Abstract
The exact pathophysiological mechanisms responsible for gastroesophageal reflux disease (GERD) remain unclear. Recent studies have shown that mucosal immune and inflammatory responses, characterized by specific cytokine and chemokine profiles, may underlie the diverse esophageal phenotypes of GERD. Interleukin 8 (IL-8), a representative chemokine, mediates neutrophil trafficking via its receptors, mainly CXCR-1. The IL-8 mRNA and protein levels are increased in the esophageal mucosa, not only in reflux esophagitis (RE), but also in endoscopy-negative GERD (NERD), through activation of nuclear factor-κB (NF-κB), which is a pivotal transcription factor. Mucosal IL-8 concentrations have been found to parallel the endoscopic severity of RE, implying that this cytokine is a key player in the development of GERD. The mucosal levels of the C-C chemokines, macrophage chemoattractant protein 1 (MCP-1) and regulated on activation normal T-cell-expressed and presumably secreted (RANTES), which primarily attract monocytes and lymphocytes to the site of inflammation, respectively, are also elevated in RE. The secreted levels of IL-8 and IL-1β, a prototype of proinflammatory cytokine, are maximal at the proximal segment within Barrett esophagus (BE) tissue. The expression of the two pleiotrophic proinflammatory cytokines, IL-6 and tumor necrosis factor α, is enhanced in the intestinal epithelium of BE, which places this epithelium at a higher risk for developing malignancy. BE is characterized by a distinct Th-2 predominant cytokine profile (IL-4 and -10), compared to the proinflammatory nature of RE (interferone-γ). Treatment with a proton pump inhibitor, lansoprazole reduces the mucosal levels of IL-8 mRNA and protein in GERD, including RE and NERD. This may occur in part through an anti-inflammatory action of proton pump inhibitors beyond gastric acid inhibition.
Keywords: GERD, reflux esophagitis, Barrett’s esophagus, IL-8, proinflammatory cytokine
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common disorders. In the Western world, it is extremely common; over 30% of the general population suffers from at least monthly reflux episodes. GERD may lead to the development of serious complications, including ulcers, strictures, bleeding, Barrett’s esophagus, and eventually, adenocarcinoma of the esophagus [1–3]. It is currently thought that reflux of gastric contents may occur as a result of the transient relaxation of the lower esophageal sphincter (TLESR) unrelated to swallowing or secondary peristalsis, and that repeated episodes of reflux over time lead to impairment of LES tone and esophageal exposure to refluxates, which results in GERD [3, 4]. Thus, attention has been paid to the role of injurious agents in gastric reflux, such as hydrochloric acid, pepsin, and bile acid, and the mechanisms by which the esophageal epithelium fails to resist the chemical’s adverse effect have been studied [2].
However, approximately 60% of GERD patients have no mucosal lesions on endoscopy, 30% have reflux esophagitis (RE), and 10% have Barrett’s esophagus [5–8]. To date, the exact physiological mechanisms responsible for the diverse esophageal phenotypes of GERD are poorly understood. The frequency and duration of exposure to acid and bile, as evaluated by 24-h monitoring, vary widely among cases of GERD with different endoscopic findings [9–11]. Experimental acid- and pepsin-induced esophagitis in rabbits mimics human RE, but the degree of esophageal mucosal damage is not correlated with the amount of reflux material [12]. Thus, the severity and the various GERD phenotypes cannot be predicted solely on the basis of esophageal exposure to refluxates; this suggests that other factors are involved.
In experimental RE and in human subjects, it has been reported that some of the substances that are considered to be critical in the etiology of RE are classic inflammatory products, such as prostanoids and reactive oxygen species (ROS) [13, 14]. Moreover, recent studies have shown that mucosal immune and inflammatory responses, characterized by specific cytokine and chemokine profiles, may underlie the diverse esophageal phenotypes of GERD [15–19]. In fact, we found enhanced expression of interleukin 8 (IL-8), a representative chemokine, in esophageal biopsy samples of patients with non-erosive GERD (NERD), as well as RE; the IL-8 protein levels paralleled the endoscopic severity of RE [16, 17]. Of note, lansoprazole, a potent proton pump inhibitor (PPI) that is widely used in Japan, decreased IL-8 expression in the esophageal mucosa of GERD patients [16, 17].
We reviewed the current data dealing with the diverse proinflammatory cytokines implicated in the pathogenesis of GERD. This article also provides information on the effects of PPIs on immune and inflammatory responses that occur through the reduction of proinflammatory cytokines.
The Involvement of Various Cytokines Located in the Esophageal Mucosa in the Pathogenesis of GERD
IL-8 and GERD
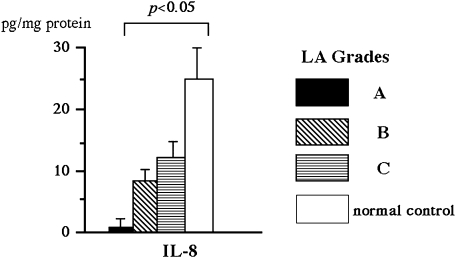
Chemokines are a group of cytokines with potent chemotactic activity towards different populations of leukocytes; they are involved in the pathogenesis of a variety of inflammatory conditions. IL-8 exhibits a potent chemotactic activity for neutrophils and plays a critical role in the induction of acute and chronic inflammatory reactions [20, 21]. For example, in patients with Helicobacter pylori (H. pylori)-related gastritis, enhanced production of IL-8 in gastric mucosa has been reported [22]. The selective infiltration into the esophagus of the polynuclear leukocyte subset suggests that this chemokine plays a role in the immune and inflammatory processes of GERD, and this is the case. Fitzgerald et al. [15] found that, compared to subjects with noninflamed or Barrett’s esophagus, GERD patients had significantly higher expression levels of IL-8 messenger ribonucleic acid (mRNA), as assessed by competitive reverse transcriptase polymerase chain reaction (RT-PCR). Our studies have also found that Japanese RE patients had increased relative IL-8 mRNA expression levels, as assessed using real-time PCR technology [17]. In addition, IL-8 protein levels measured by enzyme-linked immunosorbent assay (ELISA) were increased to a greater degree in the esophageal biopsy samples obtained from RE patients compared to normal controls [16]. The mucosal IL-8 concentrations were found to parallel the endoscopic severity of RE (Fig. 1) [16]. Based on immunohistochemical analysis with anti-IL-8 antisera, IL-8 expression was found in the epithelium of esophageal biopsy specimens (Fig. 2) [22]. In particular, GERD patients had intense immunoreactivity against anti-IL-8 antibody. Not only epithelial cells but also infiltrating leukocytes showed immunoreactivity for IL-8 antigen. In contrast, there was little or no expression of IL-8 in normal control subjects who were free of any histopathological abnormality. Recently, we demonstrated that the relative IL-8 mRNA expression levels were significantly higher in the esophageal mucosa of NERD patients than controls [17, 19, 23]. It is of clinical importance that IL-8 production is enhanced in such incipient GERD patients who do not have mucosal breaks. The study also found that NERD patients who were classified in grade M based on the modified Los Angeles (LA) system [24] had significantly higher IL-8 mRNA expression levels than those classified in grade N; this highlights the immunologic and endoscopic heterogeneity among NERD patients [23]. Collectively, IL-8, which is produced locally by esophageal epithelium and inflammatory cells, is involved in the pathogenesis of GERD, including NERD, and plays a role in the development and progression of RE.
Fig. 1.
The relationship between chemokine levels and endoscopic grading of reflux esophagitis based on the Los Angeles classification. IL-8 levels were correlated with the severity (grade). On the other hand, although MCP-1 and RANTES concentrations tended to be higher with increased RE severity, the correlations were not statistically significant. Reprinted with permission [16].
Fig. 2.
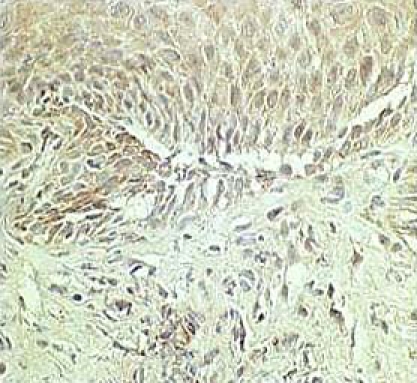
Based on immunohistochemical analysis with anti-IL-8 antisera, IL-8 expression was found in the epithelium of esophageal biopsy specimens. Reprinted with permission [22].
In patients with long segmental Barrett esophagus (BE), there was a proximal-distal gradient within the BE with respect to the IL-8 expression, as assessed by ELISA following organ culture [18]. IL-8 levels were significantly higher in the proximal than in the distal BE segment. In agreement with this finding, on light microscopy, inflammation was maximal at the new squamocolumnar junction associated with the esophagitis. The regional variation of IL-8 expression may be germane to the distribution of inflammatory and malignant complications seen with BE.
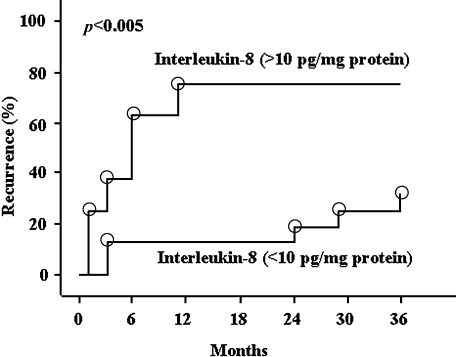
The elevated levels of IL-8 mRNA and protein levels were significantly decreased after treatment with lansoprazole in RE and NERD patients; these patients had endoscopic healing of the disease and cure of the reflux symptoms, which further highlights the important role of this chemokine in the pathogenesis of GERD [16, 17, 19]. More recently, we conducted a long-term follow-up study dealing with the association of IL-8 protein levels in the esophageal mucosa with RE relapse (Fig. 3) [25]. Thirty-one outpatients with RE, graded according to the LA classification as A or B, not treated with any anti-secretory drugs after healing with 8 weeks of lansoprazole treatment, were enrolled in this study. The estimated RE relapse within 3 years was 75% and 31.3% in patients with IL-8 levels greater than and less than 10 pg/mg protein, respectively. Using Cox’s proportional hazards regression model, RE recurrence was frequently observed in patients who had higher IL-8 levels (>10 pg/mg protein) (odds ratio, 3.5; 95% confidence interval, 1.3–12.8, p<0.01). These preliminary results highlight the fact that mucosal IL-8 levels are an indicator of RE relapse.
Fig. 3.
Kaplan-Meier analysis: incidence of reflux esophagitis recurrence in patients who had esophageal mucosal interleukin 8 levels that were greater than 10 pg/mg protein (n = 8) or less than 10 pg/mg protein (n = 23). Reprinted with permission [25].
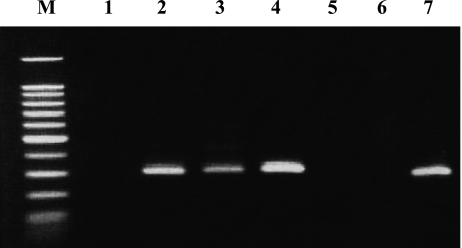
Evidence is accumulating that chemokines exert an overlapping but distinct action on specific types of leukocytes by interacting with their specific G-protein-coupled receptors that have 7 transmembrane domains [26]. To date, CXC chemokine receptor1 (CXCR-1) and -2 have been found to be two distinct receptors for IL-8 [27]. It is possible that increased IL-8 levels may facilitate trafficking of neutrophils into the mucosa affected by GERD through IL-8’s interaction with these receptors. Based on this hypothesis, we studied CXCR-1 and -2 mRNA expression using RT-PCR [28]. CXCR-1 (Fig. 4) as well as CXCR-2 mRNA was evidently expressed in esophageal mucosa of GERD patients. On the real-time PCR basis, in NERD patients, the relative CXCR-1 mRNA levels, expressed as the ratio of CXCR-1/tubulin alpha 3 (housekeeping gene) in arbitrary units using real-time PCR, were significantly higher than in controls [28]. On the other hand, the relative expression levels of CXCR-2 mRNA were comparable between GERD patients and controls. No significant correlation was noted between the mRNA expression levels of CXCR-1 and CXCR-2. As shown previously, intraepithelial neutrophils, a histopathological indicator of RE [29], were immunoreactive for CXCR-1 in the esophageal mucosa of GERD patients [17]. The enhanced expression of this specific IL-8 receptor could make the affected esophageal mucosa more responsive to locally overexpressed IL-8; thus, enhanced expression of IL-8 and CXCR-1 could mutually encourage the trafficking of neutrophils into esophageal mucosa [27, 30–32]. IL-8 is regulated in an autocrine manner [33, 34]; therefore, the interplay between IL-8 and CXCR-1 may contribute to the amplification and protraction of inflammatory responses in GERD. In this regard, on immunohistochemical analysis, epithelial cells of GERD patients were found to express abundant IL-8 antigen, and, at the same time, the epithelium was the potential cellular source of CXCR-1 protein [17]. IL-8 exerts mitogenic actions directly or by binding to its receptors on epithelial cells [35]. In fact, IL-8 mRNA and protein levels are found to be associated with basal cell hyperplasia and papillary elongation [16, 17], both of which are histopathological hallmarks of RE [29]; these findings support the notion that IL-8, possibly along with other cytokines and growth factors, could contribute to esophageal epithelial cell proliferation even in the early stage of incipient GERD and could eventually lead to carcinogenesis. Unlike CXCR-1, CXCR-2 is not specific for IL-8 and can bind to other chemokines, such as growth-related oncogene α; however, CXCR-2 has a 2- to 5-fold higher affinity for IL-8 than CXCR-1 [36]. Further research is needed to elucidate the diverse receptor-mediated signaling pathway that is part of the pathophysiological mechanisms of GERD.
Fig. 4.
The CXCR-1 mRNA transcripts were detected as 257 bp band using the reverse transcriptase polymerase chain reaction. Each lane represents the following: 1; asymptomatic normal control, 2; endoscopy-negative GERD patient, 3–5; reflux esophagitis patients, 6; non-template negative control and 7; positive control.
Proinflammatory cytokines other than IL-8 and GERD
There is limited information on the possible involvement of proinflammatory cytokines other than IL-8 in GERD [16, 37]. In our previous work, the levels of regulated on activation normal T-cell-expressed and presumably secreted (RANTES) and macrophage chemoattractant protein 1 (MCP-1) in esophageal biopsy samples of RE patients were measured [16]. In that study, the mucosal levels of RANTES and MCP-1 were significantly higher in RE patients than in normal controls. Though the mucosal MCP-1 and RANTES concentrations tended to be higher with increased LA grade severity, they were not statistically significant. RANTES selectively regulates eosinophil trafficking [38]. In line with this, the RANTES levels in esophageal mucosa containing intraepithelial eosinophils were significantly higher than in mucosa lacking the cells [16]. MCP-1, along with RANTES, constitutes the C-C chemokine class and attracts primarily monocytes and lymphocytes to the site of inflammation [38, 39]. It is accepted that the accumulation of mononuclear cells in the esophageal lamina propria, but not within the epithelium, is indicative of RE [29, 40, 41]. However, most endoscopic biopsy samples contain small amounts of lamina propria tissues. Thus, a proper assessment of the relationship between mucosal production of MCP-1 and mononuclear cell infiltration is difficult. This may also explain the discrepant results reported by Uchiyama et al. showing that the mucosal MCP-1 mRNA levels in RE patients were not correlated with endoscopic RE severity [37].
Fitzgerald et al. [18], using ELISA following organ culture, examined concentrations of Il-1β, which is a representative proinflammatory cytokine, secreted by esophageal biopsy samples obtained from BE patients. Similar to IL-8, the IL-1β levels were maximal at the proximal segment within BE. Intriguingly, pulsatile but not continuous exposure of the BE specimens to acid and bile significantly increased expression of IL-1β. In our study, there were no significant differences in mucosal IL-1β levels between the RE and control groups; however, the IL-1β levels were significantly correlated with esophageal mucosal IL-8 production [16]. In fact, a transcription factor nuclear factor κB (NF-κB), which regulates gene expression of a plethora of immune and inflammatory mediators including IL-8, IL-1β, and tumor necrosis factor α (TNF-α) [42, 43], was found to be activated in the esophageal mucosa of GERD patients [17]. Classically, the most common NF-κB dimeric complexes are the p65-p50 heterodimers or the p50 homodimers [42, 43]. The NF-κB dimers are sequestered in the cytoplasm in an inactive form by their association with inhibitory κB (I-κB). Following cellular stimulation by proinflammatory cytokines such as IL-1 and TNF-α, reactive oxygen species, and microbial infection, multiple kinase cascades lead to the phosphorylation of I-κB and its subsequent degradation; this allows NF-κB to become active and then translocate into the nucleus [42–44]. In line with this, p65 and p50 subunit immunoreactivities were mainly observed in the nuclei of epithelial cells of GERD patients, whereas the dimeric proteins were expressed at lower levels in the epithelium of asymptomatic controls [17]. We also demonstrated colocalization of activated NF-κB with the expression of IL-8, which indicates a functional role for this transcription factor [17]. Accordingly, the following changes likely take place within the esophageal mucosa of GERD: (1) activation and translocation of NF-κB to the nuclei of epithelial cells possibly in response to proinflammatory cytokines and/or direct stimulation by gastroduodenal refluxates; (2) up-regulation of IL-8 mRNA induced by the activated NF-κB system; and (3) local overexpression of the IL-8 protein.
Epithelial expression of TNF-α, as well as its receptor TNFR1, was up-regulated during the progression of BE to adenocarcinoma, based on immunohistochemistry and Western blot analysis findings [45]. In contrast, a low expression level was present in inflamed and non-inflamed squamous epithelium. Unexpectedly, in Barrett’s adenocarcinoma cells, TNF-α induced transcription of an oncogene, c-myc, via a β-catenin mediated pathway that is independent of NF-κB [45].
Th 1/Th 2 cytokine profile and GERD
Recent studies have shown that expression of interferon γ (IFN-γ), which is a representative Th 1 cytokine, as assessed by semi-quantitative RT-PCR methodology, was markedly increased in esophageal biopsy samples obtained from RE patients compared those from non-inflamed squamous mucosa or BE patients; there was a modest increase in anti-inflammatory IL-10 expression and no increase in IL-4 expression [15]. On the other hand, in BE mucosa, the expression of IFN-γ was similar to that seen in non-inflamed squamous mucosa; however, in BE mucosa, the expression of Th-2 cytokines IL-10 and IL-4 was significantly increased compared to unaffected squamous esophagus or RE mucosa. Thus, in contrast with the proinflammatory nature of RE, BE is characterized by a distinct Th-2 predominant cytokine profile [15]. Furthermore, the proportion of Th 2 effector cells (plasma cells and mast cells) was higher in esophageal biopsy specimens obtained from BE patients than from RE patients [46]. On immunohistochemistry, most plasma cells in BE and RE samples expressed IgG, but several IgE-positive plasma cells were detected in BE samples; these were rare in RE. In addition, isolated lymph follicles were commonly observed in BE patients, but not in RE patients. Thus, in BE, the inflammatory response is skewed towards a more pronounced humoral immune response [46].
IL-6, a pleiotropic cytokine associated with a Th 2 response, can be involved in malignant transformation and tumor progression [47]. Dvorakova et al. [48], using ELISA, reported that increased IL-6 levels were secreted from BE tissue in conditioned media compared with squamous mucosa; on immunohistochemical analysis, this cytokine was found to be expressed in intestinal glandular epithelium in BE tissue. The activation of signal transducer and activator of transcription 3 was observed, with subsequent increased expression of anti-apoptotic genes, such as myeloid cell leukemia 1 and Bcl-xL. These data suggest a prominent role for IL-6 in the development of BE and in apoptotic resistance. This places BE epithelium at higher risk of developing malignancy.
Lansoprazole Treatment and Proinflammatory Cytokines in GERD
In GERD patients, several reports have described the effects of lansoprazole treatment on expression levels of diverse proinflammatory cytokines. Using ELISA, we examined IL-8, MCP-1, RANTES, and IL-1β level changes in NERD patients’ esophageal mucosa after 8 weeks of lansoprazole therapy [16, 17, 19]. Pretreatment IL-8 concentrations were significantly decreased compared to posttreatment levels [16]. Furthermore, real-time PCR analysis revealed IL-8 mRNA expression levels were significantly reduced after 8-week lansoprazole therapy in NERD patients [17]. Similarly, Yoshida et al. [19] reported that lansoprazole significantly decreased both IL-8 mRNA and protein levels in GERD patients, including the range of patients from NERD to RE. To date, there have been no data published on the effects of PPIs other than lansoprazole on esophageal expression of proinflammatory cytokines in human GERD.
There are several animal GERD models, primarily involving rats. In a rat chronic acid RE model, RE was induced by ligating the transitional region between the forestomach and the glandular portion and then wrapping the duodenum near the pylorus with a catheter; using this model and real-time PCR, Fujiwara et al. [49, 50] analyzed the expression and dynamics of IL-1β, TNF-α, MCP-1, macrophage inflammatory protein 1α (MIP-1α), MIP-2, and GRO/cytokine-induced neutrophil chemoattractant-2α (CINC-2α), which corresponds to IL-8 in humans. The mRNA expression levels of each proinflammatory cytokine were significantly increased in the esophageal lesions compared to normal esophagus. In regions with esophagitis, numerous inflammatory leukocytes were present in the lamina propria, and, on immunochemistry, the submucosal layer had positive reactions for these cytokines, and the endothelial cells stained intensely for ICAM-1. The numbers of leukocyte function-associated antigen 1 (LFA-1) and CD11b/CD18-positive cells were significantly increased in rats with chronic esophagitis. These results demonstrate the importance of proinflammatory cytokines and adhesion molecules in the pathogenesis of rat chronic RE. In this model, treatment with the PPI rabeprazole almost completely inhibited the development of RE and significantly decreased the expression of cytokines [50]; this suggests that gastric acid reflux may play a role in the induction of chronic esophageal inflammation. In a rat acute RE model, which was induced by inserting a caliber ring into the duodenum, treatment with ranitidine, a histamine-2 receptor antagonist (H2RA), did not affect the severity of the macroscopic and microscopic appearance of the experimentally induced esophagitis [51]. However, compared to ranitidine treatment, a novel antioxidant agent, DA-9601, markedly attenuated the gross esophagitis and the histological degree of esophageal inflammation [51]. In addition, in a rat chronic gastroduodenal reflux model, in which a esophagogastroduodenal anastomosis was generated between the jejunum and the gastroesophageal junction, rabeprazole and nizatidine, the H2RA, did not affect the severity of mucosal hyperplastic scores or the histological parameters of esophagitis [52]. In contrast, treatment with a protease inhibitor, camostat mesilate, was associated with a significant decrease in mucosal hyperplastic and inflammatory scores, and the enhanced expression of CINC-1, another IL-8-like chemokine [37, 49] in inflamed esophageal mucosa, which was induced by the combined refluxates, was markedly inhibited in the camostat mesilate-treated group [52]. Thus, not only gastric acid but also duodenal contents, including pancreatic proteases, are involved in esophageal immune and inflammatory responses through locally enhanced production of proinflammatory mediators. In vitro, bile acid deoxycholic acid, even at neutral pH, induces IL-8 expression in Barrett’s adenocarcinoma OE 33 cells through activation of NF-κB; this is linked to transduction of downstream survival factors [53], which suggests that a PPI given alone may not be able to prevent the progression of Barrett’s epithelium to cancer.
Nevertheless, evidence is accumulating that highlights the anti-inflammatory action of PPIs, such as lansoprazole and omeprazole, which is independent of gastric acid inhibition [54–56]. In fact, lansoprazole treatment suppresses the induction of proinflammatory cytokines (IL-1β, TNF-α, and IL-6) in murine peritoneal macrophages lacking P-type ATPase [56]. More recently, it has been shown that exposure to either PPI results in a strong induction of heme oxygenase-1, which has a central role in cellular antioxidant defenses and exerts anti-inflammatory actions [57–59] at both the mRNA and protein levels; this leads to an increased activity of this enzyme in gastric and endothelial cells [55]. It is unknown whether PPIs can induce heme oxygenase-1 in esophageal cells in vitro and in vivo; however, it is tempting to speculate that the improvement in esophageal immune and inflammatory responses seen with PPI treatment may be in part mediated by beneficial anti-inflammatory effects that go beyond acid suppression [55, 60]. Further studies are warranted to establish new therapeutic avenues for PPI treatment in preventing inflammatory disorders, including GERD.
Conclusions
Diverse proinflammatory cytokines are associated with the development and progression of GERD. In particular, a chemokine, IL-8, produced in esophageal mucosa plays a pivotal role in the pathogenesis of GERD including NERD through its specific receptor. Mucosal IL-8 levels are correlated with endoscopic RE severity and provide information about the risk of disease relapse. Other chemokines, such as RANTES and MCP-1, are also involved in RE pathogenesis. The BE-dysplasia –carcinoma sequence may be regulated by the overexpression of proinflammatory cytokines, such as IL-1β, TNF-α, and IL-6. BE is characterized by a Th-2 predominant cytokine profile, in contrast to the Th-1 nature of RE. Lansoprazole treatment reduces the mucosal levels of IL-8 expression in human GERD. This may occur in part through anti-inflammatory actions of PPIs, independent of gastric acid suppression.
References
- 1.Odze R.D. Pathology of the gastroesophageal junction. Semin. Diagn. Pathol. 2005;22:256–265. doi: 10.1053/j.semdp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Moayyedi P., Talley N.J. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086–2100. doi: 10.1016/S0140-6736(06)68932-0. [DOI] [PubMed] [Google Scholar]
- 3.Cheng L., Harnett K.M., Cao W., Liu F., Behar J., Fiocchi C., Biancani P. Hydrogen peroxide reduces lower esophageal sphincter tone in human esophagitis. Gastroenterology. 2005;129:1675–1685. doi: 10.1053/j.gastro.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Dent J., Holloway R.H., Toouli J., Dodds W.J. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastrooesophageal reflux. Gut. 1988;29:1020–1028. doi: 10.1136/gut.29.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heading R. Epidemiology of oesophageal reflux disease. Scand. J. Gastroenterol. 1989;24:25–36. [PubMed] [Google Scholar]
- 6.Winters C., Jr., Spurling T.J., Chobanian S.J., Curtis D.J., Esposito R.L., Hacker J.F., 3rd, Johnson D.A., Cruess D.F., Cotelingam J.D., Gurney M.S. Barrett’s esophagus: A prevalent occult complication of gastro-esophageal reflux disease. Gastroenterology. 1987;92:118–124. [PubMed] [Google Scholar]
- 7.Spechler S.J., Zeroogian J.M., Antonioli D.A., Wang H.H., Goyal R.K. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994;344:1533–1536. doi: 10.1016/s0140-6736(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 8.Cameron A., Kamath P., Carpenter H. Prevalence of Barrett’s esophagus and intestinal metaplasia at the esophagogastric junction. Gastroenterology. 1997;112:A82. [Google Scholar]
- 9.Hirschowitz B.I. Gastric acid and pepsin secretion in patients with Barrett’s esophagus and appropriate controls. Dig. Dis. Sci. 1996;41:1384–1391. doi: 10.1007/BF02088563. [DOI] [PubMed] [Google Scholar]
- 10.Liron R., Parrilla P., Martinez de Haro L.F., Ortiz A., Robles R., Lujan J.A., Fuente T., Andres B. Quantification of duodenogastric reflux in Barrett’s esophagus. Am. J. Gastroenteol. 1997;92:32–36. [PubMed] [Google Scholar]
- 11.Avidan B., Sonnenberg A., Schnell T. Acid reflux is a poor predictor of esophageal mucosal injury. Gastroenterology. 2000;118:A2584. [Google Scholar]
- 12.Lanas A., Royo Y., Ortego J., Molina M., Sainz R. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterology. 1999;116:97–107. doi: 10.1016/s0016-5085(99)70233-7. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez P., Piazuelo E., Sanchez M.T., Ortego J., Soteras F., Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett’s esophagus. World J. Gastroenterol. 2005;11:2697–2703. doi: 10.3748/wjg.v11.i18.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh T.Y., Lee J.S., Ahn B.O., Cho H., Kim W.B., Kim Y.B., Surh Y.J., Cho S.W., Hahm K.B. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic. Biol. Med. 2001;30:905–915. doi: 10.1016/s0891-5849(01)00472-5. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald R.C., Onwuegbusi B.A., Bajaj-Elliott M., Saeed I.T., Burnham W.R., Farthing M.J. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut. 2002;50:451–459. doi: 10.1136/gut.50.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isomoto H., Wang A., Mizuta Y., Akazawa Y., Ohba K., Omagari K., Miyazaki M., Murase K., Hayashi T., Inoue K., Murata I., Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am. J. Gastroenterol. 2003;98:551–556. doi: 10.1111/j.1572-0241.2003.07303.x. [DOI] [PubMed] [Google Scholar]
- 17.Isomoto H., Nishi Y., Wang A., Takeshima F., Omagari K., Mizuta Y., Shikuwa S., Murata I., Kohno S. Mucosal concentrations of proinflammatory cytokines and chemokines at gastric cardia: implication of Helicobacter pylori infection and gastroesophageal reflux. Am. J. Gastroenterol. 2004;99:1063–1068. doi: 10.1111/j.1572-0241.2004.30847.x. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald R.C., Abdalla S., Onwuegbusi B.A., Sirieix P., Saeed I.T., Burnham W.R., Farthing M.J. Inflammatory gradient in Barrett’s oesophagus: implications for disease complications. Gut. 2002;51:316–322. doi: 10.1136/gut.51.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida N., Uchiyama K., Kuroda M., Sakuma K., Kokura S., Ichikawa H., Naito Y., Takemura T., Yoshikawa T., Okanoue T. Interleukin-8 expression in the esophageal mucosa of patients with gastroesophageal reflux disease. Scand. J. Gastroenterol. 2004;39:816–822. doi: 10.1080/00365520410006729. [DOI] [PubMed] [Google Scholar]
- 20.Baggiolini M., Loetscher P., Moser B. Interleukin-8 and the chemokine family. Int. J. Immunopharmacol. 1995;17:103–108. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- 21.Mukaida N., Harada A., Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 22.Isomoto H., Mizuta Y., Miyazaki M., Takeshima F., Omagari K., Murase K., Nishiyama T., Inoue K., Murata I., Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am. J. Gastroenterol. 2000;95:2768–2776. doi: 10.1111/j.1572-0241.2000.02304.x. [DOI] [PubMed] [Google Scholar]
- 23.Kanazawa Y., Isomoto H., Wen C.Y., Wang A.P., Saenko V.A., Ohtsuru A., Takeshima F., Omagari K., Mizuta Y., Murata I., Yamashita S., Kohno S. Impact of endoscopically minimal involvement on IL-8 mRNA expression in esophageal mucosa of patients with non-erosive reflux disease. World J. Gastroenterol. 2003;9:2801–2804. doi: 10.3748/wjg.v9.i12.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshihara Y. Endoscopic findings of GERD. Nippon Rinsho. 2004;62:1459–1464. [PubMed] [Google Scholar]
- 25.Isomoto H., Inoue K., Kohno S. Interleukin-8 levels in esophageal mucosa and long-term clinical outcome of patients with reflux esophagitis. Scand. J. Gastroenterol. 2007;43:410–1. doi: 10.1080/00365520600931469. [DOI] [PubMed] [Google Scholar]
- 26.Thompson M.D., Takasaki J., Capra V., Rovati G.E., Siminovitch K.A., Burnham W.M., Hudson T.J., Bosse Y., Cole D.E. G-protein-coupled receptors and asthma endophenotypes: the cysteinyl leukotriene system in perspective. Mol. Diagn. Ther. 2006;10:353–366. doi: 10.1007/BF03256212. [DOI] [PubMed] [Google Scholar]
- 27.Mukaida N. Interleukin-8: An expanding universe beyond neutrophil chemotaxis and activation. Int. J. Hematol. 2000;72:391–398. [PubMed] [Google Scholar]
- 28.Isomoto H., Nishi Y., Kohno S. CXC receptor 1 is overexpressed in endoscopy-negative gastroesophageal reflux disease. Scand. J. Gastroenterol. 2005;40:231–232. doi: 10.1080/00365520510012073. [DOI] [PubMed] [Google Scholar]
- 29.Ismail-Beigi F., Horton P.F., Pope C.E., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology. 1970;58:163–174. [PubMed] [Google Scholar]
- 30.Baggiolini M., Dewald B., Moser B. Human chemokines: An update. Annu. Rev. Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher C., Clark-Lewis I., Baggiolini M., Moser B. High- and low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc. Natl. Acad. Sci. USA. 1992;89:10542–10546. doi: 10.1073/pnas.89.21.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumont R.A., Car B.D., Voitenok N.N., Junker U., Moser B., Zak O., O’Reilly T. Systemic neutralization of interleukin-8 markedly reduces neutrophilic pleocytosis during experimental lipopolysaccharide-induced meningitis in rabbits. Infect. Immun. 2000;68:5756–5763. doi: 10.1128/iai.68.10.5756-5763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki S., Kobayashi M., Chiba K., Horiuchi I., Wang J., Kondoh T., Hashino S., Tanaka J., Hosokawa M., Asaka M. Autocrine production of epithelial cell-derived neutrophil attractant-78 induced by granulocyte colony-stimulating factor in neutrophils. Blood. 2002;99:1863–1865. [PubMed] [Google Scholar]
- 34.Browning D.D., Diehl W.C., Hsu M.H., Schraufstatter I.U., Ye R.D. Autocrine regulation of interleukin-8 production in human monocytes. Am. J. Physiol. Lung. Cell Mol. Physiol. 2000;279:L1129–L1136. doi: 10.1152/ajplung.2000.279.6.L1129. [DOI] [PubMed] [Google Scholar]
- 35.Venkatakrishnan G., Salgia R., Groopman J.E. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J. Biol. Chem. 2000;275:6868–6875. doi: 10.1074/jbc.275.10.6868. [DOI] [PubMed] [Google Scholar]
- 36.Chuntharapai A., Kim K.J. Regulation of the expression of IL-8 receptor A/B by IL-8: Possible functions of each receptor. J. Immunol. 1995;155:2587–2594. [PubMed] [Google Scholar]
- 37.Yoshida N. Inflammation and oxidative stress in gastroesophageal reflux disease. J. Clin. Biochem. Nutr. 2007;40:13–23. doi: 10.3164/jcbn.40.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baggiolini M., Loetscher P., Moser B. Interleukin-8 and the chemokine family. Int. J. Immunopharmacol. 1995;17:103–108. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- 39.Mukaida N., Harada A., Yasumoto K., Matsushima K. Properties of pro-inflammatory cell type-specific leukocyte chemotactic cytokines, interleukin 8 (IL-8) and monocyte chemotactic and activating factor (MCAF) Microbiol. Immunol. 1992;36:773–789. doi: 10.1111/j.1348-0421.1992.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi S., Kasugai T. Endoscopic and biopsy criteria for the diagnosis of esophagitis with a fiberoptic esophagoscope. Am. J. Dig. Dis. 1974;19:345–352. doi: 10.1007/BF01072525. [DOI] [PubMed] [Google Scholar]
- 41.Frierson H.F., Jr. Histology in the diagnosis of reflux esophagitis. Gastroenterol. Clin. North Am. 1990;19:631–644. [PubMed] [Google Scholar]
- 42.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 43.Baeuerle P.A., Baltimore D. NF-κB: Ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 44.Isomoto H., Miyazaki M., Mizuta Y., Takeshima F., Murase K., Inoue K., Yamasaki K., Murata I., Koji T., Kohno S. Expression of nuclear factor-kappaB in Helicobacter pylori-infected gastric mucosa detected with southwestern histochemistry. Scand. J. Gastroenterol. 2000;35:247–254. doi: 10.1080/003655200750024092. [DOI] [PubMed] [Google Scholar]
- 45.Tselepis C., Perry I., Dawson C., Hardy R., Darnton S.J., McConkey C., Stuart R.C., Wright N., Harrison R., Jankowski J.A. Tumour necrosis factor-alpha in Barrett’s oesophagus: a potential novel mechanism of action. Oncogene. 2002;21:6071–6081. doi: 10.1038/sj.onc.1205731. [DOI] [PubMed] [Google Scholar]
- 46.Moons L.M., Kusters J.G., Bultman E., Kuipers E.J., van Dekken H., Tra W.M., Kleinjan A., Kwekkeboom J., van Vliet A.H., Siersema P.D. Barrett’s oesophagus is characterized by a predominantly humoral inflammatory response. J. Pathol. 2005;207:269–276. doi: 10.1002/path.1847. [DOI] [PubMed] [Google Scholar]
- 47.Hirano T. Interleukin 6 and its receptor: ten years later. Int. Rev. Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 48.Dvorakova K., Payne C.M., Ramsey L., Holubec H., Sampliner R., Dominguez J., Dvorak B., Bernstein H., Bernstein C., Prasad A., Fass R., Cui H., Garewal H. Increased expression and secretion of interleukin-6 in patients with Barrett’s esophagus. Clin. Cancer. Res. 2004;10:2020–2028. doi: 10.1158/1078-0432.ccr-0437-03. [DOI] [PubMed] [Google Scholar]
- 49.Shibata F. The role of rat cytokine-induced neutrophil chemoattractants (CINCs) in inflammation. Yakugaku Zasshi. 2002;122:263–268. doi: 10.1248/yakushi.122.263. [DOI] [PubMed] [Google Scholar]
- 50.Hayakawa T., Fujiwara Y., Hamaguchi M., Sugawa T., Okuyama M., Sasaki E., Watanabe T., Tominaga K., Oshitani N., Higuchi K., Arakawa T. Roles of cyclooxygenase 2 and microsomal prostaglandin E synthase 1 in rat acid reflux oesophagitis. Gut. 2006;55:450–456. doi: 10.1136/gut.2005.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh T.Y., Lee J.S., Ahn B.O., Cho H., Kim W.B., Kim Y.B., Surh Y.J., Cho S.W., Lee K.M., Hahm K.B. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001;49:364–371. doi: 10.1136/gut.49.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naito Y., Kuroda M., Uchiyama K., Mizushima K., Akagiri S., Takagi T., Handa O., Kokura S., Yoshida N., Ichikawa H., Yoshikawa T. Inflammatory response of esophageal epithelium in combined-type esophagitis in rats: a transcriptome analysis. Int. J. Mol. Med. 2006;18:821–828. [PubMed] [Google Scholar]
- 53.Jenkins G.J., Harries K., Doak S.H., Wilmes A., Griffiths A.P., Baxter J.N., Parry J.M. The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kappaB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis. 2004;25:317–323. doi: 10.1093/carcin/bgh032. [DOI] [PubMed] [Google Scholar]
- 54.Capodicasa E., De Bellis F., Pelli M.A. Effect of lansoprazole on human leukocyte function. Immunopharmacol. Immunotoxicol. 1999;21:357–377. doi: 10.3109/08923979909052768. [DOI] [PubMed] [Google Scholar]
- 55.Becker J.C., Grosser N., Waltke C., Schulz S., Erdmann K., Domschke W., Schroder H., Pohle T. Beyond gastric acid reduction: proton pump inhibitors induce heme oxygenase-1 in gastric and endothelial cells. Biochem. Biophys. Res. Commun. 2006;345:1014–1021. doi: 10.1016/j.bbrc.2006.04.170. [DOI] [PubMed] [Google Scholar]
- 56.Hinoki A., Yoshimura K., Fujita K., Akita M., Ikeda R., Nagashima M., Nomura M., Satomi A. Suppression of proinflammatory cytokine production in macrophages by lansoprazole. Pediatr. Surg. Int. 2006;22:915–923. doi: 10.1007/s00383-006-1767-8. [DOI] [PubMed] [Google Scholar]
- 57.Stocker R., Perrella M.A. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- 58.Farombi E.O., Surh Y.J. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J. Biochem. Mol. Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 59.Becker J.C., Domschke W., Pohle T. Current approaches to prevent NSAID-induced gastropathy—COX selectivity and beyond. Br. J. Clin. Pharmacol. 2004;58:587–600. doi: 10.1111/j.1365-2125.2004.02198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeo M., Kim D.K., Chung I.S., Moon B.S., Song K.S., Hahm K.B. The novel acid antagonists for anti-secretory actions with their peculiar applications beyond acid suppression. J. Clin. Biochem. Nutr. 2006;38:1–8. [Google Scholar]