Abstract
Coronary endothelial dysfunction is a powerful prognostic marker in patients with coronary artery disease (CAD) that is centrally related to oxidative inhibition of nitric oxide (NO)-dependent vascular cell signaling. Xanthine oxidase (XO), which both binds to and is expressed by endothelial cells, generates superoxide and hydrogen peroxide upon oxidation of purines. Whether inhibition of xanthine oxidase activity results in improved coronary vasomotor function in patients with CAD, however, remains unknown. We assessed coronary and peripheral (brachial artery) endothelial function in 18 patients (pts; 65 ± 8 years, 86% male) with angiographically documented CAD, preserved left ventricular function, and nonelevated uric acid levels (233 ± 10 μM). Patients received incremental doses of intracoronary acetylcholine (ACh; 10−7 to 10−5 μM), and minimal lumen diameter (MLD) and coronary blood flow (CBF) were assessed before and after intravenous administration of oxypurinol (200 mg). Oxypurinol inhibited plasma XO activity 63% (0.051 ± 0.001 vs 0.019 ± 0.005 μU/mg protein; p < 0.01). In pts who displayed endothelial dysfunction as evidenced by coronary vasoconstriction in response to ACh (n = 13), oxypurinol markedly attenuated ACh-induced vasoconstriction (−23 ± 4 vs −15 ± 4% at ACh 10−5 μM, p < 0.05) and significantly increased CBF (16 ± 17 vs 62 ± 18% at ACh 10−5 μM, p < 0.05), whereas in patients with preserved coronary endothelial function, oxypurinol had no effect on ACh-dependent changes in MLD (+2.8 ± 4.2 vs 5.2 ± 0.7%, p > 0.05) or CBF (135 ± 75 vs 154 ± 61%, p > 0.05). Flow-mediated dilation of the brachial artery, assessed in eight consecutive patients, increased from 5.1 ± 1.5 before to 7.6 ± 1.5% after oxypurinol administration (p < 0.05). Oxypurinol inhibition of XO improves coronary vascular endothelial dysfunction, a hallmark of patients with CAD. These observations reveal that XO-derived reactive oxygen species significantly contribute to impaired coronary NO bioavailability in CAD and that XO inhibition represents an additional treatment concept for inflammatory vascular diseases that deserves further investigation.
Keywords: Superoxide, Oxidative stress, Allopurinol, Endothelium, Perfusion, Free radicals
The pathophysiology underlying coronary artery disease (CAD) has been firmly linked to reduced bioavailability of endothelial-derived nitric oxide [1]. Nitric oxide, constitutively synthesized by endothelial cells, not only prevents leukocyte and platelet aggregation, smooth muscle proliferation, and lipid oxidation, but also elicits potent vasodilatory properties by activating smooth muscle cell guanylate cyclase [2]. Vascular nitric oxide (NO) bioavailability is under exquisite control of vessel wall-generated free radicals, with oxidant-producing, enzyme-mediated consumption of NO by superoxide and hydrogen peroxide-dependent mechanisms being viewed as one of the principal mechanisms accounting for endothelial dysfunction [3,4]. Whereas the chemical reactions responsible for NO catabolism are well characterized, the biologically relevant sources for superoxide in CAD remain to be defined. Multiple vascular superoxide- and hydrogen peroxide-generating systems have been identified, such as the phagocytic and vascular NADPH oxidase and its homologues, uncoupled endothelial NO synthase, mitochondria, platelets, and xanthine oxidase (XO) [5–10]. Xanthine oxidase, a molybdopterin-containing flavoprotein, displays increased circulating levels and both readily binds to and is expressed by vascular endothelium in a variety of inflammatory diseases. At this site critical for NO-dependent signaling, XO can generate superoxide and hydrogen peroxide upon purine oxidation [11]. Experimental studies in hypercholesterolemic rabbits have demonstrated that oxypurinol improves endothelium-dependent vasodilation by reducing vascular steady-state superoxide levels [12–14]. In clinical studies, inhibition of XO by allopurinol and its XO-inhibitory metabolite oxypurinol improved forearm blood flow in smokers and patients with heart failure, diabetes mellitus, and hypercholesterolemia, suggesting that XO significantly contributes to vascular NO catabolism in human disease [15–18]. Thus, we hypothesized that XO inhibition would improve coronary and peripheral endothelial function in patients with angiographically documented CAD, preserved left ventricular function, and physiologic uric acid levels. To this end, we investigated the effects of the XO inhibitor oxypurinol on minimal lumen diameter (MLD) and coronary blood flow (CBF) in response to acetylcholine (ACh), as well as flow-dependent vasodilation of the brachial artery.
Material and methods
Study design
The study was approved by the Ethics Committee of the Hamburg Medical Board and every patient had to give written informed consent. The trial was designed as an open-label, prospective nonrandomized study, which included patients with angiographically documented CAD and preserved left ventricular function. Main exclusion criteria were unstable coronary artery disease or myocardial infarction within 2 weeks before study entry, previous coronary bypass surgery, significant valvular disease, an ejection fraction of <40%, hypotension, uncontrolled hypertension, creatinine 1.5 times the upper limit of normal, hyperuricemia (>351 μM in women and >422 μM in men), current allopurinol intake or known allopurinol intolerance, and intravenous heparin within the last 24 h before the study. The index artery (left anterior descending artery or the circumflex artery) displayed a percentage stenosis of ≤40%.
The study included 18 patients (pts) who completed the protocol and were analyzed. The majority of pts were diagnosed for hypertension and hyperlipoproteinemia, and almost 80% of the pts were on angiotensin-converting enzyme inhibitors/angiotensin receptor antagonists and/or HMG CoA reductase inhibitors (Table 1). Vasoactive medication such as nitrates or calcium antagonists was withheld for 24 h before the procedure.
Table 1.
Baseline clinical characteristics of study population (n = 18)
| Characteristic | |
|---|---|
| Age (years) | 65 ± 8 |
| Male (n) | 96% (17) |
| Ejection fraction | 62 ± 15% |
| Arterial hypertension (n) | 86% (15) |
| Hypercholesterolemia (n) | 86% (15) |
| Diabetes mellitus (n) | 18% (3) |
| Current smoker (n) | 32% (6) |
| LDL cholesterol (mg/dl) | 126 ± 26 |
| HMG CoA reductase inhibitors (n) | 76% (14) |
| ACE inhibitors (n) | 68% (15) |
| AT-1 receptor blockers (n) | 9% (2) |
After coronary angiography (n = 3) or percutaneous angioplasty (n = 19), both performed by omitting nitroglycerin, vasoactive medication, and/or glycoprotein IIb/IIIa receptor antagonists, a 3F infusion catheter and a 0.018-in. Doppler flow wire (Cardiometrics, Mountain View, CA, USA) were positioned in the proximal left anterior descending (32%) or circumflex artery (68%) over a 7F guiding catheter. The patients received 7000 ± 2000 IU of unfractionated heparin at least 10 min before study entry and no additional heparin thereafter. Acetylcholine (Miochol, Ciba Vision), dissolved in NaCl to give solutions of 10−7, 10−6, and 10−5 μM, was infused into the target vessel at 2 ml · min−1 for 3 min. At baseline and after each infusion, heart rate, blood pressure, and average peak velocity were recorded, and a further angiography of the target vessel was performed as done previously [19]. After an ACh washout phase of 10 min, oxypurinol (200 mg · 100 ml−1) was infused over the femoral vein at 13 mg · min−1. The study medication was prepared aseptically on campus by dissolving oxypurinol (Cardiome Pharmaceuticals, Vancouver, BC, Canada) in glucose 5% and sodium hydroxide (0.07 N) to a final pH of 9.7. All preparations were used within 8 h of preparation. After oxypurinol infusion, measurements were repeated as above and intracoronary administration of nitroglycerine (200 μg) was performed (Fig. 1). Immediately before and after oxypurinol infusion, blood was sampled and plasma stored at −80°C for enzyme, metabolite, and drug analysis. At day 30 after hospital discharge, all patients were followed up by telephone.
Fig. 1.
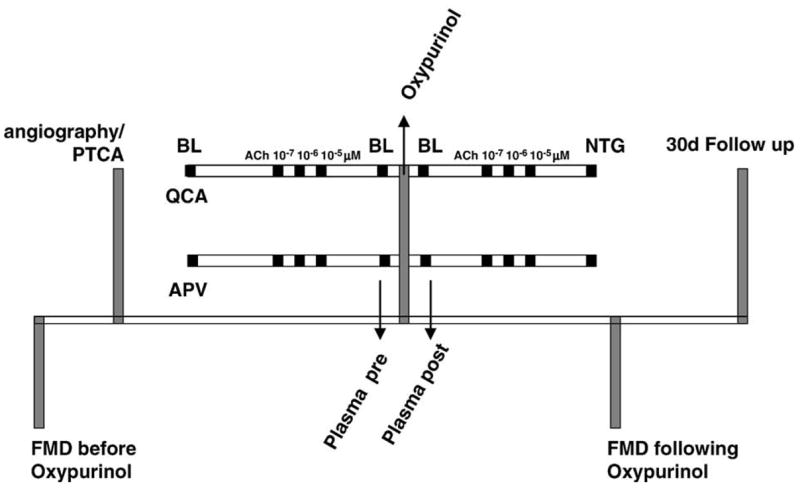
Schematic outline of the study design. Patients, who had undergone either diagnostic angiography or angioplasty, underwent assessment of coronary endothelial function by analysis of coronary lumen diameter (QCA) and average peak velocity (APV) of the coronary flow assessed by Doppler flow wire. After a baseline measurement of infused saline (BL), the patients received ACh (acetylcholine), which was infused in incremental concentrations of 10−7, 10−6, and 10−5 μM intracoronary at 2 ml • min−1 for 3 min. At each concentration, QCA and APV were recorded. Subsequently, patients received oxypurinol (intravenous infusion of 200 mg oxypurinol over 15 min) and the ACh challenge was repeated. Intracoronary administration of nitroglycerine (NTG; 200 μg) completed the study. Plasma was sampled immediately before and after oxypurinol infusion. In eight consecutive patients, noninvasive testing of forearm endothelial function by flow-mediated dilation (FMD) of the brachial artery was done immediately before and within 30 min upon termination of catheterization procedure. The 30-day follow up was performed by telephone contact.
Assessment of XO activity and plasma levels of oxypurinol, xanthine, hypoxanthine, and uric acid
Xanthine oxidase activity was determined by measurement of uric acid formation via HPLC with diode array detection. A chromatography elution scheme, using a 25 × 5-mm C18 column, was devised to permit the baseline resolution of added xanthine, uric acid, and oxypurinol. This consisted of a 30-min isocratic elution with KH2PO4 (300 mM, adjusted to pH 4.0):methanol:acetonitrile:tetrahydrofuran (97.9:1:1:0.1, v/v). The flow rate was 1 ml/min and the column temperature was maintained at 30°C. An eight-channel electrochemical CoulArray detector (ESA Chelmsford, USA) with applied potentials of 0, 120, 220, 300, 575, 700, 800, and 900 mV was used for analysis. Every five samples, an internal standard mixture of oxypurinol, xanthine, and uric acid was resolved chromatographically for calculating inhibitor and purine concentrations in patient plasma. All samples were run in duplicate and averaged and means were combined for statistical analysis. The potentially confounding effects of plasma uricase activity were separately determined by addition of known concentrations of uric acid to plasma samples. Subsequent plasma analysis by HPLC–diode array detection monitoring revealed no detectable loss of uric acid over time.
Measurement of coronary artery diameter and flow
All angiograms were evaluated by an independent blinded investigator on a Siemens workstation for computerized quantitative coronary angiography with an automated contour detection (Siemens, Erlangen, Germany). All angiograms of the patients who completed the study were analyzed. However, in accordance with previous studies assessing coronary vasomotion [19], only those pts who displayed endothelial dysfunction as evidenced by coronary vasoconstriction in response to ACh were included into the final analysis. Siemens Quantcor CCA software version 4.0 was used. Adjustments were performed by manual correction if necessary [19,20]. Analysis of vessel luminal diameter was performed at baseline, after ACh challenge, before and after oxypurinol administration, and after application of intracoronary nitroglycerine (200 μg). Calculated indices of coronary blood flow included average peak velocity and luminal diameter 5 mm distal to the tip of the flow wire, as done previously [21].
Assessment of forearm flow-mediated dilation
Endothelium-dependent, flow-mediated dilation of the brachial artery was noninvasively determined in a subset of patients as done previously [22]. In brief, images of the right brachial artery and pulsed-Doppler flow velocity signals were obtained with an ATL 7.5- to 12-MHz linear array transducer and an ATL HDI5000 ultrasound system (Philips, Da Best, The Netherlands). Assessment of brachial artery diameter and pulse-Doppler velocity signals was followed by 5 min of brachial artery occlusion and one further analysis of brachial artery diameter and pulse-Doppler velocity 60 s thereafter. Brachial artery diameters were analyzed via edge detection software (Brachial Analyzer; Medical Imaging Application, Iowa City, IA, USA), with flow-mediated dilation calculated as the percentage change in brachial artery diameter in response to hyperemia.
Statistical analysis
Changes in MLD and CBF are given as percentage change compared to baseline ± SEM. Oxypurinol plasma levels, XO activity, and xanthine, hypoxanthine, and uric acid plasma levels are given as means ± SEM. Differences in luminal diameter and coronary flow before and after oxypurinol were assessed by applying the paired Student t test. Differences of p < 0.05 were considered statistically significant.
Results
Of the 18 pts, 13 revealed coronary vasoconstriction in response to the highest dose of ACh administered (ACh 10−5 μM in n = 11 and ACh 10−6 μM in n = 2), whereas 5 pts displayed coronary vasodilation in response to ACh 10−5 μM and were analyzed separately. Administration of oxypurinol was tolerated by every patient without notification of any adverse event during the hospital stay. Within 30 days, 1 patient underwent reangiography without need for subsequent revascularization.
Plasma levels of oxypurinol and purine metabolites
After infusion of oxypurinol, plasma levels increased from 0.03 ± 0.03 to 114.4 ± 34.2 μM (Table 2; p < 0.01). Accordingly, plasma XO activity was reduced by 63% (p < 0.01, Fig. 2). There was neither a significant difference in baseline plasma XO activity between patients who revealed coronary vasoconstriction and patients who displayed vasodilation in response to ACh nor a significant difference in the extent of inhibition of the enzyme by oxypurinol (data not shown). Xanthine and uric acid levels remained unchanged before and after oxypurinol administration, whereas hypoxanthine levels increased after oxypurinol infusion (Table 2; p < 0.001).
Table 2.
Plasma levels of purine metabolites and oxypurinol before and after oxypurinol administration
| Pre-oxypurinol | Post-oxypurinol | p | |
|---|---|---|---|
| Uric acid (μM) | 233 ± 9.8 | 240 ± 10 | ns |
| Xanthine (μM) | 0.47 ± 0.1 | 0.5 ± 0.1 | ns |
| Hypoxanthine (μM) | 3.6 ± 0.3 | 6.3 ± 0.7 | <0.001 |
| Oxypurinol (μM) | 0.03 ± 0.03 | 114.4 ± 34.2 | <0.01 |
Data from n = 18 patients are presented as means ± SEM.
Fig. 2.
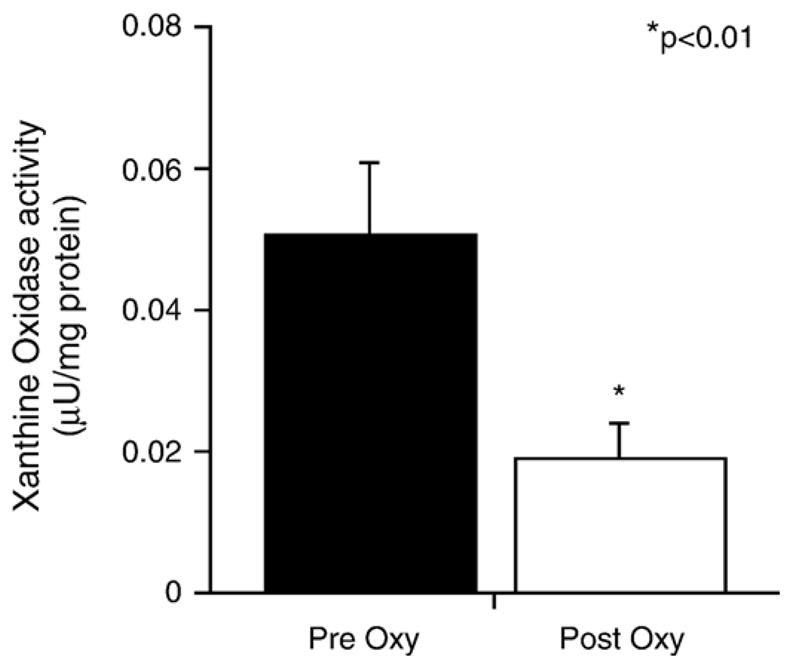
Analysis of plasma xanthine oxidase activity. After oxypurinol, xanthine oxidase activity decreased by 63% from 0.051 ± 0.001 to 0.019 ± 0.005 μU/mg protein (*p < 0.01). Data from 18 patients are presented as means ± SEM.
Coronary vascular function tests
In pts displaying vasoconstriction in response to the highest dose of ACh oxypurinol markedly attenuated ACh-dependent vasoconstriction (−23 ± 4 vs −15 ± 4% change from baseline, p < 0.05; Fig. 3a) and increased CBF (16 ± 17 vs 62 ± 18% change from baseline, p < 0.05; Fig 3b). In pts who showed vasodilation in response to ACh, oxypurinol had no effect on ACh-dependent vasoreactivity (+2.8 ± 4.2 vs 5.2 ± 0.7% change from baseline at ACh 10−5 μM, p > 0.05) nor on CBF (135 ± 75 vs 154 ± 61% change from baseline, p > 0.05). There was no effect of oxypurinol on baseline vessel diameters or blood flow in both groups and no difference in the response to nitroglycerine between patients who revealed vasoconstriction compared to those who responded to ACh by vasodilation (3.4 ± 1.8 vs 1.9 ± 1.22%; p > 0.05).
Fig. 3.
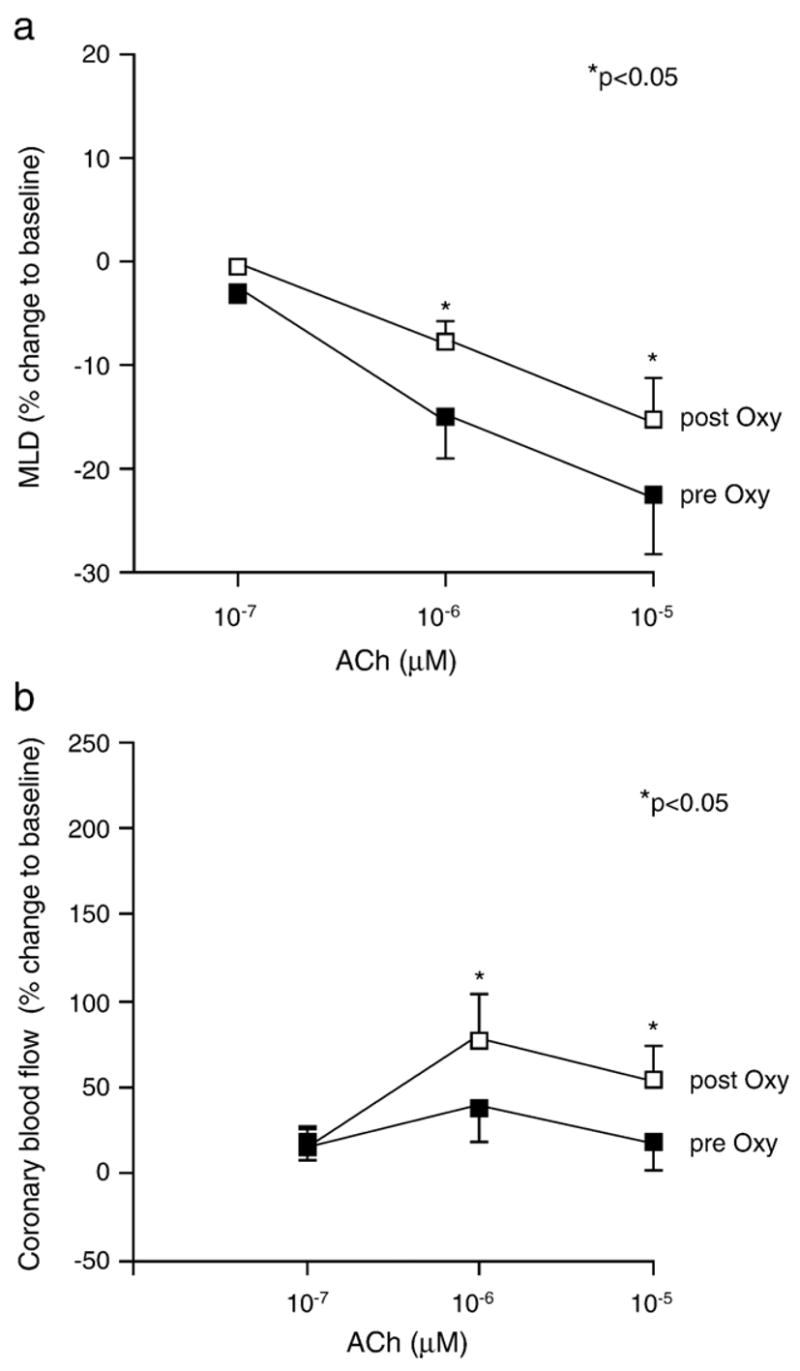
Changes in minimal coronary lumen diameter and coronary blood flow after oxypurinol administration. (a) Oxypurinol attenuated the ACh-dependent decrease in MLD in pts (n = 13) who displayed coronary vasoconstriction in response to ACh (−23 ± 4 vs −15 ± 4% at ACh 10−5 μM, p < 0.05). (b) Also, coronary blood flow increased significantly after oxypurinol infusion (16 ± 17 vs 62 ± 18% at ACh 10−5 μM, p < 0.05). In pts who showed vasodilation in response to ACh, oxypurinol had no effect on ACh-dependent vasoreactivity (+2.8 ± 4.2 vs 5.2 ± 0.7% change from baseline at ACh 10−5 μM, p > 0.05) nor on CBF (135 ± 75 vs 154 ± 61% change from baseline, p > 0.05).
Forearm flow-mediated dilation
Eight consecutive patients also underwent assessment of flow-mediated dilation and hyperemic brachial artery flow before the procedure and after the coronary intervention. Flow-dependent dilation of the brachial artery increased from 5.1 ± 1.5 before to 7.6 ± 1.5% after oxypurinol administration (p < 0.05, Fig. 4).
Fig. 4.
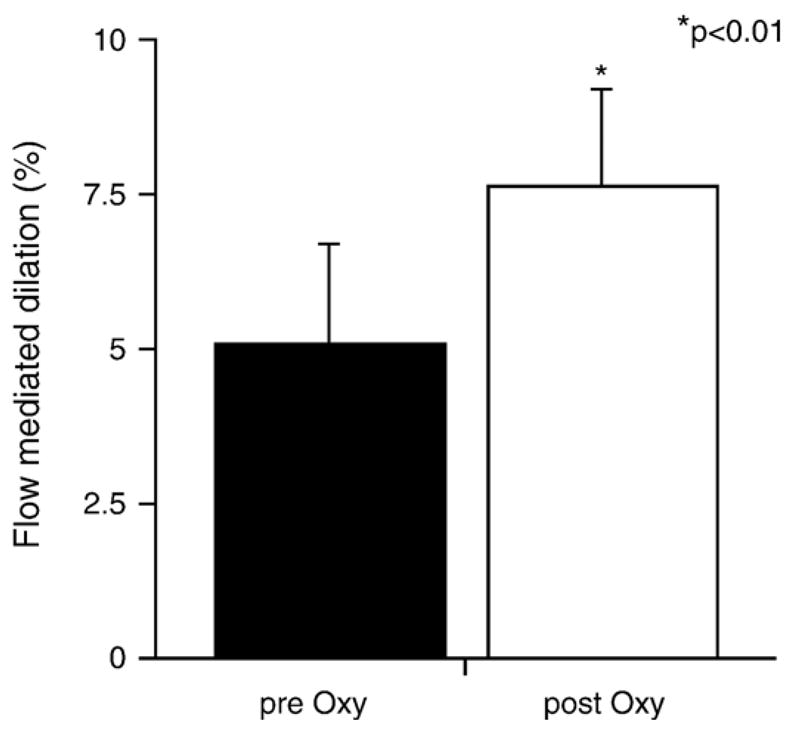
Noninvasive assessment of endothelial function in response to oxypurinol. Flow-mediated dilation of the brachial artery was measured before coronary procedure and immediately after the coronary intervention in eight consecutive patients. Forearm-mediated dilation significantly increased after acute oxypurinol treatment (5.1 ± 1.5 vs 7.6 ± 1.5%, p < 0.01).
Discussion
The present studies suggest that XO-derived reactive species contribute to impaired coronary and peripheral endothelial function of patients with stable coronary disease. Inhibition of XO via systemic administration of oxypurinol to patients attenuated ACh-induced coronary vasoconstriction (Fig. 3a) and improved myocardial perfusion (Fig. 3b).
Xanthine oxidase is abundant in vascular endothelium and plasma of CAD patients [23] (Fig. 2). Circulating XO binds avidly to endothelial cells and undergoes transcytosis into the subendothelial space [24,25]. Conditions inherent in CAD enhance XO gene expression in endothelial cells, including hypoxia and turbulent flow [26,27]. In atherosclerotic plaques, uric acid levels were found to be elevated up to sixfold, reflecting accelerated purine oxidation and suggesting that local manifestations of XO oxidant production are not necessarily reflected by systemic levels of XO metabolites [28].
When converted into its oxidase form via partial proteolysis or intramolecular thiol oxidation, xanthine oxidoreductase reduces molecular oxygen to both superoxide and hydrogen peroxide during purine oxidation [29]. The endothelial distribution of XO and the oxidative milieu that this enzyme generates predispose vascular NO catabolism. Xanthine oxidase-derived superoxide rapidly reacts at diffusion-limited rates with NO (2 × 1010 mol−1 s−1) to yield the secondary oxidant peroxynitrite [30], thus impairing NO signaling. The vasodilatory actions of oxypurinol can also reflect attenuated catalytic activity of another class of hydrogen peroxide-dependent NO-oxidizing enzymes, leukocyte myeloperoxidase and other heme peroxidases [31,32]. In particular, myeloperoxidase is distributed in the vessel wall of CAD patients in a manner similar to XO, where it can catalytically consume NO via both direct reactions and the production of secondary NO-reactive radical species. By virtue of oxypurinol-dependent inhibition of XO, local steady-state concentrations of not only superoxide, but also hydrogen peroxide, are suppressed, thus limiting multiple mechanisms of catalytic NO consumption in this vascular region critical for NO-dependent vascular relaxation [33–36].
Xanthine oxidase-dependent oxidative inhibition of vascular function has been observed in animal models of sickle cell disease, ischemia–reperfusion injury, hypertension, atherosclerosis, and hypercholesterolemia [10,11, 13,14,23,37,38]. Clinical studies of peripheral vascular blood flow corroborated these findings in patients with hypercholesterolemia, cardiomyopathy, and diabetes and in smokers [29]. However, all of these investigations studied the effects of XO inhibition in the systemic circulation, thus the clinically significant contribution of XO to impaired coronary endothelial function has remained elusive. The present observations suggest that XO-derived reactive species catalyze NO catabolism in the coronary circulation and that oxypurinol-mediated inhibition of XO attenuates ACh-dependent vasoconstriction of conductance vessels (Fig. 3a) and profoundly increases cardiac perfusion. These observations also strongly support that XO is distributed in the microcirculation (Fig. 3b), a critical determinant of resistance to cardiovascular and systemic blood flow [34].
After intravenous infusion of oxypurinol, plasma XO activity was reduced by about 65% (Fig. 2). This suggests that the net contribution of XO-derived reactive oxygen metabolites to coronary NO catabolism may be underestimated and that administration of higher levels of oxypurinol would cause a greater extent of XO inhibition and improvement of coronary endothelial function. This precept is reinforced by studies of endothelial bound, immobilized XO, a physical disposition that induces higher inhibition constants of oxypurinol for immobilized XO versus XO free in solution [39]. Moreover, all patients received intra-arterial heparin before initiation of the study. Because heparin administration induces the release of vessel-bound XO into the plasma of patients with coronary artery disease, vessel wall NO catabolism by XO may be even more pronounced in the absence of heparin [40].
Interestingly, oxypurinol still displayed vasodilatory effects in the majority of patients treated with angiotensin-converting enzyme and HMG CoA reductase inhibitors, agents which have been shown to improve vascular NO bioavailability by reducing vascular superoxide levels and stimulating NO release. Thus XO inhibition may represent an independent and additive mechanism that increases vascular NO bioavailability, an event that translates into improved endothelial function in CAD [41,42].
The current study is limited by its small size and its nonrandomized and nonblinded design. However, given the significant improvement of coronary endothelial function in a population of patients (a) managed according to current drug treatment guidelines, (b) challenged with heparin, and (c) possibly having submaximal inhibition of XO, these results affirm two key points. First, there is a pathophysiological contribution of XO-derived reactive oxygen species in vascular dysfunction of coronary disease patients and second, inhibition of XO restores more normal vascular function in coronary disease patients. In light of the powerful prognostic information gained from coronary endothelial function, larger randomized trials on chronic XO inhibition are warranted to further explore the therapeutic implications of this treatment strategy in ischemic heart disease.
Acknowledgments
This study was supported by a research grant from Cardiome Pharmaceuticals, Canada (to Drs. Baldus and Freeman), the Deutsche Forschungsgemeinschaft (to Dr. Baldus), and the NIH (to Dr. Freeman, HL58115, HL64937). The authors thank Antonia Cortes, Stefanie Beyer, and Caroline Klemme for expert technical assistance. Dr. Baldus has received lecture fees from Cardiome Pharmaceuticals, Canada.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci USA. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouloumie A, Bauersachs J, Linz W, Scholkens BA, Wiemer G, Fleming I, Busse R. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension. 1997;30:934–941. doi: 10.1161/01.hyp.30.4.934. [DOI] [PubMed] [Google Scholar]
- 4.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 5.Munzel T, Sayegh H, Freeman BA, Tarpey MM, Harrison DG. Evidence for enhanced vascular superoxide anion production in nitrate tolerance: a novel mechanism underlying tolerance and cross-tolerance. J Clin Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: II. Part, Animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 7.Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, Ullrich V, Mulsch A, Schulz E, Keaney JF, Jr, Stamler JS, Munzel T. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman JE, Keaney JF., Jr Nitric oxide and superoxide detection in human platelets. Methods Enzymol. 1999;301:61–70. doi: 10.1016/s0076-6879(99)01069-1. [DOI] [PubMed] [Google Scholar]
- 9.Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase [corrected] Circulation. 2003;108:2172–2183. doi: 10.1161/01.CIR.0000094403.78467.C3. [DOI] [PubMed] [Google Scholar]
- 10.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meneshian A, Bulkley GB. The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation. 2002;9:161–175. doi: 10.1038/sj.mn.7800136. [DOI] [PubMed] [Google Scholar]
- 12.Sobey CG, Dalipram RA, Dusting GJ, Woodman OL. Impaired endothelium-dependent relaxation of dog coronary arteries after myocardial ischaemia and reperfusion: prevention by amlodipine, propranolol and allopurinol. Br J Pharmacol. 1992;105:557–562. doi: 10.1111/j.1476-5381.1992.tb09018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White CR, Darley-Usmar V, Berrington WR, McAdams M, Gore JZ, Thompson JA, Parks DA, Tarpey MM, Freeman BA. Circulating plasma xanthine oxidase contributes to vascular dysfunction in hypercholesterolemic rabbits. Proc Natl Acad Sci USA. 1996;93:8745–8749. doi: 10.1073/pnas.93.16.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 16.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 17.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 18.Cardillo C, Kilcoyne CM, Cannon RO, III, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxypurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension. 1997;30:57–63. doi: 10.1161/01.hyp.30.1.57. [DOI] [PubMed] [Google Scholar]
- 19.ENCORE Investigators. Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease: the ENCORE I study (evaluation of nifedipine and cerivastatin on recovery of coronary endothelial function) Circulation. 2003;107:422–428. doi: 10.1161/01.cir.0000046488.52939.bf. [DOI] [PubMed] [Google Scholar]
- 20.Koster R, Vieluf D, Kiehn M, Sommerauer M, Kahler J, Baldus S, Meinertz T, Hamm CW. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet. 2000;356:1895–1897. doi: 10.1016/S0140-6736(00)03262-1. [DOI] [PubMed] [Google Scholar]
- 21.Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 22.Warnholtz A, Ostad MA, Heitzer T, Goldmann BU, Nowak G, Munzel T. Effect of tirofiban on percutaneous coronary intervention-induced endothelial dysfunction in patients with stable coronary artery disease. Am J Cardiol. 2005;95:20–23. doi: 10.1016/j.amjcard.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 23.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2003;107:1383–1389. doi: 10.1161/01.cir.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 24.Houston M, Estevez A, Chumley P, Aslan M, Marklund S, Parks DA, Freeman BA. Binding of xanthine oxidase to vascular endothelium: kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J Biol Chem. 1999;274:4985–4994. doi: 10.1074/jbc.274.8.4985. [DOI] [PubMed] [Google Scholar]
- 25.Aslan M, Ryan TM, Townes TM, Coward L, Kirk MC, Barnes S, Alexander CB, Rosenfeld SS, Freeman BA. Nitric oxide-dependent generation of reactive species in sickle cell disease: actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278:4194–4204. doi: 10.1074/jbc.M208916200. [DOI] [PubMed] [Google Scholar]
- 26.Terada LS, Piermattei D, Shibao GN, McManaman JL, Wright RM. Hypoxia regulates xanthine dehydrogenase activity at pre- and posttranslational levels. Arch Biochem Biophys. 1997;348:163–168. doi: 10.1006/abbi.1997.0367. [DOI] [PubMed] [Google Scholar]
- 27.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 28.Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, Panetta TF. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am J Cardiol. 2001;88:188–191. doi: 10.1016/s0002-9149(01)01621-6. [DOI] [PubMed] [Google Scholar]
- 29.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease—Molecular mechanisms and pathophysiologic implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ischiropoulos H, Nelson J, Duran D, Al-Mehdi A. Reactions of nitric oxide and peroxynitrite with organic molecules and ferrihorseradish peroxidase: interference with the determination of hydrogen peroxide. Free Radic Biol Med. 1996;20:373–381. doi: 10.1016/0891-5849(95)02098-5. [DOI] [PubMed] [Google Scholar]
- 32.Glover RE, Koshkin V, Dunford HB, Mason RP. The reaction rates of NO with horseradish peroxidase compounds I and II. Nitric Oxide. 1999;3:439–444. doi: 10.1006/niox.1999.0256. [DOI] [PubMed] [Google Scholar]
- 33.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 34.Brown JM, Terada LS, Grosso MA, Whitmann GJ, Velasco SE, Patt A, Harken AH, Repine JE. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J Clin Invest. 1988;81:1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 36.Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mervaala EM, Cheng ZJ, Tikkanen I, Lapatto R, Nurminen K, Vapaatalo H, Muller DN, Fiebeler A, Ganten U, Ganten D, Luft FC. Endothelial dysfunction and xanthine oxidoreductase activity in rats with human renin and angiotensinogen genes. Hypertension. 2001;37:414–418. doi: 10.1161/01.hyp.37.2.414. [DOI] [PubMed] [Google Scholar]
- 38.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci USA. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley EE, Trostchansky A, Rubbo H, Freeman BA, Radi R, Tarpey MM. Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol. J Biol Chem. 2004;279:37231–37234. doi: 10.1074/jbc.M402077200. [DOI] [PubMed] [Google Scholar]
- 40.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 41.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 42.Mancini GB, Henry GC, Macaya C, O’Neill BJ, Pucillo AL, Carere RG, Wargovich TJ, Mudra H, Luscher TF, Klibaner MI, Haber HE, Uprichard AC, Pepine CJ, Pitt B. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease: the TREND (trial on reversing endothelial dysfunction) study. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]


