Abstract
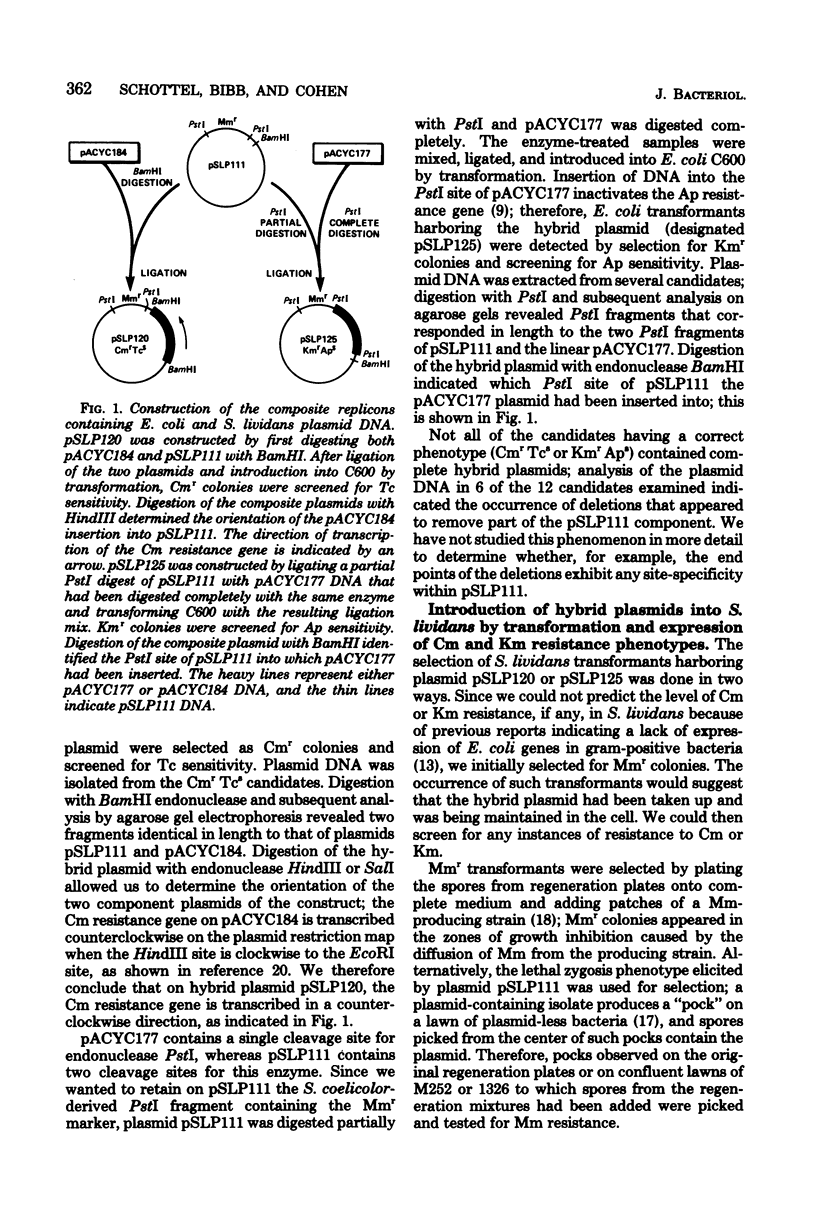
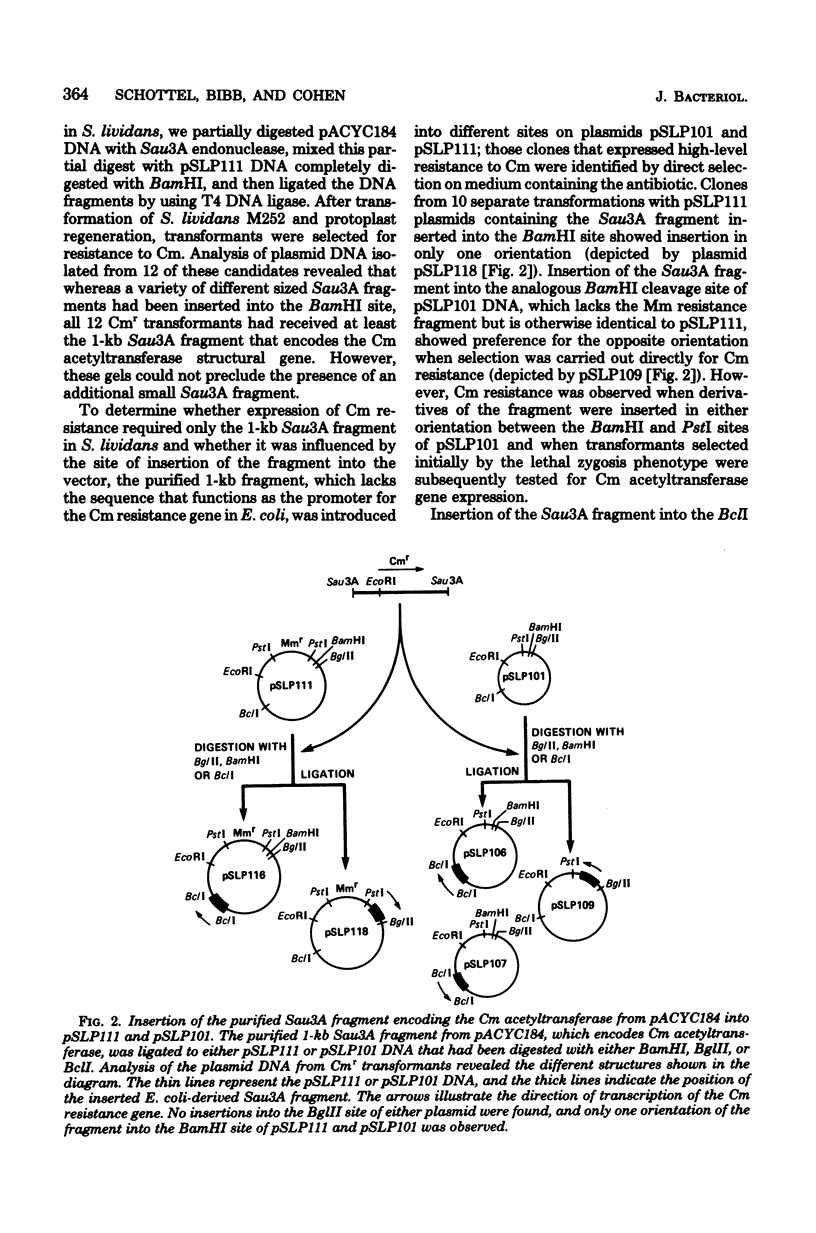
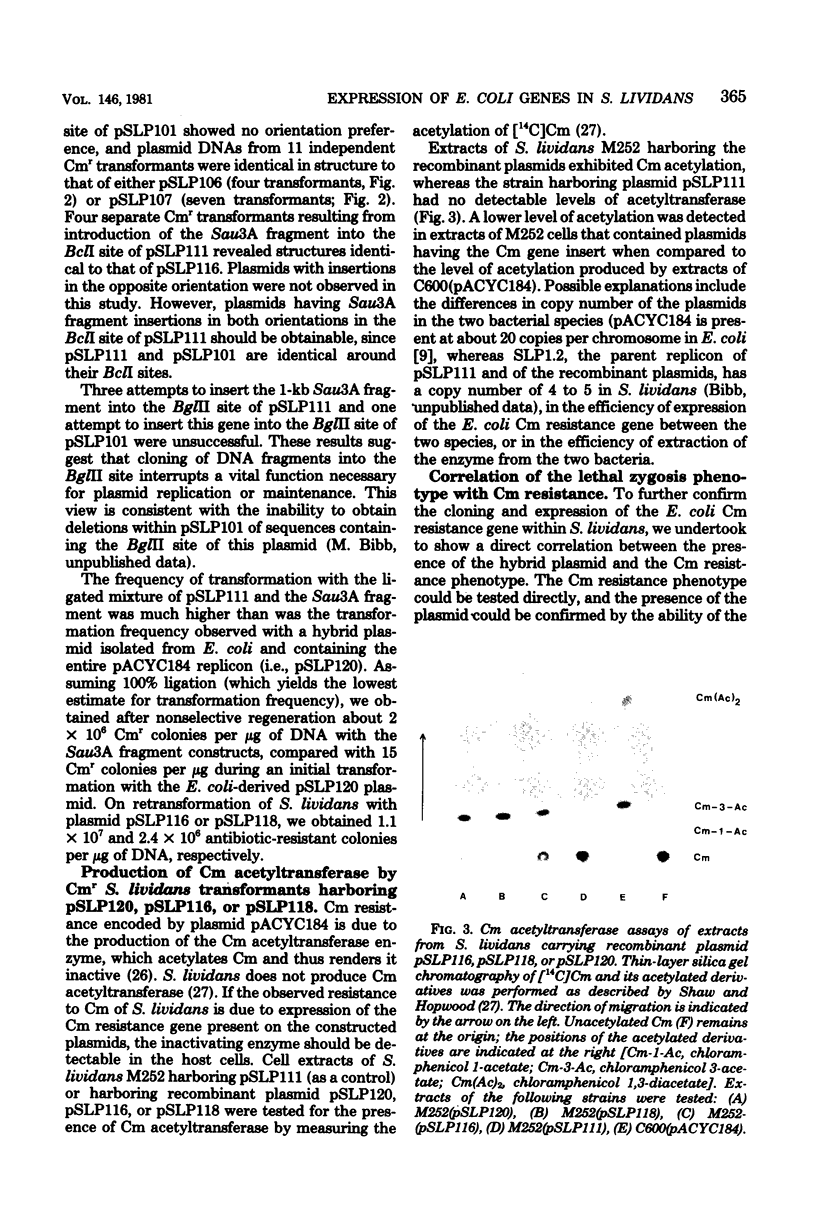
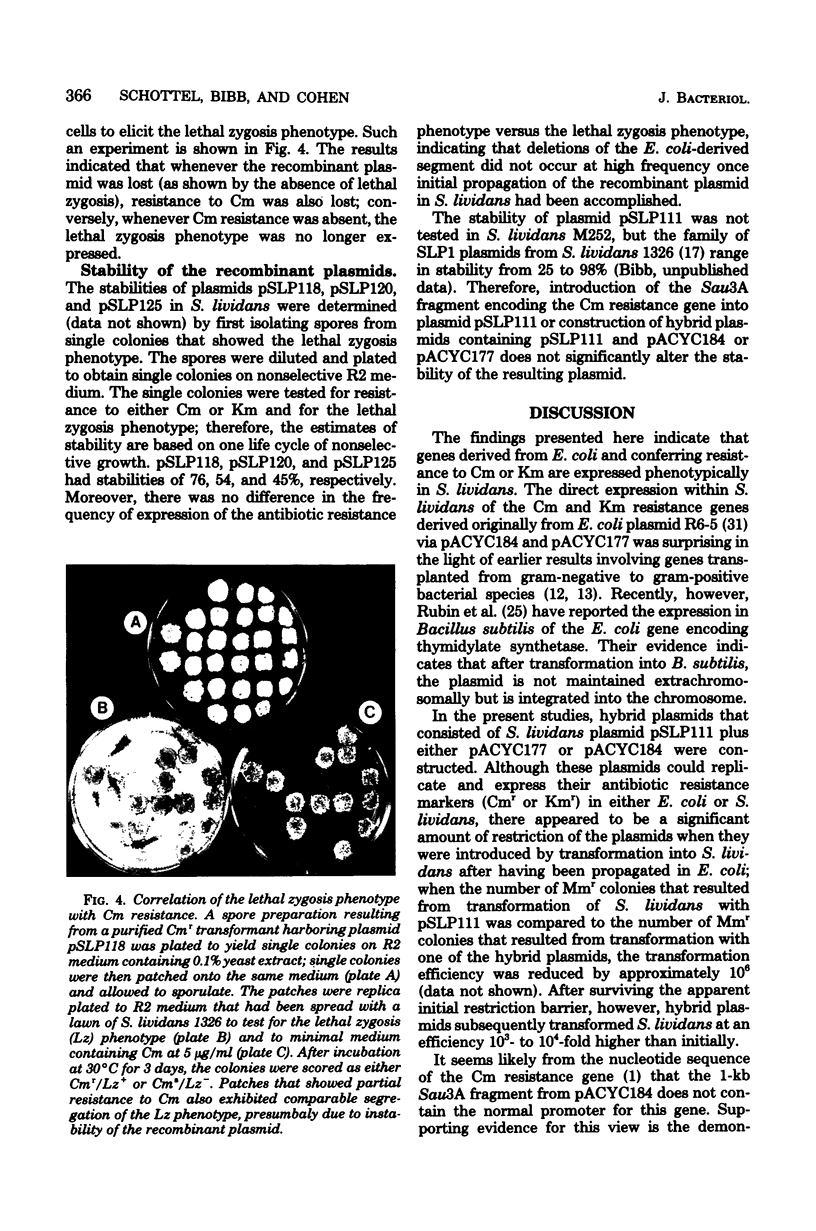
Hybrid plasmids that replicate in both Escherichia coli and Streptomyces lividans were constructed in vitro by joining the E. coli-derived plasmid pACYC184 or pACYC177, at their BamHI or PstI restriction site, respectively, to S. lividans plasmid pSLP111. After introduction of the composite replicons into S. lividans by transformation, chloramphenicol (Cm) resistance encoded by pACYC184 and kanamycin resistance encoded by pACYC177 were phenotypically expressed in the S. lividans host. A Sau3A restriction endonuclease-generated deoxyribonucleic acid fragment from pACYC184 containing the entire structural gene for the Cm acetyltransferase enzyme, but lacking the nucleotide sequence ordinarily serving as the Cm resistance gene promoter, also specified resistance to Cm when introduced in either orientation into the BamHI or BclI endonuclease cleavage site of pSLP111 or into corresponding sites of the analogous plasmid pSLP101. These findings make it unlikely that the biologically active CM acetyltransferase was being made in S. lividans as part of a fused protein, but instead indicate that the ATG start codon used for initiation of translation of the Cm resistance gene in E. coli was also utilized in S. lividans. In contrast, the synthesis of messenger ribonucleic acid that encodes the Cm acetyltransferase in S. lividans was, in at least one instance, apparently initiated at nucleotide sequences within the S. lividans plasmid vector, with resulting transcriptional read-through into the E. coli-derived deoxyribonucleic acid segment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Bibb M., Schottel J. L., Cohen S. N. A DNA cloning system for interspecies gene transfer in antibiotic-producing Streptomyces. Nature. 1980 Apr 10;284(5756):526–531. doi: 10.1038/284526a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bérdy J. Recent developments of antibiotic research and classification of antibiotics according to chemical structure. Adv Appl Microbiol. 1974;18(0):309–406. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Genome construction between bacterial species in vitro: replication and expression of Staphylococcus plasmid genes in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1030–1034. doi: 10.1073/pnas.71.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Fiandt M. Aminoglycoside-modifying enzymes of Staphylococcus aureus; expression in Escherichia coli. Gene. 1980 May;9(3-4):247–269. doi: 10.1016/0378-1119(90)90326-m. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. DNA cloning in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1433–1436. doi: 10.1073/pnas.75.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A. Genetic analysis and genome structure in Streptomyces coelicolor. Bacteriol Rev. 1967 Dec;31(4):373–403. doi: 10.1128/br.31.4.373-403.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Wright H. M., Bibb M. J., Cohen S. N. Genetic recombination through protoplast fusion in Streptomyces. Nature. 1977 Jul 14;268(5616):171–174. doi: 10.1038/268171a0. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Matzura H. Localisation of the transcription initiation site of the chloramphenicol resistance gene on plasmid pAC184. FEBS Lett. 1980 Apr 21;113(1):42–46. doi: 10.1016/0014-5793(80)80490-x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Wilson G. A., Young F. E. Expression of thymidylate synthetase activity in Bacillus subtilis upon integration of a cloned gene from Escherichia coli. Gene. 1980 Aug;10(3):227–235. doi: 10.1016/0378-1119(80)90052-9. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Hopwood D. A. Chloramphenicol acetylation in Streptomyces. J Gen Microbiol. 1976 May;94(1):159–166. doi: 10.1099/00221287-94-1-159. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J. E., Chater K. F. DNA cloning in Streptomyces: a bifunctional replicon comprising pBR322 inserted into a Streptomyces phage. Nature. 1980 Jul 31;286(5772):527–529. doi: 10.1038/286527a0. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Cabello F., Cohen S. N. Cloning and characterization of EcoRI and HindIII restriction endonuclease-generated fragments of antibiotic resistance plasmids R6-5 and R6. Mol Gen Genet. 1978 Jun 14;162(2):121–137. doi: 10.1007/BF00267869. [DOI] [PubMed] [Google Scholar]
- Wright L. F., Hopwood D. A. Identification of the antibiotic determined by the SCP1 plasmid of Streptomyces coelicolor A3(2). J Gen Microbiol. 1976 Jul;95(1):96–106. doi: 10.1099/00221287-95-1-96. [DOI] [PubMed] [Google Scholar]




