Abstract
We sought to determine if perceived depth can elicit vergence eye movements independent of binocular disparity. A flat surface in the frontal plane appears slanted about a vertical axis when the image in one eye is vertically compressed relative to the image in the other eye: the induced size effect (Ogle, 1938). We show that vergence eye movements accompany horizontal gaze shifts across such surfaces, consistent with the direction of the perceived slant, despite the absence of a horizontal disparity gradient. All images were extinguished during the gaze shifts so that eye movements were executed open-loop. We also used vertical compression of one eye’s image to null the perceived slant resulting from prior horizontal compression of that image, and show that this reduces the vergence accompanying horizontal gaze shifts across the surface, even though the horizontal disparity is unchanged. When this last experiment was repeated using vertical expansions in place of the vertical compressions, the perceived slant was increased and so too was the vergence accompanying horizontal gaze shifts, although the horizontal disparity again remained unchanged. We estimate that the perceived depth accounted, on average, for 15–41% of the vergence in our experiments depending on the conditions.
Keywords: visual perception, saccadic eye movements, vergence eye movements, induced size effect, perceived depth
1. Introduction
One function of eye movements is to bring the retinal images of objects of interest into the two foveas for detailed scrutiny where acuity is greatest. In the real world, where different objects of interest are often located at different distances from the observer, this usually requires a combination of conjugate (version) and disjunctive (vergence) eye movements. The version components consist of rapid shifts of gaze, termed saccadic eye movements, which last only tens of milliseconds (depending on their magnitude). The vergence components, which get under way before or during the saccade, are much slower and can last much longer. The rapid version components are largely preprogrammed and do not require visual feedback for completion. In fact, target displacements after saccade onset have relatively minor effects on the gaze shift, and then only when that shift is large so that it persists for at least 50 ms after the displacement (Gaveau et al., 2003). In contrast, the slower vergence components are subject to continuous negative-feedback adjustment (Collewijn & Erkelens, 1990; Erkelens, 1987; Pobuda & Erkelens, 1993). The vergence latency exceeds the duration of the gaze shift and the vergence response immediately following the saccade results from the processing of the eccentric target images prior to the onset of the saccade. Of course, any residual version and vergence errors after the primary gaze shift will be corrected by another saccade and continued vergence responses, respectively.
The present paper is concerned primarily with the vergence responses accompanying gaze shifts and, in particular, with the source of the information used to produce them. The vergence error is defined by the slight difference in the locations of the target images on the two retinas, termed the binocular disparity, which is known to be a powerful input to the vergence control mechanism [for review see Collewijn & Erkelens (1990)]. However, while binocular disparity is known to be a sufficient stimulus for eliciting vergence eye movements, it is not a necessary one. Enright found that shifts of fixation while viewing perspective drawings monocularly were accompanied by vergence changes that were “appropriate for the distance relationships implied in the illustration” (Enright, 1987a, 1987b). Ringach, Hawken, and Shapley (1996) showed that subjects experiencing the “kinetic depth effect” during monocular viewing generated vergence eye movements as though tracking the perceived motion in depth. In both of these situations, binocular disparity was absent. This suggested to us that the vergence changes accompanying gaze shifts between objects at different depths might also use the perceived difference in their depths and not rely solely on their binocular disparities.
The present study investigated this possibility using the “induced size effect” of Ogle (1938)—often termed simply “the induced effect”—to dissociate depth and disparity. In this effect, a flat surface in the frontal plane appears slanted about a vertical axis when the image in one eye is vertically compressed relative to the image in the other eye. We were interested in the vergence eye movements accompanying horizontal gaze shifts across such patterns because the horizontal disparity indicates that the surface is fronto-parallel, whereas perception indicates that it is slanted. One problem here is that any vergence responses resulting from the perceived depth will tend to be obscured by the competing vergence responses resulting from the horizontal disparity, and this problem might be expected to get progressively worse over time (i.e., after the main gaze shift). We avoided this latter problem by turning off all visual images during the saccades. In these circumstances, the version and vergence responses are based solely on the visual information available prior to the onset of the saccade and so are executed essentially open-loop. Other studies had indicated that the version and vergence eye movements linked to gaze shifts between targets that differ in their distance to the observer are still robust when the targets are flashed and hence visible only briefly before the gaze shift gets under way [for review of the extensive version literature see Becker (1989), and for quantitative documentation of the vergence changes see Krommenhoek & Van Gisbergen (1994)].
Extinguishing all images during the gaze shift also precluded any long-term adaptation of saccadic amplitudes that might otherwise have resulted from the conflict between the perceived and geometrical depth. It is well known that the oculomotor system’s ability to generate disconjugate saccades—that is, saccades of different amplitude in the two eyes—in response to imposed aniseikonia is quite limited in the short term (Bush, van der Steen, & Miles, 1994; Kapoula, Eggert, & Bucci, 1995; van der Steen & Bruno, 1995), but there are long-term adaptive mechanisms that can gradually result in substantial disconjugacy if the aniseikonia persists over time (Bucci, Gomes, Paris, & Kapoula, 2001; Bucci, Kapoula, Bernotas, & Zamfirescu, 2000; Bucci, Kapoula, & Eggert, 1999; Bucci, Paris, & Kapoula, 2003; Donnet, Kapoula, Bucci, & Daunys, 2002; Eggert & Kapoula, 1995; Erkelens, Collewijn, & Steinman, 1989; Kapoula, Bucci, Lavigne-Tomps, & Zamfirescu, 1998; Kapoula et al., 1995; Lemij & Collewijn, 1991a, 1991b, 1992; Paris, Bucci, & Kapoula, 2000; van der Steen & Bruno, 1995).
In our first two experiments, subjects saw a flat frontal pattern, and we examined the effect of horizontal and vertical compression of one eye’s image on the perceived slant of that pattern and on the horizontal vergence linked to horizontal gaze shifts across that pattern. Both types of compression resulted in horizontal vergence consistent with the direction of the perceived slant, though it was much weaker in the case of the vertical rescaling. In an additional experiment, subjects used vertical compression to null the slant resulting from prior horizontal compression of one eye’s image, and we report that this nulling also reduced the horizontal vergence linked to horizontal gaze shifts. In a variant of this nulling experiment, vertical expansions were used in place of the vertical compressions, and we report that this anti-nulling increased both the perceived slant and the horizontal vergence linked to horizontal gaze shifts, though the latter by a smaller amount. Thus, manipulations of the perceived slant by changing only the vertical disparity are sufficient to modify the horizontal vergence eye movements linked to horizontal gaze shifts, consistent with the idea that perceived depth influences vergence eye movements independently of horizontal disparity.
Preliminary results of this study were presented in abstract form elsewhere (Sheliga & Miles, 2001, 2002).
2. Experiment 1: Vergence During Horizontal Gaze Shifts When One Eye’s Image Is Compressed Horizontally or Vertically
This experiment was concerned with the horizontal vergence associated with horizontal shifts of gaze across a fronto-parallel surface whose image had been compressed in one eye horizontally or vertically. The asymmetric horizontal compressions create disparity—often termed “horizontal size disparity”—and observers perceive a surface that slants about the vertical (the geometric effect of Ogle, 1938); horizontal gaze shifts across such patterns are known to be accompanied by vergence eye movements that are appropriate for maintaining the binocular alignment of the two foveas on the slanting surface (Bush et al., 1994; Kapoula et al., 1995; van der Steen & Bruno, 1995). The asymmetric vertical compressions create so-called “vertical size disparities” and, although they do not affect the horizontal disparity, observers perceive a surface that slants about the vertical (the induced effect of Ogle, 1938); if the vergence eye movements accompanying horizontal gaze shifts across such patterns are based solely on the horizontal disparity, then the vertical compression should not affect them, but if vergence is influenced by perceived slant independent of the (horizontal) disparity, then the vergence eye movements should be influenced by the vertical compression. We now report that horizontal gaze shifts across patterns subject to vertical compression in one eye were accompanied by vergence eye movements that were consistent with the perceived slant but not with the horizontal disparity.
2.1. Methods
Experimental protocols were approved by the Institutional Review Committee concerned with the use of human subjects.
2.1.1. Subjects
The subjects were the authors (BMS and FAM) and one other subject (NPB), who was naïve as to the purpose of the recordings. Their inter-pupillary distances were 68.5, 68, and 67 mm, respectively. All subjects had normal or corrected-to-normal vision.
2.1.2. Apparatus and Stimuli
The presentation of stimuli, together with the acquisition, display, and storage of data, were controlled by a PC using a Real-time EXperimentation software package (REX) developed by Hays, Richmond, and Optican (1982). The horizontal and vertical positions of both eyes were recorded with an electromagnetic induction technique using scleral search coils embedded in silastin rings as previously described (Busettini, Miles, Schwarz, & Carl, 1994). The sampling rate was 1 kHz.
The subjects sat in a completely dark room with their heads secured in place by means of an adjustable head-and-chin rest together with a head band. Dichoptic stimuli were presented using a Wheatstone mirror stereoscope. Each eye viewed a computer monitor through a 45° mirror, creating a single binocular surface straight ahead at 38.4 cm from the eye’s center of rotation (assumed to be 13 mm behind the corneal vertex), which was also the optical distance to the monitor screens. Eight-bit grayscale images were produced using Matlab Image Processing Toolbox software and stored as tiff images with Pacbits compression. The image size and resolution matched the screen size and resolution. Images were displayed on Sony GDM-F520 CRT monitors using a PC equipped with a Nvidia GeForce3 video card. The monitor screen was 375 mm wide and 300 mm high with the brightness control set to 0% (so that the black level was as dim as possible) and contrast control set to 100%. Display resolution was 1280 by 1024 pixels, refresh rate was 100 Hz, and gamma correction was applied to achieve a linear luminance profile. Pixels subtended 2.65 min of arc at the screen center. Using a video signal splitter (Black Box Corp., AC085A-R2), the “red” video signal was connected to all three RGB inputs for the monitor viewed by the left eye, and the “green” signal was connected to all three RGB inputs for the monitor viewed by the right eye. This arrangement allowed the presentation of independent black and white images simultaneously to each eye.
The images on the two monitors were centered, random–dot patterns with an elliptical outline that was varied randomly in height and width over the range 20°–30° (at 1° intervals) from trial to trial to provide no consistent slant information. Individual dots were produced from 4×4 pixels whose luminances (8 cd/m2) were adjusted to place the center of brightness at the desired location independent of the integral pixel locations (anti-aliasing). For this, intensity values were assigned to partially covered pixels by scaling the dot intensity in accordance with the percentage coverage of the individual pixels. Dot coverage was 0.1%. The small dot size and low density were chosen to minimize the perspective cues (Backus, Banks, van Ee, & Crowell, 1999).
The patterns presented on the two monitors were identical except that one of them was randomly compressed horizontally or vertically by 0%, 3%, 4%, 5%, 6%, 9%, or 12%. Horizontal compression of one pattern created a binocular surface slanted around a vertical axis, the actual magnitude of the resulting slant depending on the inter-pupillary separation but in all subjects being very close to 0°, 10°, 13°, 16°, 19°, 28°, or 36° (clockwise or counterclockwise, viewed from above) with respect to the plane of the binocular image of the two monitor screens. The magnitude of the vertical compressions was the same as that of the horizontal compressions. It is sometimes useful to specify these compressions in terms of the horizontal (HSR) and vertical size ratios (VSR), defined as the size of the left image divided by the size of the right image.
2.1.3. Task and Procedure
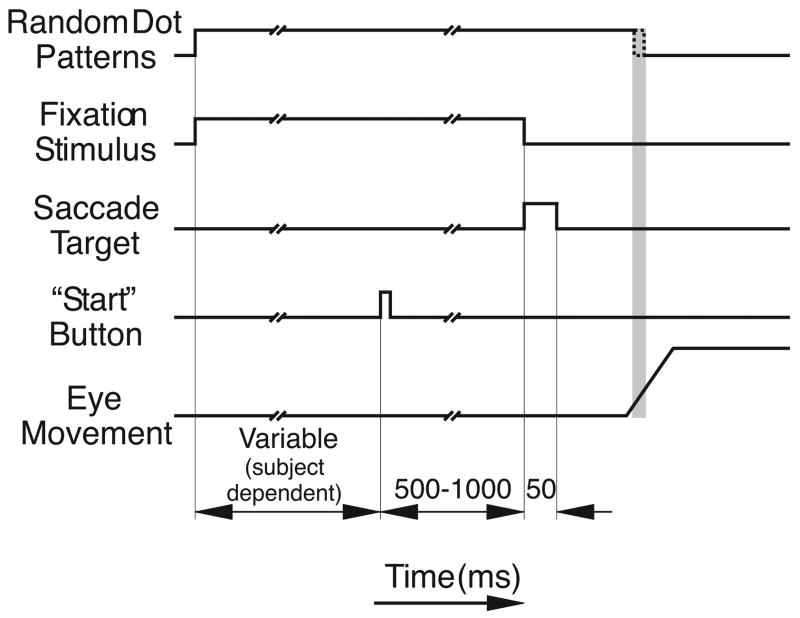
The time sequence of events during the individual trials is indicated diagrammatically in Figure 1. Each trial started with the appearance of a pair of random dot patterns, one for each eye. Also present was a pair of fixation targets, one for each eye located at the center of the screen. Each fixation target was a vertical line, nominally 0.13° × 1.00° but horizontally and vertically rescaled to accord with any rescaling of its associated pattern (as though intrinsic to that pattern). In this way the subject perceived a single target line embedded in a surface of random dots. Subjects were first required to report the direction of the slant of the binocular surface by a manual button press. The instructions were “press the left button if the surface is slanting away to the left and the right button if it is slanting away to the right”: two-alternative forced-choice (2AFC). There was no time restriction for this. Subjects were then required to look at the fixation target(s). A random period of time (500–1000 ms) after the right eye entered a 3° electronic window centered on the target seen by that eye, the pair of lines was extinguished and immediately replaced by a second pair that appeared randomly to the right or left at a cyclopean eccentricity of 7.5° for 50 ms (i.e., flashed presentation). Again, the target appeared as though embedded in the surface of random dots. The subject was required to transfer fixation to the remembered location of the flashed binocular target and, as soon as the computer detected the subject’s saccade (when eye velocity exceeded 36°/s), the screen was blanked. Thus, subjects completed their gaze shift in the dark and received no feedback about the accuracy of their (open-loop) responses. Subjects were asked to refrain from shifting their gaze again for a brief period (200 ms) to obtain a data sample free of additional saccades. The screen remained blank for 500 ms before new patterns appeared indicating the start of a new trial.
Figure 1.
Time sequence of events in Experiment 1. See text for details. The video frame rate was 100 Hz, and the blanking of the two patterns commenced within one video frame after the saccade was detected, i.e., within a 10-ms interval (shaded area).
Each experimental session consisted of 7–30 blocks, each block having 52 trials. Forty–eight of the 52 trials in the block were experimental trials: 2 (compression: horizontal vs. vertical) × 2 (eye viewing the compression: left vs. right) × 6 (amount of compression) × 2 (gaze shift: leftward or rightward). For the remaining 4 trials in the block, the patterns on both monitors were identical (controls): 2 × 2 (gaze shift: leftward vs. rightward). Trials in which an error occurred were subsequently rerun within the same block. Each subject participated in 2 recording sessions.
2.1.4. Data Analysis
The horizontal and vertical components of eye movements were recorded together with time markers for the major stimulus and response events occurring during the course of the trials. The horizontal vergence angle was computed by subtracting the right eye position signal from the left eye position signal. We used the sign convention that rightward eye movements were positive, hence increases in the vergence angle were positive. The level of statistical significance was always set at 0.05.
2.2. Results
2.2.1. Slant Perception
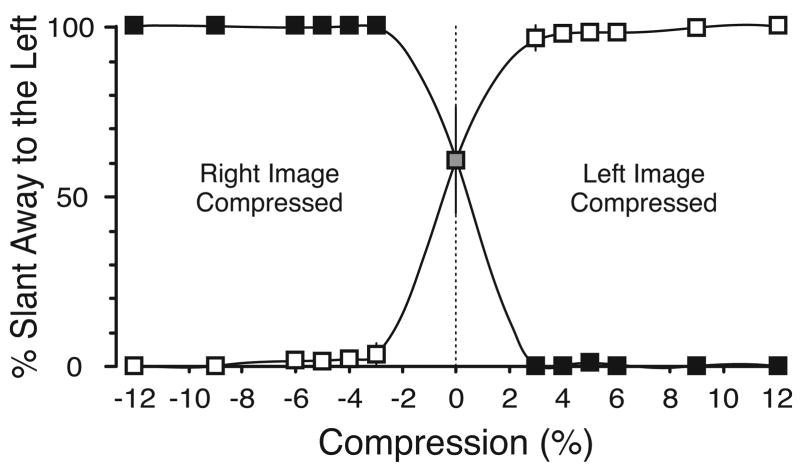
Prior to the execution of the gaze shift, subjects reported the direction of the perceived slant of the binocular surface in a 2AFC paradigm (“slanting away to the left or slanting away to the right”). There was little variability between the subjects, and Figure 2 shows the mean data for all subjects. With horizontal compression of one eye’s image (the “geometric effect” condition, filled symbols), all subjects almost invariably reported the image as slanted toward the eye that viewed the compressed pattern. With vertical compression of one eye’s image (the “induced effect” condition, open symbols), the reverse was true: the image appeared slanted away from the eye that viewed the compressed pattern. Judging the slant of control images (gray square in Figure 2) showed the most variability: on average, subjects perceived this image as being slanted away to the left in 60% (±16%, SE) of presentations.
Figure 2.
Direction of perceived slant resulting from compression of one eye’s pattern: dependence on the magnitude and direction of the compression. Ordinate: percentage of trials in which slant was judged to be away to the left. Abscissa: percentage compression, negative values indicating that compressions were applied to the image seen by the right eye and positive values that compressions were applied to the image seen by the left eye. Filled symbols, data for horizontal compressions (geometric effect). Open symbols, data for vertical compressions (induced effect). Lines are cubic spline interpolations. Data points are means of the individual means for each of our three subjects (error bars, ±1 SE).
2.2.2. Vergence Responses: Time Course
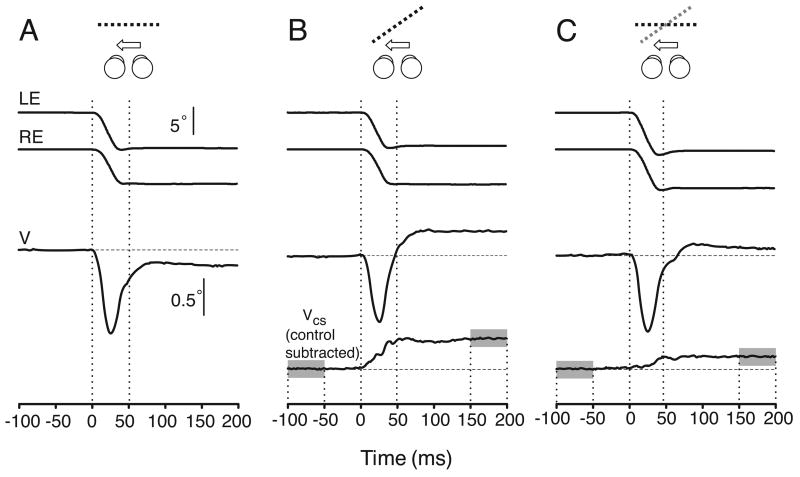
Figure 3 shows sample raw eye-movement data obtained from one subject in association with gaze shifts from a central target to one 7.5° left of center (in cyclopean coordinates). The major purpose of this figure is to show the general form of the data and our response measures. The data in Figure 3 were obtained under three different conditions: (A) the images seen by the two eyes were identical; (B) the images seen by the left eye were compressed 6% horizontally; and (C) the images seen by the right eye were compressed 12% vertically. The stimuli are shown diagrammatically in the cartoons above the traces, the black dotted lines representing the slant (seen from above) resulting from the horizontal size disparity (geometric effect), the gray dotted line representing the perceived slant caused by the vertical size disparity (induced effect), and the arrows indicating the direction of the horizontal gaze shifts. It is evident from the traces labeled “V” showing the horizontal vergence position that horizontal and vertical compressions resulted in sustained changes in the vergence angle that were in accord with the direction of the perceived slant: with horizontal compression, the vergence angle increased with gaze shifts toward the eye viewing the compressed pattern, whereas with vertical compression, the vergence angle increased with gaze shifts away from the eye viewing the compressed pattern. (Note: for clarity, the vergence traces are displayed at a gain more than 10 times greater than that used for the traces showing the movements of the individual eyes.) A complicating factor here was that during the gaze shifts, the abducting (left) eye always moved slightly more quickly than the adducting (right) eye, so that there was always a transient loss of convergence. This has been reported by many previous authors (e.g., Collewijn, Erkelens, & Steinman, 1995; Collewijn, Erkelens, & Steinman, 1997; Erkelens, Steinman, & Collewijn, 1989; Zee, Fitzgibbon, & Optican, 1992). It is also apparent that the vergence angle did not return to the pre-saccadic value in the control condition (Figure 3), consistent with the fact that the targets were presented on a fronto-parallel surface, the first at the center and the second 7.5° to the left, necessitating a slight reduction in the vergence angle in order to maintain binocular alignment on the target images. In fact, all images disappeared during the saccade so that no post-saccadic visual feedback was available to eliminate any residual version or vergence errors: the sustained change in vergence angle was planned before the saccade. To eliminate the transient effects of saccades and the sustained effects of using a tangent screen, the mean vergence profiles obtained in the control condition were subtracted from the vergence profiles obtained with the various image compressions, thereby uncovering the effects attributable to the image compressions (see the traces labeled VCS).
Figure 3.
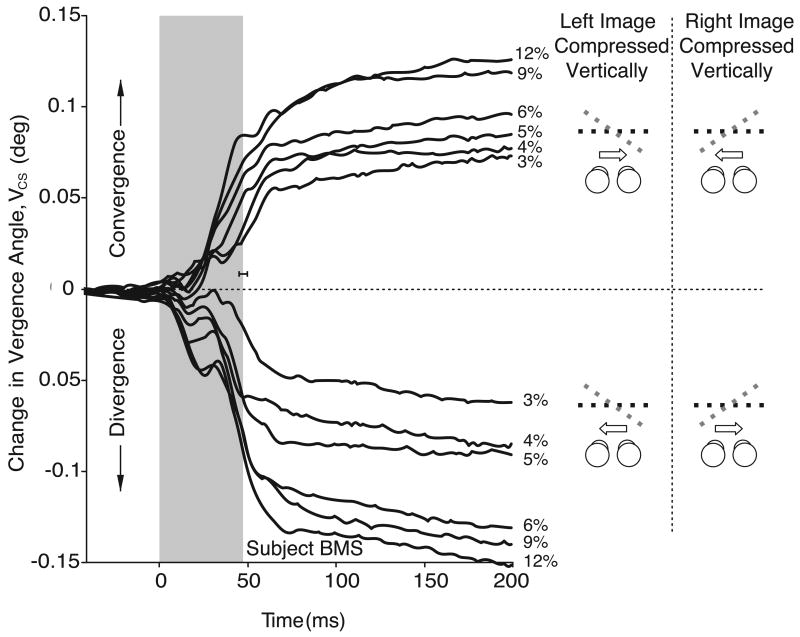
Time course of the version and vergence eye movements associated with horizontal gaze shifts (sample raw data from one subject, BMS). A. No compressions were applied so the two images are identical and the single binocular surface is fronto-parallel (control). B. A 6% horizontal compression was applied to the left image (geometric effect). C. A 12% vertical compression was applied to the right image (induced effect). Cartoons indicate the slant stimuli (seen from above): black dotted lines, slant resulting from the horizontal size disparity (geometric effect); gray dotted line, perceived slant caused by the vertical size disparity (induced effect); arrows, direction of the horizontal gaze shifts. LE and RE: horizontal position of left and right eye. V: horizontal vergence position (LE-RE). VCS: Horizontal vergence position after subtracting mean control vergence. Vertical calibration bars: 5° applies to eye position data (LE and RE traces), 0.5° applies to vergence (V and VCS traces). Time zero is the start of the saccades. Vergence changes were examined quantitatively using the difference in the value of VCS averaged over the 50-ms time intervals starting 100 ms before and 150 ms after the onset of the saccade (gray areas).
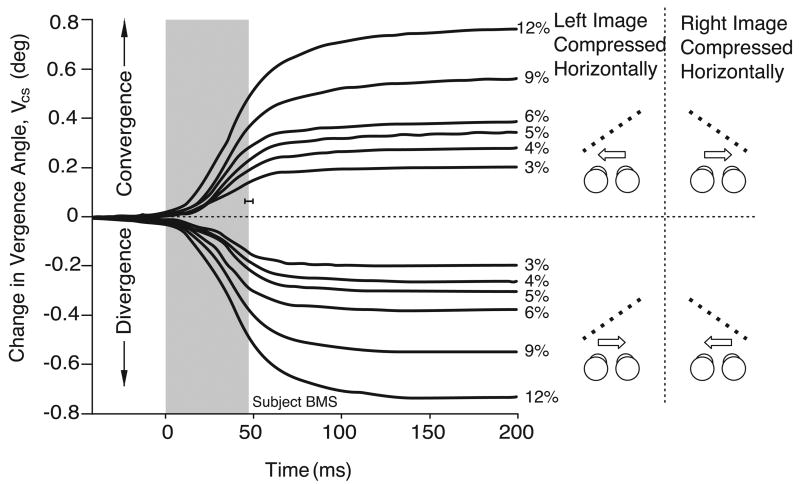
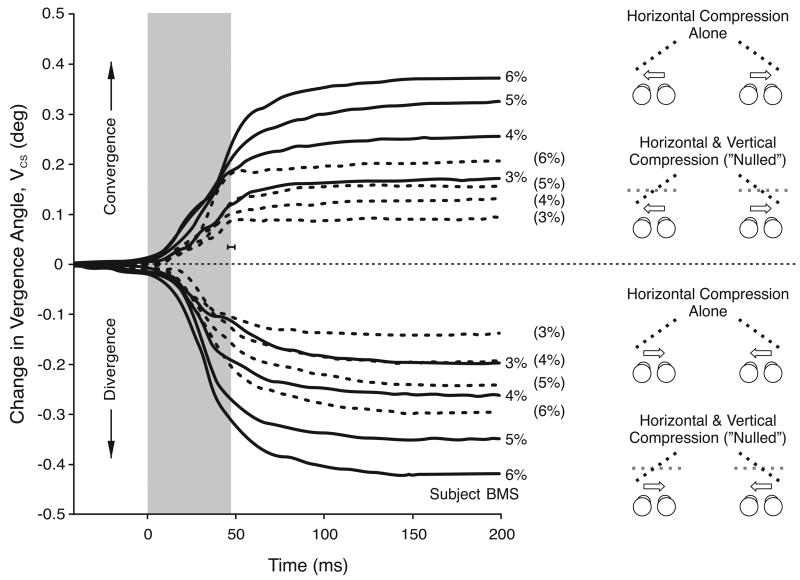
Figures 4 and 5 show the mean temporal profiles for VCS obtained from one subject, for the complete set of horizontal and vertical compressions, respectively. For these figures, the data from each trial were synchronized to the beginning of the saccade (time zero). Saccade duration averaged just under 50 ms (shown in gray), and the data for leftward and rightward gaze shifts were pooled together (as indicated in the cartoons on the right hand side). Again, there were clear vergence responses with both horizontal and vertical image compressions that were in accord with the direction of the perceived slant (see the cartoons to the right). The changes in vergence in all of our experimental situations usually did not get under way until after the start of the saccadic shift and continued for up to 100 ms after the end of the saccade, though they occasionally persisted for longer (e.g., the divergent responses with the larger compressions in Figure 5).
Figure 4.
Mean temporal profiles for the vergence responses linked to gaze shifts, VCS: dependence on horizontal compression (data for one subject, BMS). Traces are synchronized to the start of the gaze (i.e., version) shift at time zero, and each is an average of at least 58 individual responses. Cartoons depict a plan view of the single binocular images of the random-dot patterns and fixation targets as defined by the horizontal disparity (black dashed line) and the direction of the saccade (arrows). The data for leftward and rightward gaze shifts were pooled together. Convergence was observed when gaze shifted towards the side of the eye that viewed the compressed pattern (upper traces); divergence was observed when gaze shifted away from the side of the eye that viewed the compressed pattern (lower traces). Numbers at ends of traces indicate magnitude of the compression. Gray area is mean saccade duration (horizontal error bar, ±SD).
Figure 5.
Mean temporal profiles for the vergence responses linked to gaze shifts, VCS: dependence on vertical compression (data for one subject, BMS). Each trace is an average of at least 59 individual responses. Cartoons depict a plan view of the zero slant resulting from the zero horizontal size disparity (black dashed lines), and the perceived slant resulting from the vertical size disparity (gray dashed lines). Convergence was observed when gaze shifted away from the side of the eye that viewed the compressed pattern (upper traces); divergence was observed when gaze shifted towards the side of the eye that viewed the compressed pattern (lower traces). Other conventions as in Figure 4.
2.2.3. Vergence Responses: Magnitude
Figures 4 and 5 indicate that the vergence linked to horizontal gaze shifts was much weaker with vertical compression of one image than with horizontal compression (note the difference in ordinate scales in the two figures). This was examined quantitatively, estimating the change in vergence from the difference in the value of VCS averaged over the 50–ms time periods starting 100 ms before and 150 ms after the start of the saccade. These measures were then used to compute the mean vergence gains as follows.
When the slant resulted from horizontal compression, the vergence gain was computed by dividing the measured vergence response by the required vergence response, where the latter was given by the difference in the horizontal disparity of the two targets (corrected for the disparity in the control condition). When the slant resulted from vertical compression, this gain measure was inappropriate. For the purposes of comparison, however, it was useful to also compute the “gain” of the vergence response with vertical compression by assuming that the magnitude of the required vergence response for a given vertical compression was equal to that for the same amount of horizontal compression (ignoring the difference in sign).
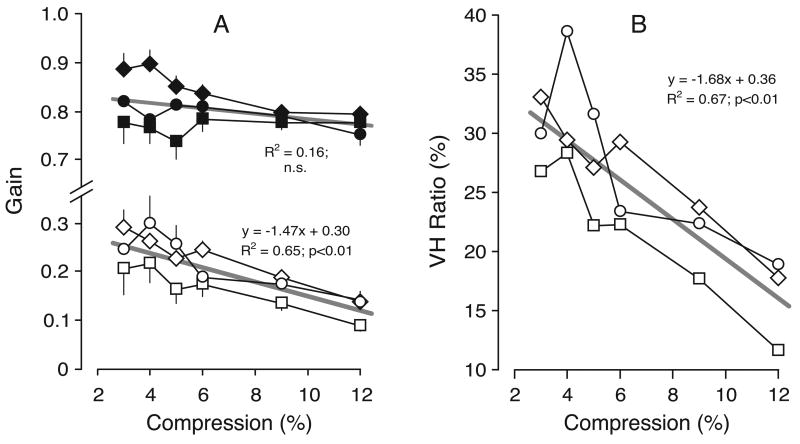
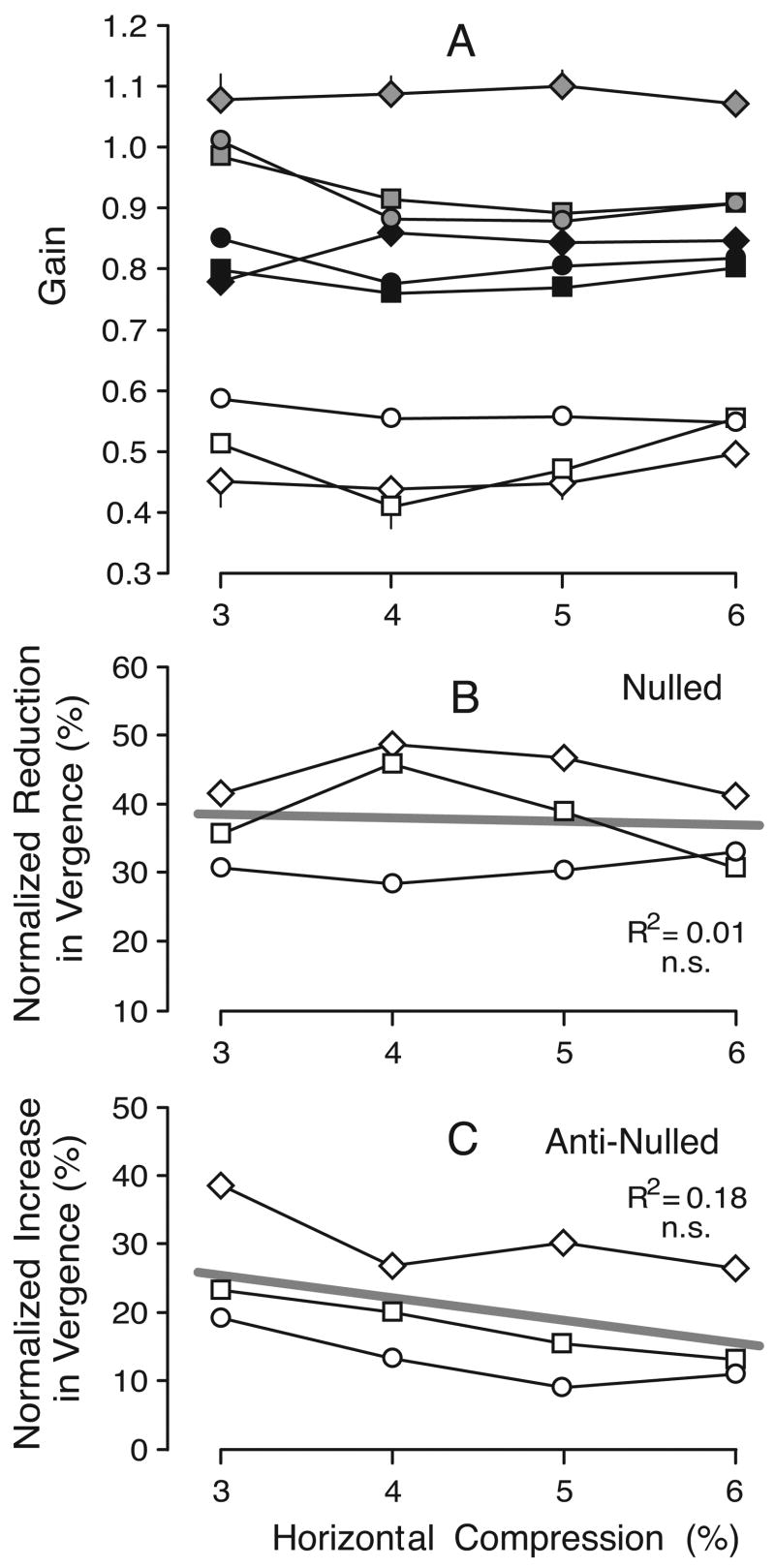
These vergence gain estimates are plotted in Figure 6A for all subjects. The data obtained with horizontal compressions are shown in filled symbols and the data obtained with vertical compressions are shown in open symbols. As there were no consistent asymmetries in the data obtained with leftward and rightward gaze shifts, or for convergent and divergent responses, all of these data were pooled together. It is clear from this plot that the vergence responses linked to horizontal compressions were always substantially greater than those linked to vertical compressions. With horizontal compressions, vergence gains fell somewhat short of unity (i.e., the change in vergence angle was slightly less than the difference in the horizontal disparity of the two targets). In fact, vergence gain here ranged from 0.74 to 0.90 (mean±SD, 0.80±0.04) and there was a slight tendency, though not significant, for the gain to decrease as the compression increased. With vertical compressions, vergence “gains” ranged from 0.09 to 0.30 (mean±SD, 0.20±0.06), and also showed a tendency—this time, significant—to decrease as compression increased. When the vergence “gains” for given vertical compressions were expressed as a percentage of the vergence gains for horizontal compressions that had the same magnitude—termed here “VH ratios”—values ranged from 12% to 39% (mean±SD, 25.2%±6.5%) but tended to decrease with increases in compression (see Figure 6B).
Figure 6.
Experiment 1: dependence of vergence linked to horizontal gaze shifts on compression (data for three subjects). A. Mean vergence gain; filled symbols, horizontal compression; open symbols, vertical compression; error bars, 1 SE; each datum point is an average of at least 118 (subject BMS), 143 (subject FAM), and 65 (subject NPB) individual responses. B. Mean VH Ratios (vergence gain with vertical compression expressed as a percentage of the vergence gain with horizontal compression). Straight gray lines are regression lines whose defining coefficients are shown together with the coefficients of determination (R2). Symbols denote subjects: diamonds, BMS; squares, FAM; circles, NPB.
2.3. Discussion of Experiment 1
It is clear that vertical compression of one eye’s image influenced the horizontal vergence responses linked to horizontal gaze shifts despite the fact that this compression did not influence the horizontal disparity. The direction of these vergence responses was appropriate for the perceived slant, but their magnitude was relatively small, especially when compared with those recorded when the slant resulted from horizontal compression of one eye’s image—as indicated by the VH ratio. Note that, despite our best efforts, there were residual monocular cues in our setup—perspective, texture, size, and luminance, for example—that were all consistent with a fronto-parallel surface. This could mean that our experiments underestimate the vergence gain associated with the induced effect and the geometric effect [for discussion, see Banks & Backus (1998)]. However, these monocular cues to depth are constant across conditions so that normalizing the data obtained with vertical compressions with respect to the data obtained with horizontal compressions (as in the “VH ratios”) tends to factor out the effect of the unwanted fronto-parallel depth cues in our setup.
One concern here was that the change in vergence with the vertical compressions might have been smaller than with the horizontal compressions because the shifts in gaze were smaller: when the surface was perceived to be slanting because of vertical compression, perhaps the version and vergence were appropriate for acquiring a point some distance short of the location of the (flashed) target.
We examined this question by estimating the saccadic (version) amplitude from the difference in the version position averaged over the 50-ms periods starting 100 ms before and 150 ms after saccade onset. Mean saccadic version gains (measured version amplitude divided by required version amplitude) for each subject in the different conditions ranged from 0.92 to 0.98 (mean±SD, 0.95±0.01). Thus, saccades in our experiments were slightly hypometric, which is in line with previous reports for centrifugal saccades between targets on a tangent screen (Becker, 1989). However, our major concern is whether the saccadic amplitudes were different with the two kinds of compression. The mean saccadic gains were subjected to a univariate analysis of variance (ANOVA) with two within-subject factors: (a) type of compression (horizontal or vertical), (b) magnitude of compression (3%, 4%, 5%, 6%, 9%, or 12%). Post hoc comparisons were made using the Newman-Keuls test and revealed that no factors or interactions were significant at the 0.05 level. We conclude that differences in the amplitude of the gaze shifts with horizontal and vertical compressions were negligibly small (overall means, 7.08° vs. 7.06°, respectively) and did not contribute significantly to the differences in vergence with the two kinds of compression.
3. Experiment 2: Perceived Slant When One Eye’s Image Is Compressed Horizontally or Vertically
The vergence eye movements recorded in Experiment 1 in association with horizontal gaze shifts when one eye’s image was compressed vertically were clear and consistent with the direction of the perceived slant. However, these vergence responses were rather modest in amplitude, especially compared with those when the compression was horizontal. We were interested in the possibility that this difference in the effects of horizontal and vertical compressions simply reflected differences in the magnitudes of the perceived slants associated with them. With this in mind, we obtained quantitative estimates of the perceived slants associated with the visual stimuli used in Experiment 1. We report that the perceived slants with vertical compressions were indeed smaller than those with horizontal compressions of comparable magnitude—as generally reported by others—but these perceptual differences were substantially less than the vergence differences reported in Experiment 1.
3.1. Methods
The subjects and visual stimuli were exactly as in Experiment 1.
3.1.1. Task and Procedure
Our objective was to estimate the perceived slant of the binocular surfaces used in Experiment 1. It has been well documented that the slant percepts associated with changes in HSR and VSR (in the absence of any visual reference, as here) generally develop over periods of many seconds, though perhaps more quickly in experienced subjects (Allison, Howard, Rogers, & Bridge, 1998; van Ee & Erkelens, 1996, 1998). For this reason, in the present experiment, subjects viewed the same selection of images as they had in Experiment 1, and each for exactly the same period of time (up to a maximum of 5 s). Subjects were asked to manually align an unseen horizontal metal bar with the perceived slant of the screen images. The bar (1×1×20 cm) was mounted so as to rotate horizontally about the center of its long axis and was attached to a calibrated rotary potentiometer. A DC voltage was applied to the end terminals of the potentiometer and the wiper was connected to one of the A/D inputs of the PC controlling the experiment, providing a direct measure of the bar’s orientation about the vertical axis. This pivoting bar was mounted immediately above a second identical bar that acted as a reference and was secured to the surface of a horizontal platform attached to the subject’s chair. This reference bar was coplanar with the binocular single image of the two computer screens (i.e., it was oriented approximately in the subject’s fronto-parallel plane), and was located within easy reach at waist level in front of the sitting subject (beneath the virtual image created by the stereoscope). At the start of each trial, the subject aligned the pivoting bar with the reference bar. When the random-dot patterns appeared, the subject aligned the pivoting bar with the perceived slant of the binocular surface and then pressed a button indicating that he had completed the task. Two seconds later, the next image appeared for the next trial in the sequence and the subject repeated the task.
3.1.2. Data Analysis
The potentiometer voltage was calibrated with the aid of a protractor to yield the orientation of the bar (in degrees) with respect to the plane of the binocular single image of the two computer screens. These measures of bar orientation provided estimates of the perceived slant of the random-dot patterns, and were then used to compute the gain of the open-loop slant estimates as follows. When the perceived slant resulted from horizontal compression, the slant gain was computed by dividing the measured slant by the required slant estimated from the HSR. When the slant resulted from vertical compression, the usual gain measure was inappropriate as discussed earlier in Experiment 1. For the purposes of comparison, however, it was useful to estimate a “gain” for the induced effect by assuming that the required slant for a given vertical compression was equal to that for the same amount of horizontal compression (ignoring the difference in sign). Thus, our “gains” are directly comparable with the “normalized slants” of Allison et al (1998) and of van Ee and Erkelens (1998).
3.2. Results
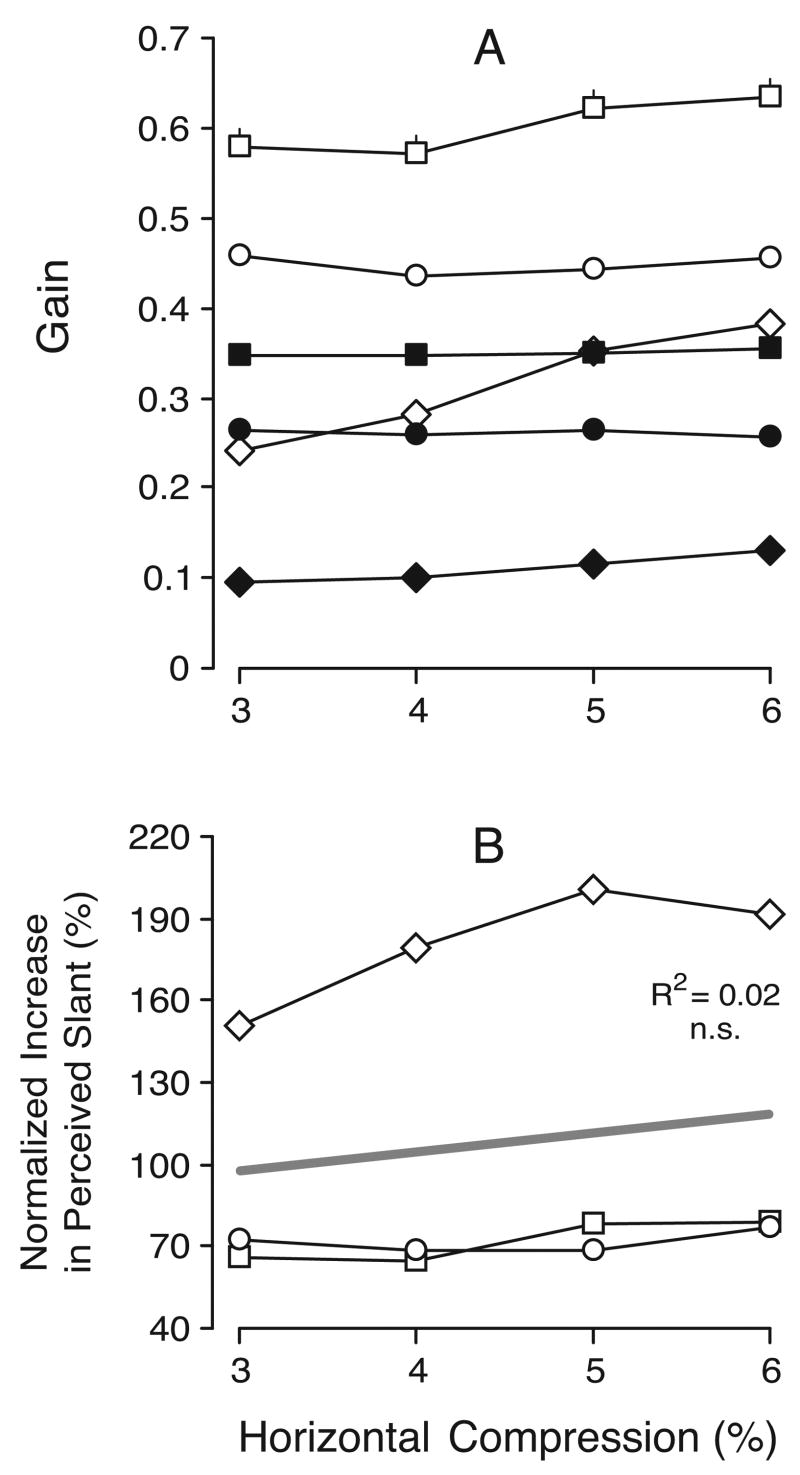
The gains of the perceived slants for each of the three subjects are plotted in Figure 7A; the data obtained with horizontal compressions (geometric effect) are shown in filled symbols, and the data obtained with vertical compressions (induced effect) are shown in open symbols. The data obtained when the perceived slants were clockwise and counterclockwise (seen from above) have been pooled together. The gain of the geometric effect varied widely between subjects, ranging from 0.09 to 0.45 (mean±SD, 0.26±0.11) and tended to increase with compression. The gain of the induced effect was invariably lower than that of the geometric effect (for a given level of compression), ranging from 0.06 to 0.24 (mean±SD, 0.16±0.07), and showed only minor dependence on compression. When the slant gains for given vertical compressions were expressed as a percentage of the slant gains for horizontal compressions that had the same magnitude—yielding VH ratios as in Section 2.2.3.—values were greatest with the smallest compression and, on average, decreased roughly linearly with increases in compression: see Figure 7B, which shows the data for all three subjects. The regression line in Figure 7B has a value of ~75% when the compression is 3% and a value of ~40% when the compression is 12%.
Figure 7.
Experiment 2: dependence of perceived slant on compression (data for three subjects). A. Mean slant gain; filled symbols, horizontal compression; open symbols, vertical compression; error bars, 1 SE; each datum point is an average of at least 120 (BMS), 147 (FAM), and 67 (NPB) individual responses. B. Mean VH ratios (slant “gain” with vertical compression expressed as a percentage of the slant gain with horizontal compression). Other conventions as in Figure 6.
3.3. Discussion of Experiment 2
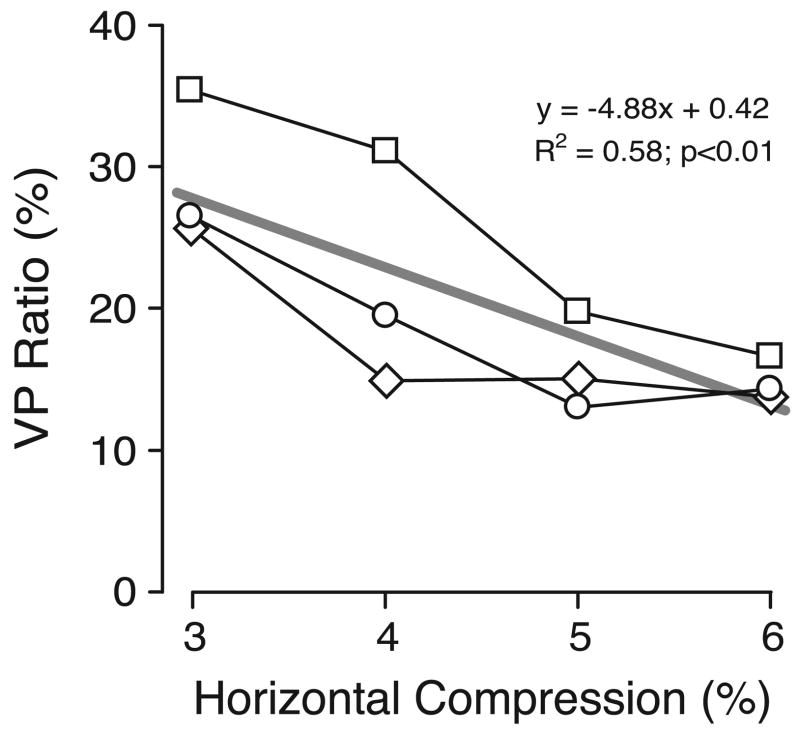
Our measures of the perceived slant associated with the geometric effect and the induced effect showed substantial variation between subjects. Reports in the literature indicate that such variation is not unusual (e.g., Allison et al., 1998; Berends & Erkelens, 2001; Gillam, Chambers, & Lawergren, 1988; van Ee & Erkelens, 1996, 1998). Some of the variation in our study might have been due in part to our open-loop methodology: subjects were given no practice and were denied any feedback about their performance. However, our main interest was in the magnitude of the induced effect compared with the magnitude of the geometric effect—for a given magnitude of compression—and this showed much greater consistency between subjects, with a clear, and roughly linear, dependence on the amount of compression: see the VH ratios plotted in Figure 7B. The regression line in this plot indicates that, on average, the induced effect approached ~75% of the geometric with our smallest compression and fell to almost half that with our largest compression, values roughly comparable with those in the literature. Thus, the induced effect could be appreciably weaker than the geometric effect, especially with higher compressions [cf. Ogle (1938)]. These data are qualitatively similar to those of Kaneko and Howard (1996), who also used a manual matching task and a similar range of image compressions. However, these VH ratios for perceived slant are all substantially higher than those for vergence in Experiment 1 and this point is made clear by Figure 8, in which the VH ratios from Experiment 1 (for vergence) have been normalized with respect to the VH ratios from Experiment 2 (for perceived slant) and are plotted as a function of compression. This plot of the “vergence-perception ratios,” or VP ratios as these normalized gain estimates will be referred to here, shows the mean values for each of the three subjects, and these range from 28% to 55% (mean±SD, 41.4%±7.5%).
Figure 8.
VP ratios: dependence on compression (data for three subjects). The VH ratios for vergence in Experiment 1 were expressed as a percentage of the VH ratios for perceived slant in Experiment 2. Other conventions as in Figure 6.
We suggest that the VP ratio provides an estimate of the proportion of the vergence linked to gaze shifts that can be attributed to the perceived slant. Let us refer to the contribution to the vergence due to perceived slant and to horizontal disparity in condition X as KP ·PX and KD ·DX, respectively, where PX is the perceived slant signal in condition X, KP is a coefficient specifying the extent to which the PX signal is utilized by the vergence system, DX is the horizontal disparity signal provided by the visual system in condition X, and KD is a coefficient specifying the extent to which the DX signal is utilized by the vergence system. The VH ratio in Experiment 1, which expresses the vergence responses for given vertical compressions as a percentage of the vergence responses for horizontal compressions of the same magnitude, is then given by
| (1) |
where I and G refer to the induced effect condition and the geometric effect condition, respectively. In Experiment 1, the vertical compression does not affect the horizontal disparity so that DI=0, hence the VH ratio simplifies to
| (2) |
In Experiment 2, only perception is involved (i.e., DI=DG=0) and so the VH ratio, which expresses the slant measures for given vertical compressions as a percentage of the slant measures for horizontal compressions of the same magnitude, is given by
| (3) |
where KM is a coefficient specifying the extent to which the signal provided by the perceptual system, PX, is utilized by the hand positioning system that provides the measure of perceived slant. The VP ratio can now be derived as follows:
| (4) |
Thus, the VP ratio gives an estimate of the proportion of the vergence response attributable to the perceived depth.
The duration of the exposure to the patterns in our study averaged 2.0 s (range, 1.1–5 s), and other studies have shown that the percept of slant associated with horizontal or vertical size disparities develops rather gradually, especially when no visual reference is present, often continuing to increase over periods of many seconds (Allison et al., 1998; van Ee & Erkelens, 1996, 1998). This suggests that our subjects might have reported larger perceived slants if longer exposure times had been used.
4. Experiment 3: Vergence During Horizontal Gaze Shifts When Perceived Slant Resulting From Horizontal Compression of One Eye’s Image Is Nulled by Vertical Compression of That Image
This experiment employed a variant of a nulling technique often used by others (Backus et al., 1999; Banks & Backus, 1998; Berends & Erkelens, 2001; Ogle, 1938, 1939a; 1939b, 1940). Subjects were asked to apply vertical compression (or expansion) to one eye’s image to cancel the perceived slant resulting from horizontal compression (or expansion) of that same eye’s image. Thus, the induced effect was used here to cancel the geometric effect without changing the horizontal disparity so that, again, horizontal disparity and perceived depth were dissociated. Note that in the nulled situation—in contrast to the situations in Experiments 1 and 2—the monocular cues signaling a fronto-parallel surface (such as perspective, texture, size, and luminance) are in agreement with the perceived slant resulting from the horizontal and vertical compressions. We report that when vertical compression of one image was used to null the perceived slant resulting from prior horizontal compression of that image, it also reduced the vergence linked to horizontal gaze shifts, even though it did not alter the horizontal disparity, again consistent with the idea that perceived depth has a role in producing the vergence.
4.1. Methods
The subjects, experimental setup, visual stimuli, and data analyses were essentially the same as in Experiment 1.
4.1.1. Task and Procedure
At the start of the trial, elliptically shaped random-dot patterns appeared on the two monitor screens. The pattern seen by one or other eye was compressed horizontally by 3%, 4%, 5%, or 6% (randomly selected each trial), and subjects were required to use button presses to rotate the binocular surface until it appeared to be fronto-parallel: the “nulled” condition. With each button press, a new pair of patterns appeared with a new elliptical outline (randomized in height and width as in Experiment 1) whose HSR remained the same but whose VSR differed from that of the previous pair by 1%. Pressing the left button decreased the VSR by 1% (tending to rotate the percept of the pattern clockwise, as seen from above), and pressing the right button had the opposite effect. These changes in the VSR were always accomplished by applying the vertical compressions or expansions to the image that had been horizontally compressed at the outset. However, the randomization of outlines meant that the subject could not know which of the two patterns was vertically compressed or expanded by his button presses. To prevent the subject from being able to associate the slant resulting from a particular horizontal compression with the need for a particular number of button presses, the horizontally compressed pattern started out with a vertical compression or expansion of 0–6% (equivalent to 0–6 button presses), randomly selected. Once satisfied that the binocular surface was fronto-parallel, the subject pressed a third, central, button, which resulted in the appearance of a pair of fixation targets, one for each eye, located at the center of the screen. As in Experiment 1, these targets were vertical lines, nominally 1° long but each vertically and horizontally rescaled in accord with the compression or expansion of its associated pattern as though intrinsic to that pattern. The remaining part of the trial was almost exactly as in Experiment 1. Briefly, a specified random time period after the right eye entered a 3° electronic window centered on the line seen by that eye (1000–1500 ms), the pair of target lines was extinguished and replaced by a second pair that appeared randomly to the right or left at an eccentricity of 7.5° for 50 ms. The subject was required to transfer fixation to the remembered location of the new binocular target; and, the screen was blanked during the saccade and remained so for the rest of the trial.
Randomly interleaved with these experimental trials were control trials in which the pattern seen by one or other eye was compressed horizontally by 0%, 3%, 4%, 5%, or 6%. The VSR in these control trials was always unity and no nulling was required of the subject. This was made apparent to the subject by having the binocular fixation target—the usual pair of vertical lines positioned at the screen center—appear immediately at the start of the trial along with the random-dot patterns. Once the subject had fixated the binocular target line for a specified random period (1000–1500 ms), the line was extinguished and briefly replaced by another on the right or left, exactly as in the experimental trials; from this point on, these control trials were the same as the experimental trials.
Each experimental session consisted of 6–15 blocks, each block having 36 trials. Thirty-two of the 36 trials in the block were experimental trials: 2 (compression: horizontal alone vs. “nulled” condition) × 2 (eye viewing the compression: left vs. right) × 4 (amount of horizontal compression) × 2 (gaze shift: leftward vs. rightward). For the remaining 4 trials in the block, the patterns on the two monitors were identical (controls): 2 × 2 (gaze shift: leftward vs. rightward). Trials in which an error occurred were subsequently rerun within the same block. Each subject participated in 2–3 recording sessions.
4.2. Results
4.2.1. Amount of Vertical Rescaling Needed to Null the Perceived Slant Resulting From Prior Horizontal Rescaling
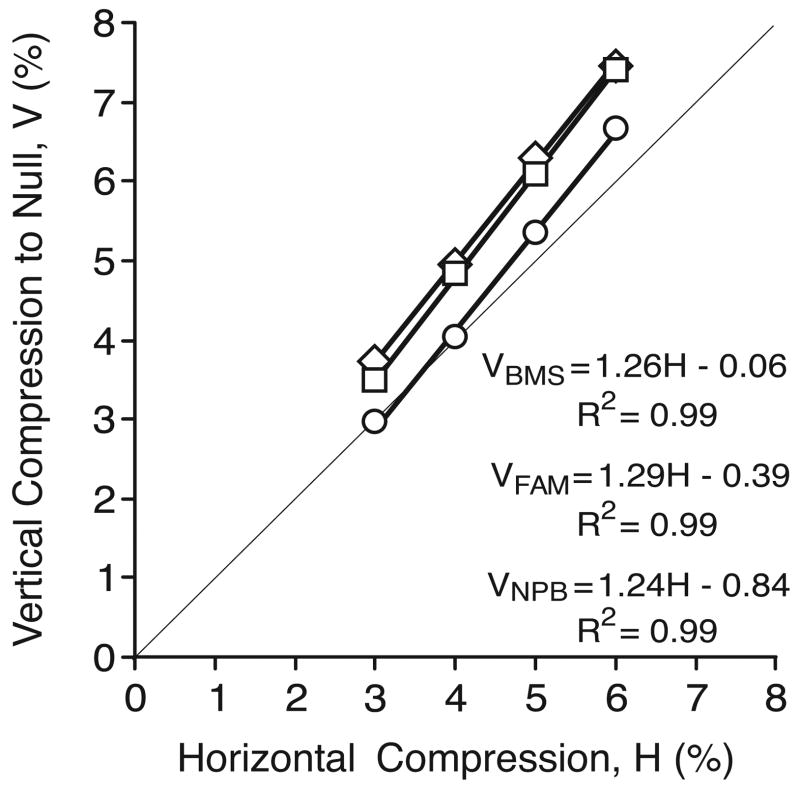
In order to null the perceived slant resulting from horizontal compression of the image seen by one eye, all subjects consistently applied vertical compression to that same image (i.e., increases in the HSR were nulled by increases in the VSR and vice versa). This was in accord with the fact that the induced effect and the geometric effect have the opposite sign. The amount of vertical compression required to accomplish nulling was always linearly related to the applied horizontal compression (R2>0.998 in all cases) over the range of compressions used (see Figure 9, which shows the data for all three subjects). The regression lines in Figure 9 all have slopes that exceed unity (range, 1.24 to1.29) and vertical offsets that are always small (range, –0.06% to –0.84%). The reciprocal of the vertical compression required for nulling provides a direct estimate of the gain of the induced effect with respect to the geometric effect and, based on the means in Figure 9, yielded values ranging from 0.79 to 1.02 (mean±SD, 0.87±0.02).
Figure 9.
Experiment 3: the vertical compression (ordinate, V) required to null the slant resulting from horizontal compression (abscissa, H), for each of the three subjects. Each datum point is an average of at least 119 (BMS), 116 (FAM), and 59 (NPB) individual responses. The thin diagonal line through the origin has unity slope. Thick lines represent regression lines whose defining coefficients are shown together with the coefficients of determination (R2).
4.2.2. Vergence Linked to Horizontal Gaze Shifts in the Nulled Condition
When the perceived slant resulting from horizontal compression of one eye’s pattern was nulled with vertical compression of that same pattern, the vergence linked to horizontal gaze shifts was consistently smaller than when there was horizontal compression alone, even though the horizontal disparity of the targets was the same in the two cases. This result is clear from Figure 10, which has a layout very similar to Figures 4 and 5, and shows the mean time course of the vergence responses, VCS, linked to horizontal gaze shifts, with (dashed line) and without (continuous line) nulling, for one subject for the complete set of compressions. Once again, the data for leftward and rightward gaze shifts are pooled together, as indicated in the cartoons on the right hand side of Figure 10, in which the black dashed lines represent the slant resulting from horizontal compression and the gray dashed lines represent the perceived slant after nulling with vertical compression. (The cartoons in the left column depict the situations in which the compressions were applied to the left image and the cartoons in the right column when the compressions were applied to the right image.) The numbers at the ends of the traces indicate the magnitude of the horizontal compression with (in parentheses) and without (no parentheses) nulling. The data without nulling are directly comparable with the data in Figure 4: increases in vergence occurred when the gaze shifts were toward the eye seeing the horizontally compressed image (shown in upper half of Figure 10) and decreases in vergence occurred when the gaze shifts were away from the eye seeing the horizontally compressed image (shown in lower half of Figure 10). Nulling clearly reduced the amplitude of the vergence responses and had little effect on their time course, as though causing pure attenuation with only a minor impact on dynamics.
Figure 10.
Experiment 3: effect of nulling on the mean temporal profiles of the vergence linked to gaze shifts, VCS (data for one subject, BMS). Each trace is an average of at least 59 individual responses. Continuous-line traces show the data with horizontal compression alone (magnitude indicated by numbers at ends of traces). Dashed-line traces show the data after vertical compression has been applied to null the perceived slant resulting from the horizontal compression (magnitude of latter indicated by numbers in parentheses at ends of traces). Cartoons depict a plan view of the slant resulting from the horizontal size disparity alone (black dotted lines), and the zero perceived slant after the nulling (gray dotted lines). Other conventions as in Figure 4.
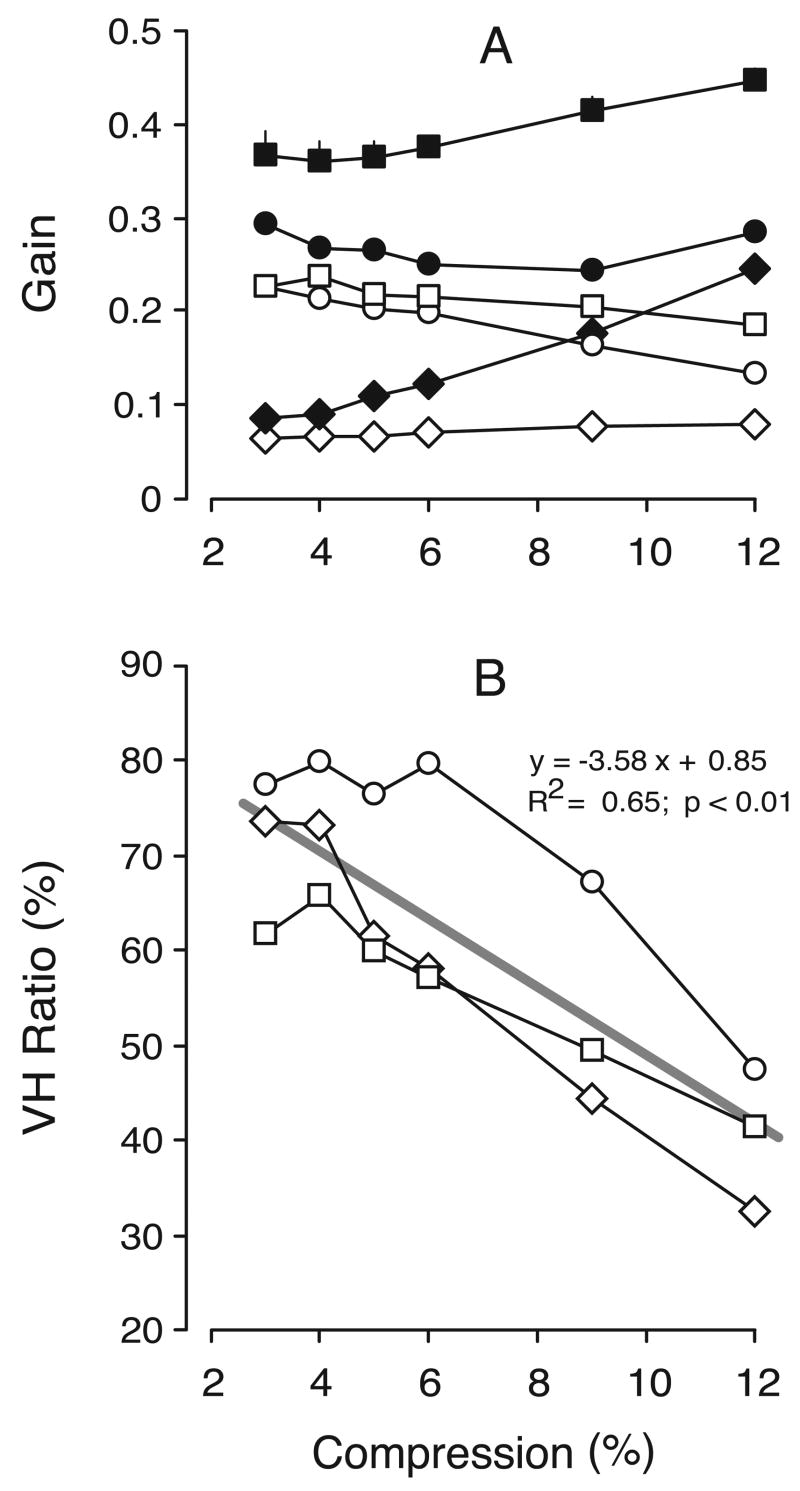
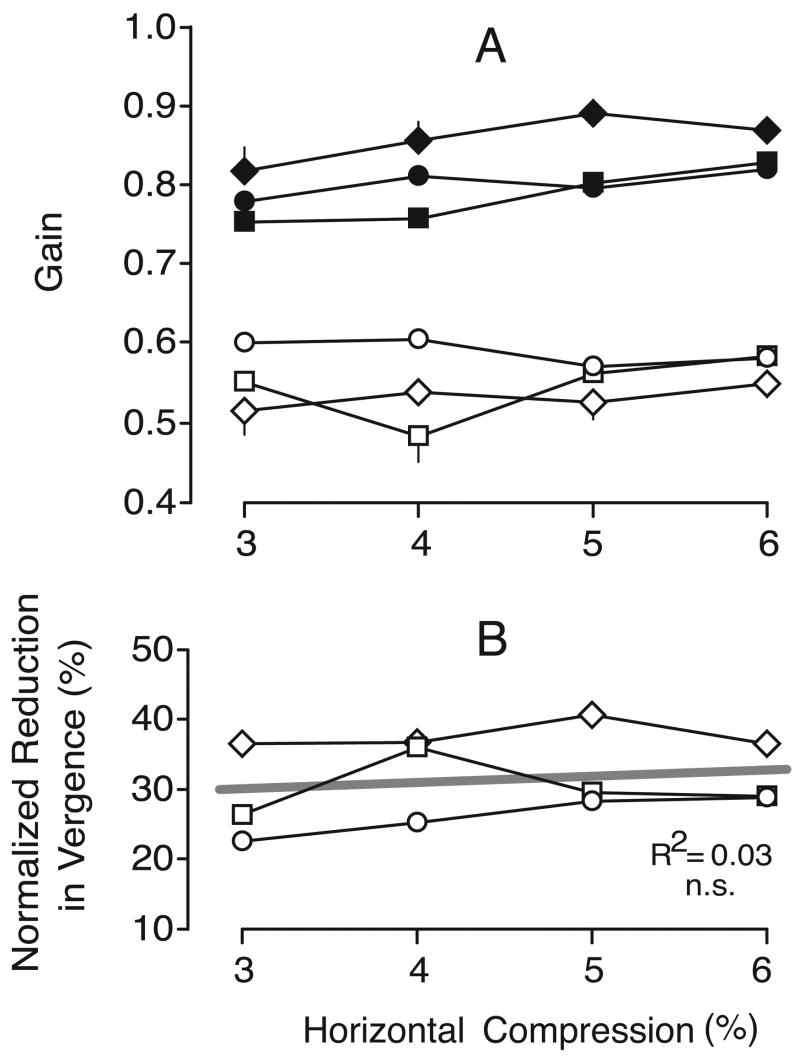
This effect of nulling was examined quantitatively by computing the mean vergence gains, dividing the measured vergence response by the required vergence response, where the latter was given by the difference in the horizontal disparity of the two targets corrected for the disparity of the control condition. These vergence gains are plotted in Figure 11A for all subjects. The data obtained without nulling (i.e., horizontal compression alone) are shown in filled symbols and the data obtained after nulling are shown in open symbols. Once again, there were no consistent asymmetries in the data obtained with leftward and rightward gaze shifts, or for convergent and divergent responses; hence, all of these data were pooled together. It is evident from this plot that, for any given level of horizontal compression, nulling always reduced the vergence responses linked to horizontal gaze shifts, and this effect was statistically significant for every subject (p < .001, two-tailed paired t test; d.f.=3 for each subject). Without nulling, vergence gains ranged from 0.75 to 0.89 (mean±SD, 0.82±0.04), and with nulling, vergence gains ranged from 0.48 to 0.61 (mean±SD, 0.56±0.04). The reductions in vergence due to nulling, when normalized with respect to the vergence gain without nulling, ranged from 23% to 41% (mean±SD, 31.6%±5.7): see Figure 11B, in which these normalized reductions are plotted as a function of the horizontal compression for all three subjects. There is a slight tendency in this plot for the reduction to increase with horizontal compression, but overall this was not statistically significant.
Figure 11.
Experiment 3: effect of nulling on vergence gain (data for three subjects). A. Mean vergence gain; filled symbols, horizontal compression alone; open symbols, nulled data; error bars, 1 SE; each datum point is an average of at least 119 (BMS), 116 (FAM), and 59 (NPB) individual responses. B. Mean percentage reduction in vergence gain with nulling normalized with respect to the gain without nulling. Other conventions as in Figures 6 and 8.
4.3. Discussion of Experiment 3
When vertical compression of one image was used to null the perceived slant resulting from prior horizontal compression of that image, it also reduced the vergence linked to horizontal gaze shifts, despite the fact that those vertical compressions did not affect the horizontal disparity of the two targets. Of course, the fact that substantial vergence changes still occurred despite the absence of perceived depth indicates that horizontal disparity alone suffices to elicit robust changes in vergence during voluntary gaze shifts.
We suggest that when the reduction in the vergence gain due to nulling is normalized with respect to the gain without nulling it provides an estimate of the VP ratio discussed earlier in Experiments 1 and 2. This normalized reduction in vergence is given by the following expression:
| (5) |
where KP·PG and KD·DG are the contributions of perceived slant and horizontal disparity, respectively, without nulling (horizontal compression alone, exactly as in the geometric effect condition in Experiments 1 and 2), and KP·PN and KD·DN are the same entities after nulling. But PN = 0 and the vertical compression used to null does not affect the horizontal disparity so that DN = DG; hence, the normalized reduction in vergence simplifies to
| (6) |
which corresponds to the VP ratio as determined in Experiments 1 and 2. Thus, based on the nulling technique, the perceived depth was responsible, on average, for ~32% of the vergence linked to gaze shifts. This estimate is somewhat smaller than that from Experiments 1 and 2, which was based on the vergence linked to gaze shifts with horizontal and vertical compressions alone (mean, ~41%).
Mean saccadic version gains in Experiment 3 ranged from 0.93 to 0.98 (mean±SD, 0.96±0.01) and were subjected to ANOVA with two within-subject factors—type of compression (horizontal alone or nulled) and magnitude of compression (3%, 4%, 5%, or 6%)—exactly as in Experiment 1, and no factors or interactions were significant at the 0.05 level. Thus, differences in the amplitude of the gaze shifts did not contribute significantly to our findings.
5. Experiment 4: “Anti-Null” and Other Controls
There were two major concerns about Experiment 3 that needed to be addressed. First, the adjustments needed to achieve nulling could take several seconds to complete and subjects were free to move their eyes during this period, perhaps providing an opportunity for adaptation to reduce the impact of the nulling on vergence eye movements because the perception here is in conflict with the binocular disparity that specifies the vergence error. Second, the reduced vergence in the nulled condition might have been a response decrement secondary to some disruption of the depth-sensing mechanism guiding vergence rather than a positive response to the change in perceived slant per se: perhaps the mere existence of vertical disparity is sufficient to interfere with the extraction of a depth signal for vergence.
The first concern about the possibility of adaptation during the nulling adjustments was addressed by having “nulled” trials in which the combinations of horizontal and vertical compressions used in the nulling trials in Experiment 3 were exactly replicated and applied at the beginning of the trial along with the fixation target so that the trial could proceed immediately without the subject having to make any adjustments. We will show that the resulting vergence data linked to horizontal gaze shifts were very similar to those in Experiment 3.
To address the second concern, so-called “anti-nulled” trials were included in which the conditions used in the nulling trials in Experiment 3 were again replicated except that the vertical compressions were replaced with vertical expansions of the same magnitude (i.e., the vertical rescaling had the opposite sign). If the reduced vergence in the nulled condition reflects a disruptive influence of vertical disparity per se, then one might expect to see a similar effect in the anti-nulled condition; on the other hand, if the reduced vergence response in the nulled condition was due to the reduction in the perceived slant then one might expect to see an increase in the vergence linked to gaze shifts in the anti-nulled condition. We report that anti-nulling had the reverse effect of nulling, increasing rather than decreasing the vergence responses linked to horizontal gaze shifts.
5.1. Methods
The subjects, experimental setup, visual stimuli, and data analyses were essentially the same as in Experiment 3.
5.1.1. Task and Procedure
The screen was initially blank and random-dot patterns appeared, together with a central fixation target, when the subject pressed a start button. After a random fixation period of 1000–1500 ms, the fixation target was replaced by a second one located 7.5° right or left of center, and the trial then proceeded as in Experiments 1 and 3. Three kinds of trials were randomly interleaved: (1) “nulled” trials, in which the same combinations of horizontal and vertical compressions were applied to the two images as in the nulling trials in Experiment 3; these stimulus combinations were specific to each subject and the vertical rescalings were exactly as selected by the subject in Experiment 3 to achieve nulling; (2) “anti-nulled” trials, in which the same selection of horizontal compressions was used as in the nulled trials but the vertical compressions were replaced by vertical expansions of the same magnitude; and (3) control trials, in which horizontal compressions alone were applied to one image exactly as in Experiment 3.
Each experimental session consisted of 5–30 blocks, each block having 52 trials. Forty-eight of the 52 trials in the block were experimental trials: 3 (compression: horizontal alone vs. nulled condition vs. anti-nulled condition) × 2 (eye viewing the compression: left vs. right) × 4 (amount of horizontal compression) × 2 (gaze shift: leftward vs. rightward). For the remaining 4 trials in the block the patterns on the two monitors were identical (controls): 2 × 2 (gaze shift: leftward vs. rightward). Trials in which an error occurred were subsequently rerun within the same block. Each subject participated in 1–2 recording sessions.
5.2. Results
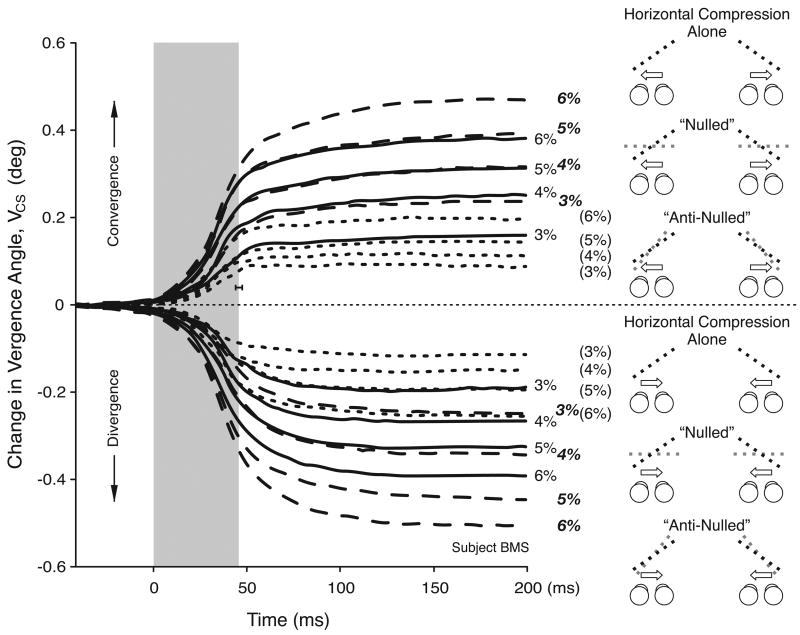
When the combinations of horizontal and vertical compression were exactly the same as those used in the nulling trials in Experiment 3, the changes in horizontal vergence linked to horizontal gaze shifts were again consistently smaller than when there was horizontal compression alone. This is apparent from Figure 12, which has a layout very similar to Figure 10 and shows the mean time course of the vergence responses, VCS, linked to horizontal gaze shifts, with (short-dash line) and without (continuous line) nulling, for one subject for the complete set of compressions. Figure 12 also includes the data from the anti-nulled trials (long-dash lines), which indicate that reversing the sign of the vertical rescaling reversed the vergence effects: in all cases in Figure 12, the vergence responses linked to horizontal gaze shifts were now larger than with horizontal compression alone. Effects on the time course of the vergence responses were again minor. The magnitude of these effects of nulling and anti-nulling are evident from the vergence gain plots in Figure 13A, which shows the data for all three subjects: the data obtained with nulling are shown in open symbols, the control data obtained with horizontal compression alone are shown in black symbols, and the data obtained with anti-nulling are shown in gray symbols.
Figure 12.
Experiment 4: Effect of prior nulling and anti-nulling on the mean temporal profiles for the vergence linked to gaze shifts, VCS (data for one subject, BMS). Each trace is an average of at least 59 individual responses. Continuous-line traces show the data with horizontal compression alone (magnitude indicated by numbers at ends of traces). Short-dash-line traces show the data after vertical compression has been applied to null the perceived slant that ordinarily results from the horizontal compression (magnitude of latter indicated by numbers in parentheses at ends of traces). Long-dash-line traces show the data after vertical expansion (anti-nulling) has been applied to increase the perceived slant that ordinarily results from the horizontal compression (magnitude of latter indicated by bold numbers at ends of traces). Cartoons depict a plan view of the slant resulting from the horizontal size disparity alone (black dashed lines), and the perceived slants after the nulling and anti-nulling (gray dashed lines). Other conventions as in Figure 10B.
Figure 13.
Experiment 4: effect of nulling and anti-nulling on vergence gain (data for three subjects). A. Mean vergence gain; black symbols, horizontal compression alone; open symbols, nulled data; gray symbols, anti-nulled data; each datum point is an average of at least 119 (BMS), 119 (FAM), and 59 (NPB) individual responses. B. Mean percentage reduction in vergence with nulling, normalized with respect to the gain without nulling or anti-nulling. C. Mean percentage increase in vergence with anti-nulling, normalized with respect to the gain without nulling or anti-nulling. Other conventions as in Figure 6.
The control and nulled data in Figure 13A look very similar to those in Figure 11A. The vergence gains for the control data in Experiment 4 ranged from 0.76 to 0.86 (mean±SD, 0.81±0.03), whereas the vergence gains for the nulled data in this experiment ranged from 0.41 to 0.59 (mean±SD, 0.50±0.06). These mean values are both a little smaller—by ~1% and ~6%, respectively—than those in Experiment 3. The differences between the control and nulled data in Experiment 4 were significant for all subjects (p < .001, two-tailed paired t test; d.f.=3 for each subject). The reductions in vergence due to nulling, when normalized with respect to the vergence gain without nulling (or anti-nulling), ranged from 29% to 49% (mean±SD, 37.9%±7.1%), and were plotted as a function of the horizontal compression: see Figure 13B, which shows the data for all three subjects and should be compared with Figure 11B. For all three subjects, these normalized reductions were significantly larger in Experiment 4 than in Experiment 3 (p < .05, two-tailed paired t test; d.f.=3 for each subject), though the difference averaged only 6.3%.
It is very clear from Figure 13A that the anti-nulled stimuli resulted in the highest vergence gains, values ranging from 0.88 to 1.10 (mean±SD, 0.98±0.09), and every subject showed an increase above their control level (i.e., with horizontal compression alone) that was significant (p < .05, two-tailed paired t test; d.f.=3 for each subject). The increases in vergence due to anti-nulling, when normalized with respect to the vergence gain without anti-nulling (or nulling), ranged from 9% to 39% (mean±SD, 20.6%±8.9%): see Figure 13C, in which these normalized increases are plotted as a function of the horizontal compression for all subjects.
5.3. Discussion of Experiment 4
The effects of nulling on the vergence linked to gaze shifts were only slightly greater in Experiment 4 than in Experiment 3. The normalized reduction in vergence, which we argued in Section 4.3 provides an estimate of the VP ratio and hence of the proportion of vergence attributable to the perceived slant, differed on average by ~6%. This indicated that the more prolonged (closed- loop) exposure to the stimuli necessitated by the manual adjustments in the earlier experiment had had almost negligible impact on the data. Thus, our concern that there might have been adaptation effects in the earlier experiment was not supported by these data. Of course, it is also possible that the small observed differences were related to the fact that, of necessity, Experiment 4 was done after Experiment 3. In fact, there is evidence which suggests that experience can influence (increase) the magnitude of the perceived slant associated with vertical size disparity (van Ee & Erkelens, 1998).
The other major finding in this experiment was that anti-nulling—involving vertical rescaling of the same magnitude but opposite sign to that used in nulling—increased the vergence linked to gaze shifts. Clearly, the vertical disparity here did not compromise the depth signals guiding vergence. This result reduces the likelihood that the reduction in vergence caused by nulling is due to the disruption of the depth-sensing mechanism used for vergence, and strengthens our hypothesis that it is the differences in the perceived slant that are responsible for the differences in the magnitude of vergence in our three experimental conditions. Note that by normalizing the anti-nulled data to the control data, the monocular depth cues that signal a fronto-parallel surface again tend to be factored out because they are constant across conditions.
Mean saccadic version gains in Experiment 4 ranged from 0.94 to 0.99 (mean±SD, 0.96±0.02) and were again subjected to ANOVA with two within-subject factors—type of compression (horizontal alone or nulled) and magnitude of compression (3%, 4%, 5%, or 6%)—exactly as in Experiment 1, and no factors or interactions were significant at the 0.05 level. Thus, again differences in the amplitude of the gaze shifts did not contribute significantly to our findings.
6. Experiment 5: Perceived Slant With the Anti-Null Stimuli
The object of this experiment was to obtain quantitative estimates of the perceived slants associated with the control and anti-nulled stimuli used in Experiment 4. We report that anti-nulling increased the perceived slant substantially, proportionately much more than it had increased the vergence eye movements linked to horizontal shifts of gaze in Experiment 4.
6.1. Methods
The subjects and visual stimuli were exactly as in Experiment 4.
6.1.1. Task and Procedure
Subjects viewed the same selection of control and anti-nulled images as they had in Experiment 4, each for exactly the same periods of time (up to a maximum of 5 s). Subjects were asked to manually align an unseen horizontal bar with the perceived slant of the screen images, exactly as in Experiment 2.
6.1.2. Data Analysis
As in Experiment 2, measures of bar orientation provided estimates of the perceived slant of the random-dot patterns, and were then used to compute the gain of the open-loop slant estimates as described in Section 3.1.2., except that here the concern was with vertical expansions—rather than compressions—of an image that had been previously compressed horizontally.
6.2. Results
The gains of the perceived slants for each of the three subjects are plotted in Figure 14A, the data obtained with the control stimuli—horizontal compressions alone (geometric effect)—being shown in filled symbols, and the data obtained with anti-nulled stimuli—vertical expansions—being shown in open symbols. As in Experiment 2, the gain of the geometric effect varied widely between subjects, ranging from 0.10 to 0.36 (mean±SD, 0.24±0.10). (Note that the horizontal compressions in the present experiment ranged only from 3% to 6%, whereas those in Experiment 2 ranged from 3% to 12%). The gain in the anti-nulled condition was invariably higher than in the control condition (for a given level of compression), ranging from 0.24 to 0.63 (mean±SD, 0.45±0.13). The increases in perceived slant due to anti-nulling, when expressed in terms of the perceived slant without anti-nulling, showed a great deal of scatter, values ranging from 64% to 201% (mean±SD, 108.0%±55.0%): see Figure 14B, which shows these normalized increases for all three subjects. No consistent dependence on compression is evident in the data of Figure 14B.
Figure 14.
Experiment 5: effect of anti-nulling on perceived slant (data for three subjects). A. Mean slant gain; filled symbols, horizontal compression alone; open symbols, anti-nulled data; each datum point is an average of at least 120 (BMS), 117 (FAM), and 60 (NPB) individual responses. B. Mean percentage increase in perceived slant with anti-nulling, normalized with respect to the gain without anti-nulling. Other conventions as in Figure 7.
6.3. Discussion of Experiment 5
Anti-nulling increased the perceived slant substantially, despite having no effect on horizontal disparity, although there was considerable variability between subjects and no consistent dependence on compression. The normalized increases in perceived slant with anti-nulling were proportionately greater than the normalized increases in vergence in Experiment 4, sometimes substantially. We suggest that the ratio of these normalized increases in the vergence and in the perceived slant provides an estimate of the VP ratio described earlier in Experiments 2 and 3. The normalized increase in vergence with anti-nulling in Experiment 4 is given by the following expression:
| (7) |
where KP·PG and KD·DG are the contributions of perceived slant and horizontal disparity, respectively, without nulling or anti-nulling (horizontal compression alone, exactly as in the geometric effect condition in Experiments 1, 2, and 3), and KP·PA and KD·DA are the same entities after anti-nulling. But, the vertical expansion used in anti-nulling does not affect the horizontal disparity so that DA =DG, hence the normalized increase in vergence with anti-nulling in Experiment 4 simplifies to
| (8) |
The normalized increase in perceived slant with anti-nulling in Experiment 5 is given by the following expression:
| (9) |
where KM is a coefficient specifying the extent to which the signal provided by the perceptual system is utilized by the hand positioning system that provides the measure of perceived slant. The ratio, (Expression 8)/(Expression 9), is then given by
| (10) |
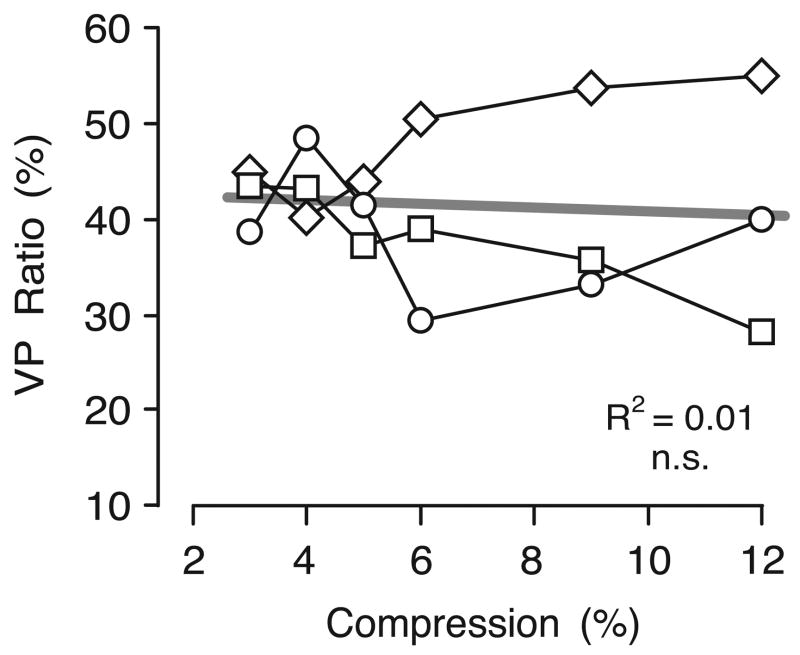
which corresponds to the VP ratio as determined in Experiments 1 and 2 and provides an estimate of the contribution of perceived depth to the vergence linked to gaze shifts. The VP ratios were therefore estimated by dividing the normalized increases in vergence (from Experiment 4) by the normalized increases in perceived slant (from Experiment 5). The plot in Figure 15 shows these estimates of the VP ratios for all three subjects, which range from 13% to 35% (mean±SD, 20.5%±7.5%). The VP ratios showed a significant tendency to decrease with horizontal compression, on average, roughly halving over the range of compressions studied.
Figure 15.
VP Ratios: dependence on compression (data for three subjects). The normalized increases in vergence in Experiment 4 are expressed as a percentage of the normalized increases in the perceived slant in Experiment 5. Other conventions as in Figure 8.
As already mentioned in discussing Experiment 2, inter-subject variability is a well-known characteristic of the geometric and induced effects (Allison et al., 1998; van Ee & Erkelens, 1996, 1998) and it is a feature of our data too (Figures 7A and 14A). When compared over the same range of horizontal compressions (3–6%), the geometric effects in Experiment 5 were almost identical to those in Experiment 2. In subject BMS, values in Experiment 5 were on average ~0.9% greater than in Experiment 2, whereas in the other two subjects, the opposite was true: the gains in Experiment 5 were ~0.8% and ~1.7% lower than in Experiment 2 for subjects NPB and FAM, respectively.
7. Summary and General Discussion
Experiment 1 showed that horizontal open-loop gaze shifts across a flat surface that appeared slanted because its image had been compressed horizontally or vertically in one eye were accompanied by vergence eye movements—albeit much weaker with the vertical rescaling—that were consistent with the direction of the perceived slant. The most important point here is that the vertical rescaling influenced the vergence even though it did not alter the horizontal disparity of the fixation targets. In Experiment 2, subjects manually adjusted the orientation of a bar to match the perceived slants of stimuli exactly matching those used in Experiment 1. This indicated that vertical rescaling induced proportionately greater changes in the perceived slant than in the vergence linked to gaze shifts. We estimated that perceived depth was responsible, on average, for ~41% of the vergence linked to horizontal gaze shifts in Experiment 1.
In Experiment 3, subjects saw a pattern that appeared to be slanted because one eye’s image had been compressed horizontally and they used button presses to apply vertical compression to that image to null the perceived slant; subjects then executed horizontal gaze shifts as in Experiment 1. This vertical compression not only reduced the perceived slant but also reduced the vergence associated with the gaze shifts even though it did not alter the horizontal disparity resulting from the prior horizontal compression. Once more, however, the change in the perceived slant was proportionately much greater than the change in the vergence linked to gaze shifts. The normalized reduction in vergence due to nulling suggested that perceived depth was responsible, on average, for ~32% of the vergence linked to horizontal gaze shifts in this paradigm.
One of the potential problems with Experiment 3 was that it involved prolonged exposure to the patterns during the nulling adjustments, perhaps providing an opportunity for the system to show adaptive modification of the vergence linked to horizontal gaze shifts and thereby attenuating any apparent effects of perceived slant on the vergence linked to gaze shifts. In Experiment 4, the combinations of horizontal and vertical compressions that had been applied in Experiment 3 were applied again, but this time at the very beginning of the trial, thereby avoiding the prolonged exposure to the patterns. The vergence data were essentially the same as in Experiment 3, the normalized reduction in vergence due to nulling suggesting that perceived depth was responsible, on average, for ~38% of the vergence linked to horizontal gaze shifts. This indicated that the prolonged exposure in Experiment 3 was not an important factor.
Another concern with Experiment 3 was that vertical disparity per se might have adversely affected the depth-sensing operations guiding the vergence linked to gaze shifts and that this—rather than the reduction in the perceived slant per se—might have been responsible for the observed attenuation of the vergence response. For this reason, Experiment 4 also included trials in which the vertical compressions that had been used in Experiment 3 were now replaced with vertical expansions of the same magnitude. This anti-nulling resulted in an increase in the vergence linked to horizontal gaze shifts, despite the fact that the horizontal disparity was unchanged, strongly suggesting that the decreased vergence resulting from vertical compression in Experiment 3 was not due simply to degradation of the depth signals guiding vergence.
In Experiment 5, subjects manually adjusted the orientation of a bar to match the perceived slants of stimuli exactly like those used in Experiment 4. This indicated that vertical expansions applied to one eye’s image increased the perceived slant by a much greater margin than they had increased the vergence linked to gaze shifts, especially with the larger compressions. The increase in the vergence due to anti-nulling suggested that perceived depth was responsible, on average, for only ~15% of the vergence linked to horizontal gaze shifts with the largest compression (6%) but almost twice that with the lowest compression (3%).
Our estimates of the contribution of perceived depth to the vergence linked to gaze shifts in the various experiments ranged, on average, from ~15% to ~41%. Thus, although perceived depth makes a clear contribution to the vergence, the binocular disparity is generally the more important factor. As pointed out in earlier discussions, our apparatus had monocular cues to depth (such as perspective, texture, size, and luminance) that were all consistent with a fronto-parallel surface and so would have reinforced the slant perceived in the nulled situation but competed with the slants perceived in all other paradigms. This could mean that our experiments underestimate the vergence gains associated with the induced and geometric effects in Experiments 1 and 4 [for discussion, see Banks & Backus (1998)]. However, these monocular cues to depth are constant across conditions and all of our estimates of the contribution of perceived depth to the vergence responses relied on data that were always normalized with respect to the data obtained with horizontal magnification alone (as in the “VH ratios”, for example). This would tend to factor out the effect of the unwanted fronto-parallel depth cues resulting from our apparatus. Thus, we think that such problems had little impact on our estimates of the contribution of perceived slant to the vergence linked to horizontal gaze shifts.
A number of binocular factors are known to contribute to the perceived slant and a recent extensive analysis concluded that “there are four other signals that, in combination with horizontal disparity, could in principle allow an unambiguous estimate of slant: the vergence and version of the eyes, the vertical size ratio (VSR), and the horizontal gradient of VSR” (Backus et al., 1999). A discussion of these factors is beyond the scope of the present paper, but it is clear from the study of Backus et al. that the influence of any given signal or cue is context dependent and has a weight that can vary across viewing conditions.
Another article in this special issue of Journal of Vision (Both, van Ee, & Erkelens, 2003) reports on the vergence eye movements that accompany gaze shifts across a flat surface whose perceived slant was altered by manipulating the slant of flanking surfaces situated above and below (Werner’s illusion). The vergence responses specifically attributable to the perceived slant in this study were generally much weaker than in the present study. This might have been due to the use of depth contrast to dissociate perceived depth and horizontal disparity, and/or to procedural differences. Apropos these differences, the vergence response measures of Both et al. (2003) either followed a fixation period lasting hundreds of milliseconds—permitting the closed-loop disparity vergence mechanism to override the perceived slant—or, were restricted to the intrasaccadic period—so that only the initial portion of the vergence response was considered.
There are vivid examples in the literature showing clear dissociation between depth perception and vergence eye movements (Erkelens, 2001; Erkelens & Collewijn, 1985a, 1985b; Masson, Busettini, & Miles, 1997). These studies indicate clearly that binocular disparity is sufficient to produce vergence and the vergence responses in our nulling paradigm provide additional support for this. In addition, the studies of Enright (1987a, 1987b) and Ringach et al (1996), which reported vergence eye movements during monocular viewing of images with implied depth, indicate that binocular disparity is not necessary for vergence, and the vergence responses associated with the induced effect in our study indicate that this remains true during binocular viewing. Depth perception relies on our ability to develop an internal representation of three-dimensional space, and it has been known for a long time that eye movements are under the control of both bottom-up and top-down factors [for reviews of the latter, see Kowler (1990) and Findlay & Walker (1999)]. Vergence eye movements are no exception.
Acknowledgments
We thank Tom Ruffner, Nick Nichols, and Lee Jensen for technical assistance, Ed FitzGibbon, John McClurkin, Kelvin Chen, and Art Hays for software support, and Jean Steinberg for secretarial assistance. Commercial relationships: none.
Contributor Information
B. M. Sheliga, Laboratory of Sensorimotor Research, National Eye Institute, Bethesda, MD, USA
F. A. Miles, Laboratory of Sensorimotor Research, National Eye Institute, Bethesda, MD, USA
References
- Allison RS, Howard IP, Rogers BJ, Bridge H. Temporal aspects of slant and inclination perception. Perception. 1998;27:1287–1304. doi: 10.1068/p271287. [PubMed] [DOI] [PubMed] [Google Scholar]
- Backus BT, Banks MS, van Ee R, Crowell JA. Horizontal and vertical disparity, eye position, and stereoscopic slant perception. Vision Research. 1999;39(6):1143–1170. doi: 10.1016/s0042-6989(98)00139-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- Banks MS, Backus BT. Extra-retinal and perspective cues cause the small range of the induced effect. Vision Research. 1998;38(2):187–194. doi: 10.1016/s0042-6989(97)00179-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Becker W. The neurobiology of saccadic eye movements: Metrics. Reviews of Oculomotor Research. 1989;3:13–67. [PubMed] [PubMed] [Google Scholar]
- Berends EM, Erkelens CJ. Strength of depth effects induced by three types of vertical disparity. Vision Research. 2001;41(1):37–45. doi: 10.1016/s0042-6989(00)00215-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- Both MH, van Ee R, Erkelens CJ. Perceived slant from Werner’s illusion affects binocular saccadic eye movements. Journal of Vision. 2003;3(11):685–697. doi: 10.1167/3.11.4. [Article] [DOI] [PubMed] [Google Scholar]
- Bucci MP, Gomes M, Paris S, Kapoula Z. Disconjugate oculomotor learning caused by feeble image-size inequality: differences between secondary and tertiary positions. Vision Research. 2001;41(5):625–637. doi: 10.1016/s0042-6989(00)00293-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Bucci MP, Kapoula Z, Bernotas M, Zamfirescu F. Disconjugate memory-guided saccades to disparate targets: Temporal aspects. Experimental Brain Research. 2000;134(1):133–138. doi: 10.1007/s002210000478. [PubMed] [DOI] [PubMed] [Google Scholar]
- Bucci MP, Kapoula Z, Eggert T. Saccade amplitude disconjugacy induced by aniseikonia: role of monocular depth cues. Vision Research. 1999;39:3109–3122. doi: 10.1016/s0042-6989(99)00064-4. [PubMed] [DOI] [PubMed] [Google Scholar]
- Bucci MP, Paris S, Kapoula Z. Oculomotor consequences of feeble image size inequality at near reading distance. Experimental Brain Research. 2003;149(2):252–259. doi: 10.1007/s00221-003-1372-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- Busettini C, Miles FA, Schwarz U, Carl JR. Human ocular responses to translation of the observer and of the scene: Dependence on viewing distance. Experimental Brain Research. 1994;100:484–494. doi: 10.1007/BF02738407. [PubMed] [DOI] [PubMed] [Google Scholar]
- Bush GA, van der Steen J, Miles FA. When the two eyes see patterns of unequal size they produce saccades of unequal amplitude. In: Delgado-Garcia JM, editor. Information processing underlying gaze control. Oxford & New York: Elsevier Science Ltd; 1994. pp. 261–267. [Google Scholar]
- Collewijn H, Erkelens CJ. Binocular eye movements and the perception of depth. Reviews of Oculomotor Research. 1990;4:213–261. [PubMed] [PubMed] [Google Scholar]
- Collewijn H, Erkelens CJ, Steinman RM. Voluntary binocular gaze-shifts in the plane of regard: Dynamics of version and vergence. Vision Research. 1995;35(23–24):3335–3358. doi: 10.1016/0042-6989(95)00082-p. [PubMed] [DOI] [PubMed] [Google Scholar]
- Collewijn H, Erkelens CJ, Steinman RM. Trajectories of the human binocular fixation point during conjugate and non-conjugate gaze-shifts. Vision Research. 1997;37(8):1049–1069. doi: 10.1016/s0042-6989(96)00245-3. [PubMed] [DOI] [PubMed] [Google Scholar]
- Donnet SP, Kapoula Z, Bucci MP, Daunys G. Vertical memory-based disconjugate learning for downward saccades at a viewing distance of 70 cm: Relation to horizontal vergence and to vertical phoria. Experimental Brain Research. 2002;146(4):474–480. doi: 10.1007/s00221-002-1194-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- Eggert T, Kapoula Z. Position dependency of rapidly induced saccade disconjugacy. Vision Research. 1995;35:3493–3503. doi: 10.1016/0042-6989(95)00147-7. [PubMed] [DOI] [PubMed] [Google Scholar]
- Enright JT. Art and the oculomotor system: perspective illustrations evoke vergence changes. Perception. 1987a;16:731–746. doi: 10.1068/p160731. [PubMed] [DOI] [PubMed] [Google Scholar]
- Enright JT. Perspective vergence: Oculomotor responses to line drawings. Vision Research. 1987b;27:1513–1526. doi: 10.1016/0042-6989(87)90160-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- Erkelens CJ. Adaptation of ocular vergence to stimulation with large disparities. Experimental Brain Research. 1987;66(3):507–516. doi: 10.1007/BF00270683. [PubMed] [DOI] [PubMed] [Google Scholar]
- Erkelens CJ. Organisation of signals involved in binocular perception and vergence control. Vision Research. 2001;41:3497, 3503. doi: 10.1016/s0042-6989(01)00004-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- Erkelens CJ, Collewijn H. Eye movements and stereopsis during dichoptic viewing of moving random-dot stereograms. Vision Research. 1985a;25:1689–1700. doi: 10.1016/0042-6989(85)90141-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Erkelens CJ, Collewijn H. Motion perception during dichoptic viewing of moving random-dot stereograms. Vision Research. 1985b;25:583–588. doi: 10.1016/0042-6989(85)90164-6. [PubMed] [DOI] [PubMed] [Google Scholar]
- Erkelens CJ, Collewijn H, Steinman RM. Asymmetrical adaptation of human saccades to anisometropic spectacles. Investigative Ophthalmology and Visual Science. 1989;30(6):1132–1145. [PubMed] [PubMed] [Google Scholar]
- Erkelens CJ, Steinman RM, Collewijn H. Ocular vergence under natural conditions. II. Gaze shifts between real targets differing in distance and direction. Proceedings of the Royal Society of London. Series B Biological Sciences. 1989;236:441–465. doi: 10.1098/rspb.1989.0031. [PubMed] [DOI] [PubMed] [Google Scholar]
- Findlay JM, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behavioral and Brain Sciences. 1999;22(4):661–674. doi: 10.1017/s0140525x99002150. discussion 674–721. [PubMed] [DOI] [PubMed] [Google Scholar]
- Gaveau V, Martin O, Prablanc C, Pelisson D, Urquizar C, Desmurget M. On-line modification of saccadic eye movements by retinal signals. Neuroreport. 2003;14(6):875–878. doi: 10.1097/00001756-200305060-00020. [PubMed] [DOI] [PubMed] [Google Scholar]
- Gillam B, Chambers D, Lawergren B. The role of vertical disparity in the scaling of stereoscopic depth perception: An empirical and theoretical study. Perception & Psychophysics. 1988;44(5):473–483. doi: 10.3758/bf03210433. [PubMed] [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conference Proceedings. 1982;2:1–10. [Google Scholar]
- Kaneko H, Howard IP. Relative size disparities and the perception of surface slant. Vision Research. 1996;36:1919–1930. doi: 10.1016/0042-6989(95)00258-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Bucci MP, Lavigne-Tomps F, Zamfirescu F. Disconjugate memory-guided saccades to disparate targets: Evidence for 3D sensitivity. Experimental Brain Research. 1998;122(4):413–423. doi: 10.1007/s002210050529. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kapoula Z, Eggert T, Bucci MP. Immediate saccade amplitude disconjugacy induced by unequal images. Vision Research. 1995;35:3505–3518. doi: 10.1016/0042-6989(95)00150-d. [PubMed] [DOI] [PubMed] [Google Scholar]
- Kowler E. The role of visual and cognitive processes in the control of eye movement. Reviews of Oculomotor Research. 1990;4:1–70. [PubMed] [PubMed] [Google Scholar]
- Krommenhoek KP, Van Gisbergen JA. Evidence for nonretinal feedback in combined version-vergence eye movements. Experimental Brain Research. 1994;102:95–109. doi: 10.1007/BF00232442. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lemij HG, Collewijn H. Long-term nonconjugate adaptation of human saccades to anisometropic spectacles. Vision Research. 1991a;31:1939–1954. doi: 10.1016/0042-6989(91)90189-c. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lemij HG, Collewijn H. Short-term nonconjugate adaptation of human saccades to anisometropic spectacles. Vision Research. 1991b;31:1955–1966. doi: 10.1016/0042-6989(91)90190-g. [PubMed] [DOI] [PubMed] [Google Scholar]
- Lemij HG, Collewijn H. Nonconjugate adaptation of human saccades to anisometropic spectacles: Meridian-specificity. Vision Research. 1992;32:453–464. doi: 10.1016/0042-6989(92)90237-d. [PubMed] [DOI] [PubMed] [Google Scholar]
- Masson GS, Busettini C, Miles FA. Vergence eye movements in response to binocular disparity without depth perception. Nature. 1997;389:283–286. doi: 10.1038/38496. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ogle KN. Induced size effect. I. A new phenomenon in binocular space perception associated with the relative sizes of the images of the two eyes. Archives of Ophthalmology. 1938;20:604–623. [Google Scholar]
- Ogle KN. Induced size effect. II. An experimental study of the phenomenon with restricted fusion stimuli. Archives of Ophthalmology. 1939a;21:604–625. [Google Scholar]
- Ogle KN. Induced size effect. III. A study of the phenomenon as influenced by horizontal disparity of the fusion contours. Archives of Ophthalmology. 1939b;22:613–635. [Google Scholar]
- Ogle KN. Induced size effect with the eyes in asymmetric convergence. Archives of Ophthalmology. 1940;23:1023–1038. [Google Scholar]
- Paris S, Bucci MP, Kapoula Z. Disconjugate vertical memory-guided saccades to disparate targets. Experimental Brain Research. 2000;135(2):267–274. doi: 10.1007/s002210000548. [PubMed] [DOI] [PubMed] [Google Scholar]
- Pobuda M, Erkelens CJ. The relationship between absolute disparity and ocular vergence. Biological Cybernetics. 1993;68(3):221–228. doi: 10.1007/BF00224855. [PubMed] [DOI] [PubMed] [Google Scholar]
- Ringach DL, Hawken MJ, Shapley R. Binocular eye movements caused by the perception of three-dimensional structure from motion. Vision Research. 1996;36:1479–1492. doi: 10.1016/0042-6989(95)00285-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Miles FA. The induced effect of Ogle (1938) influences horizontal gaze shifts of humans [Abstract] Society for Neuroscience Abstracts. 2001;27:575.2. [Google Scholar]
- Sheliga BM, Miles FA. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2002. Perception can influence the vergence responses associated with horizontal gaze shifts in 3D [Abstract]. Program No. 265.5, 2002. [Google Scholar]
- van der Steen J, Bruno P. Unequal amplitude saccades produced by aniseikonic patterns: Effects of viewing distance. Vision Research. 1995;35:3459–3471. doi: 10.1016/0042-6989(95)00138-5. [PubMed] [DOI] [PubMed] [Google Scholar]
- van Ee R, Erkelens CJ. Temporal aspects of binocular slant perception. Vision Research. 1996;36:43–51. [PubMed] [PubMed] [Google Scholar]
- van Ee R, Erkelens CJ. Temporal aspects of stereoscopic slant estimation: An evaluation and extension of Howard and Kaneko’s theory. Vision Research. 1998;38:3871–3882. doi: 10.1016/s0042-6989(97)00445-8. [PubMed] [DOI] [PubMed] [Google Scholar]
- Zee DS, Fitzgibbon EJ, Optican LM. Saccade-vergence interactions in humans. Journal of Neurophysiology. 1992;68:1624–1641. doi: 10.1152/jn.1992.68.5.1624. [PubMed] [DOI] [PubMed] [Google Scholar]