Abstract
Coenzyme Q10 (CoQ10) has been widely commercially available in Japan as a dietary and health supplement since 2001 and is used for the prevention of lifestyle-related diseases induced by free radicals and aging. We evaluated CoQ10 supplements to ensure that these supplements can be used effectively and safely. Commercially available products were selected and assessed by the quality control tests specified in the Japanese Pharmacopoeia XV. When the disintegration time of CoQ10 supplements was measured, a few tested supplements did not completely disintegrate even after incubation in water for an hour at 37°C. In the content test, many samples were well controlled. However, a few supplements showed low recovery rates of CoQ10 as compared to manufacturer’s indicated contents. Among soft capsule and liquid supplements, the reduced form of CoQ10 (H2CoQ10), as well as the oxidized form, was detected by HPLC with electrochemical detector. The results for experimental formulated CoQ10 supplements demonstrated that H2CoQ10 was produced by the interaction of CoQ10 with vitamins E and/or C. From these results, we concluded that quality varied considerably among the many supplement brands containing CoQ10. Additionally, we also demonstrated that H2CoQ10 can be detected in some foods as well as in CoQ10 supplements.
Keywords: coenzyme Q10, ubiqinol-10, quality control, dietary and health supplement, food
Introduction
It is well known that coenzyme Q (CoQ) serves as an essential carrier for electron transport and proton translocation in the mitochondrial respiratory chain [1]. Besides its role in electron-transfer reactions, CoQ10 serves as a free radical scavenger, thereby preventing oxidative damage in the human body. Several researchers have pointed out that the reduced form of CoQ10 (H2CoQ10) is an efficient scavenger of lipid radicals and inhibitors of lipid peroxidation in low-density lipoprotein [2, 3], biomembrane [4], and liposomes [5, 6]. CoQ in the bodies of human beings is thought to be provided by both dietary intake, from foods or dietary supplements [7, 8], and de novo biosynthesis [9, 10]. Therefore, decreases in the biosynthesis or intake of CoQ10 might affect the physiological action of CoQ10. In fact, decreased serum levels of CoQ10 were observed in patients undergoing total parenteral nutrition therapy without dietary intake [11], and in patients [12, 13] and animals [14, 15] receiving HMG-CoA reductase inhibitor (statin) administration.
CoQ10 was introduced into clinical therapy in 1974 in Japan. Its generic name is ubidecarenone. Many clinical trials showed that oral administration to patients was effective for mild congestive heart failure symptoms such as edema, lung congestion, and swollen liver. On the basis of these clinical findings, CoQ10 was classified in the group of cardiovascular drugs for metabolic disturbances. In 1991, CoQ10, whose generic name is ubiquinone-10, was also made available as an over-the-counter drug in pharmacies.
While sales of dietary and health supplement products have been rapidly increasing in Japan, it is vital to supply quality-controlled products for consumers. In 2001, the Ministry of Health, Labour and Welfare in Japan permitted the use of CoQ10 as a food additive as long as no claims were made about its pharmacological effectiveness and application. Currently, it is estimated that more than 200 kinds of CoQ10-containing dietary and health supplements (CoQ10 supplements), produced by more than 100 different manufacturers, are available. In particular, CoQ10 supplements are used for the prevention of lifestyle-related diseases induced by free radicals and aging. However, the products may vary in quality. Because CoQ10 has a rather complicated chemical structure and possesses several physical properties, such as low melting point, hydrophobic nature, and light sensitivity, that do not favor large-scale commercial production, highly sophisticated techniques should be employed at all production stages to obtain a satisfactory product [16]. Moreover, many Japanese CoQ10 supplements also contain other components, e.g., vitamins, minerals, amino acids, antioxidative compounds, and enzymes, in the same tablet, soft capsule, or other product. Therefore, the chemical stability of CoQ10 supplements is also an important point to be considered.
In this study, we evaluated the quality of commercially available CoQ10 supplements to ensure that these supplements can be used effectively and safely. In addition, to investigate the distribution of CoQ10 in foods from a nutritional point of view, we also measured both CoQ10 and H2CoQ10 contents in selected foods.
Materials and Methods
Reagents
Authentic CoQ homologues from CoQ7 to CoQ11 were kindly supplied by Nisshin Pharma Inc., Tokyo. High performance liquid chromatography (HPLC) solvents and ethanol were purchased (HPLC grade) from Wako Pure Chemical Industries, Ltd., Osaka, Japan. All other chemicals used were of analytical grade, available from commercial suppliers.
CoQ10 supplements
CoQ10 supplements were commercially purchased from pharmacies, health food stores, department stores, convenience stores, and sport goods shops in the Kobe city area. Forms tested in this study included tablet (TB), hard capsule (HCP), soft capsule (SCP), granule (GN), liquid (LQ), jelly (JL), and inclusion complex with κ-dextrin (ICD). The quality control tests of CoQ10 supplements were completed within 6 months before the product’s consume-by date. CoQ10 supplements obtained for testing were preserved in tight, light-resistant containers at room temperature until the quality control tests were conducted.
Experimental manufacturing preparations for SCPs, a LQ, and an HCP of “Formulas 1 to 5”
Experimental manufacturing products for 3 SCP forms, a LQ form, and an HCP form of formulas 1 to 5 were kindly provided by Shiseido Medical, Co., Ltd., Tokyo, Japan. The detailed formulas of their components are shown in Table 1.
Table 1.
Experimental formulas of CoQ10 supplement forms of soft capsule, liquid, and hard capsule
| Contents | “Formula 1” | “Formula 2” | “Formula 3” | “Formula 4” | “Formula 5” |
|---|---|---|---|---|---|
| Intake form | Soft capsule | Soft capsule | Soft capsule | Liquid | Hard capsule |
| Based Oil | Safflower oil | Safflower oil | Olive oil | — | — |
| CoQ10 | 30 mg | 10 mg | 30 mg | 30 mg | 30 mg |
| Vitamin E | 10 mg | 30 mg | 10 mg | 10 mg | 10 mg |
| Vitamin C | 30 mg | — | 30 mg | 30 mg | 30 mg |
Quality control tests
Disintegration test—This test was performed using the apparatus (Model TMB-81, Toyama Sangyo Co., Ltd., Osaka, Japan) and test conditions specified in the Japanese Pharmacopoeia XV (J.P.XV). Water and the first (pH 1.2) and the second (pH 6.8) fluids of the J.P.XV method were used as the immersion solution at 37 ± 2°C. The time required for each TB, HCP, and SCP to completely disintegrate was recorded, and the mean disintegration time was calculated. Complete disintegration is defined by the J.P.XV as that state in which any residue of the unit, except fragments of insoluble coating or capsule shell, remaining on the screen (0.25–0.31 mm) of the test apparatus is a soft mass having no palpably firm core.
Content test—All procedures for the content test of CoQ10 supplements were carried out in the dark.
i) Pre-preparation of CoQ10 supplements
TB and ICD products were crushed carefully in a mortar to obtain a homogenous fine powder. This powder was transferred completely to a 50-ml brown volumetric flask, and about 30 ml of ethanol was added to the flask.
HCP and SCP products were carefully opened, the contents were transferred completely to a brown 50-ml volumetric flask, and about 30 ml of ethanol was added to the flask. If ethanol-insoluble contents and/or fragments were observed, these were sonicated on ice for 1 min with output 3 to 5 (Ultrasonic Distruptor, Model UR-200P, Tomy Seiko Co, Ltd., Tokyo, Japan).
In the case of LQ products, an aliquot of 5 ml of drink solution was pipetted into a 50-ml brown volumetric flask directly, and about 30 ml of ethanol was added to the flask. For JL and GN products, an equivalent of 5 mg was weighed accurately and dissolved in 10 ml of water and about 20 ml of ethanol in a mortar to obtain a homogenous solution. The solution was completely transferred to a brown 50-ml volumetric flask.
ii) Preparation of CoQ10 supplements
After the ethanol solution was prepared as described above, it was incubated for 10 min at 50°C and allowed to stand for 10 min at room temperature. Then, the ethanol solution was added to the flask again to scale up to 50 ml. The solution was filtered with filter paper (No. 3) and diluted with ethanol to adjust to a 10 µg/ml CoQ10-containing ethanol solution. Finally, except for SCP products, an aliquot of 10 µl of the ethanol solution was injected into the column to determine H2CoQ10.
In the case of SCP products only, an aliquot of 0.5 ml of 10 µg/ml CoQ10-containing ethanol solution was pipetted into a brown glass-stoppered centrifuged tube, and then 0.5 ml of distilled water, 1.5 ml of ethanol, and 5 ml of n-hexane were added in turn. The mixture was shaken vigorously reciprocally at a rate of 80 times per minute for 10 min and centrifuged at 500 g for 10 min. This extraction procedure was repeated three times. Subsequently, the combined n-hexane layer was concentrated in vacuo under a stream of nitrogen. The resulting residue was dissolved in 0.5 ml of ethanol, and an aliquot of 10 µl of the ethanol solution was injected into the column to determine H2CoQ10.
Separately, an aliquot of 10 µl of 0.25% sodium borohydride solution (0.25 mg/ml water) was added to 0.4 ml of the ethanol and the mixture allowed to stand for 10 min at room temperature. An aliquot of 10 µl of the solution was injected into the column to determine total CoQ10, the sum of reduced and oxidized CoQ10.
iii) HPLC conditions for determination of CoQ10 and H2CoQ10
The content of CoQ10 supplements was determined by an HPLC method with electrochemical detection (ECD), as previously described [17]. Authentic CoQ homologues from CoQ7 to CoQ11 were prepared by dissolution in ethanol to yield concentrations of 10 µg/ml. Authentic CoQ homologues were freshly prepared from the corresponding CoQ, adding 25 µg of sodium borohydride (10 µl of a 0.25% solution of sodium borohydride in water) to give a concentration of 1 µg/ml prior to HPLC-ECD analysis.
Food samples
Food samples were obtained from local food stores or supermarkets in the Kobe city area. All food items were analyzed raw, without freezing. In some cases, samples of the same food were collected on separate days.
Measurement of CoQ homologue contents in foods
Food samples were homogenized with distilled water at 4°C using a Polytron homogenizer (Type PT 10/35; Kinematica, Lucerne, Switzerland) at a setting of 7 to 30 seconds. The final volume of the homogenate was adjusted so as to contain about 1 to 2 µg of H2CoQ10. An aliquot of 0.5 ml of the homogenate was pipetted into a brown glass-stoppered centrifuged tube, and then 2 ml of ethanol and 5 ml of n-hexane were added. The solution was extracted as described above for SCP products, and the contents of H2CoQs and CoQs were determined by HPLC-ECD [17].
Results
Internal profile for SCP forms of CoQ10 supplements
Among CoQ10 supplements, SCP is the most common commercially available intake form in Japan. According to the United States Pharmacopoeia, SCPs (sometimes called soft gelatin capsules or softgels) are filled with liquid contents in most cases. Typically, CoQ10 is dissolved or suspended in a liquid vehicle such as vegetable oil or the lower-molecular-weight polyethylene glycols.
First, we assessed the internal profile of different SCP CoQ10 supplement products. Most samples showed the typical internal profile of SCPs. However, as shown in Fig. 1, in a few SCPs, CoQ10 crystallized itself inside and existed in the solid state but not the lipid-soluble form. Moreover, a certain SCP did not show the uniform distribution of CoQ10 in the capsule.
Fig. 1.
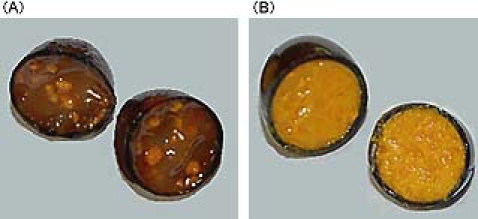
Internal appearance of soft capsule forms of CoQ10-supplements. (A) Crystallized CoQ10; the yellowed compound is crystallized CoQ10. (B) Typical soft capsule appearance; CoQ10 distributes uniformly in the soft capsule.
Disintegration tests for TB, HCP, SCP, and ICD forms of CoQ10 supplements
According to the disintegration test specified in the J.P.XV, TB supplements should disintegrate with water as the immersion fluid within 30 min after operating the apparatus. For both HCP and SCP supplements, the time limit is 20 min. Although ICD is the inclusion complex form of CoQ10 and γ-dextrin, the disintegration time is not specified by J.P.XV.
When we measured the disintegration time of 2 kinds of CoQ10-containing over-the-counter (OTC) drugs as controls, their disintegration times were 3.8 min for OTC-TB and 4.6 min for OTC-SCP, respectively. OTC drugs were well controlled and met the necessary requirements specified in the J.P.XV (Table 2).
Table 2.
The disintegration time of CoQ10 supplements, tablets, hard capsules, soft capsules, and inclusion complex with γ-dextrin
| Samples* | Disintegration Time (min) |
||
|---|---|---|---|
| Water | First fluid (pH 1.2, J.P.XV) | Second fluid (pH 6.8, J.P.XV) | |
| OTC-TB | 3.8 ± 0.2 | 4.1 ± 0.4 | 6.0 ± 0.4 |
| OTC-SCP | 4.6 ± 0.1 | 6.8 ± 0.1 | 9.8 ± 0.5 |
| TB-1 | 11.3 ± 0.9 | 16.5 ± 0.6 | 11.1 ± 0.7 |
| TB-2 | 3.2 ± 0.4 | 3.6 ± 1.0 | 2.4 ± 0.1 |
| TB-3 | 10.7 ± 0.4 | 12.8 ± 0.6 | 13.1 ± 0.3 |
| TB-4 | 11.1 ± 0.1 | 12.1 ± 0.1 | 13.4 ± 0.1 |
| TB-5 | >60 | >60 | >60 |
| TB-6 | 55.2 ± 0.9 | 56.4 ± 0.4 | 55.9 ± 0.8 |
| TB-7 | 4.6 ± 0.6 | 3.3 ± 0.0 | 4.1 ± 0.8 |
| TB-8 | 31.5 ± 2.6 | 32.1 ± 13.1 | 33.7 ± 3.9 |
| TB-9 | 23.4 ± 0.7 | 32.8 ± 0.5 | 21.4 ± 0.4 |
| TB-10 | 11.8 ± 3.2 | 11.8 ± 1.9 | 14.8 ± 4.2 |
| TB-11 | 17.5 ± 2.8 | 19.6 ± 1.3 | 16.5 ± 2.0 |
| TB-12 | >60 | >60 | >60 |
| TB-13 | 18.3 ± 0.2 | 18.6 ± 0.5 | 18.8 ± 0.6 |
| TB-14 | 56.7 ± 1.3 | 53.4 ± 0.8 | 58.7 ± 1.6 |
| TB-15 | 19.6 ± 1.4 | 18.9 ± 1.5 | 18.7 ± 1.6 |
| TB-16 | 9.3 ± 1.0 | 11.3 ± 0.3 | 11.6 ± 0.5 |
| TB-17 | 18.8 ± 1.0 | 18.6 ± 0.8 | 25.1 ± 1.9 |
| HCP-1 | 6.9 ± 1.9 | 7.2 ± 1.5 | 17.6 ± 0.4 |
| HCP-2 | 10.8 ± 0.6 | 9.9 ± 0.3 | 11.9 ± 0.7 |
| HCP-3 | 3.2 ± 0.2 | 4.0 ± 0.4 | 3.6 ± 0.3 |
| HCP-4 | 23.8 ± 3.0 | 22.5 ± 3.8 | 13.9 ± 0.9 |
| HCP-5 | 4.9 ± 0.9 | 3.2 ± 0.1 | 5.3 ± 0.9 |
| SCP-1 | 10.2 ± 1.0 | 12.9 ± 1.0 | 11.2 ± 1.0 |
| SCP-2 | 5.6 ± 0.2 | 5.6 ± 0.5 | 10.7 ± 0.5 |
| SCP-3 | 47.2 ± 1.5 | 54.9 ± 2.8 | 57.2 ± 0.9 |
| SCP-4 | >60 | >60 | >60 |
| SCP-5 | >60 | 44.0 ± 2.5 | 52.7 ± 2.0 |
| SCP-6 | >60 | >60 | >60 |
| SCP-7 | >60 | >60 | >60 |
| SCP-8 | 41.0 ± 0.9 | 40.7 ± 2.5 | 32.6 ± 1.9 |
| SCP-9 | 19.0 ± 1.5 | 21.5 ± 0.5 | 13.8 ± 0.9 |
| SCP-10 | >60 | >60 | >60 |
| SCP-11 | 14.8 ± 0.7 | 16.9 ± 1.5 | 14.0 ± 1.6 |
| SCP-12 | >60 | >60 | >60 |
| SCP-13 | 11.4 ± 0.7 | 7.8 ± 0.3 | 8.0 ± 0.7 |
| ICD-1 | 32.5 ± 3.4 | 30.8 ± 2.7 | 41.6 ± 2.1 |
| ICD-2 | 29.9 ± 2.1 | 41.3 ± 1.1 | 28.1 ± 1.5 |
| ICD-3 | 12.1 ± 0.8 | 15.6 ± 1.9 | 11.6 ± 0.9 |
| ICD-4 | 30.0 ± 2.2 | 29.8 ± 0.7 | 31.4 ± 2.4 |
| ICD-5 | 26.4 ± 3.3 | 36.4 ± 2.7 | 25.1 ± 2.8 |
| ICD-6 | 8.8 ± 0.7 | 12.1 ± 0.9 | 7.6 ± 0.6 |
*OTC, over-the-counter drug; TB, tablet; HCP, hard capsule; SCP, soft capsule; ICD, inclusion complex with γ-dextrin. Data are expressed as means ± SD (n = 6).
For CoQ10 supplements, as shown in Table 2, the disintegration time was 3.2 min to >60 min for TBs, 3.2 min to 23.8 min for HCPs, 5.6 min to >60 min for SCPs, and 8.8 min to 32.5 min for ICDs; considerable differences were observed among the many supplements. In particular, the disintegration time of about half of the SCPs was more than 60 min. Among the different forms of CoQ10 supplements, the disintegration performance of ICDs was not always faster than that of other forms. Moreover, as tested supplements might be enteric-coated tablets, we also carried out the disintegration tests again for the first fluid, simulated gastric fluid (pH 1.2), and the second fluid, simulated intestinal fluid (pH 6.8). However, the results did not differ from those of water as the immersion fluid.
Content tests for CoQ10 supplements
Although the J.P.XV specifies the HPLC-UV method as the identification and content test for ubidecarenone, we applied the HPLC-ECD method as described in Materials and Methods instead because this method can measure both the reduced and oxidized forms of CoQ10 and shows higher sensitivity than the HPLC-UV method does.
Of course, the content of the over-the-counter drugs used as controls was well controlled within an extremely limited range (99–100%). Most CoQ10 supplements showed a content value of more than 80%. However, a few supplements exhibited low recovery rates of CoQ10 as compared to manufacturers’ indicated contents. Moreover, in several SCP and LQ products, H2CoQ10 as well as CoQ10 was detected. Interestingly, in LQ-6, as shown in Table 3, CoQ10 was recovered entirely as the reduced form, H2CoQ10.
Table 3.
Content test of CoQ10 supplements, tablets, hard capsules, soft capsules, liquids, jellies, granules, and inclusion complex with γ-dextrindextrin
| CoQ10-supplements* | Content (mg) |
||
|---|---|---|---|
| Labeled CoQ10 | H2CoQ10 (%)** | Total CoQ10 (%)*** | |
| OTC-TB | 10 | ND**** | 100.5 ± 0.1 (100) |
| OTC-SCP | 10 | ND | 99.4 ± 0.2 (99) |
| TB-1 | 10 | ND | 9.6 ± 0.3 (96) |
| TB-2 | 6.3 | ND | 3.4 ± 0.1 (54) |
| TB-3 | 11.1 | ND | 9.4 ± 0.5 (85) |
| TB-4 | 1.7 | ND | 1.6 ± 0.1 (94) |
| TB-5 | 5 | ND | 4.6 ± 0.2 (92) |
| TB-6 | 16.7 | ND | 17.2 ± 0.4 (103) |
| TB-7 | 10 | ND | 9.3 ± 0.4 (93) |
| TB-8 | 60 | ND | 55.6 ± 1.4 (93) |
| TB-9 | 20 | ND | 20.0 ± 0.5 (100) |
| TB-10 | 0.34 | ND | 0.4 ± 0.01 (118) |
| TB-11 | 3.75 | ND | 3.7 ± 0.3 (99) |
| TB-12 | 0.83 | ND | 0.9 ± 0.1 (108) |
| HCP-1 | 90 | ND | 92.3 ± 0.5 (103) |
| HCP-2 | 80 | ND | 4.2 ± 0.1 (5) |
| HCP-3 | 30 | ND | 28.7 ± 1.47 (96) |
| HCP-4 | 60 | ND | 59.3 ± 1.3 (99) |
| HCP-5 | 30 | ND | 30.8 ± 0.9 (103) |
| SCP-1 | 33.3 | 5.8 ± 0.5 (17) | 33.8 ± 1.4 (101) |
| SCP-2 | 30 | 14.1 ± 1.0 (46) | 30.6 ± 0.9 (102) |
| SCP-3 | 30 | 7.0 ± 0.8 (22) | 31.0 ± 1.2 (103) |
| SCP-4 | 30 | 13.5 ± 0.4 (44) | 31.1 ± 0.7 (104) |
| SCP-5 | 30 | 13.7 ± 0.7 (52) | 26.4 ± 1.0 (88) |
| SCP-6 | 35 | 1.6 ± 0.5 (4) | 35.8 ± 0.8 (102) |
| SCP-7 | 30 | 1.8 ± 0.03 (6) | 28.0 ± 0.6 (94) |
| SCP-8 | 30 | 25.3 ± 0.7 (88) | 28.8 ± 0.6 (96) |
| SCP-9 | 150 | 6.2 ± 0.4 (5) | 120.8 ± 3.8 (80) |
| SCP-10 | 30 | 1.4 ± 0.1 (4) | 30.7 ± 0.3 (102) |
| SCP-11 | 30 | 3.8 ± 0.7 (12) | 30.7 ± 0.9 (102) |
| SCP-12 | 50 | ND | 49.6 ± 1.0 (99) |
| SCP-13 | 30 | ND | 31.1 ± 0.4 (104) |
| SCP-14 | 30 | 5.9 ± 0.7 (19) | 31.0 ± 0.5 (103) |
| SCP-15 | 10 | ND | 10.1 ± 0.1 (101) |
| SCP-16 | 10 | ND | 10.0 ± 0.1 (100) |
| SCP-17 | 10 | 1.1 ± 0.2 (14) | 8.0 ± 0.6 (80) |
| SCP-18 | 30 | 5.2 ± 0.4 (16) | 31.4 ± 0.4 (105) |
| SCP-19 | 30 | 2.6 ± 0.7 (9) | 30.4 ± 0.6 (101) |
| SCP-20 | 45 | 3.9 ± 0.2 (8) | 46.5 ± 0.7 (103) |
| SCP-21 | 15 | 8.0 ± 0.5 (53) | 14.9 ± 0.6 ( 99) |
| SCP-22 | 30 | 3.4 ± 0.2 (12) | 27.2 ± 0.9 ( 91) |
| SCP-23 | 20 | 0.9 ± 0.1 (4.2) | 20.2 ± 1.1 (101) |
| SCP-24 | 60 | ND | 58.0 ± 4.1 (97) |
| SCP-25 | 0.25 | 0.04 ± 0.1 (16) | 0.2 ± 0.1 (96) |
| LQ-1 | 40 | ND | 41.4 ± 1.9 (104) |
| LQ-2 | 30 | ND | 31.7 ± 1.4 (106) |
| LQ-3 | 30 | ND | 32.5 ± 0.3 (108) |
| LQ-4 | 1 | ND | 1.0 ± 0.03 (100) |
| LQ-5 | 50 | 12.3 ± 2.4 (24) | 52.1 ± 0.8 (104) |
| LQ-6 | — | 5.2 ± 0.1 (—) | 5.2 ± 0.1 (—) |
| JL-1 | 30 | ND | 29.3 ± 0.1 (98) |
| JL-2 | 50 | ND | 61.5 ± 4.6 (123) |
| GN-1 | 30 | ND | 28.5 ± 0.3 (95) |
| GN-2 | 30 | ND | 30.4 ± 0.2 (101) |
| GN-3 | 30 | ND | 30.2 ± 0.2 (101) |
| GN-4 | 30 | ND | 30.1 ± 0.1 (100) |
| ICD-1 | 3.75 as ICD | ND | 1.0 ± 0.01 (27) |
| ICD-2 | 84 as ICD | ND | 10.4 ± 0.3 (12) |
| ICD-3 | 18 as ICD | 2.0 ± 0.6 (50) | 3.9 ± 0.72 (22) |
| ICD-4 | 100 as ICD | ND | 21.8 ± 1.4 (22) |
| ICD-5 | 12.5 as ICD | ND | 0.9 ± 0.1 (7) |
| ICD-6 | 100 as ICD | ND | 22.8 ± 0.1 (23) |
*OTC, over-the-counter drug; TB, tablet; HCP, hard capsule; SCP, soft capsule; LQ, liquid; JL, jelly; GN, granule; ICD, inclusion complex with k-dextrin. Data are expressed as means ± SD (n = 6).
** [H2CoQ10/total CoQ10 (sum of CoQ10 and H2CoQ10)] × 100.
*** [Sum of CoQ10 and H2CoQ10] × 100.
****Not detected.
ICD is the inclusion complex form of CoQ10 and γ-dextrin. The purposes of developing this inclusion complex with CoQ10 seem to be reduction of instability to light and enhancement of bioavailability. However, the manufacturer’s indicated contents were mentioned and listed on the package only as “CoQ10 inclusion complexes with γ-dextrin.” Thus, the CoQ10 content of the product was uncertain. As shown in Table 3, we estimated that the inclusion ratio of CoQ10 to γ-dextrin in the tested ICD will be in the range from 5% to 30%.
H2CoQ10 production in the SCP and LQ forms of CoQ10 supplements
We recognized that all CoQ10 supplements in which H2CoQ10 was detected contained vitamin E (α-tocopherol) and/or vitamin C (ascorbic acid). Therefore, we presumed that H2CoQ10 might be produced by the interaction of CoQ10 with vitamin E and/or vitamin C. To confirm this possibility, we prepared the experimental formulas listed in Table 1.
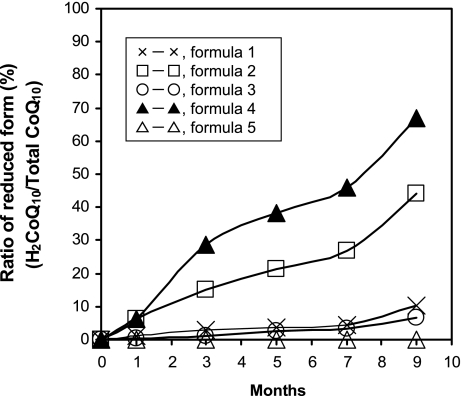
As shown in Fig. 2, co-existence of CoQ10 and vitamin E and/or vitamin C produced reduction of CoQ10 to H2CoQ10 in SCPs and a LQ. The content ratio of vitamin E to CoQ10 was also important. The existence of safflower oil in SCPs was also an essential condition for production of H2CoQ10 from CoQ10 in SCPs. In particular, a LQ form of CoQ10 that co-existed with vitamin E and vitamin C exhibited a high ratio of the reduced form to total CoQ10 in a time-dependent manner. In an HCP, however, the reduction of CoQ10 to H2CoQ10 was not observed even if CoQ10 co-existed with vitamin E and C.
Fig. 2.
H2CoQ10 produced by interaction of CoQ10 with vitamins C and/or E in soft capsule and liquid. Each formula is shown in Table 1.
H2CoQ10 and CoQ10 contents in foods
To determine the dietary intake of H2CoQ10 as well as CoQ10 in human beings, we analyzed the CoQ10 contents of raw foods. Table 4 shows both the CoQ10 and H2CoQ10 contents of meats and vegetables. The greater part of the CoQ homologues in meats and vegetables was CoQ10. Moreover, H2CoQ10 as well as CoQ10 was detected in all examined samples. In particular, squid and eel meats showed high ratios of the reduced form, H2CoQ10, as compared to other animal products. H2CoQ9 and CoQ9 were also detected in small amounts in beef, pork, and chicken.
Table 4.
H2CoQs and CoQs contents in foods
| Samples | CoQ9 |
(µg/g wet weight) |
CoQ10 |
(µg/g wet weight) |
|---|---|---|---|---|
| H2CoQ9 (%)* | Total CoQ9** | H2CoQ10 (%)* | Total CoQ10** | |
| Meats: | ||||
| Beef | ||||
| sirloin | 0.1 ± 0.1 (17) | 0.6 ± 0.1 | 1.8 ± 0.2 (6) | 30.6 ± 1.4 |
| tenderloin | ND*** | ND | 1.8 ± 0.2 (7) | 26.5 ± 1.2 |
| Pork | ||||
| sirloin | 0.1 ± 0.1 (14) | 0.7 ± 0.2 | 0.6 ± 0.2 (4) | 14.0 ± 0.6 |
| heart | ND | ND | 6.7 ± 1.9 (6) | 118.1 ± 12.2 |
| liver | 0.3 ± 0.1 (17) | 1.8 ± 0.3 | 11.1 ± 1.6 (21) | 54.0 ± 5.7 |
| Chicken | ||||
| chest | ND | ND | 0.9 ± 0.1 (5) | 16.6 ± 1.6 |
| heart | ND | ND | 5.0 ± 1.2 (4) | 123.2 ± 7.2 |
| liver | 0.2 ± 0.1 (12) | 1.7 ± 0.4 | 18.9 ± 3.0 (16) | 116.2 ± 6.2 |
| Salmon | ND | ND | 1.2 ± 0.3 (16) | 7.6 ± 0.9 |
| Eel | ND | ND | 4.6 ± 1.6 (62) | 7.4 ± 1.8 |
| Squid | ND | ND | 2.3 ± 0.2 (60) | 3.8 ± 0.8 |
| Vegetables: | ||||
| Chinese cabbage | ND | ND | 0.4 ± 0.1 (15) | 2.7 ± 0.4 |
| Eggplant | ND | ND | 0.2 ± 0.1 (9) | 2.2 ± 0.6 |
| Parsley | ND | ND | 7.5 ± 0.8 (28) | 26.4 ± 1.9 |
Data are expressed as means ± SD (n = 5).
* [H2CoQn/total CoQn (sum of CoQn and H2CoQn)] × 100 (%).
**Sum of CoQn and H2CoQn.
***Not detected.
Discussion
Although CoQ10 supplements have not been required to meet the same rigorous product quality performance standards as medicines do, impaired product performance, such as failure to disintegrate in the gastrointestinal tract, might limit the absorption of CoQ10. However, standardized guidelines and methods for assessing the quality control of dietary and health supplements have not been established in Japan. So, we applied the Japanese Pharmacopoeial Convention General Tests to evaluate the quality of CoQ10 supplements in this study.
Externally administered CoQ10 appears to be converted to H2CoQ10 in the human body and then to act as an antioxidant against lipid peroxidation. In fact, a CoQ10 supplement given to a human increases the serum level of H2CoQ10 as well as CoQ10 [18]. The enzymes responsible for the reduction of CoQ10 to H2CoQ10 have been reported to be not only mitochondrial respiratory enzymes but also NADPH-CoQ reductase [19–21], NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase) [22, 23], thioredoxin reductase [24], and lipoamide dehydrogenase [25, 26]. CoQ in the human body is thought to be provided by both dietary intake from foods and/or dietary supplements and biosynthesis de novo.
In this study, we tested two quality control tests, disintegration and content tests. The disintegration profile and content test are thought to be essential parameters relative to absorption and intake efficacy. Many commercially available CoQ10 supplements in Japan were well controlled and met the requirements specified by J.P.XV. However, a few products exhibited insufficient quality performance. In particular, the disintegration of many SCPs required more than 60 min. The gelatin shell of an SCP is somewhat thicker than that of an HCP shell and is plasticized by the addition of a polyol such as sorbitol or glycerol. The ratio of dry plasticizer to dry gelatin determines the hardness of the shell and may be varied to accommodate environmental conditions as well as the nature of the contents. Therefore, the disintegration time of SCPs may have a higher score than that of HCPs. In this study, we demonstrated that the quality of the many supplement products containing CoQ10 in Japan varies considerably, and thus, we recommend introducing simple quality control tests for CoQ10 supplements.
Among CoQ10 supplements, in SCPs and LQs, H2CoQ10 as well as CoQ10 was detected by the HPLC-ECD method. The results for experimentally formulated CoQ10 supplements suggested that H2CoQ10 was produced by the interaction of CoQ10 with vitamins E and/or C. Moreover, although H2CoQ10 is unstable when exposed to air, and can easily be oxidized into oxidized CoQ, chemically unstable H2CoQ10 is thought to exist in the body to serve as an antioxidant. Recently, Yan et al. have reported [27] that dietary supplementation with H2CoQ10 decreased the degree of senescence in middle-aged SAMP1 mice. H2CoQ10 produced by the interaction of CoQ10 with vitamins E and/or C, therefore, may exert beneficial effects in the human body.
Many investigators have reported that exogenous CoQ10 exhibits useful health effects. If humans consume H2CoQ10 as well as CoQ10 in meals every day, foods containing H2CoQ10 and CoQ10 might have some physiological activities. Therefore, it is important to investigate the distribution of these substances in foods. Some investigators have reported the CoQ homologue contents in foods. However, they could not analyze reduced forms of CoQ homologues because of the HPLC-UV method and insufficient sensitivity. In our study, we measured the distribution of both CoQ10 and H2CoQ10 in food items. The results clarify that humans consume not only CoQ10 but also H2CoQ10 from foods. In other words, the present study confirms the human intake of H2CoQ10 in daily foods and dietary CoQ10 supplements.
Acknowledgment
This work was supported in part by a Grant-in-Aid for Scientific Research (No. 16500429) from the Ministry of Education, Science and Culture, Japan, and a Grant-in-Aid for Health Science Research as well as a Grant-in-Aid for Cooperative Research (A) from Kobe Gakuin University, Japan. This research was carried out in Coenzyme Q10 Biofunctional Research Center endowed by Shiseido Pharmaceutical Co. Ltd., Tokyo, Japan.
References
- 1.Crane F.L., Hatefi Y., Lester R.L., Widmer C. Isolation of a quinine from beef heart mitochondria. Biochim. Biophys. Acta. 1957;25:220–221. doi: 10.1016/0006-3002(57)90457-2. [DOI] [PubMed] [Google Scholar]
- 2.Frei B., Kim M.C., Ames B.N. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc. Natl. Acad. Sci. USA. 1990;87:4879–4883. doi: 10.1073/pnas.87.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocker R., Bowry V.W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc. Natl. Acad. Sci. USA. 1991;88:1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kagan V.E., Serbinova E.A., Koynova G.M., Kitanova S.A., Tyurin V.A., Stoytchev T.S., Quinn P.J., Packer L. Antioxidant action of ubiquinol homologues with different isoprenoid chain length in biomembranes. Free Radic. Biol. Med. 1990;9:117–126. doi: 10.1016/0891-5849(90)90114-x. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y., Komuro E., Niki E. Antioxidant activity of ubiquinol in solution and phosphatidylcholine liposome. J. Nutr. Sci. Vitaminol. 1990;36:505–511. doi: 10.3177/jnsv.36.505. [DOI] [PubMed] [Google Scholar]
- 6.Landi L., Cabrini L., Fiorentini D., Stefanelli C., Pedulli G.F. The antioxidant activity of ubiquinol-3 in homogeneous solution and in liposomes. Chem. Phys. Lipids. 1992;61:121–130. doi: 10.1016/0009-3084(92)90004-9. [DOI] [PubMed] [Google Scholar]
- 7.Kamei M., Fujita T., Kanbe T., Sasaki K., Oshiba K., Otani S., Matsui-Yuasa I., Morisawa S. The distribution and content of ubiquinone in foods. Int. J. Vitam. Nutr. Res. 1986;56:57–63. [PubMed] [Google Scholar]
- 8.Weber C., Bysted A., Hϕlmer G. The coenzyme Q10 content of the average Danish diet. Int. J. Vitam. Nutr. Res. 1996;67:123–129. [PubMed] [Google Scholar]
- 9.Szkopinska A. Ubiquinone. biosynthesis of quinine ring and its isoprenoid side chain. Intracellular localization. Acta Biochim. Pol. 2000;47:496–480. [PubMed] [Google Scholar]
- 10.Crane F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001;20:591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T., Fukui K., Nakamoto M., Kishi T., Kanamori R., Kataoka K., Nishii S., Kishi H., Hiraoka E., Okada A. Serum levels of coenzyme Q and lipids in patients during tatal parenteral nutrition. J. Nutr. Sci. Vitaminol. 1986;32:1–12. doi: 10.3177/jnsv.32.1. [DOI] [PubMed] [Google Scholar]
- 12.Folkers K., Langsjoen P., Willis R., Richardson P., Xia L.J., Ye C.Q., Tamagawa H. Lovastatin decreases coenzyme Q levels in humans. Proc. Natl. Acad. Sci. USA. 1990;87:8931–8934. doi: 10.1073/pnas.87.22.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghirlanda G., Oradei A., Manto A., Lippa S., Uccioli L., Caputo S., Greco A.V., Littarru G.P. Evidence of plasma CoQ-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J. Clin. Pharmacol. 1993;33:226–229. doi: 10.1002/j.1552-4604.1993.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 14.Willis R.A., Folkers K., Tucker J.L., Ye C.Q., Xia L.J., Tamagawa H. Lovastatin decreases coenzyme Q levels in rats. Proc. Natl. Acad. Sci. USA. 1990;87:8928–8930. doi: 10.1073/pnas.87.22.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kettawan A., Takahashi T., Kongkachuichai R., Charoenkiatkul S., Kishi T., Okamoto T. Protective effects of coenzyme Q10 on decreased oxidative stress resistance induced by simvastatin. J. Clin. Biochem. Nutr. 2007;40:194–202. doi: 10.3164/jcbn.40.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishi H., Nishii S., Nishikawa K., Maruta E., Hiraoka E. In: Clinical application of coenzyme Q10 and the quality control of its preparations in Japan, in Biomedical and Clinical Aspects of Coenzyme Q, Vol. 3. Folkers K., Yamamura Y., editors. Elsevier/North-Holland Biomedical Press; Amsterdam: 1981. pp. 45–50. [Google Scholar]
- 17.Okamoto T., Fukunaga Y., Ida Y., Kishi T. Determination of reduced and total ubiquinones in biological materials by liquid chromatography with electrochemical detection. J. Chromatogr. 1988;430:11–19. doi: 10.1016/s0378-4347(00)83129-1. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto T., Matsuya T., Fukunaga Y., Kishi T., Yamagami T. Human serum ubiquinol-10 levels and relationship to serum lipids. Int. J. Vitam. Nutr. Res. 1989;59:288–292. [PubMed] [Google Scholar]
- 19.Takahashi T., Shitashige M., Okamoto T., Kishi T., Goshima K. A nevel ubiquinone reductase activity in rat cytosol. FEBS Lett. 1992;314:331–334. doi: 10.1016/0014-5793(92)81499-c. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T., Yamaguchi T., Shitashige M., Okamoto T., Kishi T. Reduction of ubiquinone in membrane lipids by rat liver cytosol and its involvement in the cellular defense system against lipid peroxidation. Biochem. J. 1995;309:883–890. doi: 10.1042/bj3090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishi T., Takahashi T., Mizobuchi S., Mori K., Okamoto T. Effect of dicumarol, a NAD(P)H:quinine acceptor oxidoreductase 1 (DT- diaphorase) inhibitor on ubiquinone redox cycling in cultured rat hepatocytes. Free Radic. Res. 2002;36:413–419. doi: 10.1080/10715760290021261. [DOI] [PubMed] [Google Scholar]
- 22.Beyer R.E., Segura-Aguilar J., Di Bernardo S., Cavazzoni M., Fato R., Fiorentini D., Galli M.C., Setti M., Landi L., Lenaz G. The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proc. Natl. Acad. Sci. USA. 1996;93:2528–2532. doi: 10.1073/pnas.93.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landi L., Fiorentini D., Galli M.C., Segura-Aguilar J., Beyer R.E. DT-diaphorase maintains the reduced state of ubiquinones in lipid vesicles thereby promoting their antioxidant function. Free Radic. Biol. Med. 1997;22:329–335. doi: 10.1016/s0891-5849(96)00294-8. [DOI] [PubMed] [Google Scholar]
- 24.Xia L., Nordan T., Olsson J.M., Damdimopoulos A., Bjorkhem-Bergman L., Nalvarte I., Eriksson L.C., Arner E.S., Spyrou G., Bjornstedt M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J. Biol. Chem. 2003;278:2141–2146. doi: 10.1074/jbc.M210456200. [DOI] [PubMed] [Google Scholar]
- 25.Olsson J.M., Xia L., Eriksson L.C., Bjornstedt M. Ubiquinone is reduced by lipoamide dehydrogenase and this reaction is potently stimulated by zinc. FEBS Lett. 1999;448:190–192. doi: 10.1016/s0014-5793(99)00363-4. [DOI] [PubMed] [Google Scholar]
- 26.Xia L., Bjornstedt M., Nordman T., Eriksson L.C., Olsson J.M. Reduction of ubiquinone by lipoamide dehydrogenase. An antioxidant regenerating pathway. Eur. J. Biochem. 2001;268:1486–1490. doi: 10.1046/j.1432-1327.2001.02013.x. [DOI] [PubMed] [Google Scholar]
- 27.Yan J., Fujii K., Yao J., Kishida H., Hosoe K., Sawashita J., Takeda T., Mori M., Higuchi K. Reduced coenzyme Q10 supplementation decelerates senescence in SAMP1 mice. Exp. Gerontol. 2006;41:130–140. doi: 10.1016/j.exger.2005.11.007. [DOI] [PubMed] [Google Scholar]