Abstract
Despite the acknowledged advantages of CsI:Tl in many scintillator applications, a characteristic property that undermines its use in high-speed radiographic and radionuclide imaging is the presence of a strong afterglow component in its scintillation decay. This causes pulse pileup in high count-rate applications, reduced energy resolution in radionuclide imaging, and reconstruction artifacts in computed tomography applications. The research outlined here addresses the specific issue of suppressing the afterglow in CsI:Tl crystals by modifying them with codopants. In previous work we reported that one specific codopant, Eu2+, was particularly effective in this regard, lowering the normalized intensity of the afterglow in the time range of 10 μs – 100 ms by almost two orders of magnitude compared to conventional material. We also found, however, that the extent of the suppressive effect was significantly influenced by the presence of additional additives, some of which were inadvertently introduced by the very material that provided the primary Eu codopant itself. The effects of these secondary codopants, which include elemental iodine and various oxidic species, are addressed in the present investigation.
Keywords: Cesium iodide, Scintillation, Afterglow, Additives
1. Introduction
While no single scintillator can be expected to satisfy the needs of all medical and industrial applications, CsI:Tl probably comes closest to the ideal. Its desirable properties include: the highest conversion efficiency of any known scintillator (64,000 photons/MeV), rapid initial decay (680 ns); emission in the visible range (540 nm), and adequate density (4.53 g/cm3) and atomic number (Z = 54). Moreover, it is relatively inexpensive, widely available (http://www.bicron.com/), and readily fabricable as both single crystals and microcolumnar films. Consequently, CsI:Tl has been the material of choice for a wide range of radiological applications, including medical imaging (Rodnyi, 1997; Knoll, 1989), x-ray and gamma ray spectroscopy, homeland security, and nuclear medicine applications such as intra-operative surgical probes and SPECT. Despite its obvious advantages, however, CsI:Tl suffers from an intrinsic shortcoming that has hindered its use in CT and many other high-speed imaging applications: the presence of a persistent afterglow component in its scintillation decay. Although the initial decay has a characteristic time of 680 ns (Rodnyi, 1997), its residual afterglow at 2 ms after the excitation can be as high as 5% of the peak value, depending on the intensity and duration of the excitation pulse. This causes pulse pileup in high count rate applications, reconstruction artifacts in CT applications, and problems of reduced contrast and image blurring in high speed x-ray imaging (Rodnyi, 1997; Knoll, 1989; Siewerdsen et al., 1999). If only this drawback could be reduced, CsI:Tl would find extensive use in many important modalities such as medical cone-beam or spiral CT, providing improved performance with substantial reduction in cost. Consequently, we have been exploring the feasibility of suppressing this afterglow by chemical means. We have found that by codoping the CsI:Tl with Eu2+, the afterglow in the time range of 10 μs–100 ms can be lowered by almost two orders of magnitude (Nagarkar et al., 2005). Through an extensive investigation we have been able to characterize this unusual effect (Brecher et al., 2006), and derive a detailed mathematical model that explains the underlying kinetic processes (Bartram et al., 2006). Nevertheless, and despite the insight that this comprehensive effort has provided, some important questions about the phenomenon remain unanswered, particularly with regard to additive chemistry. In an earlier paper (Nagarkar et al., 2005) we dealt with issues related to the conditions of fabrication. In this paper we address the effects of secondary codopants.
2. Experimental
The CsI:Tl, Eu crystals for this investigation were all grown under similar conditions, differing only in content of the secondary codopant. The starting material (CsI crystals containing 0.08 mol percent Tl) was purchased from either Amcrys (http://www.amcrys-h.com/) or Aldrich (http://www.sigma-aldrich.com), which showed no measurable difference in the grown crystals. The anhydrous EuI2 providing the primary Eu codopant came from Aldrich, and was added to the starting material at levels of 0.01 and 0.2 mol percent. On the basis of the results reported by Nagarkar et al. (2005), two secondary codopants, elemental iodine and oxygen, were chosen for study in the present investigation. Addition of the former was straightforward, in the form of granular crystalline iodine, but given the size of the I2 molecule, we would expect the iodine to enter the CsI lattice as I0 (neutral) atoms, which should fit comfortably into the Cs+ vacancies that must be present to compensate for the excess charge introduced by 2+ (Nagarkar et al., 2005). This would assume the form of stable VK centers, in which a single negative charge is shared by two iodine atoms. Addition of oxygen is a more difficult challenge. Since crystal growth was to take place under vacuum, we did not feel that we could introduce the requisite small amounts of oxygen gas with adequate precision and reproducibility. Nor could we use the alkali metal oxide, which is both extremely hygroscopic and highly corrosive to quartz. For these reasons we chose to use anhydrous sodium iodate, relying on its thermal decomposition to provide the desired levels of oxide:
The introduction of oxide into the lattice provides an extra negative charge to serve as compensation for the Eu2+ codopant ion. While this necessarily introduces elemental iodine as well, which can complicate the interpretation of the results, our ability to independently characterize the effect of iodine alone will enable us to separate the two effects. The crystals were grown in evacuated quartz ampoules using the vertical Bridgman technique, producing boules 10 mm in diameter and 35 mm long. From these boules, disks were cut and polished to transparency, with a finished thickness of 2.5 mm. To prevent any artifacts that might arise from the inevitable concentration gradient along the axis of the boule, all parametric comparisons were made on specimens cut from the same position in the boule. Similarly, the masses of the crystals and the conditions of their growth were maintained the same, to assure that the concentration relationships would be internally consistent. Spectroscopic measurements were performed under steady-state excitation by 8-keV Cu Kα x-rays, while the decay measurements were made after excitation by square pulses of 100 ms duration. The scintillation light yield was obtained from the position of the photopeak in the scintillation energy spectrum, which was measured by counting the photons emitted by the specimen at each scintillation event during irradiation with 137Cs source (662 keV photons). The various measurement processes are described in more detail in Brecher et al., 2006.
3. Results and Discussion
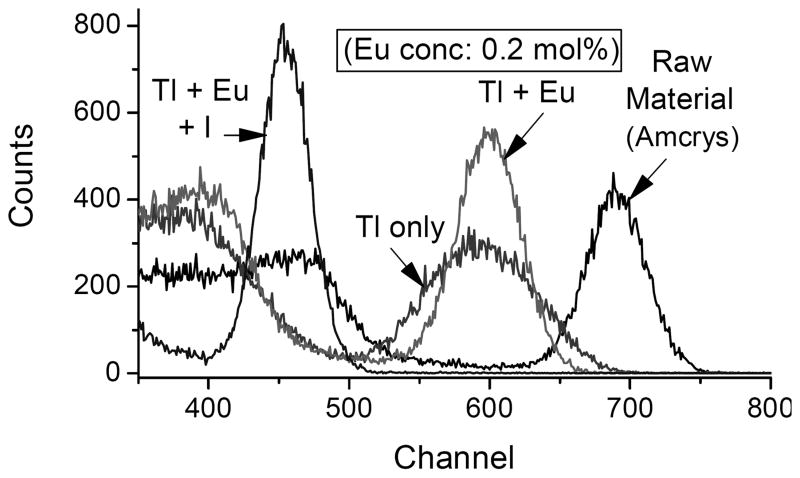
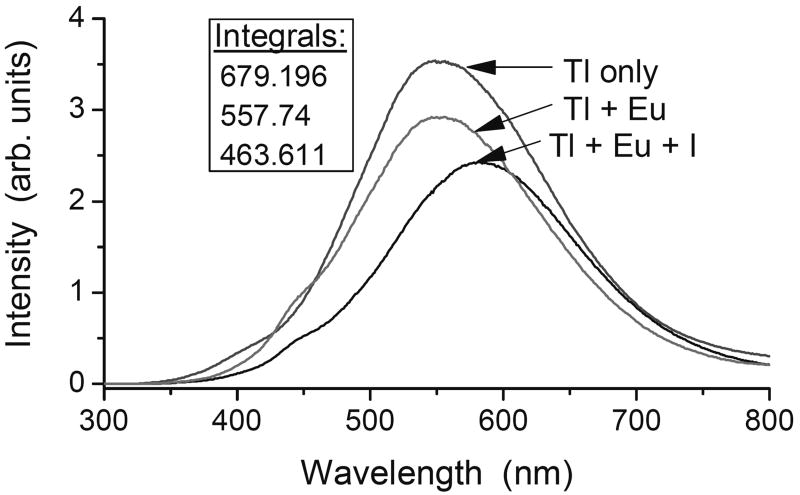
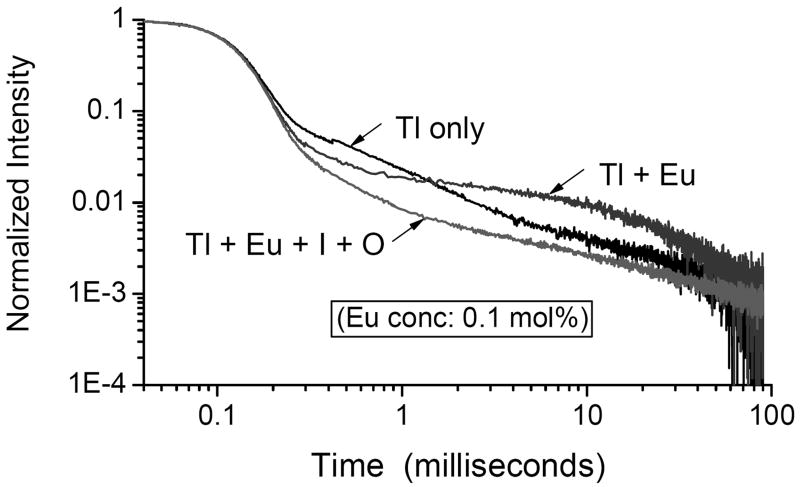
In this study, we focus our attention on two scintillation properties of the various specimens, their light yield and the shape of their decay traces after pulse excitation. The former is summarized in Figure 1, which illustrates a number of relevant points. First, we see that none of our codoped specimens, whether with Eu2+ alone or accompanied by one of the secondary modifiers, provides a light yield as high as that of the unchanged source material from Amcrys. This should not be surprising, since the light yield of the best CsI:Tl already approaches the theoretical maximum that can be achieved (Lempicki et al., 1999), and there is no place to go but down. But the light yields as measured here can clearly be improved, since even the specimens without any codopant fall short of the starting material. This has been reported previously (Brecher et al., 2006), and is attributed to a substantial loss of the luminescent Tl+ dopant, an inevitable result of the Bridgman growth technique. Since the Tl+ content of the raw material started out close to optimal (≈0.1 mol%), any significant diminution in the grown specimens, exacerbated by lattice imperfections, should result in lower light output, as we observe. Indeed we have confirmed that when additional TlI is added to the starting material, the light output of the resulting crystal goes up. However, for the purposes of this study, we chose not to introduce yet another variable parameter into the mix, concentrating instead on the effect of the secondary modifiers. Another point of note is that the presence of elemental iodine in the lattice is significantly more deleterious to the light yield than had been expected (Nagarkar et al., 2005). This is true regardless of whether Eu2+ is also present. Much of this shortfall can be attributed to the strong absorption in the blue-violet that the iodine introduces; this absorption substantially overlaps the broad Tl+ emission, providing a major loss mechanism and a significant red shift of the remaining emission (Fig. 2). And this does not take into account any hindrance to the scintillation kinetics, which will be discussed later. Perhaps the most significant observation, however, is that oxygen is not nearly as harmful to the emission as is iodine. As seen in Fig. 3*, the specimens containing oxygen, as introduced by the decomposition of the iodate anion, have light yields substantially higher than those grown with elemental iodine, even though iodine is also produced by the same reaction that produced the oxide. Indeed, despite the residual iodine the presence of oxide appears to restore the light yield to almost the level reached with only the Eu2+ codopant. Now let us consider the decay kinetics. As we have emphasized in earlier work (Nagarkar et al., 2005; Brecher, et al., 2006), quantitative comparison of afterglow levels from different specimens is impossible to achieve unless the conditions of excitation are identical. Afterglow is not an intrinsic property of the material, but can vary by many orders of magnitude depending on the duration and energy of the excitation pulse and even the excitation history of the specimen. For the purposes of this study we measured the decay traces from the various specimens following excitation by a square X-ray pulse of 100 ms duration; these are presented in Figs. 4 and 5. In the former we see the effect of elemental iodine on CsI:Tl that does not contain the Eu2+ afterglow-suppressing codopant. Here we see that the long slow decay characteristic of conventional CsI:Tl is made even worse by iodine, with almost twice the intensity in the critical 2 μs – 20 ms time range relevant to CT applications. This uncharged species can trap both holes and electrons, thereby effectively competing with the Tl+ emitting center and impeding its excitation. In Fig. 4 we see that the presence of europium has reduced the afterglow intensity in that time range by around 50%; this is consistent with results reported for the high-purity (Aldrich) material in Nagarkar et al., 2005, but falls well short of the improvement seen in the lower-purity material. Here again, however, the addition of elemental iodine makes matters worse, contrary to our expectations expressed in that paper. With the use of iodate, however, we see in Fig. 5 just the opposite result. With this ion (or, more accurately, the decomposition products of this ion), we see that the afterglow-suppressive effect of Eu2+ is enhanced by about a factor of three, bringing the afterglow down almost to the lowest level achieved with ESPI material, reported earlier. Thus it would appear that, contrary to Nagarkar et al., 2005, it is oxygen, not iodine, that is the desired modifying codopant, and that if the former were introduced without adding to the latter, the afterglow-suppressive effect could be even greater.
Fig. 1.
Effect of iodine on the energy spectrum of CsI:Tl single crystals containing Eu2+ codopant, when excited with a 137Cs source. For clarity, the lower channels have been truncated and the intensity normalized to the Compton peak. The diminished light output of the crystal specimens without additives is attributed to a loss of some of the Tl+ that had been present in the raw material.
Fig. 2.
Effect of iodine on the optical spectrum of CsI:Tl single crystals containing 0.2 mol% Eu2+ codopant, under steady-state excitation by 8-keV Cu Kα x-rays. Note the red shift associated with the presence of iodine in the crystal.
Fig. 3.
Effect of oxide on energy spectrum of CsI:Tl single crystals containing Eu2+ codopant, when excited with a 137Cs source. Note that oxide restores most of the light yield that had been lost because of iodine alone. (Na content about same as in Fig. 1.)
Fig. 4.
Effect of iodine on the decay kinetics of CsI:Tl single crystals containing Eu2+ codopant, excitation by square X-ray pulses of 100 ms duration. Note that the presence of iodine increases the afterglow with or without europium.
Fig. 5.
Effect of oxide on the decay kinetics of CsI:Tl single crystals containing Eu2+ codopant, after excitation by square X-ray pulses of 100 ms duration. Note that the presence of oxide decreases the afterglow even in the presence of iodine.
For a phenomenological feel for the processes that underlie our observations, we must first revisit the chemical issues addressed in Nagarkar et al., 2005. One of the prime motivations of that work was to reconcile the disparate behavior of specimens whose Eu2+ codopant had different chemical origins. The argument identified the critical factor governing the shape of the decay as being the manner in which lattice compensates for the charge imbalance introduced by the dipositive cation. In the absence of other foreign ions, there are only three possibilities: insertion of an interstitial I− anion; removal of a Cs+ cation; or reduction of Tl+ to the neutral state. Given the huge ionic radius of I− (2.2 Å) and the high packing density of the lattice, we can effectively disregard the first option, leaving only the cation vacancy and Tl+ reduction for further consideration. And both of these point defects are known to occur in the CsI:Tl lattice. The key issue here is that the trapping of holes at Tl+ sites to form Tl0 species plays a major role in the scintillation kinetics, and anything that can change the concentration of that species, such as the presence of an oxidizing agent, will have a major impact on the decay. As originally envisioned, the hypothetical process involved an oxidized contaminant reacting with the iodide to form elemental iodine, which in turn would suppress the concentration of Tl0:
This would force charge compensation to occur largely by Cs+ vacancies. The diminished content of Tl0 would alter the decay kinetics, while the increased abundance of cation vacancies would alter the crystal field at the Tl+ emitting centers, shifting and reshaping the spectrum of the emission. We have already observed both of these effects. However, as this work demonstrates, the addition of elemental iodine does not provide the anticipated benefits. Not only does it decrease the luminescence but it introduces new trapping sites that interfere with the scintillation kinetics. According to the present results it is not iodine but oxygen itself that is the relevant oxidizing agent. This apparently results in two salutary effects, both providing charge compensation for the Eu2+ and minimizing the concentration of Tl0. Although this runs counter to conventional wisdom regarding oxidic contamination in halide crystals, it does appear that, at least in this one material, a small amount of oxide can be beneficial rather than detrimental.
4. Conclusion
We have found that the effect of Eu2+ in suppressing the afterglow of CsI:Tl can be altered in either direction through the addition of selected secondary modifying additives. Contrary to expectations, elemental iodine turned out to be deleterious to both light yield and afterglow suppression. Oxygen, on the other hand, substantially improves the afterglow suppression with much smaller penalty to light yield. The results are consistent with the earlier model involving minimization of the concentration of Tl0, but with oxygen rather than iodine as the oxidizing agent.
Acknowledgments
We thank the Public Health Service (NIH), DHHS for Grant R44-EB003382-02, and the Medical Sciences Div., DOE, for Grant DE-FG02-04ER84054, which provided partial support for parts of this work.
Footnotes
The Amcrys commercial material also contains about 0.04 mol percent of sodium to impart greater radiation hardness, but our previous studies (Brecher et al., 2005) reveal that this ion has only a minimal effect on both light yield and afterglow. Nevertheless, in Figures 3 and 5, we used Aldrich (which does not contain Na) as raw material, so that the Na added as iodate would not be excessive.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartram RH, Kappers LA, Hamilton DS, Lempicki A, Brecher C, Gaysinskiy V, Ovechkina EE. Suppression of afterglow in CsI:Tl by codoping with Eu2+ II. Theoretical model. Nucl Instr & Meth A. 2006;558:458–467. [Google Scholar]
- Brecher C, Lempicki A, Miller SR, Ovechkina EE, Gaysinskiy V, Nagarkar VV, Bartram RH. Suppression of afterglow in CsI:Tl by codoping with Eu2+ I. Experimental. Nucl Inst & Meth A. 2006;558:450–457. [Google Scholar]
- Brecher C, Nagarkar VV, Gaysinskiy V, Miller SR, Lempicki A. Time-Resolved Studies of the Nonexponential Decay of CsI:Tl after Short-Pulse X-ray Excitation. Nucl Inst & Meth A. 2005;537:117–124. [Google Scholar]
- Rodnyi PA. Physical Processes in Inorganic Scintillators. CRC Press; Boca Raton, New York: 1997. [Google Scholar]
- Knoll GF. Radiation Detection and Measurement. 2 1989. [Google Scholar]
- Lempicki A, Wojtowicz AJ, Brecher C. Inorganic Scintillators. In: Rotman SR, editor. Wide Gap Luminescent Materials: Theory and Applications. Kluwer Academic Press; Norwell MA USA: 1999. [Google Scholar]
- Nagarkar VV, Ovechkina EE, Miller SR, Gaysinskiy V, Brecher C, Lempicki A. Luminescence Properties of CsI:Tl Crystals Doped with Eu. Functional Materials. 2005;12:645–652. [Google Scholar]
- Siewerdsen JH, Jaffray DA. A ghost story: Spatio-temporal response characteristics of an indirect-detection flat panel imager. Med Phys. 1999;26(8) doi: 10.1118/1.598657. [DOI] [PubMed] [Google Scholar]