Abstract
By using the quail–chicken chimera system, we have previously shown that during development of the spinal cord, floor plate cells are inserted between neural progenitors giving rise to the alar plates. These cells are derived from the regressing Hensen’s node or cordoneural hinge (HN-CNH). This common population of HN-CNH cells gives rise to three types of midline descendants: notochord, floor plate, and dorsal endoderm. Here we find that HNF3β, an important gene in the development of the midline structures, is continuously expressed in the HN-CNH cells and their derivatives, floor plate, notochord, and dorsal endoderm. Experiments in which the notochord was removed in the posterior region of either normal chicken or of quail–chicken chimeras in which a quail HN had been grafted showed that the floor plate develops in a cell-autonomous manner in the absence of notochord. Absence of floor plate observed at the posterior level of the excision results from removal of HN-CNH material, including the future floor plate, and not from the lack of an inductive signal of notochord origin.
Keywords: quail–chicken chimeras/avian organizer/Hensen’s node/cordoneural hinge/HNF3β and Shh genes
In amniotes, where the blastula is formed of a flat multilayered sheet of cells constituting the epiblast, gastrulation proceeds through the primitive streak, whose lateral borders correspond to the lateral lips of the amphibian blastopore. Hensen’s node, located at the tip of the primitive streak, is considered as the homologue of the dorsal blastoporal lip, which contains the organizer.
Cell marking studies using a variety of labeling procedures such as carbon particles (1, 2), tritiated thymidine (3), quail–chicken transplantations (4), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) deposition or lysine–rhodamine–dextran (LRD) intracellular injection (5, 6) have been carried out on chicken embryos cultured at the primitive streak stage for periods of time ranging from 24 to 48 hr. These experiments have shown that the notochord arises from cells contained in Hensen’s node. The latter undergoes a rostrocaudal movement also designated as “regression” (7, 8), which takes place as the embryo elongates along the anteroposterior axis. Similar conclusions have been reached for Xenopus (9), mouse (10), and zebrafish (11) embryos.
The notochord is considered as the source of signals involved in the dorsoventral patterning of the spinal cord (see ref. 12 for a review). This idea was initially based on experiments performed in the chick in which the notochord was removed in the posterior region of the embryo. Removal of the notochord results in the absence of floor plate and motoneurons on a short segment located in the caudalmost part of the operated territory (13–15). The conclusion was drawn that the notochord is inducing a floor plate in the midline of the neural plate. This induction is thought to be mediated by a signaling molecule produced by the notochord and encoded by one of the vertebrate homologs of Drosophila hedgehog called Vertebrate hedgehog (Vhh) or Sonic hedgehog (Shh) (16–21). Thus, the Shh-encoded protein was shown to induce HNF3β, a marker of floor plate cells in in vitro cultured early neural epithelium (21). According to this view, floor plate cells would thereafter become able to produce Shh, which plays a critical role in the specification of motoneurons (20, 21). This view was further supported by the fact that grafts of notochord laterally to the neural tube resulted in the induction of a floor plate-like structure, together with that of extramotoneurons in the alar plate (13, 15, 22–25).
Certain aspects of this model were recently challenged by experiments, performed in this laboratory, showing that Hensen’s node in fact generates not only the notochord but also the floor plate (26). In other words, these two midline structures share the same embryonic origin. During Hensen’s node regression, the floor plate becomes inserted within the neural plate, thus joining medioventrally the bilateral primordia of the neural tube. The ectoderm located caudally to Hensen’s node is thus split and the presumptive basal plates of the future neural tube are transiently joined in the midline by a mass of cells which, for a while, includes both the notochord and the floor plate and corresponds to the structure designated by Pasteels (7, 8) as the cordoneural hinge (CNH). In fact, the CNH corresponds to the Hensen’s node or avian trunk–tail organizer. This structure will be designated as HN-CNH throughout this article. By using the quail–chicken chimera system, we showed that the process of floor plate insertion continues throughout the primary and secondary neurulation. Cells forming the floor plate and the notochord become progressively separated from each other by the formation of a basement membrane. Then the dorsalmost part of HN-CNH-derived cells become definitively incorporated into the neural plate (26, 27).
The notochord extirpation experiments, which suggested that floor plate differentiation was the result of an induction, were generally performed in chicken embryos at stages ranging from 10- to 20-somite stage (ss) (13–15, 28–30). The notochord was removed either mechanically or enzymatically from the level of the last formed somites down to Hensen’s node, which was in principle excluded from the operation. In a certain number of cases, the neural tube lacked a floor plate as well as motoneurons, whereas the somites were fused on the midline ventrally to the neural tube. The only somitic derivatives that further differentiated in these conditions were the myotomes. The neural crest cells migrated ventrally and formed dorsal root ganglia which, like the myotomes, often fused in one mass ventrally to the “unpolarized” neural tube. A particularity of these extirpation experiments is that the neural tube defect concerned only the posteriormost part of the excised domain. This abnormal region extended for a short length corresponding to 4–5 somites. Rostrally, in spite of the absence of notochord, the neural tube developed normally with a floor plate and motoneurons. Caudally the regression of Hensen’s node that was, for its major part, not included in the operation, led to normal development.
In view of the observation just reported that during Hensen’s node regression, notochord and floor plate are joined in a common cellular population, we thought that the notochord extirpation experiments had to be revisited. This constitutes the subject of the present work. In a first step, the expression patterns of HNF3β and Shh were scrutinized in detail during the primary and secondary neurulation in control quail and chicken embryos and compared with the quail cell distribution in quail–chicken chimeras in which Hensen’s node and its derivatives were of quail origin as previously described (26). These Hensen’s node chimeras revealed that extirpation of the notochord per se does not prevent the development of a normal floor plate and the differentiation of motoneurons. We found that absence of floor plate and failure of motoneuron development together with the lack of a normal dorsoventral polarization of the neural tube are because of the surgical removal of the progenitors of the floor plate itself at the level where they are still intimately associated with the notochordal material in the rostralmost portion of the HN-CNH structure.
MATERIALS AND METHODS
Embryos.
Chicken and quail eggs from commercial sources were incubated at 38°C in a humidified atmosphere. Embryos were staged by using the developmental time table of Hamburger and Hamilton (HH) (31) or by counting the number of somites. Control and operated embryos were sacrificed at different stages for either in toto in situ hybridization or immunocytochemistry.
Microsurgery.
Excision of the notochord. Excision was performed either ex ovo or in ovo. In the first case, chicken embryos were removed from the egg at the 10–15ss and pinned ventral side up in a dish. A solution of pancreatin (GIBCO) (1/3 in PBS) was deposited on the endoderm at the level where the paraxial mesoderm is not segmented. The endoderm was then peeled off together with the notochord from the level of the last formed somite down to the HN-CNH. These embryos were immediately fixed for in situ hybridization with the HNF3β probe. The in ovo notochordectomy was performed as previously described (29) in embryos at 15–25ss. The neural tube/notochord complex was surgically removed from the level of the last formed somite down to the HN-CNH. Then the neural tube and notochord were enzymatically dissociated in vitro by using pancreatin. The isolated neural tube was back-grafted in its normal anteroposterior and dorsoventral orientations. The operated chicken embryos were reincubated for various time periods before fixation for in toto in situ hybridization with the HNF3β probe.
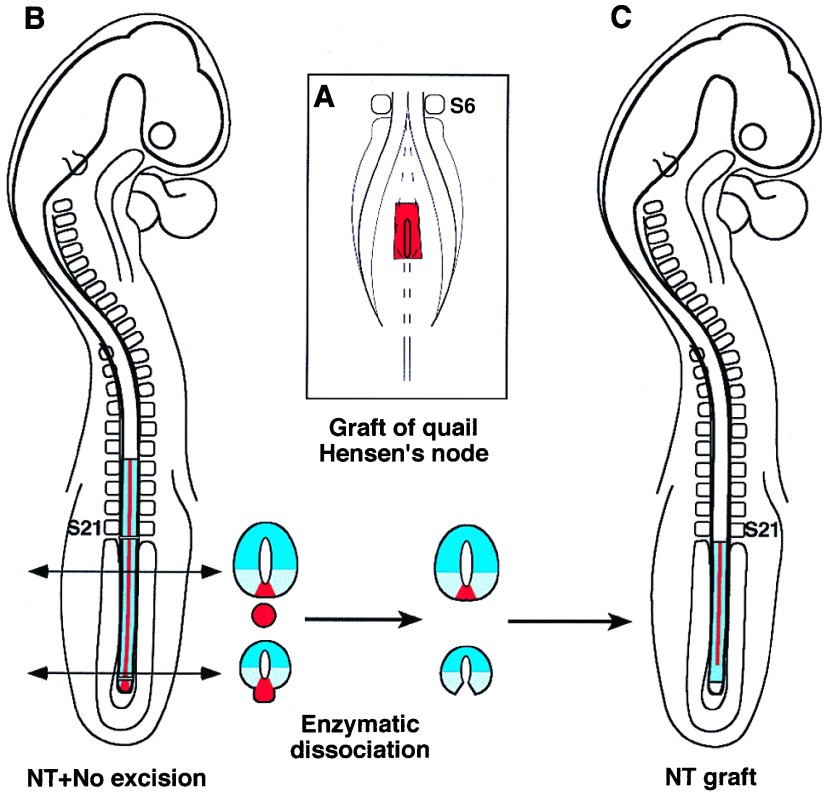
Quail–chicken chimeras. Quail–chicken chimeras were constructed according to the previously described method (26). Hensen’s node was removed in 5–6ss chicken embryos and replaced by its counterpart taken from a stage-matched quail (Fig. 1A). Embryos were either fixed after 20 hr for QCPN monoclonal antibody (mAb) staining to make evident the quail cells or were subjected to notochordectomy (Fig. 1B) as described above for control embryos. Then the neural tube, whose floor plate is derived from the grafted quail HN-CNH, was reimplanted into the embryo in the absence of notochord (Fig. 1C). These embryos were reincubated for 3 additional days before treatment on transverse histological sections with QCPN mAb.
Figure 1.
(A) Construction of an HN-CNH chimeric embryo by grafting a quail Hensen’s node into a 6ss chicken embryo; thereafter, the floor plate and the notochord are made up of quail cells from thoracic level to the tail (see B). (B) One day after the graft, the chimeric neural tube and the quail notochord are microsurgically excised posteriorly to the last formed somites down to the cordoneural hinge. The neural tube–notochord complex is submitted to enzymatic dissociation and the neural tube alone is back-grafted in another chicken embryo of the same stage and deprived of its own neural tube/notochord complex at the same level (C). Note that quail cells (red) are absent in the posterior part of the back-grafted neural tube.
In Situ Hybridization.
Hybridization was performed on embryos in toto according to an already described procedure (32): control chicken embryos from primitive streak stage (4 HH) to 25ss and operated embryos of 10–25ss were dissected in PBS/EGTA and fixed in 4% formaldehyde in PBS/EGTA. Digoxigenin-UTP-labeled RNA probes were synthesized according to the supplier’s protocol (Promega). The chicken Shh probe was kindly provided by R. Riddle and was prepared as already described (33). HNF3β probe was a gift of A. Ruiz i Altaba (25). Stained embryos were photographed, then embedded in albumin-gelatin medium and transversally or sagitally sectioned (30–50 μm) by using a Vibratome (Leica VT 1000E). Sections were observed on a Leica DMRB microscope with interference contrast optics and were photographed.
Immunocytochemistry.
Chimeric embryos were fixed at embryonic day 2 (E2) and E6 in Carnoy’s fluid. Serial 5-μm paraffin sections were immunostained with the QCPN mAb (Hybridoma Bank), which recognizes a quail perinuclear antigen.
RESULTS
Expression Patterns of HNF3β and Shh, in the Midline Cells of Chicken Embryos, from Stage 4 HH to 25ss.
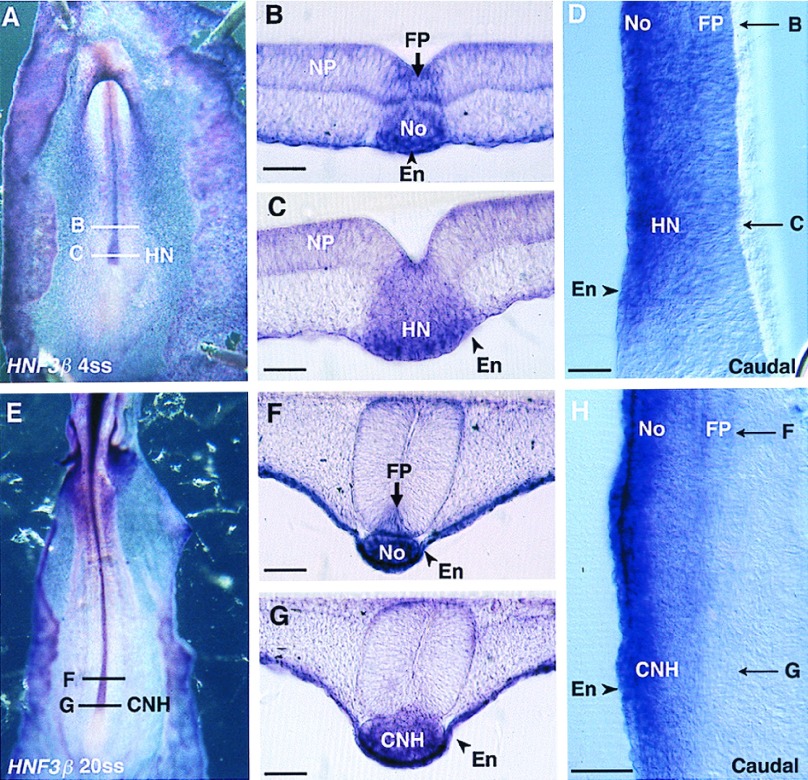
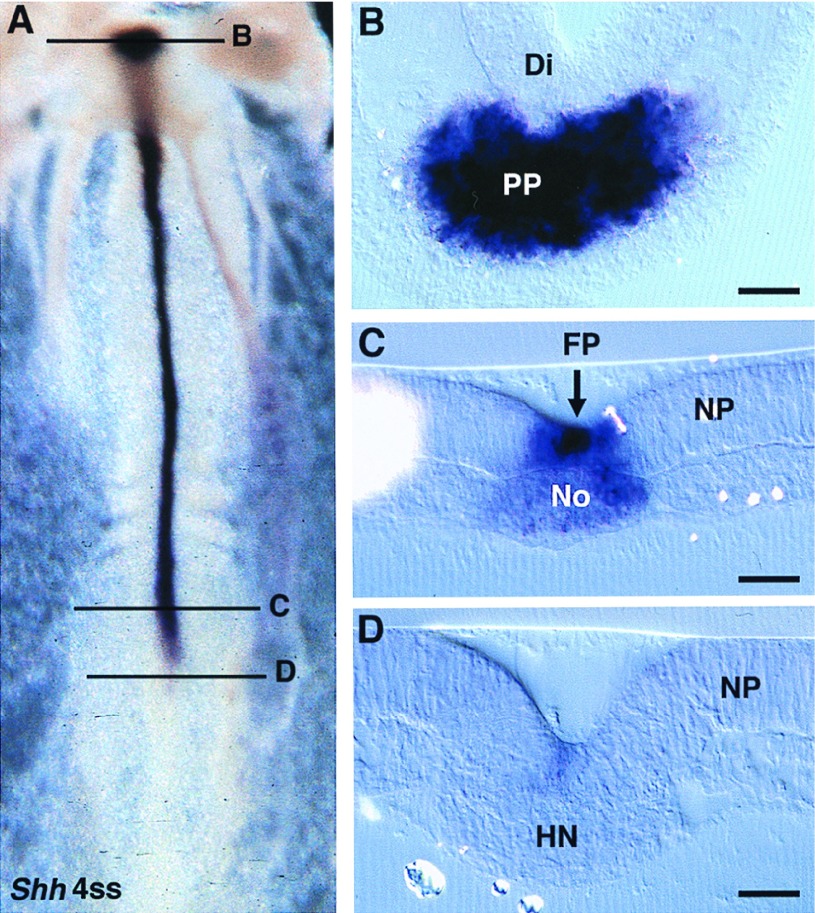
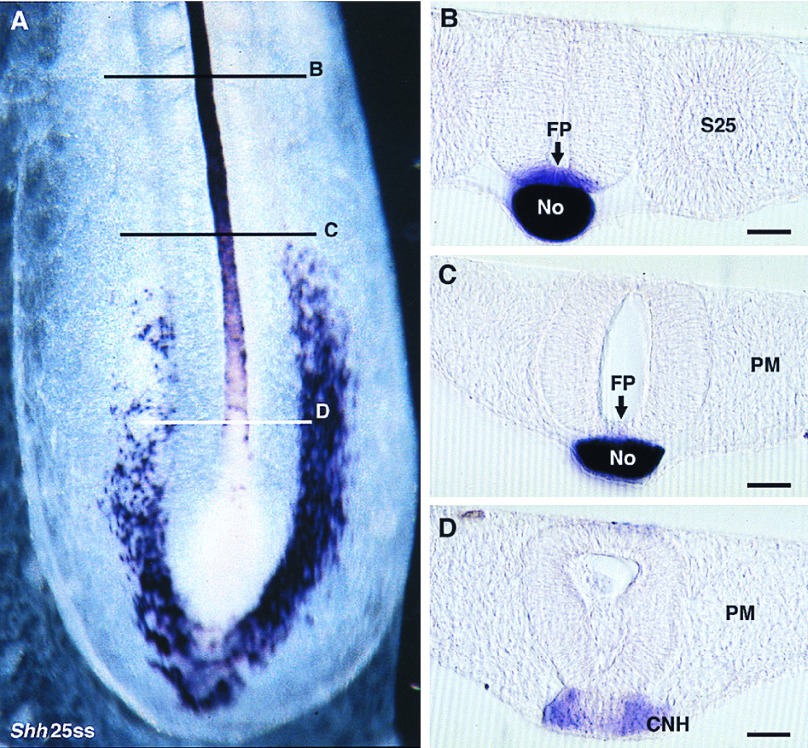
Embryos were observed in ventral view in toto and in sagittal and transverse sections. We found that the cells of HN-CNH express HNF3β throughout all steps of primary and secondary neurulation (Fig. 2). The HN-CNH structure was found inserted between the two lateral pieces of the neural plate during primary neurulation (Fig. 2C) and underlying the medullary cord during secondary neurulation (Fig. 2G). Gene expression is slightly stronger in the ventral region of HN-CNH in contact with the underlying endoderm, which is also strongly stained. Immediately rostral to HN-CNH, in the region where the notochord is segregated from the ventral part of the forming neural tube (i.e., the future floor plate), HNF3β is expressed both in the notochord and in the midline cells of the ventral neural tube whatever the stage considered up to 25ss, the last stage observed (Fig. 2B, D, F, and H). In contrast, Shh is not massively expressed in the center of the HN-CNH region (Figs. 3 and 4) but shows a bilateral distribution in the rostral part of this structure (Figs. 3 A and D and 4 A and D). Immediately rostral to it, in the region where notochord and floor plate cells have just become separated, Shh transcripts are present in the notochord but not in the floor plate (Fig. 4C). As already described (34), Shh transcripts were generally detected in the floor plate cells only at somitic levels (Fig. 4B). Expression of this gene is thus delayed in the midline cells of the future neural tube except in embryos at head-process to 4ss, in which Shh is expressed in the midline cells of the future neural tube more strongly than in the notochord and along their whole length, including in the region immediately rostral to Hensen’s node (Fig. 3C).
Figure 2.
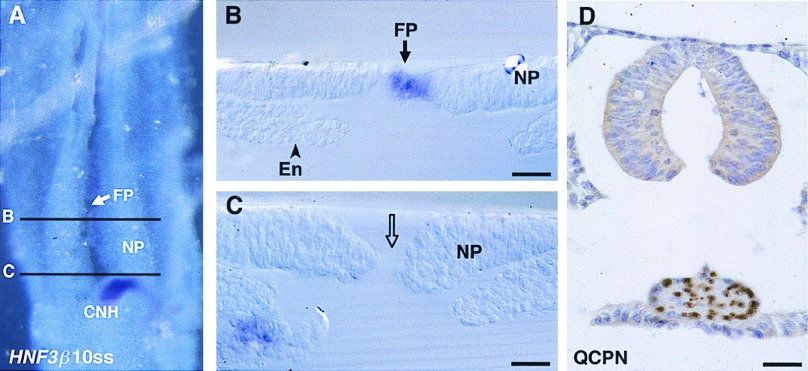
HNF3β expression pattern in 4ss (A–D) and in 20ss (E–H) chicken embryos. (A and E) In toto ventral views. (B, C, F, and G) Transverse sections. HNF3β transcripts are present in HN-CNH and in the floor plate (FP) and notochord (No) immediately rostral to HN-CNH. The gene is slightly more strongly expressed in the vicinity of the endoderm (En), where it is also expressed. Sagittal sections show the continuity between Hensen’s node (HN) and FP/No at 4ss (D) and between CNH and FP/No at 20ss (H). NP, neural plate. (Bars = 80 μm.)
Figure 3.
Shh expression pattern in 4ss chicken embryos. (A) Whole mount ventral view. (B) Transverse section at the diencephalon level (Di) shows the prechordal plate (PP) strongly expressing Shh. (C) Immediately rostral to Hensen’s node, the floor plate (FP) is more strongly positive than the notochord (No). (D) In Hensen’s node region (HN), Shh is slightly and evenly expressed in the superficial layer, the future floor plate. NP, neural plate. (Bars = 80 μm.)
Figure 4.
Shh expression pattern in 25ss chicken embryos. (A) Whole mount ventral view shows that Shh is only slightly expressed in the notochord segment immediately rostral to the node. (B) On a transverse section at the level of the last formed somite (S25) the floor plate (FP) and the notochord (No) strongly express the gene. (C) In the region where the paraxial mesoderm (PM) is not segmented, FP does not show the transcripts. (D) The cordoneural hinge (CNH) exhibits a weak and irregular expression of the gene. (Bars = 100 μm.)
The chimeric embryos that had received a quail Hensen’s node graft at 5–6ss were examined for chimerism at 25ss by using the QCPN mAb. The sections were compared with those of normal chicken embryos on which HNF3β and Shh expression patterns were studied by in situ hybridization. On sagittal sections, the quail floor plate and notochord cells that were derived from the graft (see Fig. 1) were found in continuity with the mass of quail cells constituting the HN-CNH (Fig. 5A). These labeled cells corresponded exactly to the above described HNF3β-expressing cells of control embryos (see Fig. 2H). Transverse sections showed, from caudal to rostral, a mass of quail cells underlying the chicken medullary cord (Fig. 5B) representing the CNH. More rostrally, this mass had split into a notochord and a floor plate (Fig. 5C). The patterns of quail cells in the chimeras and of HNF3β-expressing cells in the normal embryos were superimposable (compare Fig. 5 A–C to Fig. 2 F–H).
Figure 5.
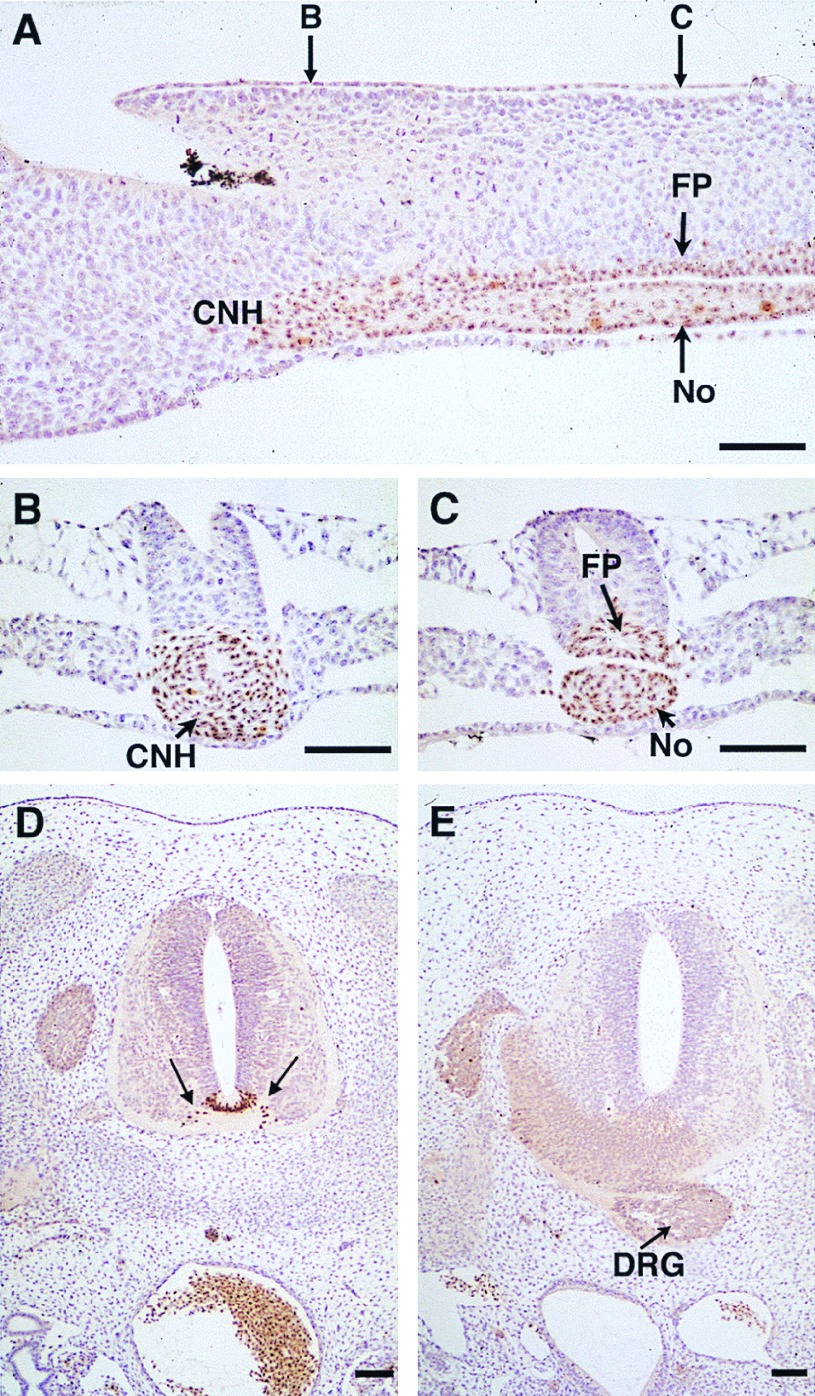
Chimeric embryos resulting from the graft of a quail Hensen’s node in a 6ss chicken embryo (see Fig. 1 A and B). (A–C) The day after the graft (25ss), the floor plate (FP), the notochord (No), and the underlying endoderm are formed of quail cells from the thoracic region down to the CNH. This is shown on sagittal (A) and transverse sections at the CNH level (B) and at the No/FP level (C). Note that in B the FP material is not yet inserted into the future neural tube. Compare with the HNF3β+ cells of Fig. 2 G and H. Section shown in C corresponds to Fig. 2F where the FP material is inserted in the neural tube formed by secondary neurulation. (D and E) Transverse sections of an equivalent embryo four days after the notochord excision in the region where the paraxial mesoderm was not segmented (see Fig. 1C). (D) The quail floor plate (arrows) is morphologically differentiated in the absence of the notochord. (E) In the most caudal region of the operation, quail cells are absent and the floor plate is lacking. Level D corresponds to the region of the back-transplanted neural tube containing the quail FP cells (see Fig. 1C). Level E corresponds to its posterior region where the midline quail cells have been removed (see Fig. 6D). A dorsal root ganglion (DRG) is located on the midline ventrally to the neural tube. (QCPN mAb and hematoxylin staining. Bars = 150 μm.)
HNF3β and Shh Expression Patterns in Notochordectomized Chicken Embryos.
With the aim of clarifying the fate of ventral midline cells of the neural tube after removal of the notochord, we performed notochord excisions according to two methods (see Materials and Methods). First, the notochord was removed in 10–15ss chicken embryos ex ovo. The HNF3β expression pattern observed immediately after the operation showed strong HNF3β expression in the midline cells of the neural tube forming the floor plate (Fig. 6 A and B)). However, at the posterior level of the neural tube, rostrally to the HN-CNH, removal of the notochord resulted in the separation of the neural plate into two lateral moieties separated by a slit into which the rostral part of the HN-CNH was previously inserted. The latter was in fact partially removed together with the notochord with which it is in continuity (Fig. 6C, see also 6D). Second, 15–25ss embryos were deprived of their notochord at the level of the entire nonsegmented region in ovo and were fixed 2 hr after the operation. In these conditions, we also observed the presence of HNF3β-positive cells on the length of the ventral midline of the neural tube except for its caudalmost part, near the CNH, which had been partly removed (not shown). Operated embryos reincubated up to E3 (35ss) were hybridized with the Shh probe. They displayed a lack of transcripts only on a short length corresponding to the region previously described as lacking HNF3β-expressing cells (data not shown). The HNF3β midline cells observed immediately after the notochord excision, which did not express Shh at the time of the operation (see Fig. 4C), had become Shh positive in the absence of the notochord.
Figure 6.
Excision of the notochord. (A–C) HNF3β expression pattern after enzymatic removal of the notochord in a 10ss chicken embryo ex ovo. (B and C) Transverse sections were performed rostrally to the CNH. (B) The HNF3β-expressing floor plate (FP) is inserted between the bilateral portions of the neural plate (NP). (C) Immediately rostral to the CNH, the FP cells have been removed and the two parts of the neural plates are separated by a hole (arrow). En, endoderm. (D) Transverse section at the level of the CNH of a 20ss chimeric embryo grafted with a quail Hensen’s node at 6ss (see Fig. 1). Separation of the notochord from the neural tube results in the removal of the anterior part of the quail CNH, which is shown detached from the chicken lateral walls of the neural tube. (QCPN mAb and hematoxylin staining. Bars = 80 μm.)
Correlation Between Floor Plate Differentiation and the Presence of Quail Cells in Notochordectomized Quail–Chicken Chimeras.
The observations reported above indicated that the absence of a floor plate after removal of the notochord did not result from a lack of induction of the former by the latter but rather from the excision of the rostral part of the CNH together with the notochord. This finding was confirmed by the fact that, when the notochordectomy was restricted to a region excluding the HN-CNH level, the neural tube developed normally with a floor plate and motoneurons (data not shown). This idea was further tested by the following experimental paradigm (see Fig. 1): a chicken embryo received the graft of a quail HN-CNH at 5–6ss. The day after (20–25ss), the neural tube/notochord complex of this chimeric embryo was removed in the region posterior to the last formed somite and submitted to an enzymatic dissociation to discard the notochord. The isolated neural tube was back-grafted into another chicken embryo of the same stage deprived of its own neural tube/notochord at the same level. The grafted embryo was reincubated for 4 more days. According to our previous observations, the only portion of the neural tube devoid of floor plate and of motoneurons should correspond to a level where no quail cells are present because they have been removed at the rostralmost part of HN-CNH that is in continuity with the notochord (Fig. 6D and Fig. 1C). Such embryos (n = 3) were examined on serial sections treated with the anti-quail QCPN mAb. In the region where the quail floor plate was present, the neural tube exhibited normal development with fully differentiated ventral horns containing motoneurons despite the absence of notochord (Fig. 5D). In contrast, the segment of the neural tube lacking quail cells and lying posteriorly in the operated region showed no motoneurons and no floor plate. Dorsal root ganglia were ventrally located at this level (Fig. 5E).
These results therefore indicate that once the floor plate material has been inserted in the neural tube during Hensen’s node regression, normal growth and patterning of the neural tube take place even in the absence of notochord. In contrast, removal of HN-CNH material that includes both notochord and floor plate results in the impaired development of the ventral neural tube.
DISCUSSION
By using the quail–chicken chimera system, we have shown that the group of cells constituting the avian organizer—i.e., Hensen’s node or cordoneural hinge—yields both the floor plate and the notochord. This material, associated with the corresponding endodermal area, moves from rostral to caudal as the embryo elongates along the anteroposterior axis, depositing in its wake three midline structures that eventually become independent from each other as they respectively become incorporated into one of the three germ layers: the floor plate dorsally in the ectoderm, the notochord in the mesoderm, and a rostrocaudal stripe of endoderm.
The fate map of the ectoderm of the sinus rhomboidalis in the 5–6ss chicken embryo revealed that posteriorly to Hensen’s node the more superficial cell layer, destined to participate in neural plate formation, is cut into two parts by regression of the organizer material. These two parts constitute the lateral walls of the neural tube joined together by the future floor plate (Fig. 7). Therefore, in amniotes, regression of Hensen’s node is an essential event in two developmental processes—i.e., gastrulation, since it participates in the segregation of the three germ layers, and neurulation, since it completes the formation of the neural plate.
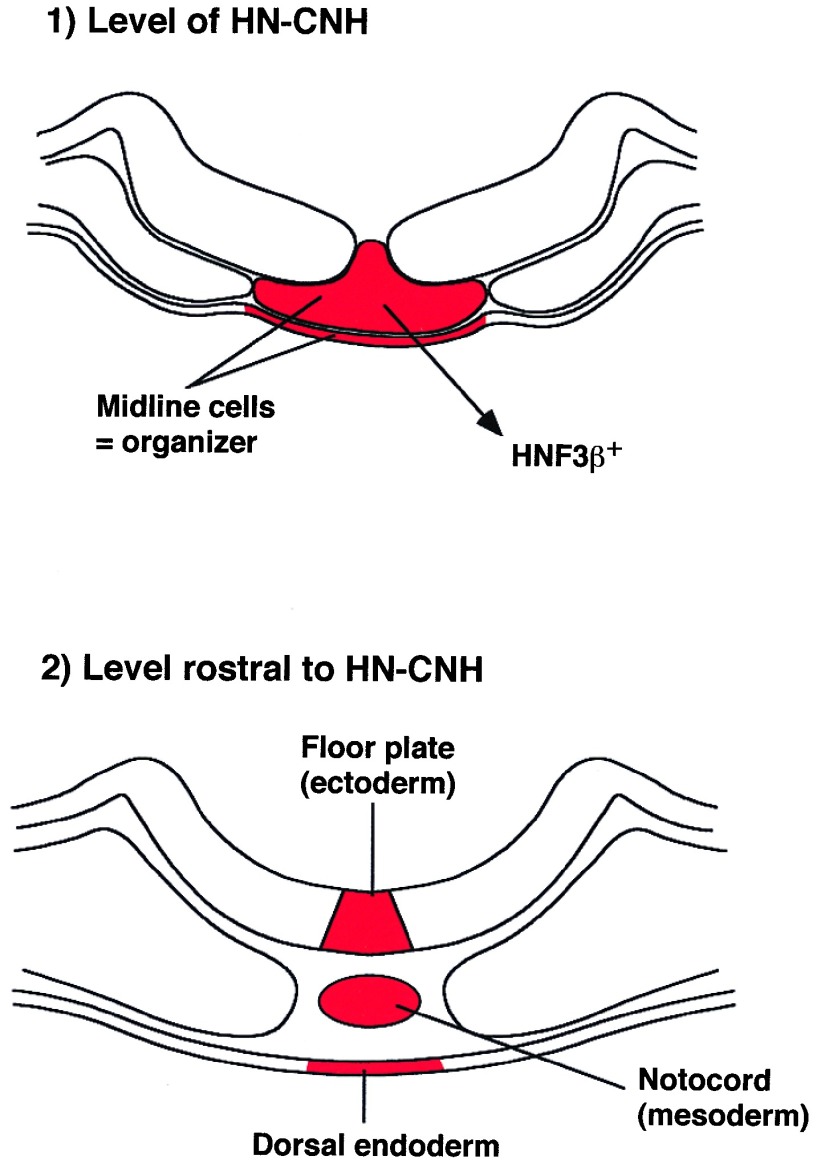
Figure 7.
Model showing the formation of the midline structures: floor plate in the ectoderm, notochord in the mesoderm, and medial endoderm from the organizer region—i.e., HN-CNH. Two levels are indicated. One is that of the organizer (HN-CNH), which undergoes a rostrocaudal movement and joins together the two lateral primordia of the neural plate. At the other level, rostral to the HN-CNH, gastrulation (that is, formation of the three germ layers) progresses in the midline by separating floor plate, notochord, and endoderm.
Our cell lineage analysis of gastrulation and neurulation (26, 27) thus leads to revision of the model previously proposed, according to which the neural plate forms first as a continuous sheet of epithelial cells in which the notochord induces the overlying ectodermal cells to become the floor plate (e.g., refs. 12, 15, and 19). In fact, the neural plate forms according to a more complex process, since its midline component, the floor plate, has an embryonic origin different from its lateral ones that yield the basal and alar plates of the neural tube and the neural crest.
The quail–chicken marker system was used to analyze the process of neurulation in the avian embryo, including not only the one going on in the cephalic and cervicotruncal part of the body (primary neurulation) but also the secondary neurulation taking place in the tail bud in which the neural tube forms from a solid cord of cells similar to the neural keel of the fish embryo. By replacing Hensen’s node of a 5–6ss chicken embryo with its stage-matched quail counterpart, we show here that, as proposed by Pasteels (7, 8), the group of cells located at the posterior tip of the notochord and floor plate in the developing tail bud is the equivalent of Hensen’s node at earlier developmental stages (see Fig. 5A)—that is, to the organizer. This is why it was designated HN-CNH (for Hensen’s node–cordoneural hinge) in this article.
We have examined in the present work some of the molecular features of the HN-CNH area and of the notochord and floor plate that it generates. We show that the winged-helix transcription factor HNF3β that has an essential role in the development of axial structures (35, 36) is strongly expressed in HN-CNH and in the notochord and floor plate throughout the neurulation process. In chimeric embryos in which HN-CNH was labeled by quail cells in the early embryonic stages (5–6ss) the derivatives of the graft strictly coincide later on with the HNF3β-expressing territories, whatever may be the stage at which the observation is performed. Concerning the Shh gene responsible for the production of the secreted protein that has been proposed to mediate the induction of the floor plate cells by the notochord (see ref. 12 and references therein), we show here that, during the early stages of neurulation (up to 4ss), it is expressed by the midline cells including floor plate and notochord, and more strongly in floor plate than in notochord immediately rostrally to the node level (see Fig. 3C). Later in development, and throughout the neurulation process, expression of Shh is down-regulated in the node and in the floor plate up to the level just posterior to the last formed somite as previously described in the chicken and other vertebrate species (18, 34). We show also that the Shh gene becomes activated to apparently normal levels in embryos notochordectomized in the region where the paraxial mesoderm is not segmented. Similar observations of expression of neural plate markers were made on the posterior region of the neural plate of stage HH10 chicken embryos explanted in a three-dimensional gel in vitro culture system, after separating it from the underlying notochord (37). A segment of neural tube formed with a normal expression pattern of Pax3 and Pax6 and showed a floor plate; the segment was later (F. Pituello and M.-A.T., unpublished results) shown to express HNF3β and Shh genes.
The above mentioned observations therefore do not support the model according to which, in normal development, Shh signaling from the notochord is responsible for induction of the expression of HNF3β and Shh in the floor plate (12, 19), since HNF3β is constantly expressed in floor plate cells from the time they arise from the node and segregate from notochordal cells (see Fig. 2). Moreover, production of Shh by floor plate cells can take place in the absence of a notochord.
These experiments, however, do not challenge the fact that under experimental conditions (i.e., in cultured explants of the neural plate) the expression of HNF3β and of other floor plate markers can be elicited in the lateral walls of the neural plate/neural tube by the notochord, the floor plate, or the Shh N-peptide (19–21, 38–40).
In the present work we have reexamined the modalities and the effect of the in vivo removal of the notochord on neural tube development in chicken embryos. One of the characteristics of the embryos subjected to the extirpation of the notochord is that the effect of the operation on the neural tube is always observed on a short length located in the very posterior end of the excised area. This level corresponds to a region where the notochord and floor plate are not yet individualized from each other but are still contained in the common cell group constituting the HN-CNH.
We have labeled the HN-CNH by a quail graft in a 5–6ss chicken embryo. Then excision of the notochord was performed when the chimeras had reached the 20–25ss. It could be seen that the segment of neural tube devoid of ventral structures (i.e., floor plate and ventral horns) at E5–6 corresponds strictly to the region devoid of quail cells in the ventral midline. In contrast, the long segment endowed with a quail floor plate had developed normally in the absence of notochord. This result supports the contention that, in the experiments in which the chicken embryos are subjected to notochordectomy, the absence of floor plate in the neural tube results from the removal of the floor plate material corresponding to the rostralmost part of the HN-CNH rather than to a lack of floor plate induction by the notochord at a later stage of development.
The fact that the floor plate does not depend upon an induction from the notochord to develop is further illustrated by the floating head (flh) mutation in zebrafish. The flh mutant lacks a notochord, and its somites fuse on the midline under the neural tube that is endowed with a floor plate. Thus in this mutant, the midline cells that express the flh gene differentiate into a floor plate while notochord development is blocked (ref. 41 and references therein), thus further showing the cell-autonomous nature of floor plate differentiation.
Acknowledgments
We deeply thank Prof. E. De Robertis for very valuable comments on the manuscript and interesting discussions in the course of the work. We are grateful to O. Malnuit for in situ hybridization and F. Viala, F. Beaujean, H. San Clemente, and C. Guilloteau for their help in preparing the manuscript. We thank R. Riddle and A. Ruiz i Altaba for Shh and HNF3β probes. The QCPN hybridoma was obtained from Developmental Studies Hybridoma Bank, Baltimore, MD, and Iowa City, IA, under contract NO1-HD-6-2915 from the National Institute of Child Health and Human Development.
ABBREVIATIONS
- CNH
cordoneural hinge
- HN-CNH
Hensen’s node–cordoneural hinge
- ss
somite stage
- HH
Hamburger and Hamilton
- En
embryonic day n
References
- 1. Spratt N T. J Exp Zool. 1955;128:121–163. [Google Scholar]
- 2.Spratt N T. J Exp Zool. 1957;134:577–612. doi: 10.1002/jez.1401340309. [DOI] [PubMed] [Google Scholar]
- 3.Rosenquist G C. Anat Rec. 1983;207:349–355. doi: 10.1002/ar.1092070214. [DOI] [PubMed] [Google Scholar]
- 4.Schoenwolf G C, Garcia-Martinez V, Dias M S. Dev Dyn. 1992;193:235–248. doi: 10.1002/aja.1001930304. [DOI] [PubMed] [Google Scholar]
- 5.Selleck M A J, Stern C D. Development (Cambridge, UK) 1991;112:615–626. doi: 10.1242/dev.112.2.615. [DOI] [PubMed] [Google Scholar]
- 6.Selleck M A J, Stern C D. Development (Cambridge, UK) 1992;114:403–415. [Google Scholar]
- 7.Pasteels J. Arch Biol. 1937;48:381–488. [Google Scholar]
- 8.Pasteels J. Arch Biol. 1943;54:1–51. [Google Scholar]
- 9.Gont L K, Steinbeisser H, Blumberg B, De Robertis E M. Development (Cambridge, UK) 1993;119:991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- 10.Wilson V, Beddington R S P. Mech Dev. 1996;55:79–89. doi: 10.1016/0925-4773(95)00493-9. [DOI] [PubMed] [Google Scholar]
- 11.Kanki J P, Ho R K. Development (Cambridge, UK) 1997;124:881–893. doi: 10.1242/dev.124.4.881. [DOI] [PubMed] [Google Scholar]
- 12.Tanabe Y, Jessell T M. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- 13.Placzek M, Tessier-Lavigne M, Yamada T, Jessell T, Dodd J. Science. 1990;250:985–988. doi: 10.1126/science.2237443. [DOI] [PubMed] [Google Scholar]
- 14.Van Straaten H W M, Hekking J W M. Anat Embryol. 1991;184:55–63. doi: 10.1007/BF01744261. [DOI] [PubMed] [Google Scholar]
- 15.Yamada T, Placzek M, Tanaka H, Dodd J, Jessell T M. Cell. 1991;64:635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- 16.Echelard Y, Epstein D J, St-Jacques B, Shen L, Mohler J, McMahon J A, McMahon A P. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 17.Krauss S, Concordet J-P, Ingham P W. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- 18.Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell T M, Dodd J. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- 19.Roelink H, Porter J A, Chiang C, Tanabe Y, Chang D T, Beachy P A, Jessell T M. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 20.Marti E, Bumcrot D A, Takada R, McMahon A P. Nature (London) 1995;375:322–325. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- 21.Ericson J, Morton S, Kawakami A, Roelink H, Jessell T M. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 22.Van Straaten H W M, Thors F, Wiertz-Hoessels E L, Hekking J, Drukker J. Dev Biol. 1985;110:247–254. doi: 10.1016/0012-1606(85)90081-8. [DOI] [PubMed] [Google Scholar]
- 23.Van Straaten H W M, Hekking J W M, Wiertz-Hoessels E L, Thors F, Drukker J. Anat Embryol. 1988;177:317–324. doi: 10.1007/BF00315839. [DOI] [PubMed] [Google Scholar]
- 24.Placzek, M., Yamada, T., Tessier-Lavigne, M., Jessell, T. & Dodd, J. (1991) Development (Cambridge, U.K.) Suppl. 2, 105–122. [PubMed]
- 25.Ruiz i Altaba A, Placzek M, Baldassare M, Dodd J, Jessell T M. Dev Biol. 1995;170:299–313. doi: 10.1006/dbio.1995.1216. [DOI] [PubMed] [Google Scholar]
- 26.Catala M, Teillet M-A, De Robertis E M, Le Douarin N M. Development (Cambridge, UK) 1996;122:2599–2610. doi: 10.1242/dev.122.9.2599. [DOI] [PubMed] [Google Scholar]
- 27.Catala M, Teillet M-A, Le Douarin N M. Mech Dev. 1995;51:51–65. doi: 10.1016/0925-4773(95)00350-a. [DOI] [PubMed] [Google Scholar]
- 28.Artinger K B, Bronner-Fraser M. Neuron. 1993;11:1147–1161. doi: 10.1016/0896-6273(93)90227-i. [DOI] [PubMed] [Google Scholar]
- 29.Pourquié O, Coltey M, Teillet M-A, Ordahl C, Le Douarin N M. Proc Natl Acad Sci USA. 1993;90:5242–5246. doi: 10.1073/pnas.90.11.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monsoro-Burq A-H, Bontoux M, Teillet M-A, Le Douarin N M. Proc Natl Acad Sci USA. 1994;91:10435–10439. doi: 10.1073/pnas.91.22.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamburger V, Hamilton H L. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 32.Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Nature (London) 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- 33.Riddle R D, Johnson R L, Laufer E, Tabin C. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 34.Marti E, Takada R, Bumcrot D A, Sasaki H, McMahon A P. Development (Cambridge, UK) 1995;121:2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- 35.Ang S-L, Rossant J. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 36.Weinstein D C, Ruiz i Altaba A, Chen W S, Hoodless P, Prezioso V R, Jessell T M, Darnell J E. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 37.Pituello F, Yamada G, Gruss P. Proc Natl Acad Sci USA. 1995;92:6952–6956. doi: 10.1073/pnas.92.15.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Placzek M, Jessell T M, Dodd J. Development (Cambridge, UK) 1993;117:205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- 39.Yamada T, Pfaff S L, Edlund T, Jessell T M. Cell. 1993;73:673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- 40.Roelink H, Tanabe Y, Jessell T M. Semin Dev Biol. 1996;7:121–127. [Google Scholar]
- 41.Halpern M E. Am Zool. 1997;37:311–322. [Google Scholar]