Abstract
The inherent ability of the host immune system to distinguish between self- and non-self forms the basis of allorecognition. T lymphocytes constitute the most important effector arm of allorecognition. Here we describe the fundamentals of direct and indirect pathways by which allopeptides are presented to effector T cells. The nature of allopeptides presented along with tolerogenic strategies like altered peptide ligands and intra- or extra-thymic allopeptide inoculation are discussed. In addition, we speculate on the potential of regulatory T cells to modulate alloimmune responses.
Keywords: allopeptides, alloimmune response, MHC antigens, rejection, tolerance, regulatory T cells
Introduction
Allorecognition refers to the phenomenon by which the recipient immune system reacts with donor antigens that are considered to be “non-self”. T cells constitute the principal effector arm of allorecognition. In contrast, there exists a “tolerogenic” arm of regulatory T cells that suppresses the alloimmune response and facilitates tolerance. Nevertheless, the most common natural consequence following transplantation is allograft rejection. This suggests that the alloreactive T cells have a survival advantage following transplantation and are able to predominate. In this review, we discuss the nature of allopeptides recognized by T cells along with the different pathways of allorecognition. In addition, we speculate on the potential role of regulatory T cells in suppressing alloreactive T cells and achieving allograft tolerance.
Cellular basis of allopeptide recognition
The main targets of the recipient immune response against the allograft are the donor major (MHC) histocompatibility antigens present on the allogeneic tissue. The recognition of mismatched donor histocompatibility antigens is the primary event that ultimately leads to allograft rejection (1–3). Allorecognition occurs through two unique but not mutually exclusive pathways: called direct and indirect pathways of antigen presentation. Direct pathway involves recognition of intact donor MHC molecules on the donor cells, usually the antigen presenting cells (APC). Both CD8+ and CD4+ T cells can directly recognize donor MHC class I and class II, respectively. The indirect pathway, in contrast, involves presentation of processed donor antigens by recipient APC to recipient T cells (Figure 1). Again, both CD4+ and CD8+ T cells can mediate indirect allorecognition.
Figure 1.
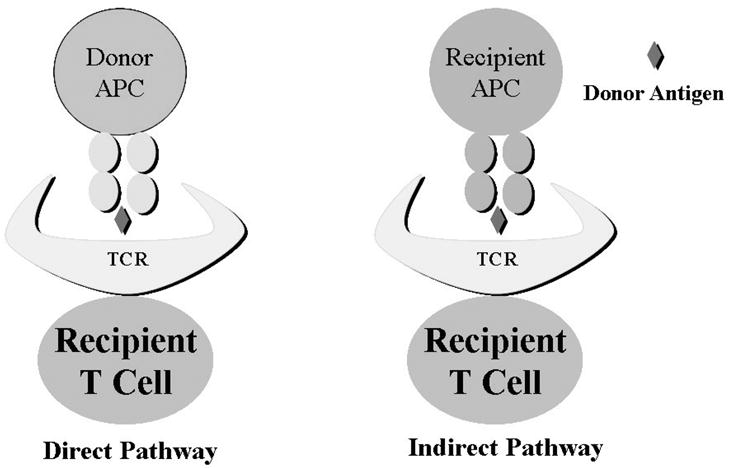
Direct and Indirect Allorecognition
Direct Allorecognition
Direct pathway involves presentation of intact donor antigens to the recipient T cells. This may seem to contradict the classic self-MHC restriction property of T cells since the peptide being recognized is presented in a non-self MHC. Two models have been proposed to explain this discrepancy (2).
The first, called the “high determinant density” model, proposes that the direct alloreactive T cells recognize amino acid polymorphisms on the MHC molecules of the donor cells and the nature of peptide in the MHC groove is not important. Therefore, all the donor cells of any given MHC act as ligands for the direct alloreactive T cells, thereby creating a very high ligand density. Consequently, the affinity of the alloreactive T cell receptors required to generate an optimal alloimmune response can be significantly lower compared to that required for self-MHC peptide complex (4).
The “multiple binary complex” model proposes that the alloreactive T cells recognize specific peptides in the donor MHC grooves. These peptides are derived from the same normal cellular proteins that are present even in the recipient. However, the differences in the allo-MHC groove causes different set of peptides to be presented from homologous proteins. These peptides can be recognized by the recipient T cells. Therefore, even a single MHC mismatch between the donor and the recipient would be able to stimulate a large number of alloreactive T cells by providinga completely different set of peptides. It is now known that the TCR contact surfaces of many MHC alleles may be similar, thereby providing a degeneracy effect with regards to MHC-restriction and allowing the recipient T cells to cross-react with donor MHC. In another version of this model, any particular cell surface MHC protein is complexed with a naturally arising peptide from the intracellular proteolytic machinery, forming a heterogeneous population of binary complexes. Such a multitude of MHC-peptide complexes could be recognized by many different T cell clones in the recipient (5, 6).
It is hypothesized that the allograft brings with it “passenger” APC that are able to stimulate recipient T cells directly. There have been several classical studies to support the concept of passenger APC. Lafferty et al demonstrated that cultured thyroid tissue has prolonged survival due to the loss of passenger APC (7). Another important set of experiments revealed that depleted passenger APC survive permanently in allogeneic recipients (8). Importantly, the recipients of the re-transplanted kidneys rapidly rejected the allografts when injected with donor APC (9). These reports also indicate that the allospecific T cells reactive through the direct pathway need to be primed by the donor passenger APC. If this is not achieved while the passenger APC are present, the direct alloreactive T cells cannot mediate rejection. More conclusive evidence of the direct pathway in allograft rejection came from studies from Pietra et al (10). They demonstrated that lymphocyte deficient, SCID or RAG1−/−, mice when reconstituted with CD4+ T cells rejected MHC class I but not MHC class II deficient cardiac allografts. Furthermore, RAG1−/− mice that were also MHC class II deficient rejected cardiac allografts when reconstituted with CD4+ T cells. This indicated that CD4+ T cells alone, directly activated by donor MHC class II bearing APC, could mediate rejection. Another interesting observation that emerges from the above studies is that the direct pathway may be of decreasing importance with time after transplantation as the passenger APC are lost.
Indirect allorecognition
The indirect pathway of allorecognition is more representative of how the immune system typically recognizes an antigen. Here, the T cells recognize the donor antigens that have been processed and presented in the context of self-MHC on the recipient APC. Using monoclonal antibodies directed against specific MHC-peptide complexes, it was demonstrated that MHC-derived peptides could be presented in the context of other (recipient) MHC molecules. One of the first such monoclonal antibodies developed was the Y-Ae (11, 12). This antibody reacts to a peptide derived from the H2-Eα chain presented in the context of H2-Ab. This antibody brightly stained dendritic cells (DC) and B cells from murine strains co-expressing H2-Ab and H2-E but not from those expressing either of these alone. When H2-E bearing DCs were injected into the H2-Ab recipients, a significant proportion of recipient DCs in the draining lymph nodes became reactive with the Y-Ae antibody. These studies clearly show that MHC molecules can be processed and presented by self- or by allogeneic MHC.
Seminal studies done by Fangmann et al demonstrated that immunization with peptides corresponding to the MHC class I molecules could accelerate rejection of renal allografts in rats (13, 14). Furthermore, CD4+ T cells from recipient mice could specifically proliferate in presence of these peptides and recipient APC. Conclusive evidence for the indirect pathway of allorecognition in organ rejection came from the reports of Auchincloss et al (15). They demonstrated that MHC class I deficient mice could reject skin grafts from MHC class II deficient donor mice. The recipient mice in this model lack CD8+ T cells capable of directly recognizing MHC class I of the donors. Furthermore, since the donor lacks MHC class II, direct allorecognition by recipient CD4+ T cells is excluded. Therefore, the rejection of the skin allografts in this model is mediated by indirect allorecognition pathway, namely, recognition of processed donor MHC class I antigens in the context of self-MHC class II molecules present on recipient APC by the recipient CD4+ T cells. Recent evidence also demonstrates that recipient DC can acquire and process intact donor MHC molecules from donor cell debris and stimulate CD8+ T cells by cross-priming (16). Therefore, both CD4+ and CD8+ T cells mediate indirect allorecognition.
While the direct pathway is more important for acute allograft rejection, the indirect pathway is postulated to play a dominant role in chronic allograft rejection (17, 18). This hypothesis originates from experiments demonstrating that inhibition of acute rejection by depleting passenger APC significantly delays but not prevent development of chronic rejection. The frequency of direct alloreactive T cells exceeds indirect alloreactive T cells especially in the early post-transplant period. Indeed, draining lymph node analysis demonstrated that more than 90% of allospecific T cells were of direct pathway while only 1–5% represented the indirect pathway of alloreactive T cells (18–20). However, the frequency of direct alloreactive T cells declines with time following transplantation while the continuous influx of the processed donor antigens by the recipient APC through the indirect pathways increases the number of indirect alloreactive T cells. It has also been shown that indirect alloreactive T cells are more resistant to conventional immunosuppression (21). Indeed, we and others, have demonstrated that indirect alloreactive T cells can be readily detected in the peripheral blood of human allograft recipients years after transplantation and are associated with allograft rejection (22–27).
In addition to the above two pathways, transfer of intact MHC molecules between cells has been observed (28–30). DC have been shown to acquire intact MHC class I and II molecules from exosomes secreted by other DC and prime both naïve CD8+ and CD4+ T cells (31–33). Reports from Knight and Lechler’s group observed (28–30, 34) proposed that this represents a third mode of allorecognition, which Lechler’s group has termed “semi-direct” pathway (34). Briefly, through this pathway, DC could simultaneously present intact MHC molecules to directly alloreactive CD8+ T cells as well as internalized and processed donor MHC peptides to indirect alloreactive CD4+ T cells. Further studies are required to establish the clinical significance of this pathway in rejection of organ allografts.
Role of MHC bound peptides in allorecognition
When considering the phenomenon of allorecognition two main questions emerge. First, are allopeptides truly required to elicit an alloimmune response? And secondly, what is the nature of such allopeptides? Interestingly, there is evidence that allorecognition can occur independent of MHC-bound peptides (35–37). Elliott et al denatured purified HLA-A2 protein, separated its heavy (α) and light (β-2 microglobulin) chains and then mixed them in the absence of any peptides. The reconstituted protein, deficient of any peptides, was indistinguishable from native A2 in its reactivity to a monoclonal antibody and ability to activate HLA-A2 specific CD8+ T cells (35). Smith et al isolated H2-Kb alloreactive CD8+ T cells clones from skin allograft recipients and demonstrated that some of those clones could react with peptide deficient H2-Kb molecules on T2 and RMA-S cell lines that have defective antigen processing and presentation (37). Furthermore, the reactivity of these CD8+ T cells was not altered by either eluting or adding the alloreactive peptides. However, empty MHC molecules may not have an important role in vivo since they are rare and highly unstable under physiological conditions. But recognition of peptide carrying intact MHC molecules on donor APC by recipient T cells, regardless of the nature of peptide, does seem to exist and support the determinant-density hypothesis. In a more recent study, Jankovic et al developed an engineered variant of the murine MHC class I molecule H-2Kb, called KbW9 (36). The variant was devoid of the central anchor pocket owing to a point mutation on the floor of the peptide binding site. This substitution drastically altered selection of bound peptides and therefore, the peptide repertoires of Kb and KbW9 were nonoverlapping. KbW9 readily served as a restriction element for a peptide-specific syngeneic CTL response suggesting that the mutation did not result in gross distortions of the TCR-interacting surface of class I. When KbW9 was used to stimulate allogeneic T cells, some of the CTL lines induced cross-reacted against the original Kb molecule and demonstrated peptide-independent MHC reactivity.
The peptide-dependent arm of allorecognition plays a role in both the direct and indirect pathways. It also forms the basis for the multiple binary complex model of direct allorecognition. While CD8+ T cells recognize MHC class I bound peptides, CD4+ T cells react with MHC class II peptides. MHC class I molecules preferentially bind peptides from intracellular cytoplasmic and nuclear proteins while class II molecules present peptides from cell surface and extracellular proteins. Importantly both MHC class I and class II antigens can also be presented in the context of self- and non-self MHC molecules. MHC derived peptides are highly represented amongst the naturally processed peptides bound to class I and II molecules, making them accessible to direct alloreative T cells (3). In addition, host APC can process donor MHC molecules and activate alloreactive T cells through the indirect pathway. We and others have previously demonstrated that development of indirect alloreactive T cells specific to donor MHC class I as well as class II antigens correlates with human lung allograft rejection (25–27, 38–40). Alloreactive T cells specific to mismatched donor HLA class I and class II were analyzed using limiting dilution or ELISPOT assay. As shown in Figure 2, patients with chronic human lung allograft rejction (bronchiolitis obliterans syndrome, BOS) were found to have a significantly higher frequency of alloreactive T cells (Figure 2). Moreover, the predominant alloreactive T cells were characterized to be of Th1-phenotype (27).
Figure 2.
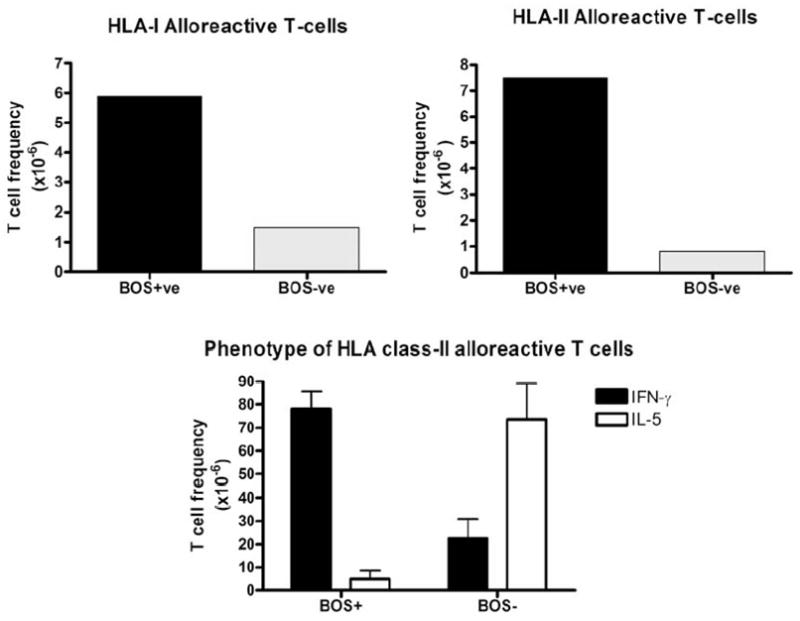
Chronic lung allograft rejection is association with an expansion of Th1-predominant mismatched donor HLA class I and II allospecific T cells
Alloreactive T cell can also recognize peptides from other (non-MHC) polymorphic loci such as HY-encoded proteins, known as minor histocompatibility antigens (MnHC). MnHC antigens are peptides derived from allelically polymorphic host proteins, other than MHC molecules. MnHC play an important role in the development of graft versus host disease after MHC-matched allogeneic bone marrow transplantation (41). MnHC have also been shown to mediate murine skin allograft (42, 43) and rat cardiac allograft rejection in MHC-matched recipients (44–46). We have previously cloned mismatched MnHC antigen specific cytotoxic CD8+ T cells from human renal allograft infiltrating cells at the time of acute rejection (47). We further characterized the role of MnHC in chronic allograft rejection using two well characterized heterotopic murine cardiac (vascularized) and tracheal (non-vascularized) allo-transplantation models. C56BL/10SnJ (H13a) cardiac allografts were transplanted into congenic B10.CE-H13b Aw(30NX)/Sn (H13b) mice (48). The H13a and H13b alleles encode the SSVVGVWYL (SVL9) and SSVIGVWYL (SIL9) MnHC antigens bound to the H2Db molecule, respectively. H13a cardiac allografts transplanted into H13b recipients were rejected while isografts survived indefinitely. Pre-transplant sensitization with donor (H13a) antigens accelerated the rejection process. Rejected cardiac allografts revealed histopathological signs of chronic rejection with diffuse mononuclear cell infiltration, concentric intimal hyperplasia, and fibrosis. While both CD4+ and CD8+ T cells were identified in the graft infiltrating cells, CD8+ T cells revealed SVL9 specific cytotoxicity under H2Db restriction and, furthermore, specifically bound to H2Db/SVL9 tetramers (Table 1). Murine heterotopic tracheal transplantation has been used as a model to investigate the pathogenesis of obliterative airway disease (OAD) following lung transplantation. H13a tracheal allografts were transplanted into congenic H13b recipients. The allografts were harvested at different times post transplantation and OAD lesions including epithelial damage, cellular infiltration, and luminal fibrosis were analyzed. In parallel experiments, mice were immunized (subcutaneous injection) or tolerized (intravenous injection) with the SVL9 or SIL9 peptide before transplantation. H13a tracheal allografts developed OAD in the H13b recipients within 90 days (Table 2). SVL9 immunization significantly accelerated the kinetics of OAD development. In contrast, tolerization using SVL9 completely abrogated the OAD development. This correlated with significant inhibition of H13a-specific CD8+ T cell cytotoxicity along with suppression of IFN-γ production (49).
Table 1.
Development of H13a cardiac allograft rejection in H13b recipients
| Group | Donor | Recipient | H13a immunization | Mean graft survival (days ± SD) |
|---|---|---|---|---|
| 1 | H13b | H13b | − | >100 |
| 2 | H13a | H13b | − | 37.0 ± 14.5 |
| 3 | H13a | H13b | + | 27.6 ± 15.9 |
Table 2.
Development of H13a tracheal allograft rejection in H13b recipients
| Donor | Recipient | Time post-Tx (days) | Treatment | OAD Lesions (% of Allografts) | ||
|---|---|---|---|---|---|---|
| Epithelial Damage | Cellular Infiltration | Lumen Occlusion | ||||
| H13a | H13b | 30 | - | 25 | 25 | 0 |
| H13a | H13b | 60 | - | 100 | 100 | 0 |
| H13a | H13b | 90 | - | 100 | 100 | 100 |
| H13b | H13b | 30 | - | 0 | 0 | 0 |
| H13b | H13b | 60 | - | 0 | 0 | 0 |
| H13b | H13b | 90 | - | 0 | 0 | 0 |
| Pre-transplant sensitization (subcutaneous injection of peptide) | ||||||
| H13a | H13b | 30 | SVL9 | 100 | 100 | 50 |
| H13a | H13b | 60 | SVL9 | 100 | 100 | 75 |
| H13a | H13b | 90 | SVL9 | 100 | 100 | 100 |
| H13a | H13b | 30 | SIL9 | 25 | 25 | 0 |
| H13a | H13b | 60 | SIL9 | 100 | 100 | 0 |
| H13a | H13b | 90 | SIL9 | 100 | 100 | 100 |
| Pre-transplant tolerization (intravenous injection of peptide) | ||||||
| H13a | H13b | 90 | SVL9 | 0 | 0 | 0 |
| H13a | H13b | 90 | SIL9 | 100 | 100 | 100 |
| BALB/c | H13b | 30 | SVL9 | 100 | 100 | 100 |
Besides the histocompatibility antigens, studies have also demonstrated the role of self-antigens in alloimmunity (50–54). Immunity against the self-antigens can again develop through the direct or indirect pathway. Each MHC allele has a very extensive array of peptides that it can present. These peptides are derived from a variety of intra- and extra-cellular protein sources. The thymic selection eliminates a significant proportion of T cells that react to self-peptides. However, different MHC alleles would present different peptide sequences from homologous proteins. Hence, the host thymus would not eliminate T cells reactive to peptides from self-proteins that are presented in the context of donor MHC. Therefore, donor passenger APC may have the potential to induce an immune response against conserved self-proteins through the direct pathway. In addition, physiological stresses (like hypoxia and ischemia-reperfusion) can significantly alter the nature of peptides presented on the MHC surface and promote the expression of rare peptides against which central tolerance may not have been achieved (55, 56). This also increases the probability of developing immunity against self-proteins. Further studies demonstrating direct alloimmune responses against peptides derived from conserved non-polymorphic proteins are required. Alternatively, the intense inflammation associated with transplantation may activate indirect auto-reactive T cells (discussed further in subsequent text). Autoreactive T cell responses to cardiac myosin (57), vimentin (58), collagen V (59–61), heat shock protein (62) have all been shown to contribute to allograft rejection. We analyzed the collagen type V (col-V) specific response longitudinally in human lung transplant patients. Col-V reactive CD4+ T cells could be detected in the peripheral blood of lung transplant recipients. Importantly, the CD4+ T recognized col-V through the indirect pathway. There was a predominance of IL-10 producing T cells (TIL-10) reactive to col-V with significantly lower levels of IFN-γ and IL-2 producing T cells (Th1-cells). The col-V specific TIL-10 cells suppressed the proliferation and expansion of col-V specific Th1-cells by IL-10 dependent but contact-independent pathways. Furthermore, during chronic lung allograft rejection there was a significant decline of TIL-10 cells with concomitant expansion of col-V-specific IFN-γ producing Th1-cells. We have also obtained evidence for the role of de novo antibodies produced against non-MHC, tissue restricted antigens in the pathogenesis of chronic allograft rejection (38, 63–66). For example, sequence analysis of a target antigen present on airway epithelial cells recognized by sera from patients with chronic lung allograft rejection identified Kα1-tubulin (manuscript under preparation) as an immunogenic protein.
Recognition of antigens by alloreactive T cells
The interactions between the T cell receptor and donor MHC during allorecognition are similar to the conventional recognition by self-MHC (67–75). The initial crystallographic studies were performed using the BM3.3 TCR and peptide pBMI bound to allogeneic class I H2-Kb (68, 69, 75). It was shown that the TCR interacted with MHC-peptide complexes in a conserved diagonal orientation across the long axis of the peptide-binding site. The interactions between the TCR and the MHC-peptide complex almost exclusively involved the β-chain CDR3. The complimentarity-determining regions CDR1 and CDR2 predominantly interacted with the α-helical portions of the MHC molecule and the contact with CDR3 segments lead to the exposure of the peptide. There seems to be degeneracy between the TCR and MHC-peptide complex interactions. This may be due to structural similarities between different MHC-peptide complexes or, alternatively, the TCR may exhibit conformational flexibility. Crystallographic studies prove that alloreactive T cells can demonstrate flexibility. For example, the 2C TCR can engage the same peptide presented in the context of self-H2Kb or H2Kbm3 by rearranging the peptide contacting CDR3 portion of TCR. Therefore, CDR3 seems to play a crucial in the reorganization of the TCR. Interestingly, it was also demonstrated that water molecules fill the empty gaps between the TCR, MHC and the peptide providing a form of “padding”. The shape of this water padding also promoted the interactions of the TCR with different MHC-peptide complexes (68, 69, 75). Allorecognition gets even more complex as conformational determinants are applied to MHC-peptide complexes (76).
Initiation of alloimmune response
The alloimmune response is postulated to be initiated in the lymphoid tissue of the recipient (77). Recipients that lacked secondary lymphoid organs were unable to reject cardiac allografts (78). However, others have challenged this view and one recent study by Zhou et al suggests that lymphoid organs may not be absolutely required for the development of alloimmunity (79). Another interesting study from Kreisel et al demonstrates that non-hemopoietic cells, vascular endothelial cells in their case, present on the allograft are sufficient to generate direct alloimmune responses and mediate allograft rejection (80).
Allorecognition, however, in the absence of an inflammatory milieu and co-stimulation may not be able to generate an alloimmune response. Following organ transplantation, activation of the recipient immune response is initiated by the surgical stress and ischemia-reperfusion injury. This is accompanied by the production of several chemokines and cytokines that leads to the recruitment of recipient immune cells into the allograft (81, 82). Several inflammatory mediators including MCP-1, IP-10, and Th1-cytokines are induced following transplantation (81, 82). These can upregulate costimulatory molecules such as CD80 and CD86, and cell adhesion molecules such as CD54 and CD58 on both donor and recipient cells including APC, epithelial, and endothelial cells (83–85). We have recently demonstrated that the early cytokine release plays a crucial role in the development of alloimmunity which may lead to allograft rejection (27). Interestingly, reports by Sayegh’s group have challenged the traditional concept of antigen presentation to T cells wherein both the MHC-peptide complex (signal 1) and co-stimulation (signal 2) are present on the same APC (86). They used B7-1/B7-2 knockout mice as donors for cardiac allografts for MHC class II knockout recipients. In this model system, the donor APC would present the MHC-peptide complex directly to the recipient T cells without any co-stimulation. In contrast, the recipient APC cannot indirectly present the donor peptides but can provide the costimulation. The cardiac allografts in these recipients were rejected as rapidly as the wild-type recipients demonstrating that the antigen and co-stimulation necessarily need not be on the same APC.
Tolerization strategies using allo-peptides
Allopeptides have been used to develop strategies to induce allograft tolerance. Some of these widely investigated include intra- or extra- thymic inoculation of the allopeptides and altered peptide ligands.
Intrathymic inoculation of allopeptides
The thymus plays a central role in the development of self-tolerance. T cells with high reactivity to antigens presented in the thymus are eliminated. On this basis it was investigated whether injection of donor antigens in the thymus would eliminate the donor alloreactive T cells. Several investigators have shown that intrathymic administration of donor antigens in various forms, including donor spleen cells, soluble MHC molecules, or allo-MHC peptides can induce prolonged tolerance to allografts. Sayegh’s group demonstrated that pre-transplant intrathymic administration of polymorphic peptides derived from the (Wistar- Furth) [WF]) RT1.Bu and RT1.Du class II MHC molecules prevented acute rejection and induced long-term graft survival in the WF-to–Lewis RT11 (LEW) kidney transplant model (87). They further demonstrated that the onset of acute rejection of cardiac allograft can be prolonged by intrathymic injection of unmodified donor splenocytes (88). Data from Hardy’s laboratory has also provided compelling evidence about the efficacy of intrathymic inoculation strategy (89–94). They demonstrated that intrathymic inoculation of donor soluble Ag (obtained from resting donor T cells using alkali-extraction) seven days prior to transplantation combined with anti-lymphocytic serum lead to indefinite and donor-specific cardiac allograft tolerance in Lewis rat recipients. The peptide inoculation was also efficacious when combined with total body radiation. The tolerized recipients specifically and permanently accepted donor-type, second-set cardiac allografts. Interestingly, thymectomy performed 7 days after peptide inoculation led to graft rejection suggesting that the early phase of induction of donor-specific tolerance is dependent on the presence of donor alloantigens in the host thymus (89–94). Flye and colleagues also demonstrated that intrathymic injection of donor antigens induces tolerance to both allo- and xeno-transplantation (95–101). Furthermore, adoptive transfer of splenocytes isolated from the tolerized recipients confers tolerance (102). Other investigators have supported these findings (103). Shirwan’s group reported that tolerization using intrathymic inoculation of donor antigens was mediated by an increased production of CD4+CD25+ regulatory T cells (104). The recipients that had received intrathymic injection of donor antigens were found to develop increase in CD4+CD25+ regulatory T cells (Tregs) within the lymphoid organs. These tolerized recipients also had significantly higher levels of IL-10. Further, Tregs from the tolerized recipients were found to suppress donor–specific proliferative responses. Importantly, depletion of Tregs from the tolerized recipients abrogated donor specific tolerance to cardiac allografts after intrathymic modulation. Other mechanisms that have been postulated to play a role in donor specific tolerance after intrathymic immunomodulation include clonal anergy, T cells deletion, and active suppression (105, 106).
Extra-thymic inoculation of allopeptides
HG Wells in 1911, made a remarkable observation that anaphylactic reactions to ovalbumin in guinea pigs could be inhibited by prior oral administration of OVA (107, 108). Subsequent systemic immunizations failed to elicit a response to OVA. Furthermore, the unresponsiveness was OVA specific. Sayegh et al tested whether oral tolerance could be achieved in the context of allo-transplantation (109, 110). They demonstrated that oral administration of MHC antigens in the form of donor splenocytes significantly inhibited the MLR reaction of unsensitized allo-recipients. Furthermore, this strategy prevented the accelerated rejection observed in cardiac allograft recipients pre-sensitized with donor skin allografts (109, 110). Kahan and Streptowski used an interesting strategy of inducing donor-specific tolerance by allochimeric MHC class I proteins bearing donor-type amino acid epitope substitutions for the host-type sequences. For example, they produced an allochimeric protein (α1H u70–77-RT1.Aa) by superimposing the nucleotides encoding last four (His70, Val73, Asn74, and Asn77) of nine (Arg62, Glu63, Gln65, Gly66, Gly69, His70, Val73, Asn74, and Asn77) polymorphic amino acids in the α1-helical region of donor-type RT1.Au onto the host RT1.Aa backbone using gene splicing and overlap extension. Intraportal injection of this allochimeric protein significantly prolonged the survival of Wistar-Furth (WF, RT1u) cardiac allografts in ACI (RT1a) rats (111, 112). They have further used allochimeric proteins via the oral route to induce allo-specific tolerance (113). More recently, Wilkes’ group reported that tolerance against fully-mismatched lung allografts could be achieved using a self-protein, collagen type V (col-V) (114). Administration of donor antigens has also been found to be effective using intragastric (115), intra-jejunal (116), transrectal, and intratracheal routes (107). The tolerance induced using such transmucosal routes has been shown to be T cell dependant as T cells, but not serum, from tolerized recipients could transfer tolerance. The regulatory T cells induced have been classified into TH3 and Tr1 that secrete TGF-β and IL-10, respectively. However, the success from using these strategies in humans has been limited due to the genetic diversity, complex tolerogenic mechanism, and differences in the pathogenesis of allograft rejection. Nevertheless, this phenomenon appears real and may have some clinical utility in the future (107).
Altered peptide ligands
In general, a peptide has one MHC binding site and another that comes in contact with the TCR. In 1991, Paul Allen’s laboratory reported a seminal observation that a cognate murine hemoglobin peptide with a TCR site-specific single amino acid alteration caused a reversible suppression of T cells (117). Subsequent studies from the same group provided compelling evidence that such altered peptide analogues (or ligands) could result in partial or complete T cells anergy (118, 119). Altered peptide ligands contain alterations in the sites of the wild-type peptide that comes in contact with the TCR. Therefore, there is no difference in the MHC binding capacity of the peptide but the alterations significantly change the outcome of TCR recognition. APL can be classified as antagonistic or partial agonistic depending upon the response they elicit. Antagonistic APL fail to activate the T cells and rather suppress the T cells response when presented with the wild-type peptide. However, the T cell response is fully reversible after re-stimulation with the wild-type agonistic peptide in the absence of antagonistic APL. In contrast, partial agonistic APL partially activate T cells, resulting in induction of anergy.
Unlike the antagonistic peptides, T cells that have been stimulated with partial agonistic peptides do not proliferate on re-stimulation with the wild-type agonistic peptide. Suciu-Foca’s group substituted multiple amino acids in the TCR binding site of wild type peptides to test the ability to generate APL (120). They examined whether analogues of the dominant determinant of HLA-DRpl*0101 molecule (peptide DR1/22–35), recognized in the context of HLA-DRj31*1101 protein, could modulate the T cell response against the wild-type peptide Ag. Using this strategy they were able to generate both antagonistic and partial agonistic APL that suppressed the T cell response to the wild-type agonistic peptide. The efficacy of APL in suppressing T cells responses has been well documented in vitro and in some autoimmune models in vivo (121). However, their efficacy in inducing transplantation tolerance in vivo needs to be further examined.
A unified model of Treg mediated modulation of allorecognition
CD4+CD25+ regulatory T cells have been identified as a dominant subset of tolerogenic T cells (122). Tregs are selected in the thymus in the context of self-peptides and predominantly recognize cognate antigens through the indirect pathway. Since natural Tregs are selected in the thymus in the context of “self-proteins”, the natural presence of allo-specific Tregs in naïve hosts is difficult to explain. Nevertheless, using animal models, allospecific Tregs have been demonstrated in naïve recipients, although at low frequency. However, considering the diversity of TCR expressed by Tregs cross-reactivity of Tregs with alloantigens cannot be excluded. Other mechanisms that may be involved in the generation of allo-specific Tregs following transplantation may include epitope-spreading from auto- to allo-antigens, TCR-affinity maturation or expansion of cross-reactive Tregs (122). In addition, as shown in Figure 3, tolerogenic properties can be imparted by Tregs into conventional T-cells (59, 60, 123, 124). Tregs impart tolerance by working through the indirect pathway and have been shown to contribute to indirect allospecific hyporesponsiveness, and more recently by us towards direct allospecific hyporesponsiveness using anti-donor MLR (59, 60, 122). In addition, autospecific Tregs were found to suppress anti-donor as well as mitogen induced proliferation of bystander host T cells thereby providing a powerful tolerogenic mechanism (59).
Figure 3.
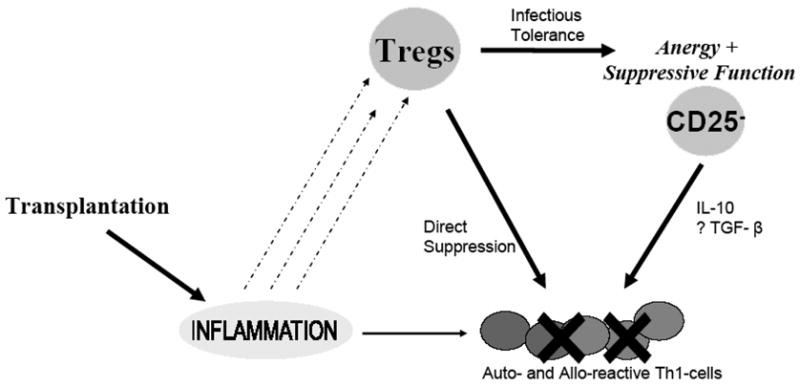
Regulation of alloimmunity by Tregs following transplantation
It has always been intriguing why, despite the presence of such a dominant subset of regulatory T cells, the most common natural consequence following transplantation is rejection. Multiple hypotheses can be postulated to explain the natural rejection of allografts in the absence of immunosuppression. Alloreactive T-cells may “overpower” Tregs in the absence of immunosuppression probably due to rapid proliferation (125), as alloreactive Tregs are present in low frequencies in the recipient compared to direct alloreactive T cells (126). Further, graft infiltrating alloreactive T-cells may induce apoptosis via CD95 in freshly recruited Tregs (127). This would mediate acute allograft rejection. However contemporary immunosuppression is highly effective on the directly alloreactive T cells and therefore has significantly reduced the incidence of acute rejection. It is interesting that immunosuppression has had a much less impact on chronic allograft rejection. It is noteworthy here that indirect alloreactive T cells are more resistant to immunosuppression. In fact, as previously discussed, we have demonstrated that indirect alloreactive T cells can be detected in human lung allograft recipients years after transplantation and correlate with the development of chronic lung allograft rejection (Figure 2). At the same time there is a constant influx of new Tregs in the recipients. However, Tregs are more sensitive to contemporary immunosuppression, specially the calcineurin inhibitors like cyclosporine and tacrolimus, since the function and survival of Tregs is dependent on interleukin-2 (128). Calcineurin inhibitors not only inhibit the development of long-term allograft tolerance (129) but can actually induce autoimmunity (130). Demirkiran et al (131) and Ciancio et al (132) demonstrated a decline in Tregs with calcineurin inhibitors in human liver and renal allograft recipients, respectively. Furthermore, we postulate that there may be additional mechanisms that favor the survival of effector T cells over Tregs.
Using a unique orthotopic tracheal transplant model for investigating the pathogenesis of BOS, we found that immediately following transplantation there was a significant loss of Tregs in the draining lymph nodes due to apoptosis, most likely induced by proliferating alloreactive T cells. However, as the recipient epithelium repopulated the tracheal allograft, there was a loss of allogeneic stimuli and restoration in the frequency of Tregs. Interestingly, respiratory viral infections were found to upregulate death receptors on airway epithelial cells in vitro that induced rapid apoptosis of Tregs (Bharat A et al, manuscript under preparation). It is noteworthy that respiratory viral infections predispose to chronic human lung allogaft rejection (133). Therefore, there are events that can lead to Treg cell death and favor proliferation of alloreactive T cells, thereby predisposing to chronic allograft rejection. Indeed, we recently found that patients with chronic human lung allograft rejection developed a significant loss of Tregs that was associated with concomitant expansion of both indirect alloreactive, and autoreactive, Th1-cells (59). Chronic allograft rejection may therefore be the “function of predominance” between (indirect) alloreactive effector T cells and Tregs (Figure 4).
Figure 4. Balance between Alloreactive T cells and Tregs in transplant outcome.
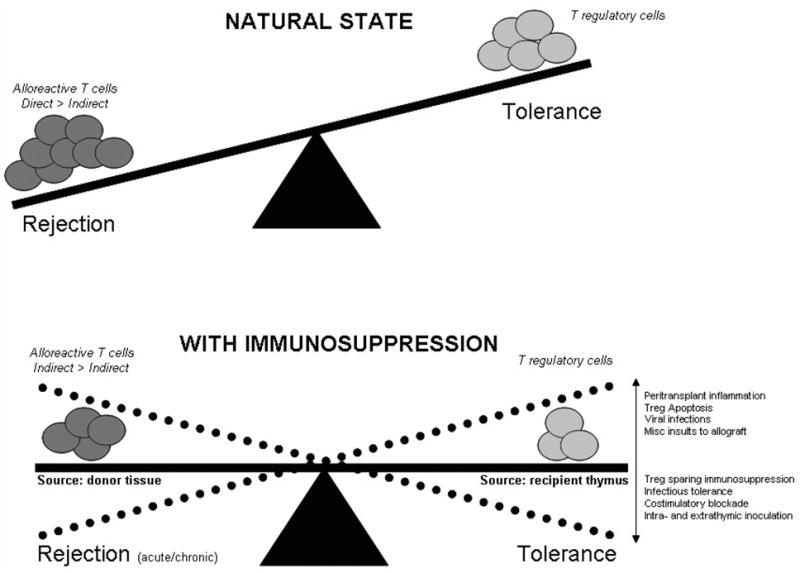
In the natural state, without any immunosuppression, there is a significantly greater frequency of directly alloreactive T cells that overcome the tolerogenic potential of Tregs and lead to allograft rejection. Administration of immunosuppression eliminates a significantly proportion of directly alloreactive T cells as well as Tregs. Nevertheless, there is influx of donor alloantigens through the indirect pathway that maintains alloreactive T cells. Since indirectly alloreactive T cells are more resistant to immunosuppression, the long-term sequelae of this influx is chronic rejection. Some important factors that can favor Tregs or alloreactive T cells following transplantation are represented.
Pathogenesis of chronic human lung allograft rejection (BOS)
We have summarized these results into a unifying hypothesis towards the immunopathogenesis of chronic rejection, ie, BOS following human lung transplantation. Following transplantation, there is an inflammatory milieu within the allograft that promotes allo-antigen presentation through both direct and indirect pathways. In addition, inflammation also leads to the release of sequestered, but immunogenic, self-antigens. Furthermore, there is epitope spreading from auto- to allo- antigens combined with activation of autoreactive “low-affinity” T cells that escaped the thymic deletion. Activation of both allo- and auto- reactive T cells promotes allograft rejection. However, inflammation also leads to the recruitment of Tregs that are suppressive in nature (Figure 3). Autospecific Tregs can inhibit both autoreactive as well as alloreactive effector T cells. In a naïve host, the combined frequency of allo- and auto-reactive T cells far exceeds Tregs and therefore, the most common natural consequence following transplantation is rejection. Immunosuppression depletes both T cells and Tregs maintaining the allograft on neutral grounds between rejection and tolerance. Following transplantation there is influx of new indirect alloreactive T cells as the recipient APC process and present the allograft antigens. Simulataneously, new Tregs originate from the central (thymic) and peripheral mechanisms. Modulating the factors that favor Treg survival would promote tolerance (Figure 4). However, if the effector T cells predominate they activate other arms of the immune system and initiate a cascade of cytokines and growth factors that ultimately lead to the development of chronic (lung) allograft rejection (Figure 5).
Figure 5.
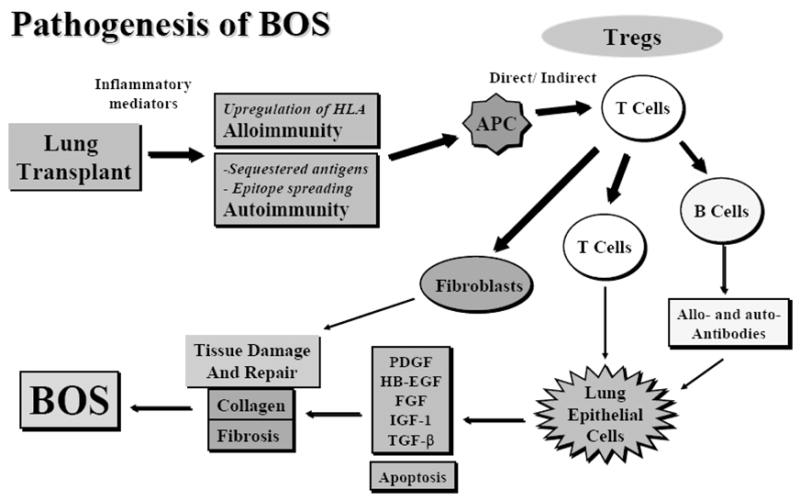
Pathogenesis of BOS
Summary
Allorecognition leads to the generation of an extremely powerful anti-donor immune response that ultimately leads to rejection of the transplanted organ. The peptides recognized by the alloreactive T cells include the polymorphic major and minor histocompatibility antigens as well as conserved self-antigens. Direct and indirect pathways form the fundamental basis of allorecognition but are not mutually exclusive. Ongoing research to further characterize the complexities within the pathways of allorecognition and the interactions between autoreactive T cells, alloreactive T cells and Tregs is imperative to promote long term success following transplantation.
Footnotes
Supported by NIH HL56543 and HL66452
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hernandez-Fuentes MP, Baker RJ, Lechler RI. The alloresponse. Rev Immunogenet. 1999;1 (3):282. [PubMed] [Google Scholar]
- 2.Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10 (2–3):101. doi: 10.1016/s0966-3274(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 3.Sherman LA, Chattopadhyay S. The molecular basis of allorecognition. Annu Rev Immunol. 1993;11:385. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 4.Portoles P, Rojo JM, Janeway CA., Jr Asymmetry in the recognition of antigen: self class II MHC and non-self class II MHC molecules by the same T-cell receptor. J Mol Cell Immunol. 1989;4 (3):129. [PubMed] [Google Scholar]
- 5.Kourilsky P, Chaouat G, Rabourdin-Combe C, Claverie JM. Working principles in the immune system implied by the “peptidic self” model. Proc Natl Acad Sci U S A. 1987;84 (10):3400. doi: 10.1073/pnas.84.10.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matzinger P, Bevan MJ. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977;29 (1):1. doi: 10.1016/0008-8749(77)90269-6. [DOI] [PubMed] [Google Scholar]
- 7.Lafferty KJ, Bootes A, Dart G, Talmage DW. Effect of organ culture on the survival of thyroid allografts in mice. Transplantation. 1976;22 (2):138. doi: 10.1097/00007890-197608000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Lechler RI, Batchelor JR. Immunogenicity of retransplanted rat kidney allografts. Effect of inducing chimerism in the first recipient and quantitative studies on immunosuppression of the second recipient. J Exp Med. 1982;156 (6):1835. doi: 10.1084/jem.156.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155 (1):31. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietra BA, Wiseman A, Bolwerk A, Rizeq M, Gill RG. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J Clin Invest. 2000;106 (8):1003. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. High levels of a major histocompatibility complex II-self peptide complex on dendritic cells from the T cell areas of lymph nodes. J Exp Med. 1997;186 (5):665. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy DB, Lo D, Rath S, et al. A novel MHC class II epitope expressed in thymic medulla but not cortex. Nature. 1989;338 (6218):765. doi: 10.1038/338765a0. [DOI] [PubMed] [Google Scholar]
- 13.Fangmann J, Dalchau R, Fabre JW. Rejection of skin allografts by indirect allorecognition of donor class I major histocompatibility complex peptides. J Exp Med. 1992;175 (6):1521. doi: 10.1084/jem.175.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fangmann J, Dalchau R, Sawyer GJ, Priestley CA, Fabre JW. T cell recognition of donor major histocompatibility complex class I peptides during allograft rejection. Eur J Immunol. 1992;22 (6):1525. doi: 10.1002/eji.1830220627. [DOI] [PubMed] [Google Scholar]
- 15.Auchincloss H, Jr, Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc Natl Acad Sci U S A. 1993;90 (8):3373. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moron G, Dadaglio G, Leclerc C. New tools for antigen delivery to the MHC class I pathway. Trends Immunol. 2004;25 (2):92. doi: 10.1016/j.it.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3 (5):525. doi: 10.1034/j.1600-6143.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 18.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162 (1):352. [PubMed] [Google Scholar]
- 19.Lindahl KF, Wilson DB. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med. 1977;145 (3):508. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166 (2):973. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 21.Sawyer GJ, Dalchau R, Fabre JW. Indirect T cell allorecognition: a cyclosporin A resistant pathway for T cell help for antibody production to donor MHC antigens. Transpl Immunol. 1993;1 (1):77. doi: 10.1016/0966-3274(93)90063-e. [DOI] [PubMed] [Google Scholar]
- 22.Susskind B, Iannotti MR, Shornick MD, Steward NS, Gorka J, Mohanakumar T. Indirect allorecognition of HLA class I peptides by CD4+ cytolytic T lymphocytes. Hum Immunol. 1996;46 (1):1. doi: 10.1016/0198-8859(95)00215-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen BP, Madrigal A, Parham P. Cytotoxic T cell recognition of an endogenous class I HLA peptide presented by a class II HLA molecule. J Exp Med. 1990;172 (3):779. doi: 10.1084/jem.172.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Essaket S, Fabron J, de Preval C, Thomsen M. Corecognition of HLA-A1 and HLA-DPw3 by a human CD4+ alloreactive T lymphocyte clone. J Exp Med. 1990;172 (1):387. doi: 10.1084/jem.172.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vella JP, Spadafora-Ferreira M, Murphy B, et al. Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation. 1997;64 (6):795. doi: 10.1097/00007890-199709270-00001. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Colovai AI, Tugulea S, et al. Indirect recognition of donor HLA-DR peptides in organ allograft rejection. J Clin Invest. 1996;98 (5):1150. doi: 10.1172/JCI118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human allograft rejection. Transplantation. 2006 doi: 10.1097/01.tp.0000250579.08042.b6. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Sharrow SO, Mathieson BJ, Singer A. Cell surface appearance of unexpected host MHC determinants on thymocytes from radiation bone marrow chimeras. J Immunol. 1981;126 (4):1327. [PubMed] [Google Scholar]
- 29.Huang JF, Yang Y, Sepulveda H, et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286 (5441):952. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 30.Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101 (7):2704. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 31.Andre F, Chaput N, Schartz NE, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172 (4):2126. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 32.Bedford P, Garner K, Knight SC. MHC class II molecules transferred between allogeneic dendritic cells stimulate primary mixed leukocyte reactions. Int Immunol. 1999;11 (11):1739. doi: 10.1093/intimm/11.11.1739. [DOI] [PubMed] [Google Scholar]
- 33.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3 (12):1156. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 34.Jiang S, Herrera O, Lechler RI. New spectrum of allorecognition pathways: implications for graft rejection and transplantation tolerance. Curr Opin Immunol. 2004;16 (5):550. doi: 10.1016/j.coi.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Elliott TJ, Eisen HN. Cytotoxic T lymphocytes recognize a reconstituted class I histocompatibility antigen (HLA-A2) as an allogeneic target molecule. Proc Natl Acad Sci U S A. 1990;87 (13):5213. doi: 10.1073/pnas.87.13.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jankovic V, Remus K, Molano A, Nikolich-Zugich J. T cell recognition of an engineered MHC class I molecule: implications for peptide-independent alloreactivity. J Immunol. 2002;169 (4):1887. doi: 10.4049/jimmunol.169.4.1887. [DOI] [PubMed] [Google Scholar]
- 37.Smith PA, Brunmark A, Jackson MR, Potter TA. Peptide-independent recognition by alloreactive cytotoxic T lymphocytes (CTL) J Exp Med. 1997;185 (6):1023. doi: 10.1084/jem.185.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaramillo A, Fernandez FG, Kuo EY, Trulock EP, Patterson GA, Mohanakumar T. Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation. Pediatr Transplant. 2005;9 (1):84. doi: 10.1111/j.1399-3046.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 39.SivaSai KS, Smith MA, Poindexter NJ, et al. Indirect recognition of donor HLA class I peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Transplantation. 1999;67 (8):1094. doi: 10.1097/00007890-199904270-00002. [DOI] [PubMed] [Google Scholar]
- 40.Lu KC, Jaramillo A, Mendeloff EN, et al. Concomitant allorecognition of mismatched donor HLA class I- and class II-derived peptides in pediatric lung transplant recipients with bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22 (1):35. doi: 10.1016/s1053-2498(02)00478-3. [DOI] [PubMed] [Google Scholar]
- 41.Goulmy E. Human minor histocompatibility antigens. Curr Opin Immunol. 1996;8 (1):75. doi: 10.1016/s0952-7915(96)80108-7. [DOI] [PubMed] [Google Scholar]
- 42.Mayer TG, Bhan AK, Winn HJ. Immunohistochemical analyses of skin graft rejection in mice. Kinetics of lymphocyte infiltration in grafts of limited immunogenetic disparity. Transplantation. 1988;46 (6):890. doi: 10.1097/00007890-198812000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Watarai Y, Koga S, Paolone DR, et al. Intraallograft chemokine RNA and protein during rejection of MHC-matched/multiple minor histocompatibility-disparate skin grafts. J Immunol. 2000;164 (11):6027. doi: 10.4049/jimmunol.164.11.6027. [DOI] [PubMed] [Google Scholar]
- 44.Koulack J, McAlister VC, MacAulay MA, Bitter-Suermann H, MacDonald AS, Lee TD. Importance of minor histocompatibility antigens in the development of allograft arteriosclerosis. Clin Immunol Immunopathol. 1996;80 (3 Pt 1):273. doi: 10.1006/clin.1996.0123. [DOI] [PubMed] [Google Scholar]
- 45.Youssef AR, Otley C, Mathieson PW, Smith RM. Role of CD4+ and CD8+ T cells in murine skin and heart allograft rejection across different antigenic desparities. Transpl Immunol. 2004;13 (4):297. doi: 10.1016/j.trim.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Karnovsky MJ, Russell ME, Hancock W, Sayegh MH, Adams DH. Chronic rejection in experimental cardiac transplantation in a rat model. Clin Transplant. 1994;8 (3 Pt 2):308. [PubMed] [Google Scholar]
- 47.Poindexter N, Shenoy S, Howard T, Flye MW, Mohanakumar T. Allograft infiltrating cytotoxic T lymphocytes recognize kidney-specific human minor histocompatibility antigens. Clin Transplant. 1997;11 (3):174. [PubMed] [Google Scholar]
- 48.Yang J, Jaramillo A, Liu W, et al. Chronic rejection of murine cardiac allografts discordant at the H13 minor histocompatibility antigen correlates with the generation of the H13-specific CD8+ cytotoxic T cells. Transplantation. 2003;76 (1):84. doi: 10.1097/01.TP.0000072013.21336.64. [DOI] [PubMed] [Google Scholar]
- 49.Higuchi T, Maruyama T, Jaramillo A, Mohanakumar T. Induction of obliterative airway disease in murine tracheal allografts by CD8+ CTLs recognizing a single minor histocompatibility antigen. J Immunol. 2005;174 (4):1871. doi: 10.4049/jimmunol.174.4.1871. [DOI] [PubMed] [Google Scholar]
- 50.Demotz S, Sette A, Sakaguchi K, Buchner R, Appella E, Grey HM. Self peptide requirement for class II major histocompatibility complex allorecognition. Proc Natl Acad Sci U S A. 1991;88 (19):8730. doi: 10.1073/pnas.88.19.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lombardi G, Sidhu S, Batchelor JR, Lechler RI. Allorecognition of DR1 by T cells from a DR4/DRw13 responder mimics self-restricted recognition of endogenous peptides. Proc Natl Acad Sci U S A. 1989;86 (11):4190. doi: 10.1073/pnas.86.11.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marrack P, Kappler J. T cells can distinguish between allogeneic major histocompatibility complex products on different cell types. Nature. 1988;332 (6167):840. doi: 10.1038/332840a0. [DOI] [PubMed] [Google Scholar]
- 53.Heath WR, Hurd ME, Carbone FR, Sherman LA. Peptide-dependent recognition of H-2Kb by alloreactive cytotoxic T lymphocytes. Nature. 1989;341 (6244):749. doi: 10.1038/341749a0. [DOI] [PubMed] [Google Scholar]
- 54.Malarkannan S, Afkarian M, Shastri N. A rare cryptic translation product is presented by Kb major histocompatibility complex class I molecule to alloreactive T cells. J Exp Med. 1995;182 (6):1739. doi: 10.1084/jem.182.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crotzer VL, Blum JS. Autophagy and intracellular surveillance: Modulating MHC class II antigen presentation with stress. Proc Natl Acad Sci U S A. 2005;102 (22):7779. doi: 10.1073/pnas.0503088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dengjel J, Schoor O, Fischer R, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci U S A. 2005;102 (22):7922. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162 (11):6836. [PubMed] [Google Scholar]
- 58.Barber LD, Whitelegg A, Madrigal JA, Banner NR, Rose ML. Detection of vimentin-specific autoreactive CD8+ T cells in cardiac transplant patients. Transplantation. 2004;77 (10):1604. doi: 10.1097/01.tp.0000129068.03900.25. [DOI] [PubMed] [Google Scholar]
- 59.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6 (8):1799. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 60.Bharat A, Fields RC, Trulock EP, Patterson GA, Mohanakumar T. Induction of IL-10 suppressors in lung transplant patients by CD4+25+ regulatory T cells through CTLA-4 signaling. J Immunol. 2006;177 (8):5631. doi: 10.4049/jimmunol.177.8.5631. [DOI] [PubMed] [Google Scholar]
- 61.Haque MA, Mizobuchi T, Yasufuku K, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169 (3):1542. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 62.Birk OS, Gur SL, Elias D, et al. The 60-kDa heat shock protein modulates allograft rejection. Proc Natl Acad Sci U S A. 1999;96 (9):5159. doi: 10.1073/pnas.96.9.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaramillo A, Naziruddin B, Zhang L, et al. Activation of human airway epithelial cells by non-HLA antibodies developed after lung transplantation: a potential etiological factor for bronchiolitis obliterans syndrome. Transplantation. 2001;71 (7):966. doi: 10.1097/00007890-200104150-00023. [DOI] [PubMed] [Google Scholar]
- 64.Carter V, Shenton BK, Jaques B, et al. Vimentin antibodies: a non-HLA antibody as a potential risk factor in renal transplantation. Transplant Proc. 2005;37 (2):654. doi: 10.1016/j.transproceed.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 65.Le Bas-Bernardet S, Hourmant M, Coupel S, Bignon JD, Soulillou JP, Charreau B. Non-HLA-type endothelial cell reactive alloantibodies in pre-transplant sera of kidney recipients trigger apoptosis. Am J Transplant. 2003;3 (2):167. doi: 10.1034/j.1600-6143.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 66.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352 (6):558. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 67.Garcia KC, Tallquist MD, Pease LR, et al. Alphabeta T cell receptor interactions with syngeneic and allogeneic ligands: affinity measurements and crystallization. Proc Natl Acad Sci U S A. 1997;94 (25):13838. doi: 10.1073/pnas.94.25.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reiser JB, Darnault C, Gregoire C, et al. CDR3 loop flexibility contributes to the degeneracy of TCR recognition. Nat Immunol. 2003;4 (3):241. doi: 10.1038/ni891. [DOI] [PubMed] [Google Scholar]
- 69.Reiser JB, Darnault C, Guimezanes A, et al. Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat Immunol. 2000;1 (4):291. doi: 10.1038/79728. [DOI] [PubMed] [Google Scholar]
- 70.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336 (6194):73. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 71.Rudolph MG, Wilson IA. The specificity of TCR/pMHC interaction. Curr Opin Immunol. 2002;14 (1):52. doi: 10.1016/s0952-7915(01)00298-9. [DOI] [PubMed] [Google Scholar]
- 72.Stewart-Jones GB, McMichael AJ, Bell JI, Stuart DI, Jones EY. A structural basis for immunodominant human T cell receptor recognition. Nat Immunol. 2003;4 (7):657. doi: 10.1038/ni942. [DOI] [PubMed] [Google Scholar]
- 73.Luz JG, Huang M, Garcia KC, et al. Structural comparison of allogeneic and syngeneic T cell receptor-peptide-major histocompatibility complex complexes: a buried alloreactive mutation subtly alters peptide presentation substantially increasing V(beta) Interactions. J Exp Med. 2002;195 (9):1175. doi: 10.1084/jem.20011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hennecke J, Wiley DC. Structure of a complex of the human alpha/beta T cell receptor (TCR) HA1.7, influenza hemagglutinin peptide, and major histocompatibility complex class II molecule, HLA-DR4 (DRA*0101 and DRB1*0401): insight into TCR cross-restriction and alloreactivity. J Exp Med. 2002;195 (5):571. doi: 10.1084/jem.20011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reiser JB, Gregoire C, Darnault C, et al. A T cell receptor CDR3beta loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity. 2002;16 (3):345. doi: 10.1016/s1074-7613(02)00288-1. [DOI] [PubMed] [Google Scholar]
- 76.Dyall R, Fremont DH, Jameson SC, Nikolic-Zugic J. T cell receptor (TCR) recognition of MHC class I variants: intermolecular second-site reversion provides evidence for peptide/MHC conformational variation. J Exp Med. 1996;184 (1):253. doi: 10.1084/jem.184.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172 (5):1483. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6 (6):686. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 79.Zhou P, Hwang KW, Palucki D, et al. Secondary lymphoid organs are important but not absolutely required for allograft responses. Am J Transplant. 2003;3 (3):259. doi: 10.1034/j.1600-6143.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 80.Kreisel D, Krupnick AS, Gelman AE, et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8 (3):233. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 81.Morita K, Miura M, Paolone DR, et al. Early chemokine cascades in murine cardiac grafts regulate T cell recruitment and progression of acute allograft rejection. J Immunol. 2001;167 (5):2979. doi: 10.4049/jimmunol.167.5.2979. [DOI] [PubMed] [Google Scholar]
- 82.el-Sawy T, Fahmy NM, Fairchild RL. Chemokines: directing leukocyte infiltration into allografts. Curr Opin Immunol. 2002;14 (5):562. doi: 10.1016/s0952-7915(02)00382-5. [DOI] [PubMed] [Google Scholar]
- 83.Smith CR, Jaramillo A, Duffy BF, Mohanakumar T. Airway epithelial cell damage mediated by antigen-specific T cells: implications in lung allograft rejection. Hum Immunol. 2000;61 (10):985. doi: 10.1016/s0198-8859(00)00175-0. [DOI] [PubMed] [Google Scholar]
- 84.Mauck KA, Hosenpud JD. The bronchial epithelium: a potential allogeneic target for chronic rejection after lung transplantation. J Heart Lung Transplant. 1996;15 (7):709. [PubMed] [Google Scholar]
- 85.Nakajima J, Ono M, Takeda M, Kawauchi M, Furuse A, Takizawa H. Role of costimulatory molecules on airway epithelial cells acting as alloantigen-presenting cells. Transplant Proc. 1997;29 (4):2297. doi: 10.1016/s0041-1345(97)00334-5. [DOI] [PubMed] [Google Scholar]
- 86.Mandelbrot DA, Kishimoto K, Auchincloss H, Jr, Sharpe AH, Sayegh MH. Rejection of mouse cardiac allografts by costimulation in trans. J Immunol. 2001;167 (3):1174. doi: 10.4049/jimmunol.167.3.1174. [DOI] [PubMed] [Google Scholar]
- 87.Sayegh MH, Perico N, Imberti O, Hancock WW, Carpenter CB, Remuzzi G. Thymic recognition of class II major histocompatibility complex allopeptides induces donor-specific unresponsiveness to renal allografts. Transplantation. 1993;56 (2):461. doi: 10.1097/00007890-199308000-00040. [DOI] [PubMed] [Google Scholar]
- 88.Binder J, Sayegh MH, Watschinger B, Hancock WW, Kupiec-Weglinski JW. Intrathymic injection of donor-specific X-irradiation-sensitive spleen cells abrogates accelerated rejection of cardiac allografts in sensitized rats. Transplantation. 1994;58 (1):80. [PubMed] [Google Scholar]
- 89.Chowdhury NC, Jin MX, Hardy MA, Oluwole SF. Donor-specific unresponsiveness to murine cardiac allografts induced by intrathymic-soluble alloantigens is dependent on alternate pathway of antigen presentation. J Surg Res. 1995;59 (1):91. doi: 10.1006/jsre.1995.1137. [DOI] [PubMed] [Google Scholar]
- 90.Ohajekwe OA, Chowdhury NC, Fiedor PS, Hardy MA, Oluwole SF. Transplantation tolerance to rat cardiac and islet allografts by posttransplant intrathymic inoculation of soluble alloantigens. Transplantation. 1995;60 (10):1139. doi: 10.1097/00007890-199511270-00014. [DOI] [PubMed] [Google Scholar]
- 91.Oluwole OO, Depaz HA, Gopinathan R, et al. Indirect allorecognition in acquired thymic tolerance: induction of donor-specific permanent acceptance of rat islets by adoptive transfer of allopeptide-pulsed host myeloid and thymic dendritic cells. Diabetes. 2001;50 (7):1546. doi: 10.2337/diabetes.50.7.1546. [DOI] [PubMed] [Google Scholar]
- 92.Oluwole SF, Chowdhury NC, Jin MX, Hardy MA. Induction of transplantation tolerance to rat cardiac allografts by intrathymic inoculation of allogeneic soluble peptides. Transplantation. 1993;56 (6):1523. doi: 10.1097/00007890-199312000-00046. [DOI] [PubMed] [Google Scholar]
- 93.Shimomura K, Hardy MA, Oluwole SF. Tolerance induction to cardiac allografts by simultaneous or sequential intrathymic inoculation of disparate alloantigens. Transplantation. 1995;60 (8):806. [PubMed] [Google Scholar]
- 94.Shimomura K, Hardy MA, Oluwole SF. Effect of simultaneous intrathymic injection of two unrelated cellular alloantigens on rat cardiac allograft survival. Transplant Proc. 1995;27 (1):127. [PubMed] [Google Scholar]
- 95.Goss JA, Nakafusa Y, Finke EH, Flye MW, Lacy PE. Induction of tolerance to islet xenografts in a concordant rat-to-mouse model. Diabetes. 1994;43 (1):16. doi: 10.2337/diab.43.1.16. [DOI] [PubMed] [Google Scholar]
- 96.Goss JA, Nakafusa Y, Uchiyama K, Flye MW. Induction of extended survival of rat skin xenografts in mice by pretreatment with intrathymic xenoantigen and antilymphocyte serum. Transplantation. 1992;54 (6):1101. [PubMed] [Google Scholar]
- 97.Nakafusa Y, Goss JA, Flye MW. Prevention by thymectomy of tolerance induced by intrathymic injection of donor splenocytes. Surgery. 1993;114 (2):183. [PubMed] [Google Scholar]
- 98.Goss JA, Nakafusa Y, Flye MW. MHC class II presenting cells are necessary for the induction of intrathymic tolerance. Ann Surg. 1993;217 (5):492. doi: 10.1097/00000658-199305010-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goss JA, Nakafusa Y, Yu S, Flye MW. Reduction in donor-specific cytotoxic T lymphocytes and prolonged cardiac allograft survival following intrathymic donor splenocyte injection. Transplant Proc. 1993;25 (1 Pt 1):286. [PubMed] [Google Scholar]
- 100.Goss JA, Nakafusa Y, Flye MW. Donor-specific cardiac allograft tolerance without immunosuppression after intrathymic injection of donor alloantigen. Transplant Proc. 1992;24 (6):2879. [PubMed] [Google Scholar]
- 101.Goss JA, Nakafusa Y, Flye MW. Intrathymic injection of donor alloantigens induces donor-specific vascularized allograft tolerance without immunosuppression. Ann Surg. 1992;216 (4):409. doi: 10.1097/00000658-199210000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takayashiki T, Asakura H, Ku G, Kataoka M, Flye MW. Infectious tolerance develops after intrathymic alloantigen-induced acceptance of rat heart allografts can be adoptively transferred. Surgery. 2005;138 (2):254. doi: 10.1016/j.surg.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Tamura S, Beck Y, Ando Y, Tahara H. Intrathymic inoculation of donor HLA class I-derived peptide generates donor-specific CD4+CD25+ regulatory T cells. Transplant Proc. 2005;37 (1):40. doi: 10.1016/j.transproceed.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 104.Koksoy S, Elpek KG, Yolcu ES, Shirwan H. Tolerance to rat heart grafts induced by intrathymic immunomodulation is mediated by indirect recognition primed CD4+CD25+ Treg cells. Transplantation. 2005;79 (11):1492. doi: 10.1097/01.tp.0000159870.01567.02. [DOI] [PubMed] [Google Scholar]
- 105.Stadlbauer TH, Schaub M, Magee CC, Kupiec-Weglinski JW, Sayegh MH. Intrathymic immunomodulation in sensitized rat recipients of cardiac allografts: requirements for allorecognition pathways. J Heart Lung Transplant. 2000;19 (6):566. doi: 10.1016/s1053-2498(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 106.Ali A, Garrovillo M, Oluwole OO, et al. Mechanisms of acquired thymic tolerance: induction of transplant tolerance by adoptive transfer of in vivo allomhc peptide activated syngeneic T cells. Transplantation. 2001;71 (10):1442. doi: 10.1097/00007890-200105270-00015. [DOI] [PubMed] [Google Scholar]
- 107.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 108.Wells HG. Studies on the chemistry of anaphylaxis III. Experiments with isolated proteins, specially those of the hen’s eff. J Infect Dis. 1911;(8):147. [Google Scholar]
- 109.Sayegh MH, Zhang ZJ, Hancock WW, Kwok CA, Carpenter CB, Weiner HL. Down-regulation of the immune response to histocompatibility antigens and prevention of sensitization by skin allografts by orally administered alloantigen. Transplantation. 1992;53 (1):163. doi: 10.1097/00007890-199201000-00033. [DOI] [PubMed] [Google Scholar]
- 110.Hancock WW, Sayegh MH, Zhang ZJ, Kwok CA, Weiner HL, Carpenter CB. Oral immunization with allogeneic splenocytes inhibits development of accelerated but not acute rejection of cardiac grafts: analysis of intragraft effector mechanisms. Transplant Proc. 1992;24 (1):250. [PubMed] [Google Scholar]
- 111.Wang M, Stepkowski SM, Yu J, Wang M, Kahan BD. Localization of cryptic tolerogenic epitopes in the alpha1-helical region of the RT1.u alloantigen. Transplantation. 1997;63 (10):1373. doi: 10.1097/00007890-199705270-00001. [DOI] [PubMed] [Google Scholar]
- 112.Stepkowski SM, Wang M, Langowski J, et al. Localization of tolerogenic epitopes in the alpha 1 helical region of the rat class I major histocompatibility complex molecule. Transplant Proc. 1997;29 (3):1663. doi: 10.1016/s0041-1345(97)00006-7. [DOI] [PubMed] [Google Scholar]
- 113.Stepkowski SM, Yu J, Wang M, Kahan BD. Induction of tolerance by oral administration of a tolerogenic allochimeric donor/recipient class I MHC protein. Transplant Proc. 1999;31 (3):1557. doi: 10.1016/s0041-1345(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 114.Yasufuku K, Heidler KM, O’Donnell PW, et al. Oral tolerance induction by type V collagen downregulates lung allograft rejection. Am J Respir Cell Mol Biol. 2001;25 (1):26. doi: 10.1165/ajrcmb.25.1.4431. [DOI] [PubMed] [Google Scholar]
- 115.Dettino AL, Duarte AJ, Sato MN. Induction of oral tolerance and the effect of interleukin-4 on murine skin allograft rejection. Braz J Med Biol Res. 2004;37 (3):435. doi: 10.1590/s0100-879x2004000300022. [DOI] [PubMed] [Google Scholar]
- 116.Ishido N, Matsuoka J, Matsuno T, Nakagawa K, Tanaka N. Induction of donor-specific hyporesponsiveness and prolongation of cardiac allograft survival by jejunal administration of donor splenocytes. Transplantation. 1999;68 (9):1377. doi: 10.1097/00007890-199911150-00026. [DOI] [PubMed] [Google Scholar]
- 117.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252 (5010):1308. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 118.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363 (6425):156. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 119.Evavold BD, Sloan-Lancaster J, Hsu BL, Allen PM. Separation of T helper 1 clone cytolysis from proliferation and lymphokine production using analog peptides. J Immunol. 1993;150 (8 Pt 1):3131. [PubMed] [Google Scholar]
- 120.Colovai AI, Liu Z, Harris PE, Cortesini R, Suciu-Foca N. Allopeptide-specific T cell reactivity altered by peptide analogs. J Immunol. 1997;158 (1):48. [PubMed] [Google Scholar]
- 121.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 122.Bharat A, Fields RC, Mohanakumar T. Regulatory T cell-mediated transplantation tolerance. Immunol Res. 2005;33 (3):195. doi: 10.1385/IR:33:3:195. [DOI] [PubMed] [Google Scholar]
- 123.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196 (2):247. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196 (2):255. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanchez-Fueyo A, Weber M, Domenig C, Strom TB, Zheng XX. Tracking the immunoregulatory mechanisms active during allograft tolerance. J Immunol. 2002;168 (5):2274. doi: 10.4049/jimmunol.168.5.2274. [DOI] [PubMed] [Google Scholar]
- 126.Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4(+)CD25(+) and CD4(+)CD25(−) regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002;168 (11):5558. doi: 10.4049/jimmunol.168.11.5558. [DOI] [PubMed] [Google Scholar]
- 127.Fritzsching B, Oberle N, Eberhardt N, et al. Cutting edge: In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand- but not to TCR-mediated cell death. J Immunol. 2005;175 (1):32. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 128.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196 (6):851. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smith CR, Mohanakumar T, Shimizu Y, et al. Brief cyclosporine treatment prevents intrathymic (IT) tolerance induction and precipitates acute rejection in an IT rat cardiac allograft model. Transplantation. 2000;69 (2):294. doi: 10.1097/00007890-200001270-00016. [DOI] [PubMed] [Google Scholar]
- 130.Sakaguchi S, Sakaguchi N. Thymus and autoimmunity. Transplantation of the thymus from cyclosporin A-treated mice causes organ-specific autoimmune disease in athymic nude mice. J Exp Med. 1988;167 (4):1479. doi: 10.1084/jem.167.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Demirkiran A, Kok A, Kwekkeboom J, Metselaar HJ, Tilanus HW, van der Laan LJ. Decrease of CD4+CD25+ T cells in peripheral blood after liver transplantation: association with immunosuppression. Transplant Proc. 2005;37 (2):1194. doi: 10.1016/j.transproceed.2004.12.095. [DOI] [PubMed] [Google Scholar]
- 132.Ciancio G, Burke GW, Gaynor JJ, et al. A Randomized Trial of Three Renal Transplant Induction Antibodies: Early Comparison of Tacrolimus, Mycophenolate Mofetil, and Steroid Dosing, and Newer Immune-Monitoring1. Transplantation. 2005;80 (4):457. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- 133.Khalifah AP, Hachem RR, Chakinala MM, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170 (2):181. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]


