Abstract
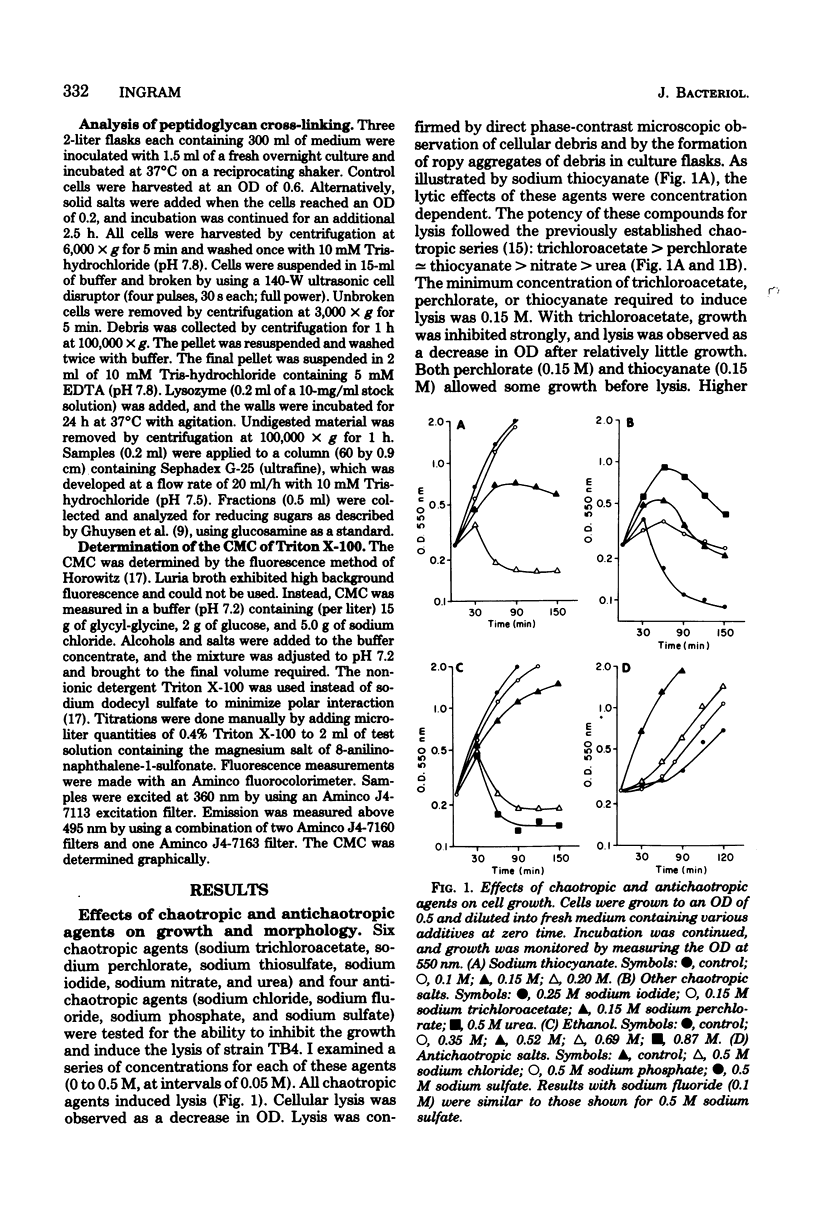
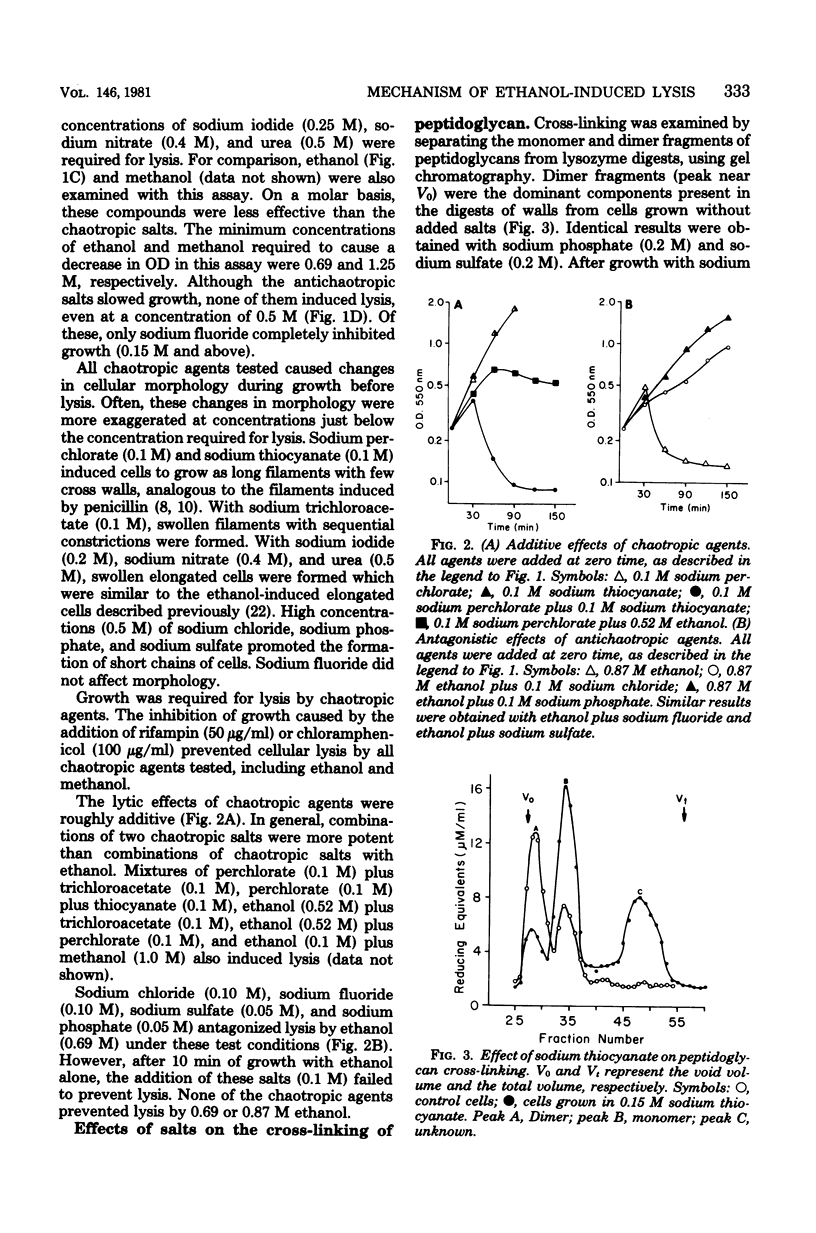
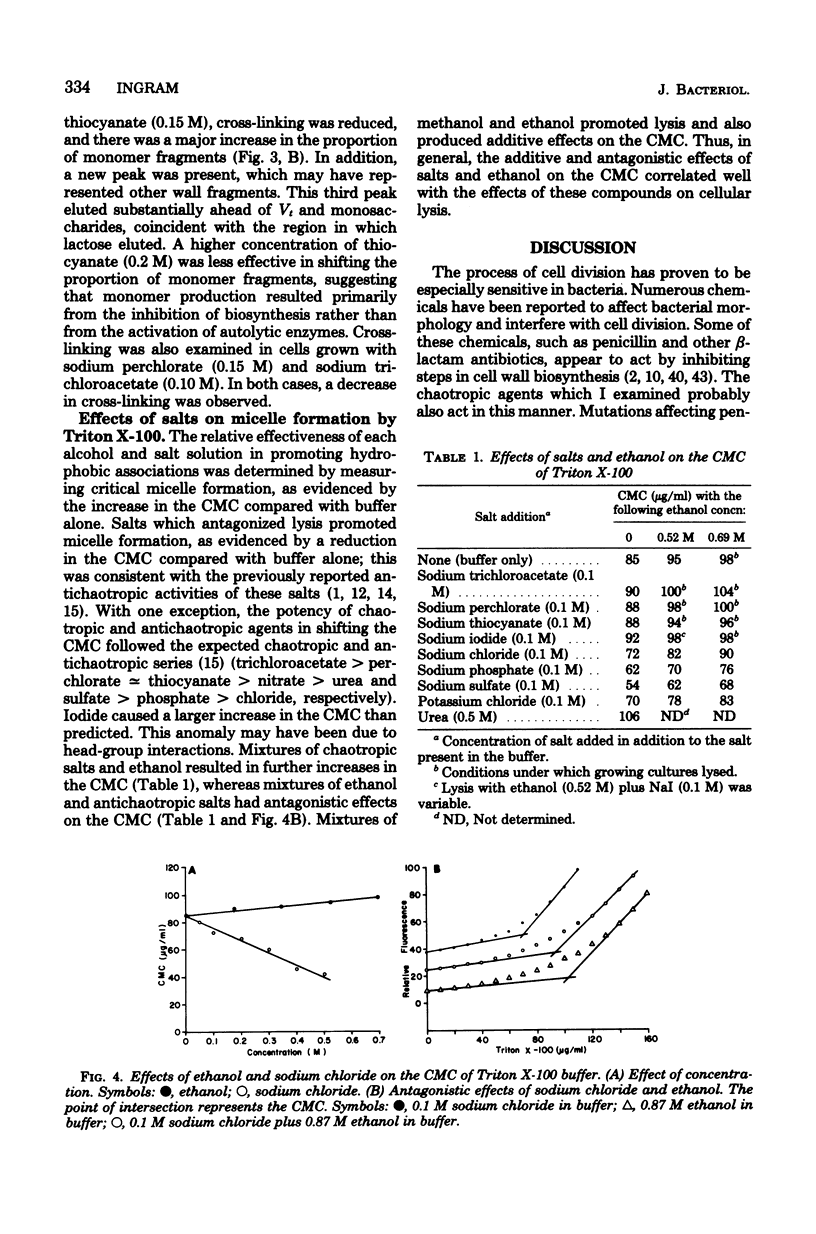
Ethanol has been shown to inhibit the assembly of cross-linked peptidoglycan and to induce cell lysis in Escherichia coli. These effects of ethanol appear to result from the weakening of hydrophobic interactions by ethanol rather than from the intercalation of ethanol into membranes. Other chaotropic agents also inhibited cross-linking and induced lysis. The potency of chaotropic anions with regard to this effect followed the expected chaotropic series. Antichaotropic agents, which strengthened hydrophobic interactions, antagonized ethanol-induced lysis. The weakening of hydrophobic interactions by ethanol is proposed as a general mechanism by which ethanol and other chaotropic agents could affect membrane-associated enzyme activities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke T. M., Ingram L. O. Ethanol-induced changes in lipid composition of Escherichia coli: inhibition of saturated fatty acid synthesis in vitro. Arch Biochem Biophys. 1980 Sep;203(2):565–571. doi: 10.1016/0003-9861(80)90213-1. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., Ingram L. O. Mechanism of ethanol-induced changes in lipid composition of Escherichia coli: inhibition of saturated fatty acid synthesis in vivo. Biochemistry. 1978 Feb 21;17(4):637–644. doi: 10.1021/bi00597a012. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. DNA denaturation in situ. Effect of divalent cations and alcohols. J Cell Biol. 1976 Jan;68(1):1–10. doi: 10.1083/jcb.68.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia III. Reversal of inhibition of cell division caused by D-amino acids, penicillin, and ultraviolet light. J Bacteriol. 1962 May;83:981–988. doi: 10.1128/jb.83.5.981-988.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. The concept of the penicillin target from 1965 until today. The thirteenth marjory stephenson memorial lecture. J Gen Microbiol. 1977 Jul;101(1):13–33. doi: 10.1099/00221287-101-1-13. [DOI] [PubMed] [Google Scholar]
- Hasan S. M., Rosen B. P. Energy transduction in Escherichia coli. The effect of chaotropic agents on energy coupling in everted membrane vesicles from aerobic and anaerobic cultures. Biochim Biophys Acta. 1977 Feb 7;459(2):225–240. doi: 10.1016/0005-2728(77)90024-x. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976 Feb;125(2):670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Fisher W. D. Stimulation of cell division by croton oil in blue-green bacteria. J Bacteriol. 1973 May;114(2):874–875. doi: 10.1128/jb.114.2.874-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Fisher W. D. Stimulation of cell division by membrane-active agents. Biochem Biophys Res Commun. 1973 Jan 23;50(2):200–210. doi: 10.1016/0006-291x(73)90827-9. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Thurston E. L. Potassium requirement for cell division in Anacystis nidulans. J Bacteriol. 1976 Jan;125(1):369–371. doi: 10.1128/jb.125.1.369-371.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Vreeland N. S. Differential effects of ethanol and hexanol on the Escherichia coli cell envelope. J Bacteriol. 1980 Nov;144(2):481–488. doi: 10.1128/jb.144.2.481-488.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Grula E. A. Cell membrane phospholipids and their constitutent fatty acids in dividing and nondividing cells of Micrococcus lysodeikticus. Can J Microbiol. 1980 Jun;26(6):658–663. doi: 10.1139/m80-115. [DOI] [PubMed] [Google Scholar]
- Kennell D., Kotoulas A. Magnesium starvation of Aerobacter aerogenes. IV. Cytochemical changes. J Bacteriol. 1967 Jan;93(1):367–378. doi: 10.1128/jb.93.1.367-378.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- LOVELESS L. E., SPOERL E., WEISMAN T. H. A survey of effects of chemicals on division and growth of yeast and Escherichia coli. J Bacteriol. 1954 Dec;68(6):637–644. doi: 10.1128/jb.68.6.637-644.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel G., Di Savino D., Starka J. Phospholipid composition and phenotypic correction of an envC division mutant of Escherichia coli. J Bacteriol. 1977 Jan;129(1):145–150. doi: 10.1128/jb.129.1.145-150.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Nunn W. D., Tropp B. E. Effects of phenethyl alcohol on phospholipid metabolism in Escherichia coli. J Bacteriol. 1972 Jan;109(1):162–168. doi: 10.1128/jb.109.1.162-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H., Yamada K., Kameyama Y., Ikezawa H., Akamatsu Y., Nojima S. Regulation of membrane lipid synthesis in Escherichia coli after shifts in temperature. Biochemistry. 1977 Jun 14;16(12):2668–2673. doi: 10.1021/bi00631a013. [DOI] [PubMed] [Google Scholar]
- Pang K. Y., Chang T. L., Miller K. W. On the coupling between anesthetic induced membrane fluidization and cation permeability in lipid vesicles. Mol Pharmacol. 1979 May;15(3):729–738. [PubMed] [Google Scholar]
- Patel L., Schuldiner S., Kaback H. R. Reversible effects of chaotropic agents on the proton permeability of Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3387–3391. doi: 10.1073/pnas.72.9.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previc E., Richardson S. Growth-physiological changes in Escherichia coli and other bacteria during division inhibition by 5-diazouracil. J Bacteriol. 1969 Jan;97(1):416–425. doi: 10.1128/jb.97.1.416-425.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J., Chapman D. The dynamics of membrane structure. CRC Crit Rev Biochem. 1980;8(1):1–117. doi: 10.3109/10409238009105466. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Biogenesis of the wall in bacterial morphogenesis. Adv Microb Physiol. 1979;19:1–62. doi: 10.1016/s0065-2911(08)60197-6. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Boyd A., Stoker N. Defective and plaque-forming lambda transducing bacteriophage carrying penicillin-binding protein-cell shape genes: genetic and physical mapping and identification of gene products from the lip-dacA-rodA-pbpA-leuS region of the Escherichia coli chromosome. J Bacteriol. 1980 Aug;143(2):569–581. doi: 10.1128/jb.143.2.569-581.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]


