Abstract
We investigated whether pretreatment with geranylgeranylacetone (GGA), a potent heat shock protein (HSP) inducer, could inhibit proinflammatory cytokine liberation and nitric oxide (NO) production in lipopolysaccharide (LPS)-treated murine macrophages. The levels of NO and tumor necrosis factor-α (TNF-α) released from murine macrophage RAW 264 cells were increased dose- and time-dependently following treatment with LPS (1 µg/ml). GGA (80 µM) treatment 2 h before LPS addition significantly suppressed TNF-α and NO productions at 12 h and 24 h after LPS, respectively, indicating that GGA inhibits activation of macrophages. However, replacement by fresh culture medium before LPS treatment abolished the inhibitory effect of GGA on NO production in LPS-treated cells. Furthermore, GGA inhibited both HSP70 and inducible NO synthase expressions induced by LPS treatment despite an HSP inducer. When it was examined whether GGA interacts with LPS and/or affects expression of Toll-like receptor 4 (TLR4) and CD14 on the cell surface, GGA inhibited the binding of LPS to the cell surface, while GGA did not affect TLR4 and CD14 expressions. These results indicate that GGA suppresses the binding of LPS to the cell surface of macrophages, resulting in inhibiting signal transduction downstream of TLR4.
Keywords: Geranylgeranylacetone, lipopolysaccharide, nitric oxide, tumor necrosis factor-α, macrophage
Introduction
Lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria is a potent activator of macrophages and a causal agent of endotoxin shock [1]. LPS is well known to induce production of proinflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, reactive oxygen species (ROS) and nitric oxide (NO), leading to death from endotoxin shock in animal models [2, 3]. Moreover, in vitro studies have demonstrated that LPS enhances cytokine and/or NO production in macrophages [4], microglial cells [5] and endothelial cells [6]. The process of these cellular responses involves recognition of LPS by Toll-like receptor (TLR) 4 on the surface of the host cells, followed by activation of nuclear factor (NF)-κB [7]. TLR is a receptor family protein related to innate immunity and to date, 13 TLRs have been identified in mice and 10 in humans [7]. TLR4 has now been established as the receptor for LPS. TLR4 consists of an extracellular protein motif called leucine-rich repeats (LRR) and a cytoplasmic signaling domain similar to type I IL-1 receptor, called Toll-IL-1 receptor (TIR) domain. LPS is recognized by TLR4 and its signal is transduced, resulting in the activation of NF-κB and the induction of interferon (IFN). NF-κB is known to regulate transcriptions of many inflammation-related proteins including cytokines [8]. It increases the expression of inflammatory cytokines such as TNF-α, IL-1β, IL-6 and IFN-κ to stimulate inflammatory reaction [8]. The intracellular signaling pathways downstream of TIR domain can be subdivided into myeloid differentiation factor 88 (MyD88)-dependent and -independent ones, which are responsible for early and later activation of NF-κB, respectively [7]. Recently, it has been revealed that the recognition of LPS by TLR4 requires myeloid differentiation 2 (MD-2) in addition to TLR4 itself [9]. As for the process upstream of TLR4, LPS-binding protein (LBP) in the serum removes LPS from the outer membrane of the bacteria and forms complexes consisting of LPS, LBP and soluble CD14 (sCD14) [9]. These complexes deliver LPS to membrane-bound CD14 (mCD14) and then LPS bound to mCD14 is transferred to TLR4-MD-2 complex.
Geranylgeranylacetone (GGA), an acylic polyisoprenoid, is an anti-ulcer drug developed in Japan [10] and known to protect against organ and cell damages via its potent HSP-inducing function [10–13]. Induction of heat shock protein (HSP) 70 has been reported to reduce the production of proinflammatory mediators [2, 14] and to protect against a variety of organ damages [15, 16] including ischemia-reperfusion injury [11] and apoptosis [17]. It is known that the overexpression of HSP70 inhibits the production of cytokines in macrophages activated by LPS [18]. Recently, we have found that oral administration of GGA to rats before LPS injection induces HSP70 in several organs, inhibits production of proinflammatory cytokines and NO, and prevents organ damages, resulting in improvement of survival rates [12]. Furthermore, GGA reportedly inhibited NF-κB activation, resulting in suppression of inducible NO synthase (iNOS) expression in IL-1β-treated vascular smooth muscle cells [19]. However, it is still unknown whether the inhibitory effect of GGA on the production of cytokines and NO in LPS-mediated endotoxin shock model involves the direct action to inflammatory mediator-producing cells such as macrophages, and if so, where GGA works in TLR4 signaling pathway.
In the present study, we investigated whether GGA inhibits LPS-induced macrophage activation, resulting in suppression of inflammation, and whether the inhibition by GGA was related to HSP induction. We demonstrated that GGA inhibited the binding of LPS to the surface of macrophages, resulting in suppression of cytokine liberation and NO production regardless of its HSP-inducing activity.
Materials and Methods
Materials
GGA was provided by Eisai Co Ltd. (Tokyo, Japan). GGA was dissolved in ethanol. Escherichia coli LPS serotype O111:B4 was purchased from Sigma Chemical Co. (St. Louis, MO). LPS was dissolved in distilled water. Lipid A (E. coli) was purchased from Peptide Institute, Inc. (Osaka, Japan). Lipid A was dissolved in dimethylsulfoxide. Fetal calf serum (FCS) was purchased from PAA Laboratories GmbH (Linz, Austria). Rabbit anti-HSP70 polyclonal antibody and Phycoerythrin (PE)-labeled anti-mouse TLR4-MD-2 complex monoclonal antibody (TLR4-MD-2-PE mAb) were purchased from Medical & Biological Laboratories Co., Ltd. (Nagoya, Japan). Mouse anti-iNOS monoclonal antibody and fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD14 monoclonal antibody (CD14-FITC mAb) were purchased from BD Biosciences Pharmingen (San Diego, CA). Anti-LPS monoclonal antibody (WN1 222-5) was purchased from HyCult biotechnology b.v. (Uden, The Netherlands). FITC-conjugated goat anti-mouse IgG antibody was purchased from American Qualex (San Clemente, CA). Cardiolipin (CL) from bovine heart was purchased from Avanti Polar Lipids Inc. (Alabaster, AL). Dulbecco’s modified eagle medium (DMEM) was obtained from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). All the other chemicals used were of analytical grade.
Cell cultures and treatments with LPS and GGA
RAW 264 murine macrophage cell line was obtained from Riken BRC Cell Bank (Tsukuba, Japan), and grown in DMEM supplemented with 10% heat-inactivated FCS, 2 mM glutamine and antibiotics (100 units/ml penicillin, 100 µg/ml streptomycin) at 37°C in a humidified incubator with 5% CO2. All the experiments were performed using DMEM containing 10% FCS unless described specifically. The cells were seeded in a 24-well plate at a cell density of 5 × 105 cells/well and incubated for 2 h at 37°C in an atmosphere of 5% CO2/95% air. After non-adherent cells were removed by washing with culture medium, attached cells were exposed to LPS at concentrations ranging from 10 to 1000 ng/ml for periods of 4, 8, 12 or 24 h at 37°C under 5% CO2/95% air. When the timing of GGA addition was preliminarily investigated, only 2-h treatment with GGA suppressed NO production in RAW 264 cells after LPS among the examined time periods (2, 4, or 8 h of incubation). Therefore, 2-h treatment with GGA was selected in the present study. GGA at concentrations of 20, 40 or 80 µM was added into the medium 2 h before LPS administration. In some experiments, the culture medium 2 h after GGA addition was replaced by fresh culture medium to investigate the potential effect of GGA on the process of LPS binding to TLR4.
To investigate the dependence of inhibitory effects of GGA on serum proteins, cells were washed several times in serum-free DMEM to completely remove soluble proteins and resuspended in serum-free medium for LPS challenge.
Determination of nitrite
The nitrite in the culture medium was measured as an index of NO production by means of Griess reaction [20]. The cells (5 × 105 cells/well) were plated on 24-well plates and incubated in the presence of LPS and/or GGA for the indicated time periods at 37°C under 5% CO2/95% air. The culture supernatant collected from each well was centrifuged, and the aliquot (100 µl) was mixed with 100 µl of Griess reagent (50 µl of 1% sulfanilamide in 5% phosphoric acid, followed by 50 µl of 0.1% N-1-naphthylethylendiamine dihydrochloride in water) and the absorbance was measured at 562 nm in a microplate reader.
Determination of TNF-α
The TNF-α in the culture supernatant was determined with an enzyme-linked immunosorbent assay (ELISA) kit (BioSource International, Camarillo, CA) according to the manufacturer’s instructions.
Cell viability
The viability of RAW 264 cells after continuous exposure to LPS and/or GGA was determined using a commercially available Cell Quanti-BlueTM Cell Viability Assay Kit (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions.
Analysis of iNOS and HSP70 contents
iNOS and HSP contents were determined by Western blotting as described previously [21, 22]. The cells harvested were homogenized and 40 µg of protein from each sample was separated by SDS polyacrylamide gel electrophoresis with 7.5% polyacrylamide gel (for iNOS) or 12.5% (for HSP70). The gels were electroblotted onto polyvinylidene difluoride membranes. The membranes were incubated with mouse anti-iNOS monoclonal antibody (1:2000) and rabbit anti-HSP70 polyclonal antibody (1:10000), washed and then incubated with horseradish-peroxidase-conjugated sheep anti-mouse (Amersham Pharmacia Biotech, Buckinghamshire, England) and goat anti-rabbit (Sigma Chemical Co., St. Louis, MO) antibody, respectively. The immunoblot was revealed with an ECLTM Western blotting analysis system (Amersham Pharmacia Biotech, Buckinghamshire, England). Western blots were quantified with NIH Image.
Determination of LPS bound to the cell surface
RAW 264 cells (2 × 106 cells/ml of DMEM) were incubated in the absence or presence of 80 µM GGA for 2 h at 37°C in 5% CO2 and further incubated for 30 min after addition of 1 µg/ml LPS. After treatments, the cells were washed with PBS twice, resuspended in staining buffer (PBS containing 2.5% FCS, 100 units/ml penicillin and 100 µg/ml streptomycin) and incubated for 15 min on ice in the presence of anti-LPS monoclonal antibody (1:100). After washing with staining buffer, the cells were incubated with goat anti-mouse IgG-FITC (1:100) for 15 min on ice in the dark. The cells were washed with PBS three times, resuspended in PBS and analyzed with an Epics cytofluorometer (Beckman Coulter, Inc., Fullerton, CA).
Flow cytometry of TLR4-MD-2 complex and CD14 on the cell surface
The cells (2 × 106 cells/ml of DMEM) were incubated in the absence or presence of 80 µM GGA for 2 h at 37°C in 5% CO2. After treatments, the cells were washed with PBS three times, resuspended in 200 µl of staining buffer (PBS containing 2.5% FCS, 100 units/ml penicillin and 100 µg/ml streptomycin) and incubated with TLR4-MD-2-PE mAb at the concentration of 1 µg/1 × 106 cells for 30 min on ice in the dark. After washing with staining buffer, the cells were incubated with CD14-FITC mAb (1 µg/1 × 106 cells) for 30 min on ice in the dark. The cells were washed with PBS, resuspended in PBS and analyzed with an Epics cytofluorometer (Beckman Coulter, Inc., Fullerton, CA).
Binding of LPS to LBP
In vitro assay for inhibition by GGA or CL of LPS-LBP binding was performed using a commercially available EndoblockTM (Cell Sciences, Inc., Canton, MA) according to the manufacturer’s instructions.
Protein assay
Protein contents were measured using BCA Protein Assay Reagent Kit (Pierce Biotechnology Inc., Rockford, IL) with bovine serum albumin as a standard.
Statistical evaluations
Data are expressed as mean ± standard error (SE). Changes in variables for different assays were analyzed by one-way ANOVA followed by post hoc Fisher’s PLSD test. Differences were considered to be significant at p<0.05
Results
Dose- and time-dependent production of NO and TNF-α in RAW 264 cells by LPS stimulation
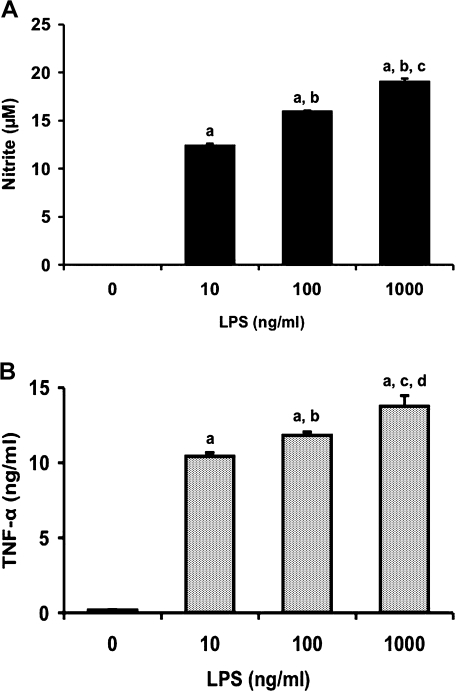
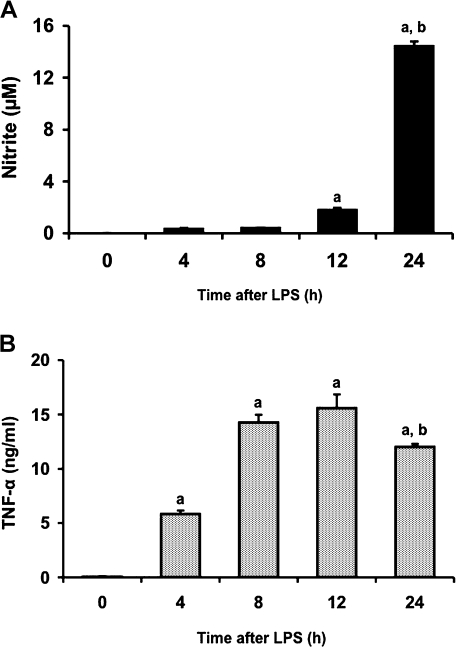
To determine the concentration of LPS required for inducing production of NO and TNF-α in RAW 264 cells, we treated the cells with LPS at concentrations ranging from 10 to 1000 ng/ml for 24 and 12 h, respectively (Fig. 1). LPS induced NO production in a dose-dependent manner (Fig. 1A). TNF-α concentration in medium also increased in a dose-dependent manner (Fig. 1B). We next examined the time course of changes in release of NO and TNF-α from RAW 264 cells following treatment with LPS (1 µg/ml) (Fig. 2). NO concentration in medium increased with time up to 24 h (Fig. 2A). TNF-α level in medium also increased time-dependently, reached a maximum at 12 h and decreased at 24 h after LPS addition (Fig. 2B). These results were consistent with those in the previous study that LPS treatment of murine macrophages resulted in the early expression of TNF-α and the subsequent synthesis of iNOS [23].
Fig. 1.
Dose-dependent production of NO (A) and TNF-α (B) in RAW 264 cells by LPS treatment. Cells were incubated with LPS (10, 100, or 1000 ng/ml) for 24 h (A) or 12 h (B) at 37°C under a 5% CO2 and 95% air atmosphere. The levels of NO and TNF-α were measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). ap<0.01 vs 0 ng/ml, bp<0.01 vs 10 ng/ml, cp<0.01 vs 100 ng/ml in (A). ap<0.01 vs 0 ng/ml, bp<0.05 vs 10 ng/ml, cp<0.01 vs 10 ng/ml, dp<0.01 vs 100 ng/ml in (B).
Fig. 2.
Time-dependent production of NO (A) and TNF-α (B) in RAW 264 cells by LPS treatment. Cells were incubated with LPS (1 µg/ml) for 4, 8, 12 or 24 h at 37°C under a 5% CO2 and 95% air atmosphere. The levels of NO and TNF-α were measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). ap<0.01 vs 0 h, bp<0.01 vs 12 h.
Effect of GGA on LPS-mediated NO and TNF-α production in RAW 264 cells
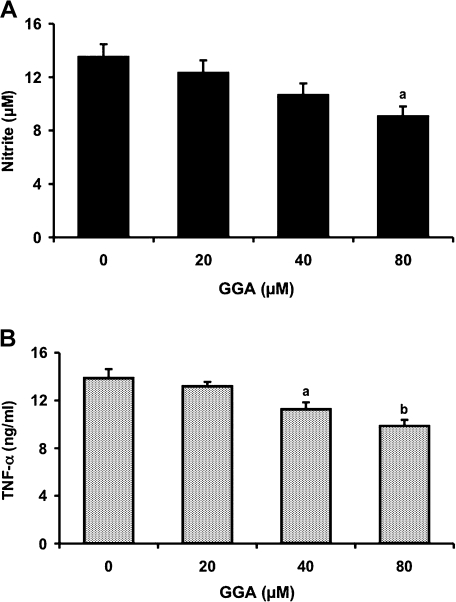
To determine whether GGA can inhibit LPS-induced NO and TNF-α production, we treated RAW 264 cells with GGA over a concentration range of 0–80 µM 2 h before 1 µg/ml LPS administration (Fig. 3). GGA inhibited LPS-induced NO production in a dose-dependent manner. Treatment with 80 µM GGA significantly reduced NO concentration 24 h after LPS by 34% in comparison with treatment with LPS alone (Fig. 3A). Pretreatment of the cells with GGA also inhibited LPS-mediated TNF-α production dose-dependently (Fig. 3B). Treatments with 40 µM and 80 µM GGA significantly inhibited TNF-α concentration by 19% and 29%, respectively, compared with LPS alone (Fig. 3B).
Fig. 3.
Inhibitory effect of GGA on LPS-mediated production of NO (A) and TNF-α (B). Cells were incubated in the presence or absence of LPS (1 µg/ml) for 24 h (A) or 12 h (B) at 37°C under a 5% CO2 and 95% air atmosphere. GGA (0, 20, 40, or 80 µM) was added 2 h before the addition of LPS. The levels of NO and TNF-α were measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). ap<0.05, bp<0.01 vs LPS alone-treated cells.
Effect of GGA treatment on cell viability
To confirm that the inhibitory effect of GGA on LPS-induced NO and TNF-α production is not due to GGA-induced cell death, we examined the cell viability 24 h after addition of GGA (20, 40 or 80 µM), LPS (10, 100 or 1000 ng/ml) or GGA (20, 40 or 80 µM) plus LPS (1 µg/ml) to RAW 264 cells. Any treatment did not decrease cell viability significantly, in comparison with untreated cell control (data not shown).
Effect of GGA on LPS-mediated iNOS induction in RAW 264 cells
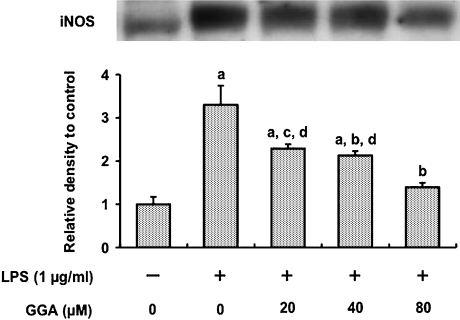
Induction of iNOS in RAW 264 cells 24 h after LPS administration (1 µg/ml) was assessed by Western blot analysis (Fig. 4). LPS increased iNOS contents 3.3-fold in comparison with untreated cells. Treatments with GGA at concentrations of 20, 40 and 80 µM 2 h before LPS addition decreased the contents by 31%, 35% and 58%, respectively in a dose-dependent manner, compared with LPS alone.
Fig. 4.
Effect of GGA on LPS-mediated iNOS induction in RAW 264 cells. Cells were incubated in the presence or absence of LPS (1 µg/ml) for 24 h at 37°C under a 5% CO2 and 95% air atmosphere. GGA (0, 20, 40, or 80 µM) was added 2 h before the addition of LPS. iNOS level was analyzed by Western blotting, quantified densitometrically and expressed as relative density to control cells. Data points represent the means ± SE (n = 3). ap<0.01 vs control, bp<0.01 vs LPS alone, cp<0.05 vs LPS alone, dp<0.05 vs LPS (1 µg/ml) + GGA (80 µM).
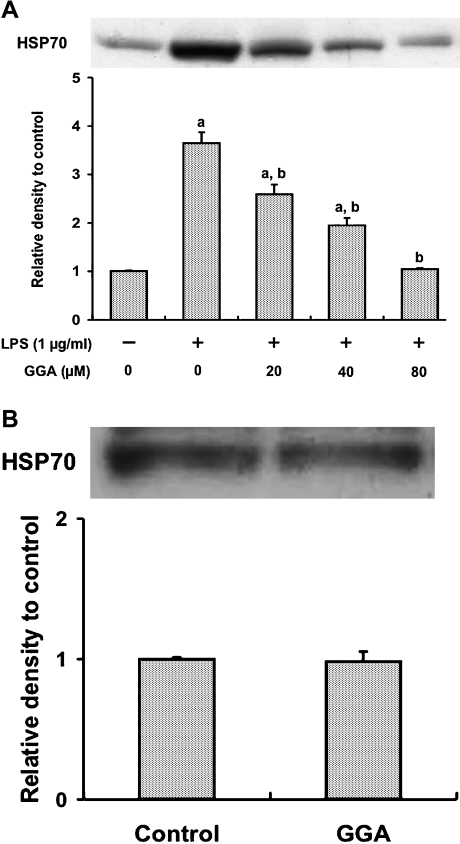
Effect of GGA on LPS-mediated HSP70 induction in RAW 264 cells
Overexpression of HSP70 reportedly inhibited the production of cytokines in macrophages activated by LPS [18]. Since GGA is well known to be a potent HSP70 inducer [12, 13], we investigated whether the inhibitory effect of GGA on LPS-induced NO and TNF-α production in RAW 264 cells was ascribed to HSP70 induction by GGA treatment (Fig. 5A). Treatment with GGA 2 h before LPS administration inhibited LPS-triggered HSP70 induction in a dose-dependent manner. Specifically, 80 µM GGA suppressed the increase in HSP70 content by LPS to the control level. Treating the cells with 80 µM GGA alone for 2 h did not enhance HSP70 protein expression (Fig. 5B).
Fig. 5.
Effect of GGA on HSP70 induction in RAW 264 cells. (A) Cells were incubated in the presence or absence of LPS (1 µg/ml) for 24 h at 37°C under a 5% CO2 and 95% air atmosphere. GGA (0, 20, 40, or 80 µM) was added 2 h before the addition of LPS. (B) Cells were incubated in the presence or absence of GGA (80 µM) for 2 h at 37°C under a 5% CO2 and 95% air atmosphere. HSP70 level was analyzed by Western blotting, quantified densitometrically and expressed as relative density to control cells. Data points represent the means ± SE (n = 3). ap<0.01 vs control, bp<0.01 vs LPS alone-treated cells.
Effect of replacement by fresh culture medium before LPS administration on inhibition by GGA of NO production in LPS-treated RAW 264 cells
When GGA (20, 40 or 80 µM) was added to the medium 2 h before LPS administration and subsequently culture medium was replaced by fresh one without GGA just before LPS addition, the inhibitory effect of GGA on NO production induced by LPS in the cells was abolished (Fig. 6).
Fig. 6.
Effect of replacement by fresh culture medium before LPS administration on inhibition by GGA of NO production in LPS-treated RAW 264 cells. GGA (0, 20, 40, or 80 µM) was added to the medium 2 h before LPS administration and subsequently culture medium was replaced by fresh one without GGA just before LPS addition. Cells were further incubated in the presence of LPS (1 µg/ml) for 24 h at 37°C under a 5% CO2 and 95% air atmosphere. The level of NO in the medium was measured as described in Materials and Methods. Data points represent the means ± SE (n = 3).
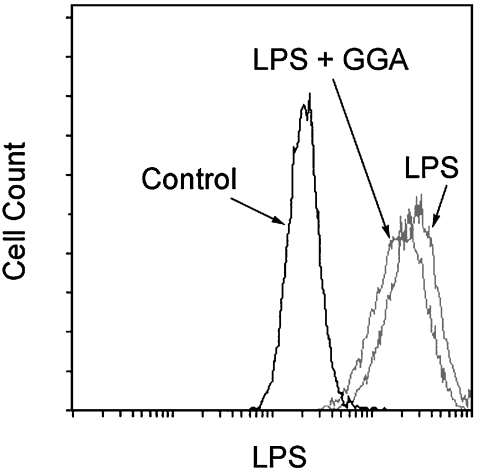
Effect of GGA on binding of LPS to the cell surface
To investigate the effect of GGA on LPS binding to the cell surface, we used flow cytometry with an anti-LPS monoclonal antibody (Fig. 7). Cell surface LPS increased after LPS stimulation. However, the LPS binding was inhibited when LPS was added to the medium in the presence of GGA.
Fig. 7.
Effects of GGA on binding of LPS to the cell surface in RAW 264 cells. Cells were incubated with 80 µM GGA for 2 h at 37°C and for further 30 min after addition of LPS (1 µg/ml). Cells were washed, and stained with anti-LPS antibody, followed by goat anti-mouse IgG-FITC. The LPS fluorescence resulting from LPS binding to cells was measured by using Epics cytofluorometer. Representative data from three separate experiments are shown.
Effect of GGA on TLR4-MD-2 complex and CD14 expressions on the surface of RAW 264 cells
We next examined the expression of TLR4-MD-2 complex and CD14 on the cell surface of RAW 264 cells in the presence or absence of GGA by using flow cytometry. Treatment of the cells with GGA did not affect the cell surface expression of TLR4-MD-2 complex and CD14 (data not shown).
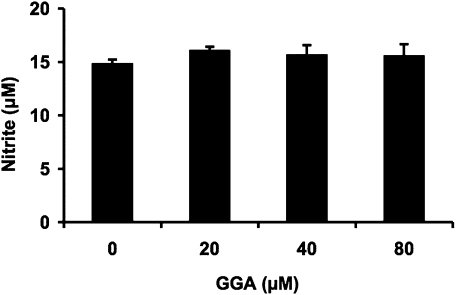
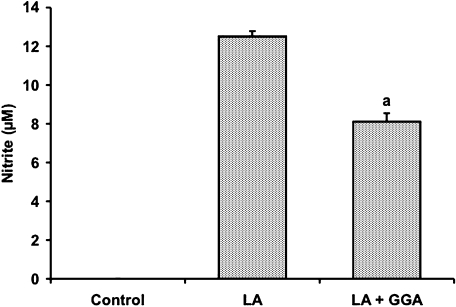
Effect of GGA on lipid A-mediated NO production in RAW 264 cells
To determine whether the inhibitory effect of GGA on LPS-mediated macrophage activation is due to its action on lipid A, we examined the effect of GGA on NO production in lipid A-treated RAW 264 cells (Fig. 8). NO contents in the medium increased dose-dependently 24 h after treatment of RAW 264 cells with lipid A at doses of 0.01–100 ng/ml (data not shown). Pretreatment of the cells with 80 µM GGA significantly decreased NO concentration by 35% at 24 h after lipid A (100 ng/ml) challenge in comparison with lipid A alone, completely consistent with inhibitory effect of GGA on LPS-mediated NO production (Fig. 8, compare with Fig. 3A).
Fig. 8.
Inhibitory effect of GGA on lipid A-mediated NO production in RAW 264 cells. Cells were incubated in the presence or absence of Lipid A (LA) (100 ng/ml) for 24 h at 37°C under a 5% CO2 and 95% air atmosphere. GGA (80 µM) was added 2 h before the addition of LA. NO level was measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). ap<0.01 vs LA alone-treated cells.
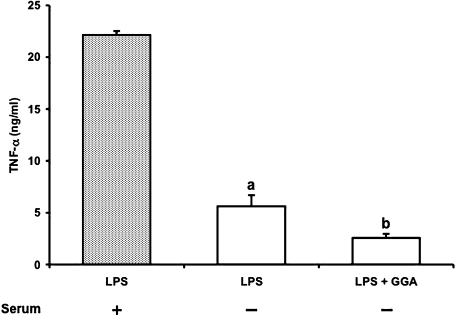
Effect of serum on LPS-induced TNF-α release
Serum proteins such as soluble LBP and soluble CD14 play important roles in cell activation induced by LPS. To investigate the involvement of serum proteins in the inhibitory action of GGA, TNF-α production by RAW 264 cells was determined in the presence or absence of serum (Fig. 9). TNF-α release at 4 h after LPS (1 µg/ml) challenge under serum-free condition reduced to one third of that in the presence of serum. However, GGA significantly inhibited TNF-α production regardless of the presence of serum. Furthermore, GGA failed to suppress the binding of LPS to LBP on in vitro study although CL as a positive control strongly inhibited LPS-LBP binding (data not shown).
Fig. 9.
TNF-α release from RAW 264 cells after LPS stimulation in the presence or absence of serum and its inhibition by GGA. Cells were incubated with LPS (1 µg/ml) in the presence (closed column) or absence (open column) of serum for 4 h at 37°C under a 5% CO2 and 95% air atmosphere. GGA (80 µM) was added 2 h before the addition of LPS. TNF-α level was measured as described in Materials and Methods. Data points represent the means ± SE (n = 3). ap<0.01 vs LPS alone-treated cells in the presence of serum. bp<0.05 vs LPS alone-treated cells in the absence of serum.
Discussion
The present study demonstrated for the first time that GGA inhibited NO and cytokine release from macrophages activated by LPS stimulation.
In our previous study, we found that GGA suppressed the liberations of proinflammatory cytokines like TNF-α, IL-6 and MIP-2 as well as NO in LPS-injected rats and this effect of GGA was related to the enhancement of HSP70 induction in several organs after LPS injection [12]. In the present study, we investigated whether anti-inflammatory effect of GGA in endotoxin shock was ascribed to the inhibition against macrophage activation in addition to the HSP induction in several organs. GGA inhibited the production of NO and TNF-α in LPS-stimulated RAW 264 murine macrophage cells (Fig. 3). The transcriptions of iNOS and TNF-α are regulated by NF-κB [8]. Recently, it has been reported that GGA suppressed IL-1β-mediated NF-κB activation in vascular smooth muscle cells, leading to the inhibition of iNOS expression and subsequent NO production [19]. Therefore, we determined the effect of GGA on iNOS expression in RAW 264 cells after LPS challenge. GGA inhibited iNOS induction as well as production of NO and TNF-α in LPS-treated RAW 264 cells (Fig. 4), suggesting that GGA suppressed NF-κB activation in LPS-stimulated macrophages as well as IL-1β-stimulated vascular smooth muscle cells [19]. It has been shown that the production of proinflammatory cytokines was inhibited by the HSP70 induction [2, 14]. Given that GGA is a potent HSP inducer, it is suggested that anti-inflammatory action of GGA might also be due to the induction of HSP70. In the present study, unexpectedly treatment of RAW 264 cells with GGA before LPS administration inhibited HSP70 induction after LPS challenge (Fig. 5). These paradoxical results for HSP inducer GGA can be explained as follows. It has been reported that the HSP-inducing activity of GGA appears to be due to enhancing the process of heat shock factor (HSF) 1 activation such as nuclear translocation, phosphorylation and DNA binding activity of HSF1 rather than due to directly stimulating HSP induction [24]. In other words, GGA can primes the cells for HSP induction, but not induce HSP expression unless stimulation is added. We examined HSP70 protein expression in RAW 264 cells at 2 h after GGA treatment. However, there was no difference in HSP70 status between GGA-treated and control cells (Fig. 5B). Even though RAW 264 cells were primed with GGA in these experiments, it is considered that the LPS stimulation signal was not transduced to the inside of the cells in the presence of GGA. Furthermore, replacement of medium before LPS addition abolished the inhibitory effect of GGA on LPS-mediated NO production in the cells. These results suggested that GGA did not exhibit anti-inflammatory action via enhancement of HSP70 induction but affected the process of LPS binding to TLR4 on the cell surface when it was in extracellular space and/or cell membrane, resulting in suppression of iNOS and HSP70 expression after LPS stimulation. The results of LPS binding assay revealed that GGA inhibited the binding of LPS to the cell surface without affecting the expression of TLR4-MD-2 complex and CD14 on the cell surface. It is known that serum proteins such as LBP and sCD14 can interact with LPS [9]. LPS from E. coli consists of the outer most O side chain with repeating sugar units, which determines the LPS antigenic specificity, a core oligosaccharide and the inner most layer, lipid A, which is responsible for LPS toxicity [9]. Hexaacylated E. coli lipid A has the highest cytokine-inducing capacity [25]. In contrast, lipid A partial structures lacking one or more fatty acids, like the lipid A precursor IVa (synthetic compound 406) which carries only four fatty acids, is endotoxically inactive, but may express strong antagonistic activity against LPS-induced cell activation [26, 27]. Recently, CL which has structural similarities to compound 406 with respect to the net negative charge (-2) and number of acyl chains (4) strongly inhibited LPS-induced TNF-α release by a blockade of the binding of LPS to LBP [25]. Because LBP has positive charges, it can bind negatively charged lipids like CL, phosphatidylserine or phosphatidylinositol [28]. On the other hand, oxidized phosphatidylcholine (PC) reportedly inhibited the interaction of LPS with LBP and CD14 although PC has a net charge of 0 [29]. Since GGA is an acylic polyisoprenoid, we determined the effect of GGA on LPS-LBP binding on in vitro study. However, GGA did not block the binding of LPS to LBP. Furthermore, GGA inhibited LPS-mediated TNF-α production in the cells even under serum-free conditions. Thus, the acting points by GGA are unlikely serum protein such as LBP and sCD14. Additionally, since GGA inhibited lipid A-induced NO production in RAW cells (Fig. 7), it seems likely that GGA acts on lipid A but not the saccharides of LPS. Taken together, the remaining potential mechanisms underlying the inhibition of macrophage activation by GGA are the inhibition of interaction of LPS (especially lipid A) with mCD14 and/or TLR4-MD2 complex. These hypotheses remain to be elucidated.
In conclusion, the present study demonstrated that the inhibition by GGA of the LPS effects on macrophages was mediated by interference of the binding of LPS to the cell surface, but not its HSP70-inducing activity, thereby inhibiting recognition of LPS by TLR4.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Akira S. Mammalian Toll-like receptors. Curr. Opin. Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 2.Klosterhalfen B., Hauptmann S., Offner F.A., Amo-Takyi B., Tons C., Winkeltau G., Affify M., Kupper W., Kirkpatrick C.J., Mittermayer C. Induction of heat shock protein 70 by zinc-bis-(DL-hydrogenaspartate) reduces cytokine liberation, apoptosis, and mortality rate in a rat model of LD100 endotoxiemia. Shock. 1997;7:254–262. doi: 10.1097/00024382-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Carrillo-Vico A., Lardone P.J., Naji L., Fernández-Santos J.M., Martín-Lacave I., Guerrero J.M., Calvo J.R. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res. 2005;39:400–408. doi: 10.1111/j.1600-079X.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama T., Fujita M., Koide N., Mori I., Yoshida T., Mori H., Yokochi T. 2-Aminopurine inhibits lipopolysaccharide-induced nitric oxide production by preventing IFN-β production. Microbiol. Immunol. 2004;48:957–963. doi: 10.1111/j.1348-0421.2004.tb03625.x. [DOI] [PubMed] [Google Scholar]
- 5.Jantaratnotai N., Utaisincharoen P., Piyachaturawat P., Chongthammakun S., Sanvarinda Y. Inhibitory effect of Curcuma comosa on NO production and cytokine expression in LPS-activated microglia. Life Sci. 2006;78:571–577. doi: 10.1016/j.lfs.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 6.Huang K.T., Kuo L., Liao J.C. Lipopolysaccharide activates endothelial nitric oxide synthase through protein tyrosine kinase. Biochem. Biophys. Res. Commun. 1998;245:33–37. doi: 10.1006/bbrc.1998.8384. [DOI] [PubMed] [Google Scholar]
- 7.Palsson-McDermott E.M., O’Neill L.A.J. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S.F., Malik A.B. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 9.Miyake K. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol. 2004;12:186–192. doi: 10.1016/j.tim.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Hirakawa T., Rokutan K., Nikawa T., Kishi K. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology. 1996;111:345–357. doi: 10.1053/gast.1996.v111.pm8690199. [DOI] [PubMed] [Google Scholar]
- 11.Ooie T., Takahashi N., Saikawa T., Nawata T., Arikawa M., Yamanaka K., Hara M., Shimada T., Sakata T. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001;104:1837–1843. doi: 10.1161/hc3901.095771. [DOI] [PubMed] [Google Scholar]
- 12.Nakada J., Matsura T., Okazaki N., Nishida T., Togawa A., Minami Y., Inagaki Y., Ito H., Yamada K., Ishibe Y. Oral administration of geranylgeranylacetone improves survival rate in a rat endotoxin shock model: administration timing and HSP70 induction. Shock. 2005;24:482–487. doi: 10.1097/01.shk.0000180980.63247.a9. [DOI] [PubMed] [Google Scholar]
- 13.Nishida T., Matsura T., Nakada J., Togawa A., Kai M., Sumioka I., Minami Y., Inagaki Y., Ishibe Y., Ito H., Ohta Y., Yamada K. Geranylgeranylacetone protects against acetaminophen-induced hepatotoxicity by inducing heat shock protein 70. Toxicology. 2006;219:187–196. doi: 10.1016/j.tox.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y., Sawa Y., Fukuyama N., Nakazawa H., Matsuda H. Preoperative glutamine administration induces heat-shock protein 70 expression and attenuates cardiopulmonary bypass-induced inflammatory response by regulating nitric oxide synthase activity. Circulation. 2002;106:2601–2607. doi: 10.1161/01.cir.0000035651.72240.07. [DOI] [PubMed] [Google Scholar]
- 15.Suganuma T., Irie K., Fujii E., Yoshioka T., Muraki T. Effect of heat stress on lipopolysaccharide-induced vascular permeability change in mice. J. Pharmacol. Exp. Ther. 2002;303:656–663. doi: 10.1124/jpet.102.035758. [DOI] [PubMed] [Google Scholar]
- 16.Sumioka I., Matsura T., Kai M., Yamada K. Potential roles of hepatic heat shock protein 25 and 70i in protection of mice against acetaminophen-induced liver injury. Life Sci. 2004; 74:2551–2561. doi: 10.1016/j.lfs.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Beere H.M., Wolf B.B., Cain K., Mosser D.D., Mahboubi A., Kuwana T., Tailor P., Morimoto R.I., Cohen G.M., Green D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 18.Ding X.Z., Fernandez-Prada C.M., Bhattacharjee A.K., Hoover D.L. Over-expression of HSP-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16:210–219. doi: 10.1006/cyto.2001.0959. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K., Sarukawa M., Ito T., Aoki H., Ichida M., Shimada K. An anti-ulcer drug, geranylgeranylacetone, suppresses inducible nitric oxide synthase in cultured vascular smooth muscle cells. J. Hypertens. 2005;23:1847–1853. doi: 10.1097/01.hjh.0000182525.74934.c0. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt H.H., Zernikow B., Baeblich S., Bohme E. Basal and stimulated formation and release of L-arginine-derived nitrogen oxides from cultured endothelial cells. J. Pharmacol. Exp. Ther. 1990;254:591–597. [PubMed] [Google Scholar]
- 21.Fujii Y., Matsura T., Kai M., Matsui H., Kawasaki H., Yamada K. Mitochondrial cytochrome c release and caspase-3-like protease activation during indomethacin-induced apoptosis in rat gastric mucosal cells. Proc. Soc. Exp. Biol. Med. 2000;224:102–108. doi: 10.1046/j.1525-1373.2000.22407.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsura T., Nishida T., Togawa A., Horie S., Kusumoto C., Ohata S., Nakada J., Ishibe Y., Yamada K., Ohta Y. Mechanisms of protection by melatonin against acetaminophen-induced liver injury in mice. J. Pineal Res. 2006;41:211–219. doi: 10.1111/j.1600-079X.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 23.Soler C., Valdes R., Garcia-Manteiga J., Xaus J., Comalada M., Casado F.J., Modolell M., Nicholson B., MacLeod C., Felipe A., Celada A., Pastor-Anglada M. Lipopolysaccharide-induced apoptosis of macrophages determines the up-regulation of concentrative nucleoside transporters Cnt1 and Cnt2 through tumor necrosis factor-α-dependent and -independent mechanisms. J. Biol. Chem. 2001;276:30043–30049. doi: 10.1074/jbc.M101807200. [DOI] [PubMed] [Google Scholar]
- 24.Ikeyama S., Kusumoto K., Miyake H., Rokutan K., Tashiro S. A non-toxic heat shock protein 70 inducer, geranylgeranylacetone, suppresses apoptosis of cultured rat hepatocytes caused by hydrogen peroxide and ethanol. J. Hepatol. 2001;35:53–61. doi: 10.1016/s0168-8278(01)00053-8. [DOI] [PubMed] [Google Scholar]
- 25.Mueller M., Brandenburg K., Dedrick R., Schromm A.B., Seydel U. Phospholipids inhibit lipopolysaccharide (LPS)-induced cell activation: a role for LPS-binding protein. J. Immunol. 2005;174:1091–1096. doi: 10.4049/jimmunol.174.2.1091. [DOI] [PubMed] [Google Scholar]
- 26.Loppnow H., Brade H., Durrbaum I., Dinarello C.A., Kusumoto S., Rietschel E.T., Flad H.D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J. Immunol. 1989;142:3229–3238. [PubMed] [Google Scholar]
- 27.Kovach N.L., Yee E., Munford R.S., Raetz C.R., Harlan J.M. Lipid IVa inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J. Exp. Med. 1990;172:77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu B., Hailman E., Wright S.D. Lipopolysaccharide binding protein and soluble CD14 catalyze exchange of phospholipids. J. Clin. Invest. 1997;99:315–324. doi: 10.1172/JCI119160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bochkov V.N., Kadl A., Huber J., Gruber F., Binder B.R., Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]