Abstract
Chemical modification of histones through a growing number of post-translational mechanisms is an integral part of transcription. A recent report provides exciting new evidence that conjugation of the Ubiquitin-like protein SUMO to histones opposes acetylation and establishes SUMOylation as important histone mark linked to transcriptional repression.
Histones are physically and figuratively central to the compact packaging of eukaryotic genomes. All DNA based transactions such as replication, transcription, recombination and repair require direct access to the nucleic acids and this is often accompanied by chemical modification of histones, many of which target lysine residues within their N and C terminal tails. Over the last few years, the list of such modifications has continued to grow not only in number but also in the size of the modifying group. The complexity of such modifications and their interrelations suggests the existence of a “histone code” where specific patterns of modification serve specific roles. Although the role of histone modification by small chemical groups such acetyl and methyl is becoming better understood, the function and mechanism of action of other modifications remain poorly defined. In general, acetylation of histone lysine residues is associated with actively transcribed chromatin and the mechanism of action of transcriptional activators often involves the recruitment of complexes harboring acetylase activity. In vertebrates and fission yeast, methylation of specific lysine residues is instrumental in the establishment of repressive heterochromatic domains by serving as a recruitment site for heterochromatin protein 1. In contrast, no such repressive mark had been identified in budding yeast. A potential answer to this apparent imbalance is provided in a recent report in the April issue of Genes and Development [1]. Through an elegant combination of chemical, biochemical and genetic approaches, Nathan and co workers provide evidence that SUMOylation of histones antagonizes their acetylation and serves as an evolutionarily conserved repressive mark.
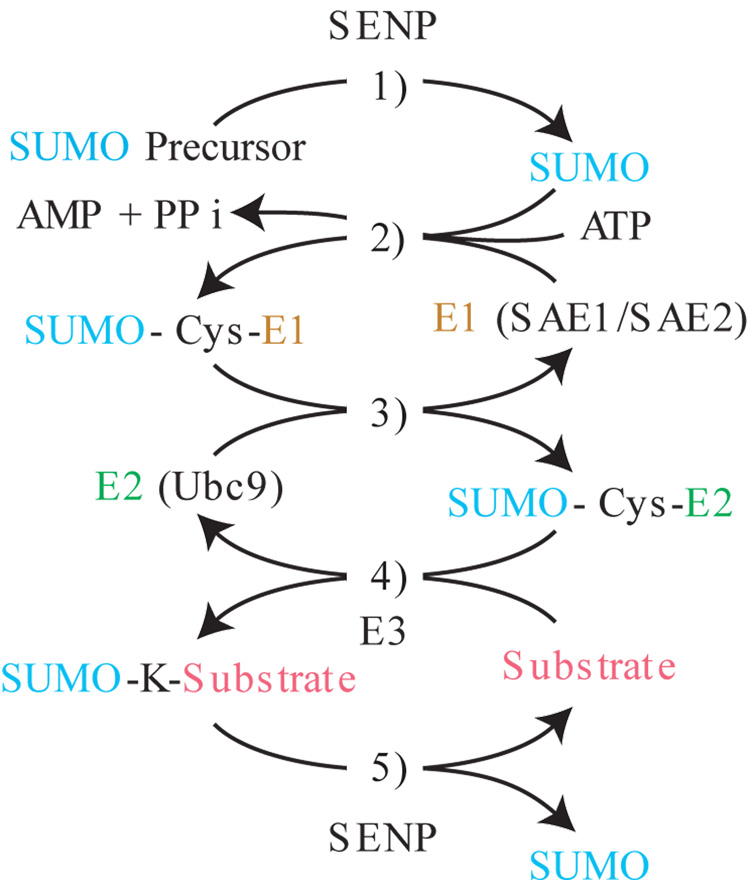
The conjugation of SUMO to target proteins follows an analogous pathway to that of ubiquitination (Figure 1) and requires dedicated E1 activating (SAE1/SAE2) and E2 conjugating (UBC9) enzymes. UBC9 interacts directly with substrates to catalyze the formation of an isopeptide bond between the C-terminus of SUMO and the amino group of the target lysine. This step is facilitated by E3 SUMO ligases. SUMOylation is reversible and specific isopeptidases release the SUMO moiety. SUMOylation appears to regulate target proteins by altering their interaction with other proteins, their intrinsic function or localization. SUMOylation can also compete with other lysine targeted modifications such as Ubiquitination or acetylation [2]. The function of SUMOylation has been substantially examined in the context of sequence-specific transcription factors and for the most part this modification leads to inhibition of transcription [3]. In many cases, SUMOylation exerts its effects in a striking promoter context dependent manner such that effective inhibition depends on recruitment of SUMOylated factors to multiple independent sites on promoters [4]. SUMOylation however, extends to other components of the transcriptional machinery and Shiio et. al described the SUMOylation of mammalian histones in 2003 [5].
Figure 1. SUMOylation pathway.
1) Initial processing of SUMO precursor by SUMO specific proteases (SENP) removes C terminal residues to generate a new GlyGly C terminus. 2) ATP dependent activation of SUMO by the SUMO specific E1 leads to the formation of a thioester bond between the C terminus of SUMO and a cysteine in the SAE2 subunit. 3) The SUMO moiety is transferred to the SUMO E2 ligase UBC9 through a trans-esterification reaction. 4) Ubc9 catalyzed conjugation of SUMO to substrate leads to the formation of an isopeptide bond between the C terminus of SUMO and the amino group of the target lysine. This step is enhanced by E3 ligases. 5) SUMO conjugation is reversible through the isopeptidase activity of SUMO-specific proteases.
The current paper by Nathan et al, provides compelling evidence that this modification plays important roles in the control of transcription by antagonizing histone acetylation (Figure 2). This group demonstrated that in budding yeast, all four core histones (H2A, H2B, H3 and H4) are SUMOylated in a manner that depends genetically on an intact sumoylation machinery. Although the extent of modification is less than a few percent, they detected multiple modified forms, suggestive of multi-site modification or poly-SUMO chain formation. Notably, the modification does not appear to be equal for all histones since the H2AZ histone variant, which is usually associated with active transcription, is SUMOylated to a substantially lower extent. Mass spectrometry analysis of protease digests identified lysines 6 and 7 in H2B and K 126 in H2A as sites of modification. Mutagenesis studies also implicated K16/17 in H2B as well as all five lysines in the N terminal region of H4. The heterogeneous pattern of modification and the lack of similarity to the canonical SUMOylation consensus indicated that histone SUMOylation can occur at multiple lysine residues without strict sequence requirements. This suggests that histone SUMOylation may rely on recruitment of UBC9 through adaptor E3 proteins. Interestingly, many of the identified SUMOylation sites are also targeted for acetylation.
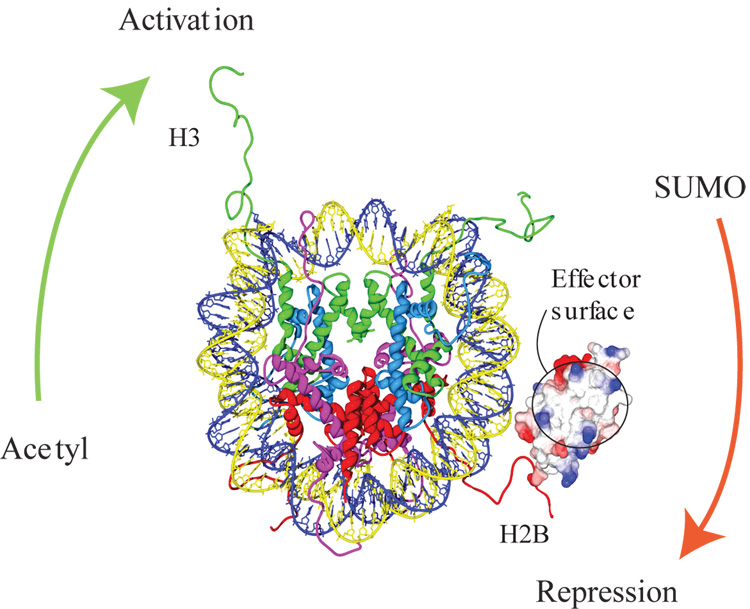
Figure 2. SUMOylation antagonizes acetylation.
Model of a nucleosome core showing a single surface rendered SUMO molecule conjugated to K6 of one of the H2B N terminal tails. The basic surface in SUMO essential for its transcriptional repressive function is circled.
Chromatin immunoprecipitation (ChIP) analysis using antibodies specific for individual modifications has allowed the mapping of the distribution and temporal evolution of acetylation and methylation histone marks in the genome. SUMOylation however, does not lend itself easily to this approach. The authors however, devised a clever sequential immunoprecipitation and affinity isolation scheme (ChDIP) to gauge the presence of SUMO conjugates associated with individual histones, which in the case of H2B, correspond mainly to sumoylation of H2B itself. Consistent with a role of histone SUMOylation in repression, this analysis revealed that SUMOylated H2B is present throughout the genome but is over-represented by ~2 fold at the transcriptionally silent telomeric regions where positive marks like acetylation and ubiquitination are less frequent.
A more direct analysis of the functional consequences of histone SUMOylation came from the expression of H2B mutants bearing alanine substitutions at the four N-terminal SUMOylation sites. These substitutions lead to a substantial but not complete loss of SUMOylation. If histone SUMOylation participates in transcriptional repression, loss of this modification should lead to derepression. Indeed, yeast expressing this H2B mutant displayed enhanced basal transcription of multiple genes under repressive conditions. By the same argument, enhanced SUMOylation of histones should lead to repression. To mimic persistently SUMOylated histones, the researchers expressed a non-cleavable colinear fusion between the C terminus of SUMO and the N terminus of H2B or H4. Consistent with an inhibitory role of SUMOylation, this manipulation led to substantial reductions in the expression of the galactose-inducible gene Gal1. The loss of activity however, could be a trivial consequence of steric hindrance or non-specific alterations in the function of the histones. To address this issue, the authors took advantage of recent data that identified a critical effector surface in SUMO essential for its transcriptional inhibitory function [6]. Substitution of basic residues by acidic ones within this region (circled in Figure 1) eliminated the repressive effects of the SUMO-H2B fusion indicating that the same effector surface of SUMO is required for its repressive effects both in the context of histones as well as transcription factors.
Since SUMOylation and acetylation sites overlap substantially, the authors examined the interplay between these modifications during the switch from repression to activation that accompanies an experimental shift in carbon source supply. A time course analysis indicated a reciprocal pattern of modifications where a transient loss of histone SUMOylation parallels enhanced acetylation both when the bulk of the histones is analyzed, or at a specific promoter. These data argue for a counterregulatory role for SUMOylation in opposition to acetylation (Figure 1). Consistent with this model, reduction on the SUMOylation capacity of the cells by inactivation of Ubc9 or genetic disruption of E3 ligases leads to enhanced bulk and gene-specific acetylation of histones.
Like many good experiments that push a field forward, these new results raise a number of questions and provide new opportunities. The mechanism of antagonism between SUMOylation and acetylation remains obscure. Although both modifications target overlapping lysine residues, acetylation is much more prevalent than SUMOylation. This argues that a direct competition mechanism is likely insufficient to account for their antagonism. This may not be so surprising since in many cases, SUMO has important regulatory effects at disturbingly low stoichiometries. Like a discreet but influential member of a royal court, SUMO appears to be uncanningly able to exert substantial influence without being detected. Nevertheless, the proteins that “read” the signal provided by SUMOylation of histones are likely to do so by binding to the critical effector surface in SUMO. Recent structural and functional data indicates that this surface is the site of binding of an emerging group of SUMO binding motifs [7].
Given the repressive role of histone SUMOylation, repressor functions within sequence-specific factors could operate by recruiting UBC9 and proteins with E3 activity towards histones. Studies in mammalian cells have indicated that UBC9 and members of the PIAS family of SUMO E3 ligases associate with multiple transcription factors that are themselves SUMOylated. In many cases though, SUMO E3’s have repressive effects that depend on their E3 activity but are independent of the SUMOylation of the factor with which they interact. Histones may be one of the targets of such E3s. Given the intrinsic repressive function of SUMO and the growing number of proteins within transcription complexes that are SUMOylated, it will be important to determine the relative contributions of histone SUMOylation to repression. In contrast to a potential role of SUMO E3s in repression, recruitment of a SUMO specific protease to erase the SUMO mark on histones could contribute to mechanisms of transcriptional activation. It is amply clear though that alterations in histone modifications have profound physiological and pharmacological consequences. Given the interplay between acetylation and SUMOylation and the potential of HDAC inhibitors in the treatment of cancer, [8] small molecule modulators of SUMOylation machinery may prove an attractive arena for pharmacochemical exploration.
Chemical complexity is at the center of biological processes and the post-translational modifications that decorate histones provides a clear example on how biological systems exploit reversible chemical variations to control dynamically complex systems. Acetylation has commanded much of the recent “action”. Given its heft, it seems appropriate for SUMO to provide the “equal and opposite reaction” to maintain a well balanced code.
REFERENCES
- 1.Nathan D, et al. Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 2006;20(8):966–976. doi: 10.1101/gad.1404206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 3.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Holmstrom S, Van Antwerp ME, Iniguez-Lluhi JA. Direct and distinguishable inhibitory roles for SUMO isoforms in the control of transcriptional synergy. Proc Natl Acad Sci USA. 2003;100(26):15758–15763. doi: 10.1073/pnas.2136933100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci U S A. 2003;100(23):13225–13330. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chupreta S, et al. A small conserved surface in SUMO is the critical structural determinant of its transcriptional inhibitory properties. Mol Cell Biol. 2005;25(10):4272–4282. doi: 10.1128/MCB.25.10.4272-4282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reverter D, Lima CD. Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. 2005;435(7042):687–692. doi: 10.1038/nature03588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond DC, et al. Clinical development of histone deacetylase inhibitors as anticancer agents. Annual Review of Pharmacology and Toxicology. 2005;45(1):495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]