Abstract
This article reviews evidence supporting the potential utility of a pharmacogenetic approach to the treatment of nicotine dependence. There is substantial evidence that nicotine dependence and smoking persistence are heritable, and are determined by a complex interplay of polygenic and environmental influences. The most robust evidence for specific genetic influences on nicotine dependence is found in studies of genetic variation in nicotine-metabolizing enzymes. Data also support the role of genes in the dopamine and opioid pathways as predictors of dependence and smoking relapse; however, the evidence for genetic associations is not always consistent. Emerging data from pharmacogenetic trials of nicotine-dependence treatment are promising, suggesting that genetic profiles of smokers someday may be used by providers to choose the type, dose, and duration of treatment for individual smokers. However, additional trials including larger and more diverse populations are needed before such data can be translated to practice to reduce smoking prevalence and tobacco-related disease.
Introduction
Despite progress made in the treatment of tobacco dependence, currently available treatments are effective for only a fraction of smokers. Although current guidelines recommend the use of nicotine patch as a firstline treatment for tobacco dependence,1 about 70%–80% of smokers treated with the patch relapse to their former smoking practices in the long-term.2,3 Bupropion has been shown to produce higher quit rates than nicotine replacement therapy,4,5 yet the large majority of smokers do not quit nor do they remain abstinent. The newly FDA-approved medication for treating nicotine dependence, varenicline, which outperforms bupropion significantly, yields a 1-year abstinence rate of 22%. However, these quit rates were achieved with behavioral counseling lasting for almost 1 year, which may not reflect real-world treatment.6,7 Thus, research is needed to identify those smokers who are at increased risk for relapse following a cessation attempt, and to tailor smoking-cessation treatments to smokers’ individual risks and needs.
Pharmacogenetics research is generating new knowledge about genetic factors that influence nicotine dependence and smoking-cessation treatment outcomes. The basic premise of this approach is that inherited differences in drug metabolism (pharmacokinetics) and drug targets (pharmacodynamics) have important effects on treatment outcome.8,9 These concepts and key variables are illustrated for nicotine-dependence treatment in Figure 1. Efforts to increase the understanding of the role that inherited variation plays in response to pharmacotherapy for nicotine dependence someday may help practitioners to individualize treatment type, dose, and duration based on genotype, thereby minimizing adverse reactions, increasing treatment compliance, and maximizing treatment efficacy.10,11 This article reviews evidence supporting the potential utility of a pharmacogenetic approach to smoking-cessation treatment. Portions of this paper were adapted from a recent book chapter on this topic.12
Figure 1.
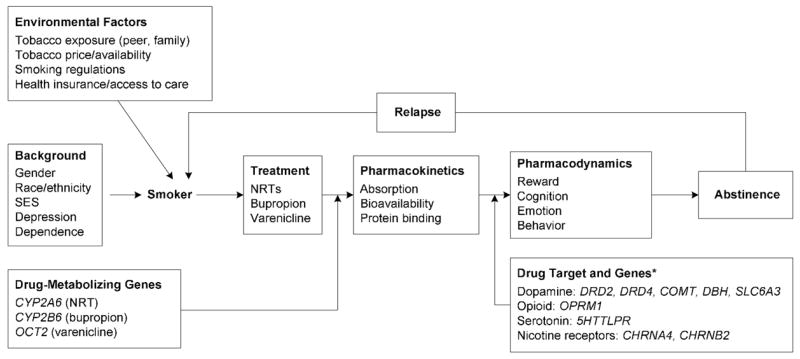
A pharmacogenetic model of nicotine-dependence treatment. * indicates that genes shown include the subset examined for pharmacogenetic effects.
nAChRs, nicotinic acetylcholine receptors; NRT, nicotine replacement therapy; SES, socioeconomic status.
Genetic Influences on Smoking Persistence and Relapse
While the initiation of tobacco use, the progression from use to nicotine dependence, and the ability to quit smoking are influenced by a range of environmental factors (e.g., parental and peer influence, depression), twin studies have shown that genes play a critical role as well.13,14 In twin studies, evidence of heritability is based on evaluating the similarity between monozygotic twins (who share 100% of their genes) on a phenotype, compared to dizygotic twins (who share 50% of their genes, similar to nontwin siblings). These studies have shown that approximately 60%–70% of the variability in nicotine dependence and smoking persistence is due to genetic influences.13,15–21 Two recent studies examined smoking-cessation data from twin pairs from the Vietnam Twin Registry22,23 and concluded that 51%–54% of the variance in the ability to quit smoking given a quit attempt, was attributable to genetic factors.
Given consistent evidence for the heritability of nicotine dependence, attention has shifted to investigations of specific genetic influences.24–26 Genetic variation in enzymes (e.g., CYP2A6) that metabolize nicotine to its inactive forms (cotinine and 3-hydroxycotinine) influence peripheral levels of nicotine and smoking behaviors.25 Genetically faster metabolizers of nicotine (two *1 alleles; ~80% of smokers)27 smoke more cigarettes per day and are more dependent on nicotine than slower metabolizers (carriers of *2 *4, *9A, and *12A alleles; ~20% of smokers)27,28 Fast metabolizers are also two times less likely to quit smoking,29 are more likely to relapse following transdermal nicotine-replacement treatment,30 and report higher levels of withdrawal symptoms following cessation.31
Candidate genes in neurobiological pathways mediating drug reward have been extensively studied for associations with nicotine dependence. Nicotine binds to neuronal nicotinic acetycholine receptors (nAChRs) expressed on dopamine and gamma-aminobutyric acid (GABA) neurons in the ventral tegmental area (VTA), resulting in increased dopamine release in the nucleus accumbens.32,33 Despite the importance of nAChRs in nicotine dependence, particularly the CHRNA4 and CHRNB2 subtypes,34 functional polymorphisms in these subunit genes have yet to be identified. Selected genetic polymorphism and haplotypes in CHRNA4 have been associated with nicotine dependence,35,36 while studies examining the role of CHRNB2 in smoking behavior have been negative.35,37,38
Given the central role of dopamine signaling in the rewarding effects of nicotine, alcohol, and other addictive drugs,39–41 many initial studies focused on the common Taq1A polymorphism, originally thought to be in the dopamine D2 receptor (DRD2) gene, but later determined to be in a neighboring gene, ANKK1.42 With respect to smoking behavior, some association studies have reported a higher prevalence of the low-activity DRD2 Taq1’A1 allele among smokers compared to nonsmokers,43,44 while other findings have been negative.45 Positive results have also been reported for associations of a variable number tandem repeat (VNTR) polymorphism in the 3″ end of the dopamine transporter (SLC6A3) gene with smoking behavior46,47; however, this has not been replicated in other studies.48 Finally, two independent studies have provided evidence for interacting effects of the DRD2 Taq1A and SLC6A3 variants on the likelihood of cessation.49,50 A separate study found associations of SLC6A3 genotypes with cessation following treatment with either nicotine replacement therapy (NRT) or bupropion.51
More robust findings have been observed for polymorphisms shown to alter protein transcription or translation. For example, the reduced-activity 7-repeat allele of the DRD4 gene VNTR has been associated with smoking persistence in African Americans.52 The high-activity (Val) allele of the catechol-o-methyl-transferase (COMT) gene, associated with more rapid degradation of dopamine, has been associated with smoking persistence in a retrospective case-control study and in a prospective smoking-cessation study.53
Nicotine also increases levels of endogeneous opioids that bind to mu opioid receptors on GABA interneurons in the VTA.41 Consistent with neurobiological evidence, the mu opioid receptor (OPRM1) Asn40Asp functional variant (low-activity Asp40 allele) has been associated with smoking persistence,54 as well as reduced nicotine reward among women.55 A recent study comparing smokers with high vs low levels of nicotine dependence did not find associations with this OPRM1 variant; however, haplotype analysis suggests that other variants, that may be in linkage disequilibrium with the Asn40Asp polymorphism, are linked with this smoking phenotype.56 Finally, despite effects of nicotine on serotonin neurotransmission, there is no strong evidence linking smoking cessation with genes in the serotonin pathway,57–59 although associations with nicotine dependence have been reported.60 Thus, it has proven difficult to identify candidate genes with robust, replicable associations with nicotine dependence and smoking persistence.
Pharmacogenetic Investigations of Treatment for Nicotine Dependence
Pharmacogenetic clinical trials, in which the type and dose of treatment are under experimental control, may provide a stronger signal for genetic effects on smoking cessation and shed light on individual differences in the efficacy of treatments for nicotine dependence (see Table 1).61 The emerging field of pharmacogenetics is based on the premise that inherited genetic variants contribute to individual variability in treatment toxicity and efficacy.8,9 Although this field is in its formative years, identifying genes related to responsiveness to treatments for nicotine addiction can lead to clinical guidelines for tailoring treatments to genetic profiles to increase treatment efficacy.10,61
Table 1.
Summary of pharmacogenetic effects in nicotine-dependence clinical trials
| Genes | Treatment | Gender interaction | Main finding | Citation |
|---|---|---|---|---|
| Pharmacokinetics/drug metabolizing enzymes | ||||
| CYP2A6 | NRT | Not reported | Genotypes related to 3-HC/cotine ratio; slow metabolizers smoke fewer cigarettes and are less nicotine dependent; in patch condition, slow metabolizers had higher plasma nicotine but equal patch use; in spray condition, fast metabolizers used more spray but had equal plasma nicotine | 28 |
| CYP2A6 (3-HC/Cotinine Ratio) | NRT | Not reported | 3-HC/cotinine ratio predicted nicotine patch efficacy butnot nasal spray; there was a 30% reduction in chance of cessation following patch therapy with each increasing quartile of metabolite ratio | 30 |
| CYP2B6 | Bupropion | Yes | Slow metabolizers had increased cravings after quitting and higher relapse rates; bupropion attenuated theses effects among females | 74 |
| Bupropion | No | Among smokers with decreased bupropion metabolism, bupropion produced significantly higher abstinence rates than placebo; bupropion was no more effective than placebo for smokers with normal bupropion metabolism | 75 | |
| Pharmacodynamics/drug target genes | ||||
| DRD2 (Taq 1A) | NRT | Yes | Quit rates from patch therapy were higher for women with the Taq1 A1 allele, but there was no genotype effect for men | 62 |
| NRT | No | Quit rates from patch therapy were significantly greater for smokers with the Taq1 A1 allele and the DBH A allele | 63 | |
| Bupropion | Yes | Women with the DRD2 A2/A2 allele were more likely to quit smoking, compared to women with A1 alleles | 80 | |
| Bupropion | No | Those with DRD2 A2/A2 alleles showed better treatment response, versus those with A1/A1 or A1/A2 alleles | 78 | |
| Venlafaxine | Smokers with the DRD2 A1 allele (A1/A1/A2) quit significantly less often than the homozygous A2s | 79 | ||
| DRD2 (-141 Ins/Del) | NRT | No | Smokers homozygous for the Del C allele responded better to NRT than carriers of the Ins C allele | 66 |
| Bupropion | No | Smokers homozygous for the Ins C allele had higher quit rates following bupropion, versus smokers with the Del C allele; smokers with the Del C allele had higher quit rates on placebo | 66 | |
| NRT | No | Smokers with at least one copy of the -141 Del allele and two copies of the FREQ rs1054879 A allele were more likely to quit smoking, compared to smokers with other alleles | 69 | |
| DRD2 (C957T) | NRT | No | Smokers with CT/CC genotypes were less than two-thirds as likely to be abstinent, versus participants with TT genotypes | 66 |
| Bupropion | No | Variants of C957T were not associated with quit rates following bupropion therapy | 66 | |
| DBH | NRT | No | Smokers with the DBH GA/AA genotype had higher quit rates, compared to those with GG alleles | 63 |
| COMT | NRT | Yes | Women with the Met/Met genotype showed higher quit rates following NRT, versus women with the Val/Val allele | 53 |
| Bupropion | No | COMT haplotype from SNPs rs165599 and rs373865 affected response to bupropion, with higher quit rates among smokers carrying the A allele of the rs165599 (A/G) SNP | 82 | |
| SLC6A3 | Bupropion | No | Smokers with DRD2-A2 genotypes and SLC6A3-9 genotypes, versus SLC6A3-10 genotypes, had significantly higher quit rates and a longer latency to relapse | 50 |
| NRT or bupropion | No | Smokers with the 9-repeat allele were more likely to quit smoking following treatment than those with 10/10 repeats | 51 | |
| Bupropion | No | There were no main effects for DRD2 and SLC6A3 genotypes on smoking cessation; those with DRD2 A1 and SLC6A3 9-repeat alleles show poorer response to bupropion | 49 | |
| OPRM1 | NRT | Yes | Quit rates following treatment were higher for carriers of the OPRM1 Asp40 variant, versus carriers of the Asn40 | 54 |
| 5-HTTLPR | NRT | No | 5-HTTLPR alleles were not related to NRT response | 58 |
| NRT | No | 5-HTTLPR alleles were not related to NRT response | 57 | |
| CHRNA4 | NRT | No | Smokers with the TC genotype were more likely to maintain abstinence on nasal spray, but not transdermal patch | 71 |
NRT, nicotine replacement therapy; 3-HC, 3-hydroxycotinin
Nicotine Replacement Therapy Trials
To date, two pharmacogenetic trials of NRT have been conducted. The first of these, conducted in the United Kingdom, compared transdermal nicotine patch to placebo patch among 755 of 1500 smokers who consented to provide DNA following the initial efficacy trial.62,63 Based on previous evidence that nicotine’s rewarding effects are mediated, in part, by dopaminergic mechanisms,64,65 initial pharmacogenetic analyses focused on genes in the dopamine reward pathway. The patch was found to be superior to placebo for carriers of the Taq1 A1 allele of the DRD2 (ANKK1) gene, but not those homozygous for the more common A2 allele.62 Further, the short-term efficacy of the transdermal nicotine patch was modulated by synonymous single-nucleotide polymorphism (SNP) in the dopamine beta hydroxylase (DBH) gene, which codes for an enzyme involved in the conversion of dopamine to norepinephrine.63 A longer-term follow-up of this analysis supported the association of the DRD2 Taq1A variant with abstinence at 6- and 12-month follow-ups; however, the effect was observed only among women.62 These findings suggest that the efficacy of pharmacotherapy may be influenced by different genetic and biological factors in men and women.
In the United States, an open-label randomized trial compared transdermal nicotine and nicotine nasal spray. A recent pharmacogenetic analysis from this trial focused on two functional genetic variants in DRD2.66 A promoter variant (–141C Ins/Del) is associated with altered transcriptional efficiency.67 Another functional SNP in DRD2 (C957T) alters mRNA stability and protein synthesis.68 Smokers carrying the reduced activity Del C allele of the –141C had statistically significantly higher quit rates on NRT compared to those homozygous for the Ins C allele, independent of NRT type. The C957T variant also was associated with abstinence following NRT. Thus, smokers carrying variants associated with reduced transcriptional efficiency or translation responded better to NRT, perhaps because of nicotine’s effects on dopamine release. Separate analyses from this trial reported that success with NRT was predicted by an interaction between the DRD2 –141 Ins/Del SNP and the NCS-1 gene, coding for a DRD2 interacting protein.69
The role of the COMT Val/Met functional polymorphism was also explored for effects on response to NRT in this trial.53 COMT is the primary enzyme involved in the degradation and inactivation of the neurotransmitter dopamine. A polymorphism in COMT results in conversion of a Val high-activity allele to a Met low-activity allele, resulting in a three- to four-fold reduction in COMT activity. In the NRT trial, the Met/Met genotype was associated with a higher probability of abstinence with either nicotine nasal spray or nicotine patch, among women, but not in men.53
The role of the OPRM1 gene was also examined in the U.S. trial.54 The Asp40 variant (G allele) is associated with reduced MRNA and protein levels70 and is carried by 25%–30% of individuals of European ancestry. In the NRT trial, smokers carrying the OPRM1 Asp40 variant were significantly more likely than those homozygous for the Asn40 variant to be abstinent at the end of the treatment phase. The differential treatment response was most pronounced among smokers receiving transdermal nicotine.54 In contrast to positive associations for DRD2 and OPRM1, there was no evidence for moderation of treatment response by the serotonin transporter (5-HTTLPR) gene in the NRT trial.58 Likewise, David et al.57 reported that response to NRT was not associated with variants of the 5-HTTLPR gene.
Finally, a recent paper reported the effects of SNPs in CHRNA4 (α4 subunit of the acetycholine nicotinic receptor) in response to NRT in the U.S. clinical trial.71 Individuals with the TC genotype for this SNP, which is associated with greater α4β2 binding and greater sensitivity to the acute effects of smoking, were more likely to maintain abstinence on nasal spray, but not transdermal patch.
Bupropion Trials
To date, three independent bupropion pharmacogenetic trials have been conducted in the U.S. An initial report from a placebo-controlled trial focused on the CYP2B6 gene, which has been implicated in bupropion kinetics72 as well as in brain metabolism of nicotine.73 Participants in this trial provided blood samples and received bupropion (300 mg/day for 10 weeks) or placebo, plus counseling. Smokers with a decreased-activity variant of CYP2B6 (slower metabolizers) reported greater increases in cravings for cigarettes following the target quit date and had significantly higher relapse rates.74 These effects were modified by a significant gender × genotype × treatment interaction, suggesting that bupropion attenuated the effects of genotype among female smokers. The absence of a genotype association with bupropion side effects suggests that the genotype effect is not due to bupropion pharmacokinetics, but may be attributable to CYP2B6-mediated differences in nicotine metabolism in the central nervous system (CNS). For example, slower metabolizers of CNS-nicotine may experience neuroadaptive changes that promote dependence and abstinence-induced craving. In a subsequent analysis of a novel functional CYP2B6 *6 variant (a genotype combining 2 SNPs), smokers with this genotype had significantly lower quit rates on placebo, and responded very well to bupropion; in contrast, smokers with the wildtype genotype performed equally well on placebo and bupropion.75
Inhibition of dopamine reuptake is one putative mechanism for the beneficial effects of bupropion.76,77 Therefore, an analysis of response to bupropion has been conducted relative to two functional genetic variants in DRD2. There was a statistically significant interaction between the DRD2 –141C Ins/Del genotype and treatment, at the end of the treatment phase, indicating a more favorable response to bupropion among smokers homozygous for the Ins C allele compared to those carrying a Del C allele.66 The C957T variant was not associated with bupropion response. Given that the –141 Ins C allele results in higher transcriptional efficiency compared to the Del (N) allele,67 individuals with the –141C Ins/Del CC genotype may have more D2 receptors available to bind dopamine, yielding a more rewarding experience of the nicotine-induced dopamine release. Blockade of dopamine reuptake by bupropion may be more effective in promoting abstinence in the Ins C genotype group due to greater ability to bind dopamine.
David et al.78 examined the DRD2, the dopamine transporter gene SLC6A3, and the CYP2B6 (C1459T) genotypes as moderators of treatment response in a placebo-controlled bupropion trial with 283 smokers of European ancestry. Smokers with the DRD2 Taq1-A2/A2 genotype had a significantly better treatment response (35% for bupropion and 12% for placebo), compared to smokers with A1/A1 or A1/A2 genotypes (21% for bupropion and 24% for placebo). Cinciripini and colleages79 examined the DRD2 Taq1A polymorphism in a placebo-controlled trial of venlafaxine (a serotonin reuptake inhibitor) and reported that smokers carrying the A1 allele were less likely to quit.
Swan and colleagues80 examined the role of the DRD2 Taq1A polymorphism in an open-label, randomized effectiveness trial comparing 150mg and 300 mg doses of bupropion. Compared to women homozygous for the A2 allele, women with at least one A1 allele were significantly less likely to quit smoking and more likely to report having stopped taking bupropion due to treatment side effects. Finally, a recent analysis from the bupropion placebo-controlled trial provides preliminary evidence for associations of a COMT haplotype with bupropion response.81
Summary, Clinical Implications, and Future Research Directions
Initial findings presented in this review support the role of genetic variation in response to bupropion and NRT for smoking cessation (see Table 1). Variations in genes in the dopamine and opioid pathways, and in nicotine-metabolizing enzymes, appear to play a role in the efficacy of nicotine-replacement therapy, while genetic variation in the dopamine pathway also appears to be important for response to bupropion. Many of these studies also provide evidence for gender heterogeneity in these genetic associations.
While the integration of genetic testing into standard clinical practice would be premature at this time, pharmacogenetic studies of treatments for nicotine dependence eventually may guide individualized smoking-cessation treatments. For instance, carriers of genetic variants that increase nicotine metabolism may not respond as well to standard NRT doses, whereas carriers of reduced-activity variants in DRD2 (e.g., Taq 1A, –141 DelC) may respond particularly well to NRTs. Selecting smokers with reduced-activity DRD2 variants for NRT or selecting smokers with genetic variants that increase nicotine metabolism for a higher dose of NRT may enhance long-term quit rates. Importantly, many of the genetic variants linked to nicotine dependence and poor response to treatments are very common, such as CYP2A6*1 (77%)27 and DRD2 A1 (43%),50 suggesting that genetically-tailored treatment approaches could have a substantial population-level impact on treatment outcomes. In addition, the effect size associated with the presence of genetic alleles linked to smoking phenotypes is typically meaningful. For instance, 35% of smokers homozygous for the Ins C allele were abstinent following bupropion treatment, compared to only 20% of smokers carrying a Del C allele.66 Likewise, at the end of NRT treatment, 23% of carriers of the Taq1 A1 allele of the DRD2 (ANKK1) gene were abstinent, compared to 13% of those homozygous for the more common A2 allele.62 Further, pharmacogenetic studies also may help researchers understand the neurobiology of nicotine dependence which, in turn, could guide the development of new treatments. Thus, given the frequency and impact of genetic alleles linked to smoking phenotypes, using genetic information to tailor the selection of treatments may ultimately have a substantial impact on overall rates of smoking.
The use of genetic information to tailor the selection of treatments for nicotine dependence is feasible, yet there are several important policy issues that must be addressed before this technology will become standard clinical practice. First, while genetic testing for smoking genotypes increasingly is becoming more affordable and efficient with advances in technology, testing largely remains confined to the context of research programs. Decisions about how the costs of testing will be covered and whether or not insurance companies will absorb these costs have yet to be bridged. Further, given the limited resources of middle- or low-income countries, it appears likely that the potential benefits of using genetic information to treat nicotine dependence would only be realized in high-income countries. Even with adequate resources in high-income countries to implement genetic testing into treatment for nicotine dependence, researchers will need to demonstrate that such a treatment approach is cost-effective. To date, cost-effectiveness analysis of genetic testing for nicotine-dependence treatment has only recently begun,82 and remains as a critical priority for future research.
Further, as genetic testing for nicotine dependence is incorporated into clinical practice, researchers and clinicians must be mindful of the role of race/ethnicity as factors that influence smoking phenotypes and genes related to nicotine dependence. To date, the vast majority of studies have been conducted with Caucasians to avoid population stratification bias. Race/ethnicity influence smoking behavior (e.g., age of initiation, smoking rate, level of dependence)83 and there are large racial differences in allele frequencies for nicotine-metabolizing genes25,84 and dopaminergic genes.85 Further, since access to healthcare and socioeconomic status vary with race/ethnicity, the potential implementation of genetic testing clinical services may need to consider race/ethnicity as an important variable if the potential for this technology is to be realized.84
Third, there is substantial comorbidity between nicotine dependence and other substance abuse conditions and psychiatric disorders, including depression, schizophrenia, attention-deficit hyperactivity disorder, anxiety, and personality disorders.33 In addition, genes linked to smoking behavior and treatment response may be related to psychiatric conditions and other addictions.87 Thus, genetic testing to tailor treatment for nicotine dependence simultaneously could identify individuals with other dependence or psychiatric disorders,88 and treatment programs may need to be prepared to provide more comprehensive interventions to ensure efficacy and to address comorbid psychiatric conditions.
Fourth, several genes and environmental factors likely combine to influence response to treatments for nicotine dependence. To date, most studies have focused on single genes and have not evaluated gene–environment interaction. The development of effective individualized treatments for nicotine dependence and moving beyond a “one size fits all” model may depend on future pharmacogenetic studies that include sufficiently large samples to evaluate gene–gene and gene–environment interactions.
Finally, if genetic testing for nicotine dependence is to be incorporated into clinical practice, primary care physicians, who are often the first point of contact for patients seeking assistance with quitting, will need to be appropriately trained. Many physicians lack confidence to provide genetic testing,89 underscoring the need for guidelines to help physicians integrate genetic testing into their practice.
Additional work is needed to validate these findings across independent trials. Meta-analytic techniques also may be applied to overcome issues related to the relatively small sample sizes of the initial trials.90 Future studies also should explore the use of more refined outcome measures that account for the longitudinal trajectories of smoking cessation, including multiple lapses, relapses, and changes in smoking rates over time.91 Increased attention to gender heterogeneity in genetic associations55 as well as ethnic heterogeneity is needed. In addition, future studies should also explore the influence of genetic variation in additional genetic pathways relevant to nicotine dependence, including GABA and glutamate. Such studies may hold great promise for the identification of novel biological targets for drug development and improvement in the delivery of nicotine-dependence treatment to reduce the morbidity and mortality caused by smoking.
Acknowledgments
This work was supported by grants from the National Cancer Institute and National Institutes on Drug Abuse (P50-CA84718), R01-CA63562, and R01-DA17555 (CL), and by Commonwealth of Pennsylvania Center of Excellence grant SAP# 4100027297 (CL).
Dr. Lerman has served as a consultant to Glaxo Smith Kline, Astra Zeneca, and Pfizer; Dr. Munafò has received consulting fees from G-nostics Ltd. No other authors reported financial disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore MC, Bailey W, Cohen S. Clinical Practice Guideline. Rockville MD: U.S. Department of Health and Human Services (USDHHS). Public Health Service; 2000. Treating tobacco use and dependence. [Google Scholar]
- 2.Fiore M, Smith S, Jorenby D, Baker T. The effectiveness of the nicotine patch for smoking cessation: a meta-analysis. JAMA. 1994;271:1940–47. [PubMed] [Google Scholar]
- 3.Transdermal Nicotine Study Group. Transdermal nicotine for smoking cessation: Six-month results from two multicenter controlled trials. JAMA. 1991;266:3133–38. [PubMed] [Google Scholar]
- 4.Gold PB, Rubey RN, Harvey RT. Naturalistic, self-assignment comparative trial of bupropion SR, a nicotine patch, or both for smoking cessation treatment in primary care. Am J Addict. 2002;11:315–31. doi: 10.1080/1055049029008811. [DOI] [PubMed] [Google Scholar]
- 5.Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–91. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 6.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–91. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 9.Poolsup N, Li Wan Po A, Knight TL. Pharmacogenetics and psychopharmacotherapy. J Clin Pharm Ther. 2000;25:197–220. doi: 10.1046/j.1365-2710.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 10.Lerman C, Niaura R. Applying genetic approaches to the treatment of nicotine dependence. Oncogene. 2002;21:7412–20. doi: 10.1038/sj.onc.1205801. [DOI] [PubMed] [Google Scholar]
- 11.Lerman C, Patterson F, Berrettini W. Treating tobacco dependence: state of the science and new directions. J Clin Oncol. 2005;23:311–23. doi: 10.1200/JCO.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 12.Lerman C, Shields A, Munafo M. Pharmacogenetic approaches to the treatment of nicotine dependence. In: George TP, editor. Medications treatments for nicotine dependence. Boca Raton FL: Taylor & Francis; 2006. [Google Scholar]
- 13.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(Suppl 2):S51–7. S69–70. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- 15.Koopmans J, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav Genet. 1999;29:382–93. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- 16.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking—a study of male twins. N Engl J Med. 1992;327:829–33. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- 17.McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. 2000;96:671–7. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.True WR, Heath AC, Scherrer JF, et al. Genetic and environmental contributions to smoking. Addiction. 1997;92:1277–87. [PubMed] [Google Scholar]
- 19.Heath A, Kirk K, Meyer J, Martin N. Genetic and social determinants of initiation and age at onset of smoking in Australian twins. Behav Genet. 1999;29:395–407. doi: 10.1023/a:1021670703806. [DOI] [PubMed] [Google Scholar]
- 20.Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Res Hum Genet. 2006;9:64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- 21.Kendler KS, Thornton LM, Pedersen NL. Tobacco consumption in Swedish twins reared apart and reared together. Arch Gen Psychiatry. 2000;57:886–92. doi: 10.1001/archpsyc.57.9.886. [DOI] [PubMed] [Google Scholar]
- 22.Xian H, Scherrer JF, Madden PA, et al. Latent class typology of nicotine withdrawal: genetic contributions and association with failed smoking cessation and psychiatric disorders. Psychol Med. 2005;35:409–19. doi: 10.1017/s0033291704003289. [DOI] [PubMed] [Google Scholar]
- 23.Xian H, Scherrer JF, Madden PA, et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob Res. 2003;5:245–54. [PubMed] [Google Scholar]
- 24.Li MD. The genetics of nicotine dependence. Curr Psychiatry Rep. 2006;8:158–64. doi: 10.1007/s11920-006-0016-0. [DOI] [PubMed] [Google Scholar]
- 25.Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clin Pharmacol Ther. 2005;77:145–58. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Munafo M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine Tob Res. 2004;6:583–97. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- 27.Benowitz NL, Swan GE, Jacob P, 3rd, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80:457–67. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006;11:400–9. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- 29.Gu DF, Hinks LJ, Morton NE, Day IN. The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit. Ann Hum Genet. 2000;64(Pt 5):383–90. doi: 10.1046/j.1469-1809.2000.6450383.x. [DOI] [PubMed] [Google Scholar]
- 30.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–8. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Kubota T, Nakajima-Taniguchi C, Fukuda T, et al. CYP2A6 polymorphisms are associated with nicotine dependence and influence withdrawal symptoms in smoking cessation. Pharmacogenomics J. 2006;6:115–9. doi: 10.1038/sj.tpj.6500348. [DOI] [PubMed] [Google Scholar]
- 32.Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 33.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–70. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 34.Lukas RJ. Pharmacological effects of nicotine and nicotinic receptor subtype pharmacological profiles. In: George TP, editor. Medication treatments for nicotine dependence. Boca Raton FL: Taylor & Francis; 2006. [Google Scholar]
- 35.Li MD, Beuten J, Ma JZ, et al. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol Genet. 2005;14:1211–9. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Niu T, Xing H, et al. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. Am J Hum Genet. 2004;75:112–21. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lueders KK, Hu S, McHugh L, Myakishev MV, Sirota LA, Hamer DH. Genetic and functional analysis of single nucleotide polymorphisms in the beta2-neuronal nicotinic acetylcholine receptor gene (CHRNB2) Nicotine Tob Res. 2002;4:115–25. doi: 10.1080/14622200110098419. [DOI] [PubMed] [Google Scholar]
- 38.Silverman M, Neale M, Sullivan P, Harris-Kerr C, Wormley B, Sadek H. Haplotypes of four novel single nucleotide polymorphisms in the nicotine acetylcholine receptor b2-subunit (CHRNB2) gene show no association with smoking initiation or nicotine dependence. Am J Med Genet (Neuropsych Genet) 2000;96:646–53. [PubMed] [Google Scholar]
- 39.Heinz A, Goldman D, Gallinat J, Schumann G, Puls I. Pharmacogenetic insights to monoaminergic dysfunction in alcohol dependence. Psychopharmacology (Berl) 2004;174:561–70. doi: 10.1007/s00213-004-1903-x. [DOI] [PubMed] [Google Scholar]
- 40.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 41.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 42.Neville MJ, Johnstone EC, Walton RT. Identification and characterization of ANKK1: a novel kinase gene closely linked to DRD2 on chromosome band 11q231. Hum Mutat. 2004;23:540–5. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 43.Spitz M, Shi H, Yang F, Hudmon K, Jiang H, Chanberlain R. Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst. 1998;90:358–63. doi: 10.1093/jnci/90.5.358. [DOI] [PubMed] [Google Scholar]
- 44.Comings D, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics. 1996;6:73–9. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Bierut L, Rice J, Edenberg H, Goate A, Foroud T, Cloninger C. Family-based study of the association of the dopamine D2 receptor gene (DRD2) with habitual smoking. Am J Med Genet. 2000;90:299–302. doi: 10.1002/(sici)1096-8628(20000214)90:4<299::aid-ajmg7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 46.Sabol S, Nelson M, Fisher C, Gunzerath L, Brody C, Hu S. A genetic association for cigarette smoking behavior. Health Psychol. 1999;18:7–13. doi: 10.1037//0278-6133.18.1.7. [DOI] [PubMed] [Google Scholar]
- 47.Lerman C, Caporaso N, Audrain J, et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol. 1999;18:14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- 48.Vandenbergh D, Bennett C, Grant M, Strasser A, O'Connor R, Stauffer R, et al. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: Failure to replicate and finding that never-smokers may be different. Nicotine Tob Res. 2002;4:333–40. doi: 10.1080/14622200210142689. [DOI] [PubMed] [Google Scholar]
- 49.Swan G, Jack LM, Valdes AM, et al. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychol. 26:361–8. doi: 10.1037/0278-6133.26.3.361. [DOI] [PubMed] [Google Scholar]
- 50.Lerman C, Shields PG, Wileyto EP, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychol. 2003;22:541–8. doi: 10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- 51.O'Gara C, Stapleton J, Sutherland G, et al. Dopamine transporter polymorphisms are associated with short-term response to smoking cessation treatment. Pharmacogenet Genomics. 2007;17:61–7. doi: 10.1097/01.fpc.0000236328.18928.4c. [DOI] [PubMed] [Google Scholar]
- 52.Shields P, Lerman C, Audrain J, Main D, Boyd N, Caporaso N. Dopamine D4 receptors and the risk of cigarette smoking in African-Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:453–58. [PubMed] [Google Scholar]
- 53.Colilla S, Lerman C, Shields P, et al. Association of Catechol-O-Methyltransferase functional variant with smoking cessation in two independent studies of women. Pharmacogenetics. 2005;15:393–98. doi: 10.1097/01213011-200506000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lerman C, Wileyto EP, Patterson F, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J. 2004;4:184–92. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- 55.Ray R, Jepson C, Patterson F, et al. Association of OPRM1 Asn40Asp variant with the relative reinforcing value of nicotine in female smokers. Psychopharmacology. 2006;188:355–63. doi: 10.1007/s00213-006-0504-2. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Kendler KS, Chen X. The μ-opioid receptor gene and smoking initiation and nicotine dependence. Behav Brain Funct. 2006;2:28. doi: 10.1186/1744-9081-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.David SP, Munafo MR, Murphy MF, Walton RT, Johnstone EC. The serotonin transporter 5-httlpr polymorphism and treatment response to nicotine patch: Follow-up of a randomized controlled trial. Nicotine Tob Res. 2007;9:225–31. doi: 10.1080/14622200601078566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munafo MR, Johnstone EC, Wileyto EP, Shields PG, Elliot KM, Lerman C. Lack of association of 5-HTTLPR genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiol Biomarkers Prev. 2006;15:398–400. doi: 10.1158/1055-9965.EPI-05-0648. [DOI] [PubMed] [Google Scholar]
- 59.Lerman C, Shields PG, Audrain J, et al. The role of the serotonin transporter gene in cigarette smoking. Cancer Epidemiol Biomarkers Prev. 1998;7:253–5. [PubMed] [Google Scholar]
- 60.Munafo M, Roberts K, Johnstone EC, Walton RT, Yidlin P. Association of serotonin transporter gene polymorphism with nicotine dependence. No evidence for an interaction with trait neuroticism. Personality Individual Diff. 2005;38:843–50. [Google Scholar]
- 61.Rutter JL. Symbiotic relationship of pharmacogenetics and drugs of abuse. AAPS J. 2006;8:E174–84. doi: 10.1208/aapsj080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yudkin P, Munafo M, Hey K, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: randomised controlled trial. BMJ. 2004;328:989–90. doi: 10.1136/bmj.38050.674826.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnstone EC, Yudkin PL, Hey K, et al. Genetic variation in dopaminergic pathways and short-term effectiveness of the nicotine patch. Pharmacogenetics. 2004;14:83–90. doi: 10.1097/00008571-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Pontieri F, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–7. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- 65.Balfour DJ. Neuroplasticity within the mesoaccumbens dopamine system and its role in tobacco dependence. Curr Drug Targets CNS Neurol Disord. 2002;1:413–21. doi: 10.2174/1568007023339076. [DOI] [PubMed] [Google Scholar]
- 66.Lerman C, Jepson C, Wileyto E, et al. The role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: Results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–42. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- 67.Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6:577–82. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 68.Duan J, Wainwright MS, Comeron JM, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–16. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- 69.Dahl JP, Jepson C, Levenson R, et al. Interaction between variation in the D2 dopamine receptor (DRD2) and the neuronal calcium sensor-1 (FREQ) genes in predicting response to nicotine replacement therapy for tobacco dependence. Pharmacogenomics J. 2006;6:194–9. doi: 10.1038/sj.tpj.6500358. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–24. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 71.Hutchison KE, Allen D, Haughey H, et al. CHRNA4 and tobacco dependence: from gene regulation to treatment outcome. Arch Gen Psychiatry. 2007;64:1078–86. doi: 10.1001/archpsyc.64.9.1078. [DOI] [PubMed] [Google Scholar]
- 72.Kirchheiner J, Klein C, Meineke I, et al. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003;13:619–26. doi: 10.1097/00008571-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45:122–32. doi: 10.1016/s0028-3908(03)00136-9. [DOI] [PubMed] [Google Scholar]
- 74.Lerman C, Shields PG, Wileyto EP, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics. 2002;12:627–34. doi: 10.1097/00008571-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 75.Lee AM, Jepson C, Hoffmann E, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry. 2007;62:635–41. doi: 10.1016/j.biopsych.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol. 1999;19:467–89. doi: 10.1023/A:1006986824213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 78.David SP, Brown RA, Papandonatos GD, et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine Tob Res. 2007;9:821–33. doi: 10.1080/14622200701382033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cinciripini P, Wetter D, Tomlinson G, et al. The effects of the DRD2 polymorphism on smoking cessation and negative affect: Evidence for a pharmacogenetic effect on mood. Nicotine Tob Res. 2004;6:229–40. doi: 10.1080/14622200410001676396. [DOI] [PubMed] [Google Scholar]
- 80.Swan GE, Valdes AM, Ring HZ, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics J. 2005;5:21–9. doi: 10.1038/sj.tpj.6500281. [DOI] [PubMed] [Google Scholar]
- 81.Berrettini WH, Wileyto EP, Epstein L, et al. Catechol-O-Methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biol Psychiatry. 2007;61:111–8. doi: 10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 82.Welton NJ, Johnstone EC, David SP, Munafò MR. A cost-effectiveness analysis of genetic testing to aid treatment choice for smoking cessation. Nicotine Tob Res. doi: 10.1080/14622200701767761. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Payne TJ, Diefenbach L. Characteristics of African American smokers: a brief review. Am J Med Sci. 2003;326:212–5. doi: 10.1097/00000441-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 84.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 85.Beuten J, Payne TJ, Ma JZ, Li MD. Significant association of catechol-O-methyltransferase (COMT) haplotypes with nicotine dependence in male and female smokers of two ethnic populations. Neuropsychopharmacology. 2006;31:675–84. doi: 10.1038/sj.npp.1300997. [DOI] [PubMed] [Google Scholar]
- 86.Shields AE, Fortun M, Hammonds EM, et al. The use of race variables in genetic studies of complex traits and the goal of reducing health disparities: a transdisciplinary perspective. Am Psychol. 2005;60:77–103. doi: 10.1037/0003-066X.60.1.77. [DOI] [PubMed] [Google Scholar]
- 87.Comings DE, Comings BG, Muhleman D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:1793–800. [PubMed] [Google Scholar]
- 88.Shields A, Lerman C, Sullivan P. Translating emerging research on the genetics of smoking into clinical practice: ethical and social considerations. Nicotine Tob Res. 2004;6:675–88. doi: 10.1080/14622200410001734058. [DOI] [PubMed] [Google Scholar]
- 89.Shields AE, Blumenthal D, Weiss KB, Comstock CB, Currivan D, Lerman C. Barriers to translating emerging genetic research on smoking into clinical practice. Perspectives of primary care physicians. J Gen Intern Med. 2005;20:131–8. doi: 10.1111/j.1525-1497.2005.30429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–44. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 91.Wileyto EP, Patterson F, Niaura R, et al. Do small lapses predict relapse to smoking behavior under bupropion treatment. Nicotine Tob Res. 2004;6:357–66. doi: 10.1080/1462220042000202463. [DOI] [PubMed] [Google Scholar]


