Abstract
Reactive oxygen species, in particular superoxide, have been closely linked to the underlying pathophysiology of ischemic cardiomyopathy: superoxide not only mediates mechanoenergetic uncoupling of the myocyte but also adversely impacts on myocardial perfusion by depleting endothelial-derived nitric oxide bioavailability. Xanthine oxidase generates superoxide upon oxidation of hypoxanthine and xanthine and has been detected in cardiac myocytes and coronary endothelial cells of patients with ischemic heart disease. Here we investigated the effects of oxypurinol, a xanthine oxidase inhibitor, on myocardial contractility in patients with ischemic cardiomyopathy. Twenty patients (19 males, 66±8 years) with stable coronary disease, severely suppressed systolic function (left ventricular ejection fraction 22±2%), and nonelevated uric acid plasma levels received a single intravenous dose of oxypurinol (400 mg). Cardiac MRI studies, performed before and 5.2±0.9 h after oxypurinol administration, revealed a reduction in end-systolic volumes (−9.7±4.2%; p=0.03) and an increase in left ventricular ejection fraction (+17.5±5.2%; p=0.003), whereas 6 patients (6 males, 63±3.8 years, ejection fraction 26±5%) who received vehicle only did not show significant changes in any of the parameters studied. Oxypurinol improves left ventricular function in patients with ischemic cardiomyopathy. These results underscore the significance of reactive oxygen species as important pathophysiological mediators in ischemic heart failure and point toward xanthine oxidase as an important source of reactive species that serve to modulate the myocardial redox state in this disease.
Keywords: Nitric oxide, Superoxide, Hydrogen peroxide, Heart failure, Endothelial function, Reactive oxygen species, Myocardial infarction, Free radicals
Impaired contractility of cardiac myocytes is a central feature of ischemic heart failure. Pathophysiologically, impaired myocyte function has been closely linked to alterations in inter- and subcellular signaling cascades in the contractile apparatus itself as well as coronary conductance and resistance vessels [1]. Reactive oxygen-containing species centrally affect myocardial homeostasis: In particular, excess generation of superoxide and hydrogen peroxide serves to mediate the pathophysiology of depressed myocardial function. For example, superoxide and hydrogen peroxide lower calcium responsiveness of stunned cardiac myofilaments by modifying redox-sensitive proteins like the L-type calcium channel, the ryanodine receptor, the calcium ATPase, and mitochondrial respiratory complexes [2–4]. Moreover, superoxide reacts at diffusion-limited rates with other free radicals, particularly with NO to form the potent oxidant peroxynitrite [5]. Peroxynitrite has been shown to activate metalloproteinases, which cause myocardial injury by degradation of troponin I [6,7]. Impairment of endothelial bioavailability by reaction of NO with superoxide also attenuates vasodilation and adversely impacts on organ perfusion [8,9].
Multiple enzyme systems emerge as potential candidates for generation of reactive oxygen species in the failing human heart, such as the leukocyte-derived NADPH oxidase and gp91phox homologues, uncoupled NO synthases, mitochondrial respiratory chain-generated superoxide, and xanthine oxido-reductase [10–13]. Xanthine oxidoreductase, when transferred into its oxidase form xanthine oxidase (XO), generates super-oxide and hydrogen peroxide upon conversion of xanthine to hypoxanthine and hypoxanthine to uric acid, respectively. Vascular and myocyte-localized XO is increased in patients with coronary artery disease, and circulating XO levels are upregulated in congestive heart failure [14,15]. XO inhibition in hyperuricemic patients with dilated and ischemic cardiomyopathy leads to an improvement of vascular NO bioavailability [16,17], and local infusion of allopurinol into the coronary circulation in patients with dilated cardiomyopathy lowered myocardial oxygen consumption [15].
Given the above observations, we hypothesized that XO inhibition by oxypurinol may translate into increased myocardial contractility in patients with ischemic cardiomyopathy. To test this, patients with coronary artery disease (CAD), severely suppressed left ventricular function, and normal uric acid levels underwent cardiac MRI studies, the accepted standard for assessment of left ventricular function [18,19], before and after intravenous administration of oxypurinol.
Materials and methods
The study was approved by the Hamburg Medical Board, the Bundesinstitut für Arzneimittel und Medizinprodukte, and the local health care department. To participate in the study every patient had to give written informed consent. The trial included patients with angiographically documented CAD and a left ventricular ejection fraction below 35%. Main exclusion criteria were unstable coronary artery disease or myocardial infarction within 2 weeks before study entry, significant valvular disease, hypotension, uncontrolled hypertension, creatinine 1.5 times the upper limit of normal, hyperuricemia (>351 μM in women and >422 μM in men), current allopurinol intake or known allopurinol intolerance, and intravenous heparin within 24 h of the study. All patients had to be eligible for cardiac MRI studies.
Oxypurinol (400 mg · 100 ml−1) was infused into the femoral vein at 26 mg · min−1. The study medication was prepared aseptically on-campus by dissolving oxypurinol (Cardiome Pharmaceuticals, Vancouver, BC, Canada) in glucose 5% and sodium hydroxide (0.07 N) to a final pH of 9.7. All solvated oxypurinol was used within 8 h of preparation. Before and after infusion of oxypurinol blood was drawn and plasma was frozen at −80°C until analysis of oxypurinol plasma levels.
MR imaging protocol
To assess left ventricular function all patients underwent cardiac MRI studies before and after oxypurinol administration. The studies were performed with a 1.5-T scanner (Magnetom Symphony; Siemens Medical Systems, Erlangen, Germany) equipped with Quantum gradients (30 mT/m) using a phased array four-element body surface coil. An ECG-gated segmented true fast imaging with steady-state precision sequence was used for cine-MRI. Typical imaging parameters were as follows: repetition time 3.6 ms, echo time 1.8 ms, flip angle 60°, slice thickness 8 mm, field-of-view 350 × 306 mm2, matrix 256×139, pixel spacing 1.4×2.2 mm2. For identification of the cardiac axes, scout images in the coronal, sagittal, and transversal planes were acquired. A cine loop in the long-axis four-chamber plane was followed by short-axis cine loops, perpendicular to the long-axis plane. Short-axis slices were obtained at 10-mm intervals, covering the entire left ventricle from the mitral valve to the apex.
Image analysis
Image analysis was performed using the public domain NIH Image program (version 1.62; U.S. National Institutes of Health) and analyzed by an independent investigator blinded to patient identity, date of examination, and treatment. Left-ventricular endocardial and epicardial contours were manually traced in end-diastole and end-systole, whereas papillary muscles were traced separately. End-diastolic and end-systolic left-ventricular volumes were calculated by summing the areas contained by the endocardial borders as previously [20,21] (Fig. 1). Stroke volume was defined as the difference between end-diastolic and end-systolic volume, ejection fraction was defined as stroke volume divided by end-diastolic volume. Left-ventricular mass was determined by multiplication by the specific gravity of the myocardium (1.05). Intraobserver variability, as assessed in six patients undergoing consecutive cardiac MRI studies with the same pathology, was 1.1%.
Fig. 1.
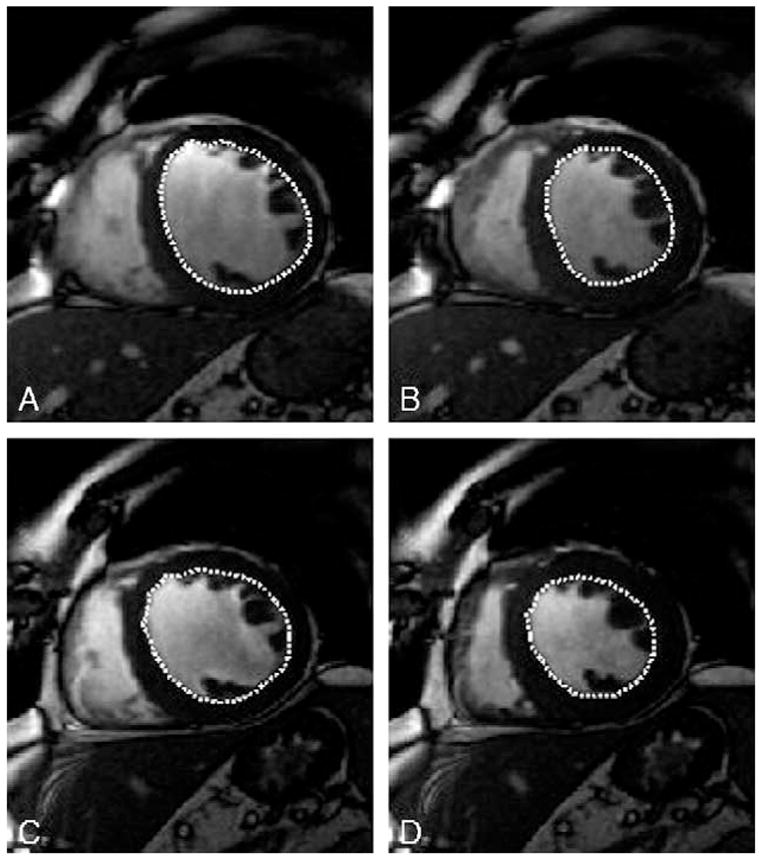
Assessment of left-ventricular volumes by cine-MRI. Endo- and epicardial borders were manually traced in all slices in end-diastole and end-systole, papillary muscles were traced separately. After administration of oxypurinol, MR imaging was repeated with corresponding slice orientations. (A) Diastolic endocardial borders in a midventricular slice before administration of oxypurinol. (B) Systolic endocardial borders in a midventricular slice before administration of oxypurinol. (C) Diastolic endocardial borders in a midventricular slice after administration of oxypurinol. (D) Systolic endocardial borders in a midventricular slice after administration of oxypurinol.
Assessment of plasma levels of oxypurinol, xanthine, hypoxanthine, and uric acid and XO activity
Xanthine oxidase activity was determined by measurement of uric acid formation via HPLC with electrochemical detection. A chromatography elution scheme, using a 25×5-mm C18 column, was devised to permit the baseline resolution of added xanthine, uric acid, and oxypurinol. This consisted of a 30-min isocratic elution with KH2PO4 (300 mM, adjusted to pH 4.0):methanol:acetonitrile: tetrahydrofuran (97.9:1:1:0.1, v/v). The flow rate was 1 ml/min and the column temperature was maintained at 30°C. An eight-channel electrochemical CoulArray detector (ESA Chelmsford, USA) with applied potentials of 0, 120, 220, 300, 575, 700, 800, and 900 mV was used for purine oxypurinol detection. Every five samples, an internal standard mixture of oxypurinol, xanthine, and uric acid was resolved chromatographically for calculating inhibitor and purine concentrations in patient plasma. All samples were run in duplicate and averaged and the means were combined for statistical analysis. The potentially confounding effects of plasma uricase activity were separately determined by addition of known concentrations of uric acid to plasma samples. Subsequent plasma analysis by HPLC–diode array detection revealed no detectable loss of uric acid over time.
Statistical analysis
All parameters are given as mean values±SEM. Differences between parameters before and after oxypurinol were assessed by applying the paired Student t test. Differences of p<0.05 were considered statistically significant.
Results
A total of 20 patients (67±2 years, 95% male) received the study medication (Table 1). All patients tolerated the study protocol and none of the patients experienced adverse reactions after oxypurinol infusion. Most patients had experienced q wave myocardial infarctions (85%) and all patients presented with NYHA class III (70%) and IV (30%), respectively. The majority of the patients were diagnosed for hyperlipoproteinemia and hypertension and 40% of the population were diabetic. The patient population was under standard therapy for heart failure with 95% taking oral diuretics including 50% receiving spironolactone, 93% receiving ACE inhibitors or AT-1 receptor blockers, and 92% on beta blockers.
Table 1.
Baseline clinical characteristics
| N=20 | (%) |
|---|---|
| Age (±SD; years) | 67±2 |
| Sex, male/female | m:19 (95); w:1 (5) |
| NYHA III; IV | 14 (70); 6 (32) |
| Q wave myocardial infarction | 17 (89) |
| Body mass index (kg/m2) | 26±4 |
| Diabetes mellitus | 8 (42) |
| Hypertension | 14 (73) |
| Hyperlipoproteinemia | 14 (73) |
| Smoker | 12 (63) |
Baseline cardiac MRI revealed highly increased end-systolic and end-diastolic volumes (247±24 and 309±25 ml, respectively; Table 2) and severely suppressed left-ventricular function (ejection fraction 22+2%).
Table 2.
Baseline hemodynamic and cardiac MRI measurements
| N=20 | |
|---|---|
| Heart rate | 74±14 |
| Ejection fraction (%) | 22±2 |
| End diastolic volume (ml) | 309±25 |
| End systolic volume (ml) | 247±24 |
| Stroke volume (ml) | 63±6 |
| End diastolic mass (g) | 227±14 |
Upon infusion of oxypurinol, plasma levels of oxypurinol increased from 1.59±1.47 to 118±8.78 μmol/L (p<0.001). No significant changes were observed in levels of purine metabolites such as xanthine (0.62±0.55 μM vs. 1.0±1.02 μM after oxypurinol, p>0.05), hypoxanthine (3.12±4.9 μM vs. 5.56± 6.02 μM after oxypurinol, p>0.05), and uric acid (27.4± 6.5 μM vs. 30.9±7.1 μM after oxypurinol, p>0.05). In addition, plasma xanthine oxidase activity remained unchanged after infusion of oxypurinol (0.06±0.01 vs. 0.09±0.02 μU/mg protein; p=0.4).
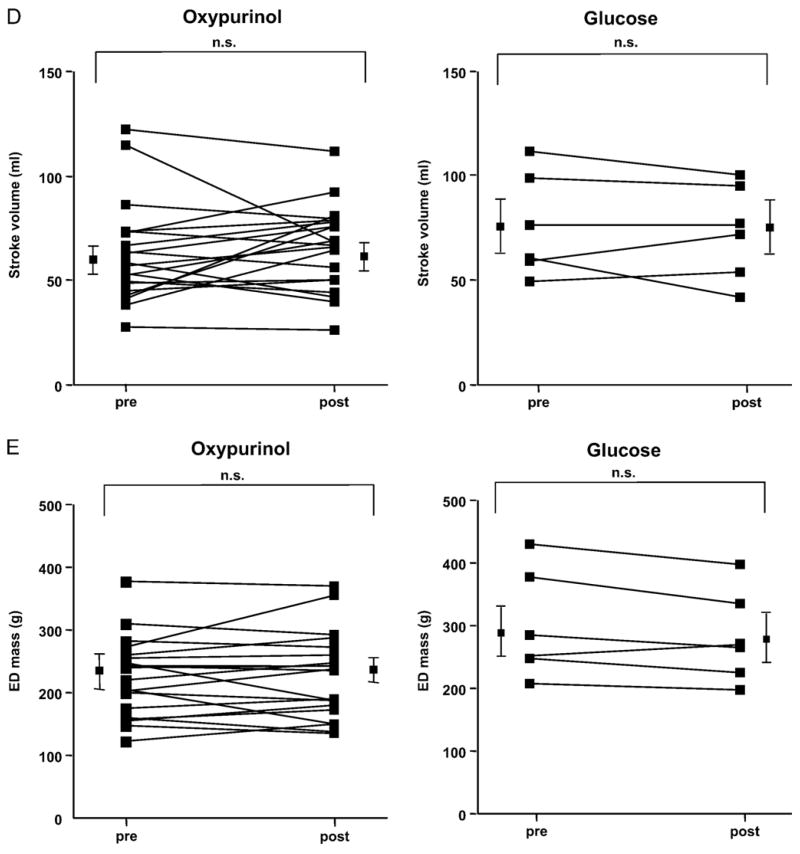
Cardiac MRI, performed 25±5.7 h after baseline MRI and 5.2 ± 1.3 h after oxypurinol administration, revealed a reduction in end-systolic volume (−9.7±4.2; p=0.03) and a nonsignificant decline in end-diastolic volume (−5.6±4.5%, p=0.2), which translated into a significantly increased left ventricular ejection fraction (+17.8±5.1%, p=0.003) in the presence of an unchanged left ventricular mass (+1.8±3.2%; p=0.6; Fig. 2). There was a trend toward an increase in mean aortic pressure after administration of oxypurinol (91.9 mm Hg vs. 97.3 mm Hg, p=0.055). The heart rate during baseline and follow-up MRI remained unchanged (77±17/min vs. 76±18/min, p>0.05).
Fig. 2.
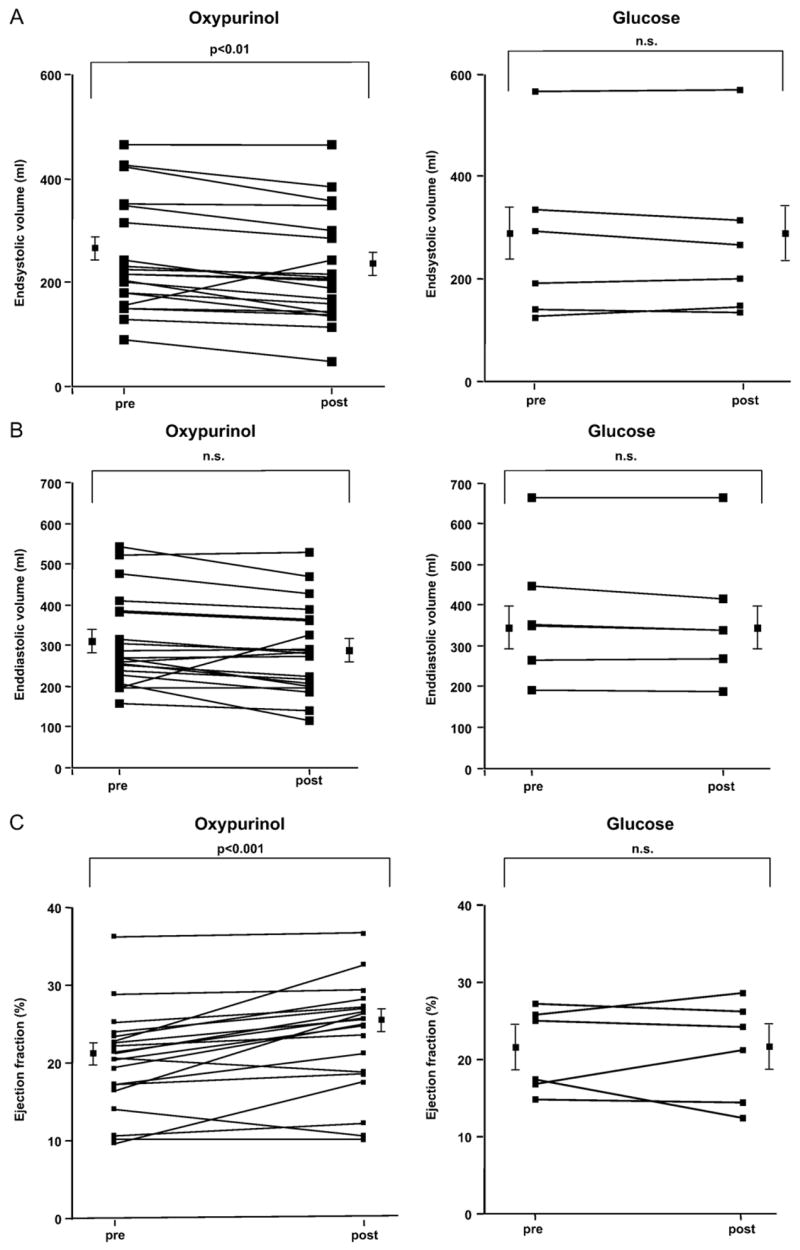
Evaluation of myocardial contractility in response to oxypurinol using cardiac MRI. (A–E) Cardiac MRI was performed in 20 patients before and after administration of oxypurinol (400 mg iv) as well as in 6 patients who received the vehicle only (glucose). Values are given for every patient before and after treatment with mean values±SEM being displayed separately.
Six consecutive patients with ischemic cardiomyopathy (male, n=6, age 63±3.8 years, ejection fraction 25.5±4.7%) who received infusion of the vehicle instead of oxypurinol revealed unchanged end- systolic (−1.4±1.9%; p=0.5) and end-diastolic volumes (−2.3±1.2%, p=0.1) with no alteration of ejection fraction (−1.1±6.3%, p=0.9) and unchanged left ventricular mass (−2.7±3.5%; p=0.4; Fig. 2).
Discussion
The principal finding of the current study is that xanthine oxidase inhibition exerts positive inotropic effects in patients with ischemic cardiomyopathy. Administration of the XO inhibitor oxypurinol lowered end-systolic volumes and increased ejection fraction by 18%.
The depression of myocardial contractility in patients with ischemic cardiomyopathy is no longer viewed as solely the consequence of a loss of structurally intact myocytes, rather is much more appreciated as a disease involving impaired myocyte and vascular redox signaling pathways. Among these, the imbalance between NO and reactive oxygen species such as superoxide and hydrogen peroxide has emerged as a central contributor to depression of myocardial function [22,23]. Xanthine oxidase has also now emerged as a potential source of superoxide and hydrogen peroxide in heart failure, given its upregulation in both vascular and myocardial compartments in this disease [14,15,24].
The modulation of myocardial contractility after xanthine oxidase inhibition has been extensively investigated in animal models of heart failure: In myocytes from a rodent model of heart failure, myocardial oxypurinol administration significantly increased twitch tension and exerted a positive inotropic effect [25]. In a canine pacing-induced heart failure model, allopurinol also increased myocardial contractility and reduced myocardial oxygen requirement [26–28].
Initial clinical studies examining the effects of XO inhibition revealed attenuated oxygen consumption and increased myocardial efficiency in individuals with dilated cardiomyopathy and decreased reperfusion injury. There was also improved endothelial function as a consequence of preserved NO bioavailability in patients with CAD and preserved left ventricular function, as well as in individuals with ischemic and dilated cardiomyopathy [9,14,15,24].
The current study now adds importantly to these initial observations by demonstrating for the first time that systemic XO inhibition by oxypurinol decreases left end-systolic and end-diastolic volumes as revealed by cardiac MRI. Importantly, these effects were seen in patients with nonelevated uric acid levels, suggesting that systemic levels of purine metabolites are not necessarily reflecting local distribution of purine-oxidizing enzymes.
In addition, the effect of oxypurinol was observed in patients, almost all of whom received standard therapy for heart failure—in particular ACE inhibitors, AT-1 blockers, and beta blockers—suggesting that XO inhibition may represent an adjunct treatment strategy in this disease. The increase in ejection fraction has to be considered modest compared to classical positive inotropic agents routinely used in current practice, such as levosimendan or dobutamine [29,30]. However, given the underlying mechanisms of XO inhibition, namely the increase in cardiac NO bioavailability, the increase in myocardial contractility may be just one aspect of net potential anti-inflammatory benefits deriving from this treatment strategy in heart failure.
The fact that infusion of the vehicle did not alter myocardial performance further reinforces the notion that the effects of oxypurinol on cardiac contractility reflect inhibition of the enzyme and reduced generation of reactive oxygen species. Of note, we did not observe changes in circulating xanthine oxidase activity. Furthermore, plasma oxypurinol levels did not exceed those measured in previous studies examining patients with coronary disease and preserved left ventricular function despite the fact that the dose of oxypurinol was doubled (400 mg iv vs. 200 mg iv in the previous trial). Potential explanations are increased body volumes in the current trial and a different extent of local XO deposition, which may have influenced plasma distribution of the drug.
Importantly, the effects of oxypurinol were not accompanied by an increase in heart rate, as opposed to other inotropic agents such as dobutamine, phosphodiesterase inhibitors, and levosimendan [31,32]. This underscores the concept that the positive inotropic effects do not simply reflect endogenous release of catecholamines, but rather are linked to inducing a reduced bioavailability of reactive oxygen species. Given recent ex vivo observation of a markedly attenuated capacity of oxypurinol to inhibit cell-associated and glycosaminoglycan-immobilized XO [33], the role of XO in the depression of cardiac function during ischemic heart failure may still be underestimated.
Certainly, the current study is limited by its nonrandomized design and its overall small size. However, given the significant decrease in end-systolic volumes and the profound increase in ejection fraction upon a single dose of oxypurinol, this pharmacological intervention emphasizes the significance of reactive oxygen species as critical mediators of impaired contractility in patients with ischemic cardiomyopathy. Moreover, xanthine oxidase emerges as a primary source of oxidant stress in the failing heart. Whether these “reverse remodeling” effects of XO inhibition will translate into an adjunct, antioxidant treatment strategy in patients with ischemic heart disease remains to be evaluated in larger clinical trials.
Acknowledgments
This study was supported by a research grant from Cardiome Pharmaceuticals, Canada (to Drs. Baldus and Freeman), the Deutsche Forschungsgemeinschaft (to Dr. Baldus), the Deutsche Herzstiftung (to Dr. Baldus), and the NIH (to Dr. Freeman). The authors thank Antonia Cortes for expert technical assistance and Brian Mangal for statistical support. Dr. Baldus has received lecture fees from Cardiome Pharmaceuticals, Canada.
References
- 1.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease—molecular mechanisms and pathophysiologic implications. J Physiol. 2006;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes: dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 4.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 5.Beckmann JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro: implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia–reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- 8.Bauersachs J, Schafer A. Endothelial dysfunction in heart failure: mechanisms and therapeutic approaches. Curr Vasc Pharmacol. 2004;2:115–124. doi: 10.2174/1570161043476447. [DOI] [PubMed] [Google Scholar]
- 9.Baldus S, Koster R, Chumley P, Heitzer T, Rudolph V, Ostad MA, Warnholtz A, Staude HJ, Thuneke F, Koss K, Berger J, Meinertz T, Freeman BA, Munzel T. Oxypurinol improves coronary and peripheral endothelial function in patients with coronary artery disease. Free Radic Biol Med. 2005;39:1184–1190. doi: 10.1016/j.freeradbiomed.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of NOX family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 11.Dixon LJ, Morgan DR, Hughes SM, McGrath LT, El-Sherbeeny NA, Plumb RD, Devine A, Leahey W, Johnston GD, McVeigh GE. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation. 2003;107:1725–1728. doi: 10.1161/01.CIR.0000066283.13253.78. [DOI] [PubMed] [Google Scholar]
- 12.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 13.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, Reepschlager N, Hornig B, Drexler H, Harrison DG. Electron spin resonance characterization of vascular xanthine and NAD (P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2003;107:1383–1389. doi: 10.1161/01.cir.0000056762.69302.46. [DOI] [PubMed] [Google Scholar]
- 15.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 16.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 17.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 18.Higgins CB. Which standard has the gold? J Am Coll Cardiol. 1992;19:1608–1609. doi: 10.1016/0735-1097(92)90626-x. [DOI] [PubMed] [Google Scholar]
- 19.Rumberger JA, Behrenbeck T, Bell MR, Breen JF, Johnston DL, Holmes DR, Jr, Enriquez-Sarano M. Determination of ventricular ejection fraction: a comparison of available imaging methods. The Cardiovascular Imaging Working Group. Mayo Clin Proc. 1997;72:860–870. doi: 10.4065/72.9.860. [DOI] [PubMed] [Google Scholar]
- 20.Semelka RC, Tomei E, Wagner S, Mayo J, Caputo G, O’Sullivan M, Parmley WW, Chatterjee K, Wolfe C, Higgins CB. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am J Heart. 1990;119:1367–1373. doi: 10.1016/s0002-8703(05)80187-5. [DOI] [PubMed] [Google Scholar]
- 21.Lund GK, Stork A, Saeed M, Bansmann MP, Gerken JH, Muller V, Mester J, Higgins CB, Adam G, Meinertz T. Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology. 2004;232:49–57. doi: 10.1148/radiol.2321031127. [DOI] [PubMed] [Google Scholar]
- 22.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1996;93:1107–1113. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- 23.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation–contraction coupling. Proc Natl Acad Sci USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 25.Kogler H, Fraser H, McCune S, Altschuld R, Marban E. Disproportionate enhancement of myocardial contractility by the xanthine oxidase inhibitor oxypurinol in failing rat myocardium. Cardiovasc Res. 2003;59:582–592. doi: 10.1016/s0008-6363(03)00512-1. [DOI] [PubMed] [Google Scholar]
- 26.Amado LC, Saliaris AP, Raju SV, Lehrke S, StJohn M, Xie J, Stewart G, Fitton T, Minhas KM, Brawn J, Hare JM. Xanthine oxidase inhibition ameliorates cardiovascular dysfunction in dogs with pacing-induced heart failure. J Mol Cell Cardiol. 2005;39:531–536. doi: 10.1016/j.yjmcc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Saavedra WF, Paolocci N, St M, John E, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA, Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 28.Ekelund UE, Harrison RW, Shokek O, Thakkar RN, Tunin RS, Senzaki H, Kass DA, Marban E, Hare JM. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ Res. 1999;85:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- 29.Husedzinovic I, Barisin S, Bradic N, Barisin A, Sonicki Z, Milanovic R. Levosimendan as a new strategy during off-pump coronary artery bypass grafting: double-blind randomized placebo-controlled trial. Croat Med J. 2005;46:950–956. [PubMed] [Google Scholar]
- 30.Ramahi TM, Longo MD, Cadariu AR, Rohlfs K, Slade M, Carolan S, Vallejo E, Wackers FJ. Dobutamine-induced augmentation of left ventricular ejection fraction predicts survival of heart failure patients with severe non-ischaemic cardiomyopathy. Eur J Heart. 2001;22:849–856. doi: 10.1053/euhj.2001.2654. [DOI] [PubMed] [Google Scholar]
- 31.Benotti JR, McCue JE, Alpert JS. Comparative vasoactive therapy for heart failure. Am J Cardiol. 1985;56:19B–24B. doi: 10.1016/0002-9149(85)91191-9. [DOI] [PubMed] [Google Scholar]
- 32.Slawsky MT, Colucci WS, Gottlieb SS, Greenberg BH, Haeusslein E, Hare J, Hutchins S, Leier CV, LeJemtel TH, Loh E, Nicklas J, Ogilby D, Singh BN, Smith W. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation. 2000;102:2222–2227. doi: 10.1161/01.cir.102.18.2222. [DOI] [PubMed] [Google Scholar]
- 33.Kelley EE, Trostchansky A, Rubbo H, Freeman BA, Radi R, Tarpey MM. Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol. J Biol Chem. 2004;279:37231–37234. doi: 10.1074/jbc.M402077200. [DOI] [PubMed] [Google Scholar]