Abstract
Species diversity is correlated with structural complexity in many animal communities; however, experimental tests of the mechanisms underlying this important relationship are rare, especially in terrestrial communities. We manipulated physical features of the habitat of gecko lizards and measured the effect on exploitation competition for insects. Increasing both the dispersion of food resources and microhabitat topography dramatically reduced interspecific competition. Adding topographic structure reduced the advantages of the larger, faster, invasive species. Interindividual spacing decreased, but intraspecific agonistic interference increased in the more territorial, resident species. Human structural alterations of the environment facilitate invasion and competitive displacement in this system. Physical microhabitat structure can potentially affect species interactions through a variety of complex mechanisms.
Physical habitat structure has been implicated as an important ecological factor nearly as often as competition (1). Animal species diversity is positively correlated with habitat structural complexity in many terrestrial and aquatic communities (2–7). Although this relationship is widespread, intuitively appealing, and in agreement with theory, manipulative experiments investigating the mechanisms causing this pattern are uncommon.
We investigate how physical habitat structure affects interspecific competition for food resources. Competition for food is often determined by relative foraging success, and habitat structure is known to affect foraging dynamics in a wide variety of animal communities [examples include fish (8, 9), lizards (10), birds (2, 11), mammals (12, 13), and insects (14)]. However, the effects of habitat structure are not limited solely to foraging dynamics. For instance, habitat structure affects the social behavior of many animals (14–18). Because of the potential for complex interactions of habitat structure and behavior, a mechanistic approach (19, 20) is ideally suited for interpreting studies of habitat structure. Although the conceptual links between habitat structure and competition are clear, the causal links have not been experimentally tested in a single system.
By using large-scale manipulative experiments, we find direct evidence that physical habitat structure can determine the intensity of exploitation competition by differentially affecting the foraging success of competing species. Habitat structure manipulations also had unexpected effects on intraspecific interference competition in one species by altering the dynamics of territoriality. These results help to interpret the scope and pattern of an ongoing invasion.
The common house gecko, Hemidactylus frenatus (Hf) is currently invading and displacing resident gecko species across the Pacific, including the mourning gecko Lepidodactylus lugubris (Ll; ref. 21). Many resident geckos (including Ll) are all-female parthenogens; with human facilitation, these geckos have invaded the more remote islands of the Pacific hundreds to thousands of years before the arrival of the sexual Hf (22, 23). With large-scale seminatural experimental manipulations, we have previously shown that the mechanism of displacement hinges on the ability of Hf to forage more efficiently and exploitatively outcompete Ll (24, 25). The competitive displacement is most severe (and often complete) in Pacific urban habitats, and experiments have verified that competition depends on the presence of lights that create aggregations of insects, the main food resource of geckos (24). Other studies show that apparent competition (26) through parasite transmission and differential susceptibility cannot explain the decline of resident species (27, 28).
We considered two aspects of habitat structure that can affect gecko encounter rates with insects. First, the spatial distribution of prey in a microhabitat will affect encounter rates of predators with prey, which in turn can affect the foraging success of predators. Second, physical topography on a small scale can impede sight, which can affect predator/prey encounter rates as well as intraspecific communication. We tested two hypotheses regarding exploitation competition in this context: the competitive superiority of Hf depends on clumping of insect resources, and the advantage of Hf is enhanced by the structural simplicity of building walls.
The hypotheses imply that the advantages of Hf may depend on high insect encounter rates; these hypotheses stem from a number of previous findings (21, 24, 25). First, although the competitive displacement is complete in many urban habitats, it is incomplete or absent in more rural and forested areas on the same islands. These habitats are more structurally complex and have less clumping of insect resources than urban habitats. Second, quantified foraging behavior suggests that Hf are more pursuit-oriented predators and are more successful at long-distance foraging strikes than Ll. We predict that this advantage should be enhanced when the physical habitat is structurally simple. Third, Hf are larger and faster than Ll (29); thus, they should have an advantage when the two species are equidistant from a catchable insect. This may contribute to the superior harvesting ability of Hf when insects are clumped into high-density foraging patches. Finally, Hf show less intraspecific agonism than Ll while foraging (and interspecific interference is minimal; ref. 25). Because agonism in Ll begins with visual detection of conspecifics, we hypothesize that a structurally complex environment will reduce agonistic encounters and interindividual distances, and more Ll will have access to high-density resource clumps.
METHODS
We used 16 aircraft hangars (revetments) built during World War II on Oahu, Hawaii, as experimental enclosures (25). Hangars were modified to enclose experimental populations of geckos; lights (8 W fluorescent) were used to attract naturally occurring insects. All experimental geckos were collected on Oahu from environments in which both species and lights were present, and Hf was stocked at an equal sex ratio. Because geckos can deplete insect standing crops (25), insect abundance was measured in the absence of geckos to determine whether the number of insects attracted to foraging areas differed among treatments. To sample insects, a single sticky trap (25) was placed 0.1 m above each light facing down. A second trap was affixed 0.8 m above the light and mounted directly on the wall. Termite alates and moths, which comprise 63% and 17% of the diet for both species, respectively (25), were counted daily for 4 consecutive days at the end of each experiment after geckos were removed. Traps near lights were replaced daily.
We measured competition intensity at regular intervals by comparing the relative body condition of geckos across treatments. Body condition was represented as the residuals from a regression of mass (log-transformed) onto length and was calculated separately for each species. Previous experiments have shown that geckos with higher relative body condition scores produce more eggs and survive significantly longer than geckos with lower body condition scores (25). Measurements were recorded during complete censuses of all enclosures at 2- to 3-wk intervals, after which dead and missing geckos were replaced. Survivorship was not compared because differences in survival are only manifested at longer time intervals (25). Survival over the course of each experiment averaged 65%.
Observations were conducted from a blind (3 m from the wall) for 90 min during peak foraging in the early evening (≈1900–2030 h). Foraging attempts (a directed movement of a gecko toward an insect near the wall surface) and successful captures were quantified (25). All agonistic interactions (inter- and intraspecific) involving an approach by one individual and a retreat by another were recorded along with the behaviors displayed. Illuminated regions closer to the light (Fig. 1) attract significantly more catchable insects than neighboring regions that are as little as 10 cm farther from the light (25). Therefore, locations of all geckos near lights were recorded at 20-min intervals. Each experiment was observed for at least 30 h.
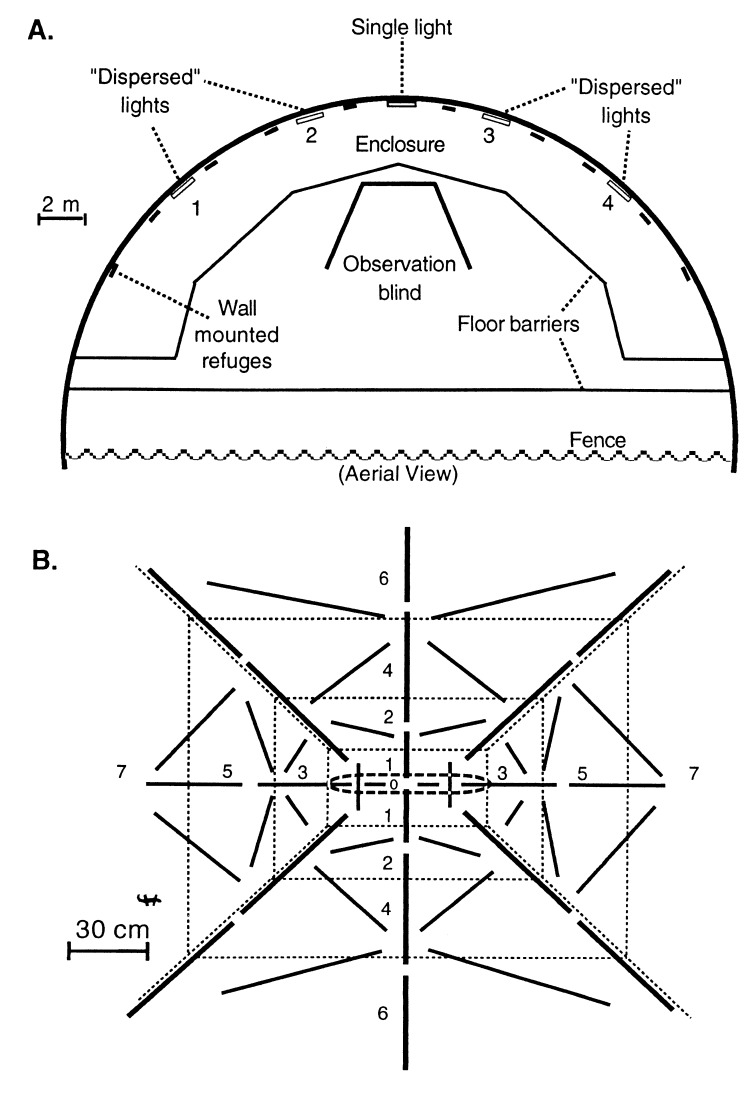
Figure 1.
Experimental enclosures. (A) Top view. Experimental geckos were kept inside enclosures with Teflon barriers, although natural insects could pass freely through the large opening. Urban control treatments contained a single light affixed to the vertical wall 1.8 m from the floor. Dispersed insect treatments contained four lights, but only one light was on at any given time (for 8–10 min). (B) Topographic structure was enhanced in a third experimental treatment by affixing aluminum baffles to the wall near the light. Diagonal barriers (thick lines) were 10 cm high (from the wall), whereas all other barriers were 5 cm high. Geckos moved freely on and around barriers. Numbers denote regions used to mark gecko positions. Region 0 contained the light, and region 8 encompassed all parts of the enclosure that were not visible.
Two types of vocalizations are common in Ll: “growls” often accompany lunges and bites; “clicks” are given before, during, or after an encounter, typically in series of 4–10. Over 80% of all agonistic interactions that involve more than a simple approach and retreat have accompanying vocalizations (K.P., unpublished data). In experiment 2, only two species enclosures were observed as above. We recorded vocalizations to estimate levels of agonism in Ll-only treatments. An audio recorder equipped with a parabolic reflector was placed 2.5 m from the wall in front of the light, and 90 min of peak foraging was recorded. To quantify individual spacing, each of eight enclosures were observed for 10 min in succession on two evenings that appeared to have moderate to high insect activity. On the second day of observation (2 wk later), the viewing order was reversed.
Experiments were conducted in succession from November 1994 to July 1995. Because of limitations in obtaining suitable numbers of geckos, 70% of the individuals in experiment 2 were retained from experiment 1. Geckos were reassigned randomly to treatments in experiment 2 with two constraints: the mean body condition of geckos was kept approximately equivalent for each enclosure, and no gecko was reintroduced into the same enclosure.
In control treatments representing the typical urban environment, a single light was affixed to the center of the enclosure. This treatment yielded strong interspecific dominance of Hf over Ll in two previous experiments (24, 25). We increased the dispersion of insects by placing four lights evenly spaced throughout the enclosure; however, to keep insect levels constant, only a single light was on at a time (Fig. 1). Topographic structure was enhanced by placing aluminum baffles projecting out from the wall around single lights (Fig. 1).
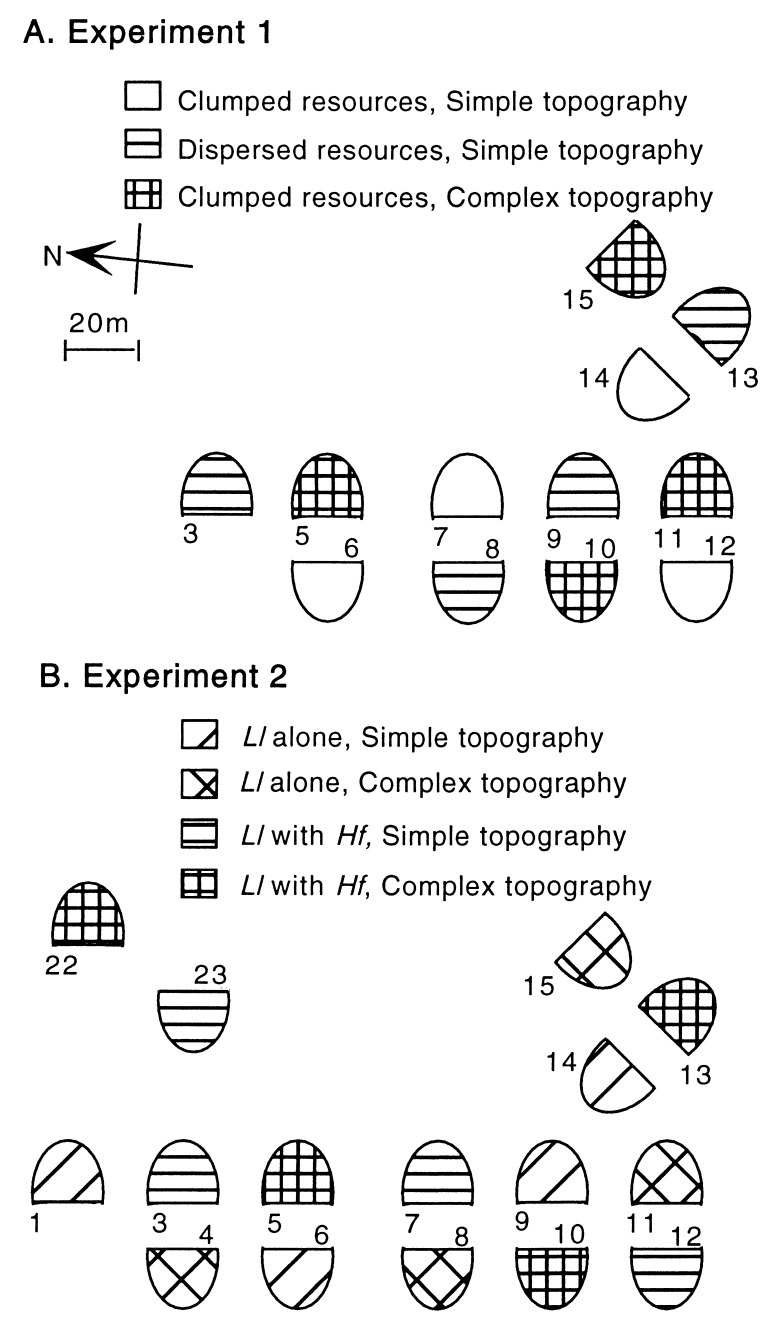
For experiment 1 (Fig. 2A), we used a nested, two-factor design (clumped/dispersed insect distribution; simple/complex topography) The cross treatment of dispersed insects and complex structure was not included. Each of four replicate enclosures for the three treatments were stocked with 10 Ll and 10 Hf. For experiment 2 (Fig. 2B), four treatments were produced by completely crossing two factors (simple/complex topographic structure; interspecific/intraspecific competitor). All four replicates of each treatment contained either 9 Ll and 9 Hf (interspecific competitor) or 18 Ll (intraspecific competitor). For all statistical tests, we treat replicate enclosures as a nested random effect; therefore, the error term in ANOVA tests is based on the enclosure degrees of freedom and not the total number of geckos.
Figure 2.
Treatments for each experiment were systematically assigned to balance for previously documented systematic patterns of insect abundance (25). (A) For experiment 1, each of three treatments had four replicate enclosures stocked with 20 geckos each (10 Ll and 10 Hf). (B) For experiment 2, two factors (inter- and intraspecific competitor) were crossed to produce four treatments, again with 4-fold replication stocked with 20 Ll or 10 Hf and 10 Ll.
RESULTS
Experimental manipulations had the intended effect on insect
resource distribution: insect abundance remained relatively constant,
whereas the distribution was substantially less clumped in dispersed
treatments. Each light in the dispersed treatment had significantly
fewer insects than each single light in the control treatments (at
0.8 m: dispersed  = 25, control
= 25, control  = 72; at 0.1 m: dispersed
= 72; at 0.1 m: dispersed  = 108, control
= 108, control
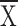 = 499). Dispersed light enclosures had more insects
than complex topography treatments overall at a distance of 0.8 m
from the lights; however, this is most likely caused by summing totals
over all four traps, which captured insects even when the closest light
was not on [at 0.8 m, ANOVA F = 4.2,
P = 0.05, Fisher’s probable least-squares difference
(PLSD) P = 0.02 for dispersed vs. structure treatments;
at 0.1 m, ANOVA F = 0.8, P >
0.4]. There were no significant differences in insect abundance among
the four treatments in experiment 2 (at 0.8 m, ANOVA
F = 0.4, P > 0.7; at 0.1 m,
F = 0.6, P > 0.6).
= 499). Dispersed light enclosures had more insects
than complex topography treatments overall at a distance of 0.8 m
from the lights; however, this is most likely caused by summing totals
over all four traps, which captured insects even when the closest light
was not on [at 0.8 m, ANOVA F = 4.2,
P = 0.05, Fisher’s probable least-squares difference
(PLSD) P = 0.02 for dispersed vs. structure treatments;
at 0.1 m, ANOVA F = 0.8, P >
0.4]. There were no significant differences in insect abundance among
the four treatments in experiment 2 (at 0.8 m, ANOVA
F = 0.4, P > 0.7; at 0.1 m,
F = 0.6, P > 0.6).
Ll relative body condition was highest in both structurally modified environments as compared with the urban control environment. In experiment 1, increasing topographic structure rapidly caused a significant increase in Ll body condition compared with populations in control treatments with flat walls (Fig. 3A). Increasing the dispersion of resources also caused a more gradual improvement in Ll condition. In experiment 2, increasing topographic structure again rapidly reduced the intensity of interspecific competition on Ll. However, topographic structure had a stronger effect on moderating interspecific competition than intraspecific competition (Fig. 3B). Consequently, Ll fared best when topographic structure and Hf were present and fared poorest when Hf were present and topographic structure was simple.
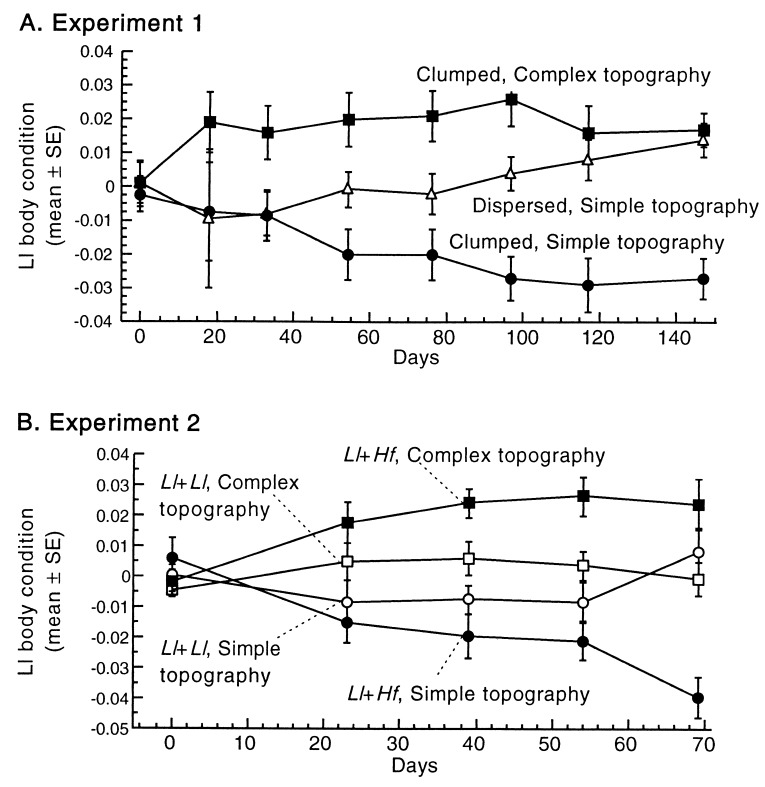
Figure 3.
Increasing resource dispersion and topographic complexity nearly eliminated competition. (A) Ll body condition showed significant differences at every census after 30 days (ANOVA all P < 0.03), but geckos with topographic structure improved more rapidly than those with dispersed resources. At census 7, both treatments were significantly different from the “urban control” (ANOVA F = 26.5, P = 0.002; Fisher’s PLSD P < 0.001). (B) More complex topographical structure reduced interspecific competition more than intraspecific competition in Ll. The interaction of competitor type (intraspecific vs. interspecific) and topographic structure (simple/complex) was significant in the last two censuses (ANOVA structure F > 13.0, P < 0.01; competitor F < 2.0, P > 0.1; structure × competitor F = 7.7, P < 0.02). The relative magnitude of inter- and intraspecific competition was completely reversed by increasing topographic complexity.
The relative body condition among Hf was not significantly affected by treatments, although by the final two censuses of experiment 1, they fared best in control treatments as expected (ANOVA F < 1.4, P > 0.3 for all censuses). In experiment 2, Hf fared consistently worse when structure was present, but not significantly so (t = 1.7, P = 0.13). The direction of the response of Hf confirms that positive effects on Ll are not a result of overall higher levels of insect abundance or generally enhanced insect catchability in structurally modified treatments.
Direct observation revealed that foraging patterns differed according
to treatment. In experiment 1, Hf made more long strike
attempts when resources were dispersed, whereas Ll made
fewer long strikes when complex structure was present (Fig.
4A). In experiment 2,
both species made fewer long strike attempts in response to increased
topographical structure (proportion of long attempts: for
Hf, control 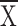 = 0.41, structure
= 0.41, structure
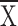 = 0.25 ± 0.1, t = 1.15,
P = 0.29; for Ll, control
= 0.25 ± 0.1, t = 1.15,
P = 0.29; for Ll, control 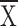 = 0.32, structure
= 0.32, structure 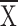 = 0.13, t =
2.6, P < 0.05). For Hf, harvest rates based
on these long strikes were significantly lower when topographic
structure was enhanced (Fig. 4B). There was no significant
decline in harvest rate attributed to long strikes for Ll;
however, Ll harvest rates are already quite low at these
distances in the presence of Hf.
= 0.13, t =
2.6, P < 0.05). For Hf, harvest rates based
on these long strikes were significantly lower when topographic
structure was enhanced (Fig. 4B). There was no significant
decline in harvest rate attributed to long strikes for Ll;
however, Ll harvest rates are already quite low at these
distances in the presence of Hf.
Figure 4.
(A) In experiment 1, the proportion of long (>20 cm) foraging attempts by Hf increased when insects were dispersed (ANOVA F = 4.8, P < 0.04; Fisher’s PLSD P < 0.02). Fewer long attempts were made by Ll when topography was complex (ANOVA F = 8.2, P < 0.01; Fisher’s PLSD P < 0.01). (B) In experiment 2, increased topographic structure caused Hf harvest rates at longer distances to decline (t = 2.8, P = 0.03), whereas Ll did not experience a decline (t = 1.2, P > 0.25). (C) In experiment 1, of those geckos that were foraging near the light, Hf showed little increased access to the light because of treatment effects (ANOVA F = 1.6, P > 0.25; regions shown in Fig. 1), whereas Ll were closer to lights in both dispersed and complex environments as compared with controls (ANOVA F = 8.5, P < 0.01; Fisher’s PLSD P < 0.03).
As predicted, both dispersed resources and more complex structure
allowed Ll greater access to the more productive foraging
areas near lights, whereas Hf were not significantly
affected (Fig. 4C). Patterns were similar in experiment 2:
Ll observed foraging are closer to the light when structure
is enhanced both with Hf present (control 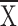 = 3.5 ± 0.3, structure
= 3.5 ± 0.3, structure 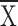 = 2.2 ± 0.3,
t = 3.1, P < 0.03) and with
Hf absent (for Ll without Hf: control
= 2.2 ± 0.3,
t = 3.1, P < 0.03) and with
Hf absent (for Ll without Hf: control
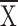 = 3.6 ± 0.2, structure
= 3.6 ± 0.2, structure 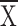 =
1.8 ± 0.2, t = 6.6, P < 0.001).
=
1.8 ± 0.2, t = 6.6, P < 0.001).
When structure was enhanced, the proportion of foraging attempts attributed to Ll in two-species enclosures increased, but not significantly. However, Ll had a significantly higher proportion of all successful insect captures with structure than without (Fig. 5A). This is direct evidence that Hf can no longer exploitatively dominate Ll at resource clumps when topographic structure is more complex.
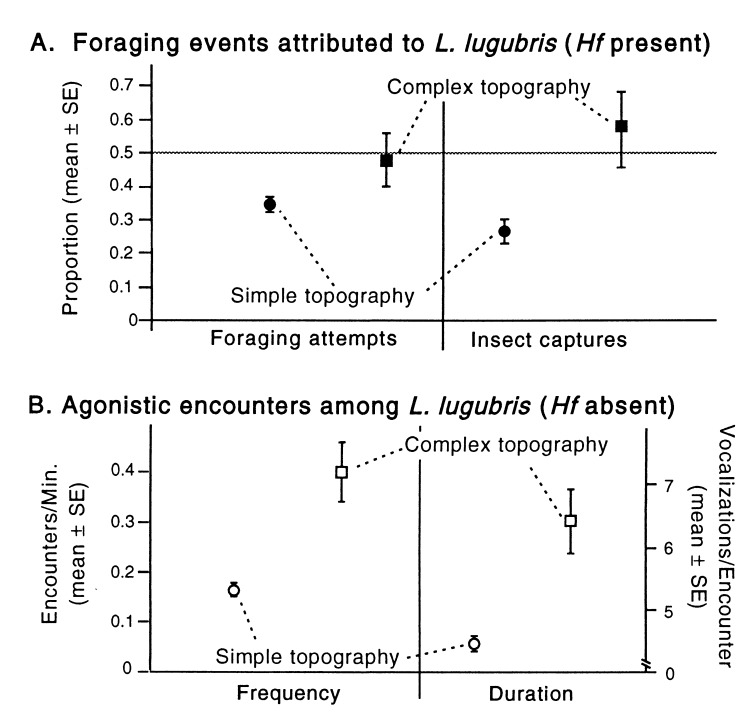
Figure 5.
(A) Ll made more foraging attempts in the presence of Hf when topography was complex, but this difference was not significant (t = 1.6, P > 1.5). However, Ll obtained a significantly greater proportion of all insects captured (t = 2.5, P < 0.05). (B) The frequency and duration of agonistic encounters among Ll increased significantly when complex topographic structure was enhanced (experiment 2, treatments without Hf).
In the more topographically structured habitat, the frequency as well as the duration of agonistic encounters increased substantially among Ll (Fig. 5B). This may partially explain why Ll intraspecific competition appears to be somewhat reduced in the structure treatments but remains stronger than interspecific competition.
DISCUSSION
Interspecific competition was significantly reduced by manipulating purely physical features of the environment in two ways: increasing the dispersion of insect food resources and increasing the topographic complexity of foraging areas. The effect of topographic structure was particularly dramatic. Each species was affected differently by structural manipulations, resulting in changes in both foraging and agonistic behavior that nearly eliminated interspecific competition. Whereas previous experiments of similar design showed that per capita interspecific competition was greater than intraspecific competition (24, 25), increasing topographic structure caused this relationship to be reversed, so that intraspecific competition was stronger than interspecific competition under these conditions (Fig. 5).
Dispersing resources may have led to improved Ll body condition through at least two mechanisms. First, the ability of Hf to consume more insects may have been diminished, because even the best foraging areas in the enclosure were only one-fourth as productive as in the single light treatments. The tendency of Hf to make more long strike attempts in dispersed treatments (Fig. 4A) may have been a result of their tendency to wander away from lights after they switched off. Second, more Ll had access to the prime foraging areas closest to the light (Fig. 4C). This was also the case in enclosures with enhanced structure; however, we would not expect the countervailing tradeoff of increased agonism that was observed when interindividual distances were reduced in structure treatments. In dispersed treatments, Ll are distributed among four different lights.
Increased topographic structure impeded the gecko’s line of sight to catchable insects, causing the speed advantage of Hf to be reduced; they were no longer able to make successful long strikes (Fig. 4B). The presumed foraging capacity advantage of Hf was also reduced because they were no longer able to obtain more insects per capita than Ll (Fig. 5A). Ll may have also benefited from topographic structure by the following mechanism: Ll made fewer long strikes (>20 cm) in structurally complex habitats when compared with structurally simple habitats (Fig. 4A). However, when Hf were present, Ll were not very successful at these long strikes in either habitat (Fig. 4B); therefore, with structure Ll may have wasted less energy making attempts that were not likely to be successful.
These results are consistent with the broad scale pattern of invasion and displacement of Ll by Hf. Hf invades and completely displaces resident geckos in most urban/suburban environments. The urban environment is characterized by clumped insect distributions and structurally simple walls. In many forested habitats throughout the Pacific, where topographic structure is more complex and insect abundance is less clumped because of the absence of electric lights, Hf rarely invades and Ll is common (21). The occasional success of Hf in some forests and the failure of Hf to colonize certain urban areas may be related to overall insect abundance. Some forests have unusually high insect abundance, enabling Hf to persist even though they may not be foraging as efficiently in this highly structured environment (K.P., unpublished observation). In forested habitats in which Hf persists, other species are not driven to local extinction (21). Therefore, the documented effects of habitat structure are best viewed as superimposed on a gradient of insect abundance.
Increased structural complexity is thought to lead to greater species diversity by reducing predation efficiency and enabling predator/prey coexistence or by increasing the number of niches available for specialization (30). This study provides direct evidence for another mechanism: differentially reduced foraging efficiency can lead to the coexistence of competitors. Among these geckos, coexistence occurs on an island-wide scale; Hf dominates urban habitats, whereas Ll remains dominant in many forest habitats. Coexistence also occurs on the within microhabitat scale in certain forest and more suburban habitats. This effect of habitat structure depends on competitors possessing different foraging strategies.
The interaction of topographic structure with the social system of Ll is more complex. Ll attempt to maintain interindividual distances and interact frequently while foraging. Conversely, Hf females show almost no intraspecific aggression, and even though Hf male–male interactions can be intense, they are usually rapid and infrequent (ref. 25; K.P., unpublished data). As predicted, increasing topographic structure did not affect spacing in Hf but had strong effects on Ll social interactions. Interindividual distances were significantly reduced, allowing foraging Ll greater access to regions with higher productivity (Fig. 4A). However, this resulted in an unanticipated increase in agonistic encounters among Ll, and these encounters lasted longer (Fig. 5B). This apparent tradeoff was only directly measured in terms of foraging attempts but may be a factor in the reduced body condition of Ll undergoing intraspecific competition as compared with interspecific competition in structurally complex environments. When resources are clumped, other species with different social systems may respond differently to increased topographic structure. We predict that visually territorial species similar to Ll, with frequent protracted encounters, may be similarly affected by topographic structure.
The current study has demonstrated that many processes can interact with habitat structure in complex ways to affect competition. Three processes known to be affected by habitat structure are commonly observed in most animals. First, predation or the threat of predation is affected by habitat structure, and species respond to this threat (31–33). Second, foraging efficiency for mobile or stationary prey is affected by habitat structure (2, 8–14). Third, social monitoring such as territoriality, interindividual spacing, or reproduction can also be influenced by habitat structure (14–18). Species respond to habitat structure by evolving adaptations, altering behavior, or changing their distribution or abundance. However for many species, the processes of foraging, predation avoidance, and social interaction are likely to be operating simultaneously. Consequently, changes in habitat structure are likely to lead to complex alterations in the interactions between animals.
The likelihood of encountering complex responses to changes in habitat structure emphasizes the importance of using a mechanistic approach (19) in future work. Here, we coupled experimental manipulation with quantified behavioral observation to untangle the mechanisms that produce higher order interactions (20). In the long term, empirical mechanistic studies will facilitate development of a theoretical framework; however, in the short term, these studies will help to evaluate similar processes in other systems. Based on the current study, changes in habitat structure that affect competition in other systems may be more accurately predicted by considering basic species attributes such as body size, foraging strategy, and social behavior.
Human-induced modifications in Pacific habitats (lights and flat walls) facilitate the invasion of Hf. Other human activities may alter microhabitat structure at different scales. For instance, habitat structure is diminished when mature forests are replaced by monotypic orchards, when underwater vegetation disappears, or when coral reefs die. Resource clumps are created by garbage dumps and heated effluent from power plants. An agricultural field may appear structured, clumped, or neither from the perspective of an herbivore, an insectivore, a small rodent, a large mammal, or a raptor. Human alterations of physical habitat structure may directly affect species diversity at higher trophic levels and may not be confined to processes cascading vertically from lower trophic levels.
Acknowledgments
We thank the U.S. Navy, Barbers Point Naval Air Station; M. Hetland and B. Davies for field assistance; and N. Tsutsui and two anonymous reviewers for comments. This work was supported by the National Science Foundation and the National Institutes of Health.
ABBREVIATIONS
- Hf, Hemidactylus frenatus
Ll, Lepidodactylus lugubris
- PLSD
probable least-squares difference
References
- 1. McCoy E D, Bell S S. In: Habitat Structure: The Physical Arrangement of Objects in Space. Bell S S, McCoy E D, Mushinsky H R, editors. London: Chapman & Hall; 1991. pp. 3–27. [Google Scholar]
- 2.MacArthur R H, MacArthur J W. Ecology. 1961;42:594–598. [Google Scholar]
- 3.Sanders H L. Am Nat. 1968;102:243–282. [Google Scholar]
- 4.Karr J R, Roth R R. Am Nat. 1971;105:423–435. [Google Scholar]
- 5.Lawton J H. Annu Rev Entomol. 1983;28:23–39. [Google Scholar]
- 6.Dean R L, Connell J H. J Exp Mar Biol Ecol. 1987;109:249–273. [Google Scholar]
- 7.McGuinness K A, Underwood A J. J Exp Mar Biol Ecol. 1986;104:97–123. [Google Scholar]
- 8.Werner E E, Hall D J. Science. 1976;199:404–406. doi: 10.1126/science.1246626. [DOI] [PubMed] [Google Scholar]
- 9.Crowder L B, Cooper W E. Ecology. 1982;63:1802–1813. [Google Scholar]
- 10.Losos J B, Warheit K I, Schoener T W. Nature (London) 1996;387:70–73. [Google Scholar]
- 11.Parrish J D. Ecology. 1995;76:1813–1820. [Google Scholar]
- 12.Price M V. Ecology. 1978;59:910–921. [Google Scholar]
- 13.Ziv Y, Kotler B P, Abramsky Z, Rosenzweig M L. Oikos. 1995;73:260–268. [Google Scholar]
- 14.Anholt B R. Ecology. 1990;71:1483–1493. [Google Scholar]
- 15.Brown J H. Ecology. 1971;52:305–311. [Google Scholar]
- 16.Ekman J. Anim Behav. 1987;35:445–452. [Google Scholar]
- 17.Snell H L, Jennings R D, Snell H M, Harcourt S. Evol Ecol. 1988;2:353–369. [Google Scholar]
- 18.Ward P I, Porter A H. Anim Behav. 1993;45:119–133. [Google Scholar]
- 19.Schoener T W. Am Zool. 1986;26:81–106. [Google Scholar]
- 20.Werner E E. Am Nat. 1992;140:S5–S32. [Google Scholar]
- 21.Case T J, Bolger D T, Petren K. Ecology. 1994;75:464–477. [Google Scholar]
- 22.Dye T, Steadman D W. Am Sci. 1990;78:207–215. [Google Scholar]
- 23.Case T J, Bolger D T. Evol Ecol. 1991;5:272–290. doi: 10.1016/0169-5347(91)90093-D. [DOI] [PubMed] [Google Scholar]
- 24.Petren K, Bolger D T, Case T J. Science. 1993;259:354–358. doi: 10.1126/science.259.5093.354. [DOI] [PubMed] [Google Scholar]
- 25.Petren K, Case T J. Ecology. 1996;77:118–132. [Google Scholar]
- 26.Holt R D. Theor Popul Biol. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- 27.Hanley K A, Vollmer D M, Case T J. Oecologia. 1995;102:220–229. doi: 10.1007/BF00333254. [DOI] [PubMed] [Google Scholar]
- 28.Hanley K A, Petren K, Case T J. Oecologia. 1998;115:196–205. doi: 10.1007/s004420050508. [DOI] [PubMed] [Google Scholar]
- 29.Huey R B, Niewiarowski P H, Kaufmann J, Heron J C. Physiol Zool. 1989;62:488–504. [Google Scholar]
- 30.Menge B A, Sutherland J P. Am Nat. 1976;110:351–369. [Google Scholar]
- 31.Lima S L. Wilson Bull. 1993;105:1–47. [Google Scholar]
- 32.Abramsky Z, Strauss E, Subach A, Kotler B P, Reichman A. Oecologia. 1996;105:313–319. doi: 10.1007/BF00328733. [DOI] [PubMed] [Google Scholar]
- 33.Vitt L J, Caldwell J P, Zani P, Titus T A. Proc Natl Acad Sci USA. 1997;94:3828–3832. doi: 10.1073/pnas.94.8.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]