 1998; Gulledge & Jaffe, 2001). In contrast, when examined at prolonged times after agonist application, D1R activation enhances PFC cell excitability in the depolarized state (Lavin & Grace, 2001) or at the resting membrane potential (Yang & Seamans, 1996; Henze et al. 2000; Seamans & Yang, 2004).
The functional impact of the D1R modulation of EPSP amplification may depend on the NMDA receptor contribution, because at depolarized potentials the NMDA EPSC might prolong the somatic EPSP duration and area (Thomson & West, 1986; Markram et al. 1997). The D1R-mediated effect described here may therefore be more significant for synaptic inputs with weaker NMDA contribution. The contribution of AMPA and NMDA receptors seems to differ for synaptic inputs onto different compartments of the pyramidal cell membrane. For example, inputs to the distal apical dendrite seem to have higher AMPA/NMDA ratio than proximal inputs (Dodt et al. 1998; Magee & Cook, 2000; Gonzalez-Burgos & Barrionuevo, 2001; Andrasfalvy & Magee, 2001; Nicholson et al. 2006), however, see (Williams & Stuart, 2002). Distal apical dendrite synapses may convey information from extrinsic sources of input, for instance cortico-cortical or thalamo-cortical connections (Cauller & Connors, 1994; Cauller et al. 1998; Zhu & Zhu, 2004; Larkum et al. 2004). Conversely, intrinsic synaptic connections between nearby pyramidal cells are predominantly located in the basal dendrites and display a robust NMDA contribution (Markram et al. 1997; Feldmeyer et al. 2002). Because D1R activation potentiates NMDA receptor currents (Seamans et al. 2001; Seamans & Yang, 2004), interactions between AMPA/NMDA ratio and D1R signalling may determine differential effects of DA on synaptic inputs onto apical versus basal dendrites. Although this idea is still a matter of speculation, it could be proposed that D1R stimulation favours coincidence detection when PFC cells receive input from extrinsic sources, thus helping to gate the information loaded into the short-term memory buffers associated with working memory (Cohen et al. 2002). On the other hand, D1R potentiation of the NMDA EPSC component at intrinsic connections could favour recurrent excitation between nearby pyramidal neurons, promoting persistent firing and thus active maintenance of information in the buffer systems during the delay period of working memory tasks (Goldman-Rakic, 1995).
EPSP integration depends in part on the membrane time constant, which may be shortened in the depolarized state in vivo, if this state is associated with a high background synaptic conductance (Destexhe et al. 2003). Although a shortened time constant in vivo may diminish the impact of EPSP amplification by Na+ channels, D1R activation may still add further constraints on the time window for temporal integration. Interestingly, in vivo tight-seal whole-cell recordings showed that background synaptic activity associated with the depolarized up states is sparser than previously thought, and that a net increase in the cells' input resistance is observed during these states (Waters & Helmchen, 2006). In such conditions, amplification of EPSPs and its modulation by D1R activation in vivo may be similar to that observed in the depolarized state in vitro.
Our results may help to understand some of the consequences of altered DA signalling in mental disorders such as schizophrenia, in which brain alterations are associated with reduced dopaminergic input to the PFC (Lewis & Gonzalez-Burgos, 2006). Reduced DA input would lead to exaggerated EPSP summation by PFC pyramidal neurons in contexts in which DA would otherwise favour coincidence detection. The modulation of EPSP summation by D1R activation may also be disturbed after repeated in vivo exposure to cocaine or amphetamine, which produce a sustained Na+ current depression and a decrease in the acute D1R-mediated down-regulation of Na+ currents in PFC neurons (Peterson et al. 2006). Our results are consistent with the idea that dysfunction of DA signalling in mental disorders may alter the flow of neural activity in PFC circuits by disturbing integration of inputs onto PFC pyramidal neurons.
Acknowledgments
We thank Dr Leonid Krimer for his support, and Drs Jeremy Seamans and Charles Yang for discussion and for their useful comments to a previous version of this manuscript. This work was funded by NIH grants MH41456, MH51234 and by a NARSAD Young Investigator Award (G.G.B).
References
- Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD. Somatic amplification of distally generated subthreshold EPSPs in rat hippocampal pyramidal neurones. J Physiol. 1999;519:85–100. doi: 10.1111/j.1469-7793.1999.0085o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol. 1998;80:1547–1551. doi: 10.1152/jn.1998.80.3.1547. [DOI] [PubMed] [Google Scholar]
- Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+ Trends Neurosci. 2005;28:422–428. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Scheuer T, Catterall WA. Voltage-dependent neuromodulation of Na+ channels by D1-like dopamine receptors in rat hippocampal neurons. J Neurosci. 1999;19:5301–5310. doi: 10.1523/JNEUROSCI.19-13-05301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Cauller LJ, Clancy B, Connors BW. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J Comp Neurol. 1998;390:297–310. [PubMed] [Google Scholar]
- Cauller LJ, Connors BW. Synaptic physiology of horizontal afferents to layer I in slices of rat SI neocortex. J Neurosci. 1994;14:751–762. doi: 10.1523/JNEUROSCI.14-02-00751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci A, Brambilla A, Duranti P, Grauert M, Grippa N, Borsini F. Effect of antipsychotic drugs and selective dopaminergic antagonists on dopamine-induced facilitatory activity in prelimbic cortical pyramidal neurons. An in vitro study. Neuroscience. 1999;93:107–115. doi: 10.1016/s0306-4522(99)00123-2. [DOI] [PubMed] [Google Scholar]
- Chen L, Bohanick J, Nishihara M, Seamans J, Yang CR. Dopamine D1/5 receptor-mediated LTP of intrinsic excitability in rat prefrontal cortical neurons: Ca2+-dependent intracellular signaling. J Neurophysiol. 2007;97:2448–2464. doi: 10.1152/jn.00317.2006. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci. 2002;5:533–538. doi: 10.1038/nn0602-857. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA, Fortin G, Zieglgansberger W. Voltage dependence of excitatory postsynaptic potentials of rat neocortical neurons. J Neurophysiol. 1991;65:371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Dodt HU, Frick A, Kampe K, Zieglgansberger W. NMDA and AMPA receptors on neocortical neurons are differentially distributed. Eur J Neurosci. 1998;10:3351–3357. doi: 10.1046/j.1460-9568.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- Dong Y, Cooper D, Nasif F, Hu XT, White FJ. Dopamine modulates inwardly rectifying potassium currents in medial prefrontal cortex pyramidal neurons. J Neurosci. 2004;24:3077–3085. doi: 10.1523/JNEUROSCI.4715-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ. Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci. 2005;25:936–940. doi: 10.1523/JNEUROSCI.4715-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, White FJ. Dopamine D1-class receptors selectively modulate a slowly inactivating potassium current in rat medial prefrontal cortex pyramidal neurons. J Neurosci. 2003;23:2686–2695. doi: 10.1523/JNEUROSCI.23-07-02686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Silver RA, Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J Physiol. 2002;538:803–822. doi: 10.1113/jphysiol.2001.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti S, Taverna S, Sancini G, Panzica F, Lombardi R, Avanzini G. Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurones. J Physiol. 2000;528:291–304. doi: 10.1111/j.1469-7793.2000.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker D, Miles R. EPSP amplification and the precision of spike timing in hippocampal neurons. Neuron. 2000;28:559–569. doi: 10.1016/s0896-6273(00)00133-1. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Geijo-Barrientos E, Pastore C. The effects of dopamine on the subthreshold electrophysiological responses of rat prefrontal cortex neurons in vitro. Eur J Neurosci. 1995;7:358–366. doi: 10.1111/j.1460-9568.1995.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Barrionuevo G. Voltage-gated sodium channels shape subthreshold EPSPs in layer 5 pyramidal neurons from rat prefrontal cortex. J Neurophysiol. 2001;86:1671–1684. doi: 10.1152/jn.2001.86.4.1671. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Yang CR. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol. 2000;84:75–87. doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- Grande LA, Kinney GA, Miracle GL, Spain WJ. Dynamic influences on coincidence detection in neocortical pyramidal neurons. J Neurosci. 2004;24:1839–1851. doi: 10.1523/JNEUROSCI.3500-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998;18:9131–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Multiple effects of dopamine on layer V pyramidal cell excitability in rat prefrontal cortex. J Neurophysiol. 2001;86:586–595. doi: 10.1152/jn.2001.86.2.586. [DOI] [PubMed] [Google Scholar]
- Henze DA, Gonzalez-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Koch C, Rapp M, Segev I. A brief history of time (constants) Cereb Cortex. 1996;6:93–101. doi: 10.1093/cercor/6.2.93. [DOI] [PubMed] [Google Scholar]
- Kole MH, Hallermann S, Stuart GJ. Single Ih channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci. 2006;26:1677–1687. doi: 10.1523/JNEUROSCI.3664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Senn W, Luscher HR. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex. 2004;14:1059–1070. doi: 10.1093/cercor/bhh065. [DOI] [PubMed] [Google Scholar]
- Lavin A, Grace AA. Stimulation of D1-type dopamine receptors enhances excitability in prefrontal cortical pyramidal neurons in a state-dependent manner. Neuroscience. 2001;104:335–346. doi: 10.1016/s0306-4522(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D1 dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci. 2000;3:895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nass SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mittmann T, Alzheimer C. Muscarinic inhibition of persistent Na+ current in rat neocortical pyramidal neurons. J Neurophysiol. 1998;79:1579–1582. doi: 10.1152/jn.1998.79.3.1579. [DOI] [PubMed] [Google Scholar]
- Mittmann T, Linton SM, Schwindt P, Crill W. Evidence for persistent Na+ current in apical dendrites of rat neocortical neurons from imaging of Na+-sensitive dye. J Neurophysiol. 1997;78:1188–1192. doi: 10.1152/jn.1997.78.2.1188. [DOI] [PubMed] [Google Scholar]
- Nasif FJ, Hu XT, White FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci. 2005;25:3674–3679. doi: 10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Neve RL. The Dopamine Receptors. Totowa, NJ, USA: Humana Press; 1997. [Google Scholar]
- Nicholson DA, Trana R, Katz Y, Kath WL, Spruston N, Geinisman Y. Distance-dependent differences in synapse number and AMPA receptor expression in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:431–442. doi: 10.1016/j.neuron.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Mermelstein PG, Wilson CJ, Surmeier DJ. Selective blockade of a slowly inactivating potassium current in striatal neurons by (±)6-chloro-APB hydrobromide ( SKF82958) Synapse. 1998;29:213–224. doi: 10.1002/(SICI)1098-2396(199807)29:3<213::AID-SYN3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J Neurosci. 2005;25:1260–1267. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Repeated amphetamine administration decreases D1 dopamine receptor-mediated inhibition of voltage-gated sodium currents in the prefrontal cortex. J Neurosci. 2006;26:3164–3168. doi: 10.1523/JNEUROSCI.2375-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex. J Neurosci. 2006;26:3229–3244. doi: 10.1523/JNEUROSCI.4333-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J Physiol. 2005;564:803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi CM, Ferreira G, Yang B, Gazula VR, Butler A, Wei A, Kaczmarek LK, Salkoff L. Opposite regulation of Slick and Slack K+ channels by neuromodulators. J Neurosci. 2006;26:5059–5068. doi: 10.1523/JNEUROSCI.3372-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J Neurophysiol. 1995;74:2220–2224. doi: 10.1152/jn.1995.74.5.2220. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie B, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Yang CR. Contributions of voltage-gated Ca2+ channels in the proximal versus distal dendrites to synaptic integration in prefrontal cortical cells. J Neurosci. 1997;17:5936–5948. doi: 10.1523/JNEUROSCI.17-15-05936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action potential initiation in neocortical pyramidal cells: evidence from whole cell axon recordings. J Neurophysiol. 2006;97:746–760. doi: 10.1152/jn.00922.2006. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Chubb MC, Crill WE. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985;53:153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Stuart G. Voltage-activated sodium channels amplify inhibition in neocortical pyramidal neurons. Nat Neurosci. 1999;2:144–150. doi: 10.1038/5698. [DOI] [PubMed] [Google Scholar]
- Stuart G, Sakmann B. Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron. 1995;15:1065–1076. doi: 10.1016/0896-6273(95)90095-0. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol. 1997;505:617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci. 1998;18:3501–3510. doi: 10.1523/JNEUROSCI.18-10-03501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B, Hablitz JJ. EPSPs in rat neocortical neurons in vitro. II. Involvement of N-methyl-D-aspartate receptors in the generation of EPSPs. J Neurophysiol. 1989;61:621–634. doi: 10.1152/jn.1989.61.3.621. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC. N-methylaspartate receptors mediate epileptiform activity evoked in some, but not all, conditions in rat neocortical slices. Neuroscience. 1986;19:1161–1177. doi: 10.1016/0306-4522(86)90130-2. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking nav1.6 sodium channels. J Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, Hu H, Graham LJ, Storm JF. Contrasting effects of the persistent Na+ current on neuronal excitability and spike timing. Neuron. 2006;49:257–270. doi: 10.1016/j.neuron.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Waters J, Helmchen F. Background synaptic activity is sparse in neocortex. J Neurosci. 2006;26:8267–8277. doi: 10.1523/JNEUROSCI.2152-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science. 2002;295:1907–1910. doi: 10.1126/science.1067903. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CE, Yang CR. Dopamine D1/D5 receptor modulates state-dependent switching of soma-dendritic Ca2+ potentials via differential protein kinase A and C activation in rat prefrontal cortical neurons. J Neurosci. 2004;24:8–23. doi: 10.1523/JNEUROSCI.1650-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackheim JA, Abercrombie ED. Decreased striatal dopamine efflux after intrastriatal application of benzazepine-class D1 agonists is not mediated via dopamine receptors. Brain Res Bull. 2001;54:603–607. doi: 10.1016/s0361-9230(01)00462-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhu JJ. Rapid arrival and integration of ascending sensory information in layer 1 nonpyramidal neurons and tuft dendrites of layer 5 pyramidal neurons of the neocortex. J Neurosci. 2004;24:1272–1279. doi: 10.1523/JNEUROSCI.4805-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsiros V, Hestrin S. Background synaptic conductance and precision of EPSP-spike coupling at pyramidal cells. J Neurophysiol. 2005;93:3248–3256. doi: 10.1152/jn.01027.2004. [DOI] [PubMed] [Google Scholar]