Abstract
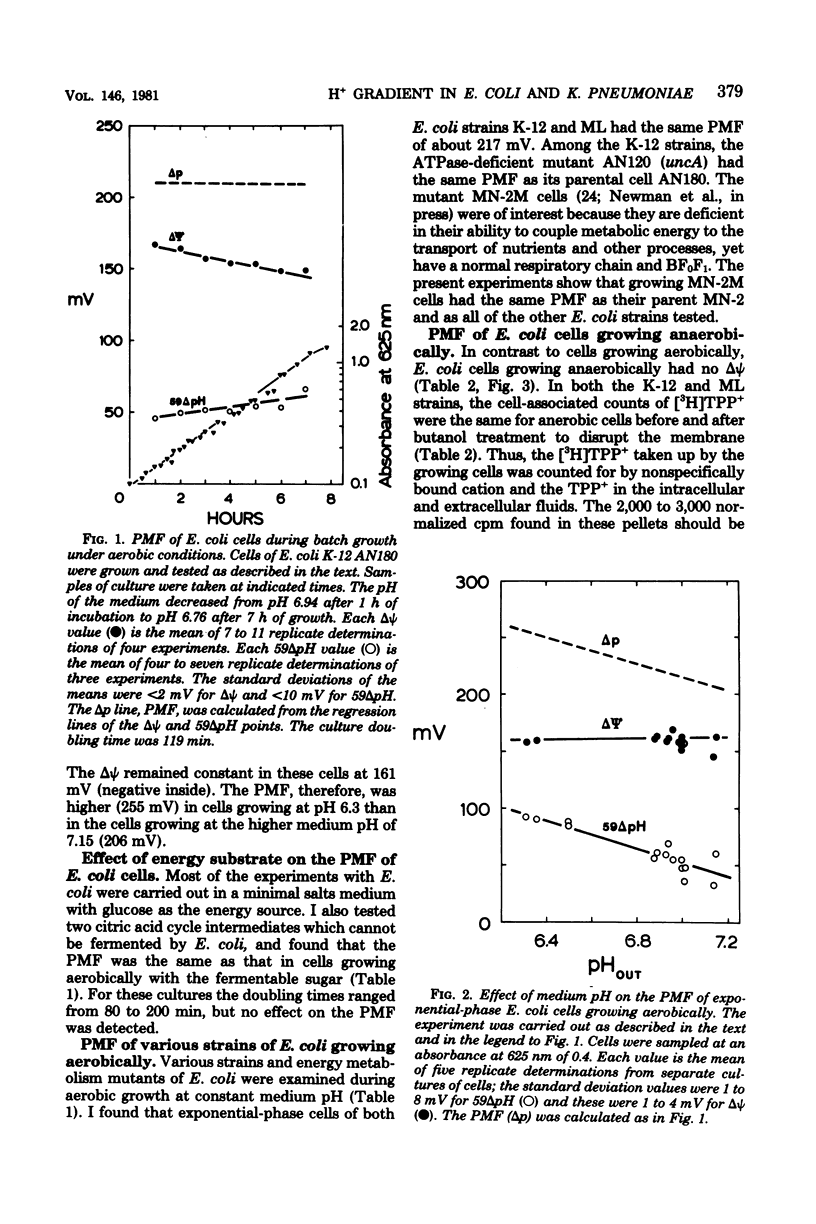
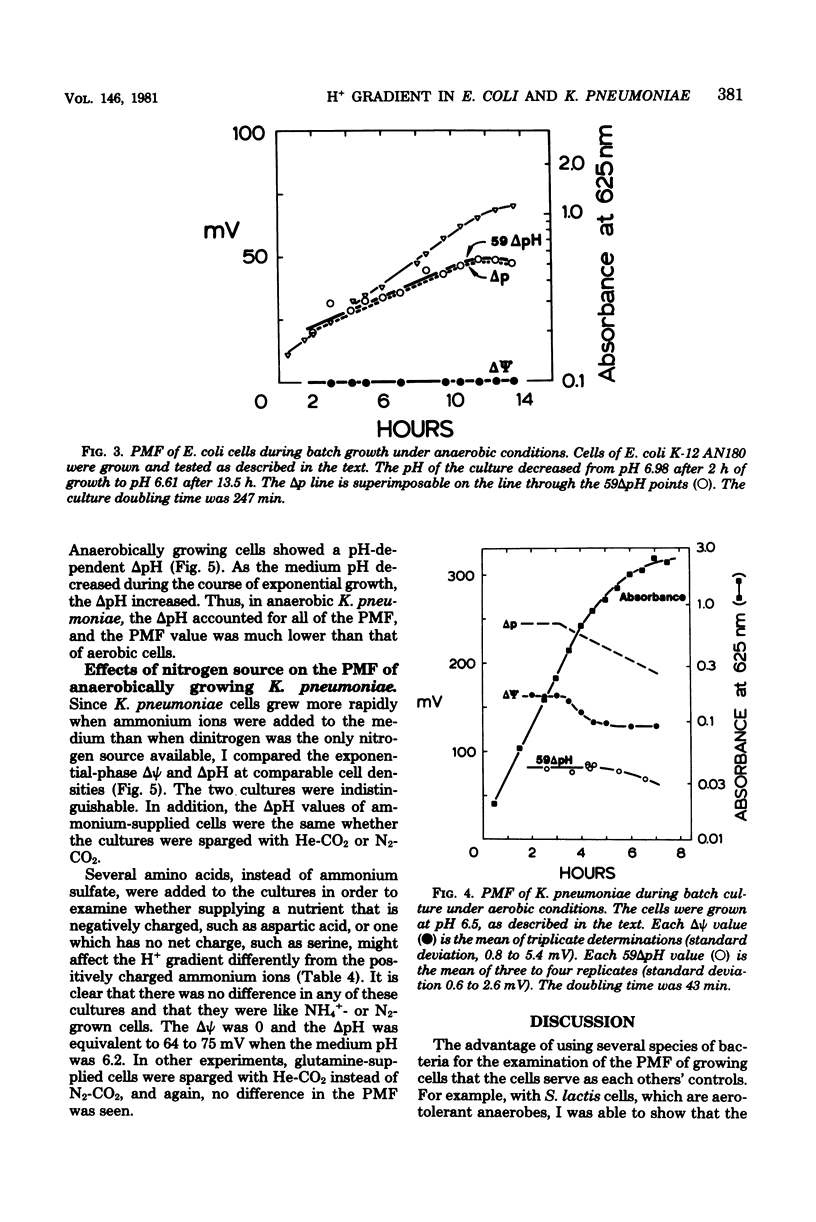
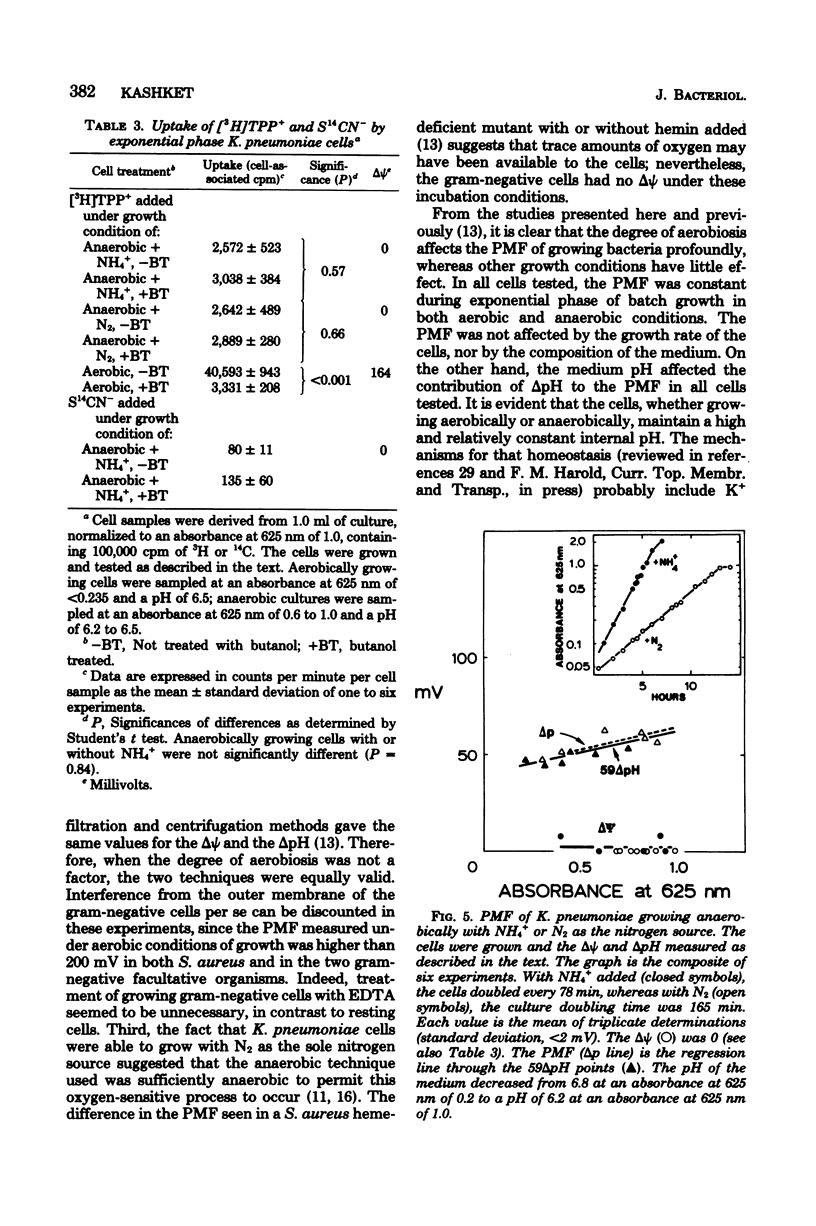
The electrochemical gradient of hydrogen ions, or proton motive force (PMF), was measured in growing Escherichia coli and Klebsiella pneumoniae in batch culture. The electrical component of the PMF (delta psi) and the chemical component (delta pH) were calculated from the cellular accumulation of radiolabeled tetraphenylphosphonium, thiocyanate, and benzoate ions. In both species, the PMF was constant during exponential phase and decreased as the cells entered stationary phase. Altering the growth rate with different energy substrates had no effect on the PMF. The delta pH (alkaline inside) varied with the pH of the culture medium, resulting in a constant internal pH. During aerobic growth in media at pH 6 to 7, the delta psi was constant at 160 mV (negative inside). The PMF, therefore, was 255 mV in cells growing at pH 6.3, and decreased progressively to 210 mV in pH 7.1 cultures. K. pneumoniae cells and two E. coli strains (K-12 and ML), including a mutant deficient in the H+-translocating ATPase and a pleiotropically energy-uncoupled mutant with a normal ATPase, had the same PMF during aerobic exponential phase. During anaerobic growth, however, both species had delta psi values equal to 0. Therefore, the PMF in anaerobic cells consisted only of the delta pH component, which was 75 mV or less in cells growing at pH 6.2 or greater. These data thus met the expectation that cells deriving metabolic energy from respiration have a PMF above a threshold value of about 200 mV when the ATPase functions in the direction of H+ influx and ATP synthesis; in fermenting cells, a PMF below a threshold value was expected since the enzyme functions in the direction of H+ extrusion and ATP hydrolysis. K. pneumoniae cells growing anaerobically had no delta psi whether the N source added was N2, NH+4 or one of several amino acids; the delta pH was unaffected. Therefore, any energy cost incurred by the process of nitrogen fixation could not be detected as an alteration of the proton gradient.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P. Accumulation of thallous ions (Tl+) as a measure of the electrical potential difference across the cytoplasmic membrane of bacteria. Biochemistry. 1978 Jul 11;17(14):2899–2904. doi: 10.1021/bi00607a031. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Harold F. M. Energy coupling to potassium transport in Streptococcus faecalis. Interplay of ATP and the protonmotive force. J Biol Chem. 1980 Jan 25;255(2):433–440. [PubMed] [Google Scholar]
- Brey R. N., Rosen B. P., Sorensen E. N. Cation/proton antiport systems in Escherichia coli. Properties of the potassium/proton antiporter. J Biol Chem. 1980 Jan 10;255(1):39–44. [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL A. Synchronization of cell division. Bacteriol Rev. 1957 Dec;21(4):263–272. doi: 10.1128/br.21.4.263-272.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Collins S. H., Hamilton W. A. Magnitude of the protonmotive force in respiring Staphylococcus aureus and Escherichia coli. J Bacteriol. 1976 Jun;126(3):1224–1231. doi: 10.1128/jb.126.3.1224-1231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H., Porter J. S., Slayman C. L., Kaback H. R. Quantitative measurements of membrane potential in Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3585–3590. doi: 10.1021/bi00556a026. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. Influence of atmospheric oxygen concentration on acetylene reduction and efficiency of nitrogen fixation in intact Klebsiella pneumoniae. J Gen Microbiol. 1976 Apr;93(2):335–345. doi: 10.1099/00221287-93-2-335. [DOI] [PubMed] [Google Scholar]
- Hill S. The apparent ATP requirement for nitrogen fixation in growing Klebsiella pneumoniae. J Gen Microbiol. 1976 Aug;96(2):297–312. doi: 10.1099/00221287-95-2-297. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell D. B., John P., Ferguson S. J. The protonmotive force in phosphorylating membrane vesicles from Paracoccus denitrificans. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J. 1978 Jul 15;174(1):257–266. doi: 10.1042/bj1740257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. Nitrogen fixation by Klebsiella grown in the presence of oxygen. Can J Microbiol. 1972 Dec;18(12):1845–1850. doi: 10.1139/m72-288. [DOI] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- Laane C., Krone W., Konings W. N., Haaker H., Veeger C. The involvement of the membrane potential in nitrogen fixation by bacteroids of Rhizobium leguminosarum. FEBS Lett. 1979 Jul 15;103(2):328–332. doi: 10.1016/0014-5793(79)81355-1. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Morris J. F., Newman E. B. Map location of the ssd mutation in Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1504–1505. doi: 10.1128/jb.143.3.1504-1505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A. Mutant of Escherichia coli defective in response to colicin K and in active transport. J Bacteriol. 1976 Feb;125(2):467–474. doi: 10.1128/jb.125.2.467-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam K. T., Valentine R. C. Microbial production of ammonium ion from nitrogen. Proc Natl Acad Sci U S A. 1975 Jan;72(1):136–139. doi: 10.1073/pnas.72.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher S., Gurney E., Valentine R. C. Transduction of the nitrogen-fixation genes in Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1174–1177. doi: 10.1073/pnas.68.6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbjarnardóttir S. H., Magnúsdóttir R. A., Eggertsson G. Mutations determining generalized resistance to aminoglycoside antibiotics in Escherichia coli. Mol Gen Genet. 1978 Apr 25;161(1):89–98. doi: 10.1007/BF00266619. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Pengra R. M. Effect of amino acids on the nitrogenase system of Klebsiella pneumoniae. J Bacteriol. 1966 Sep;92(3):618–622. doi: 10.1128/jb.92.3.618-622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]



