Abstract
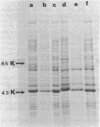
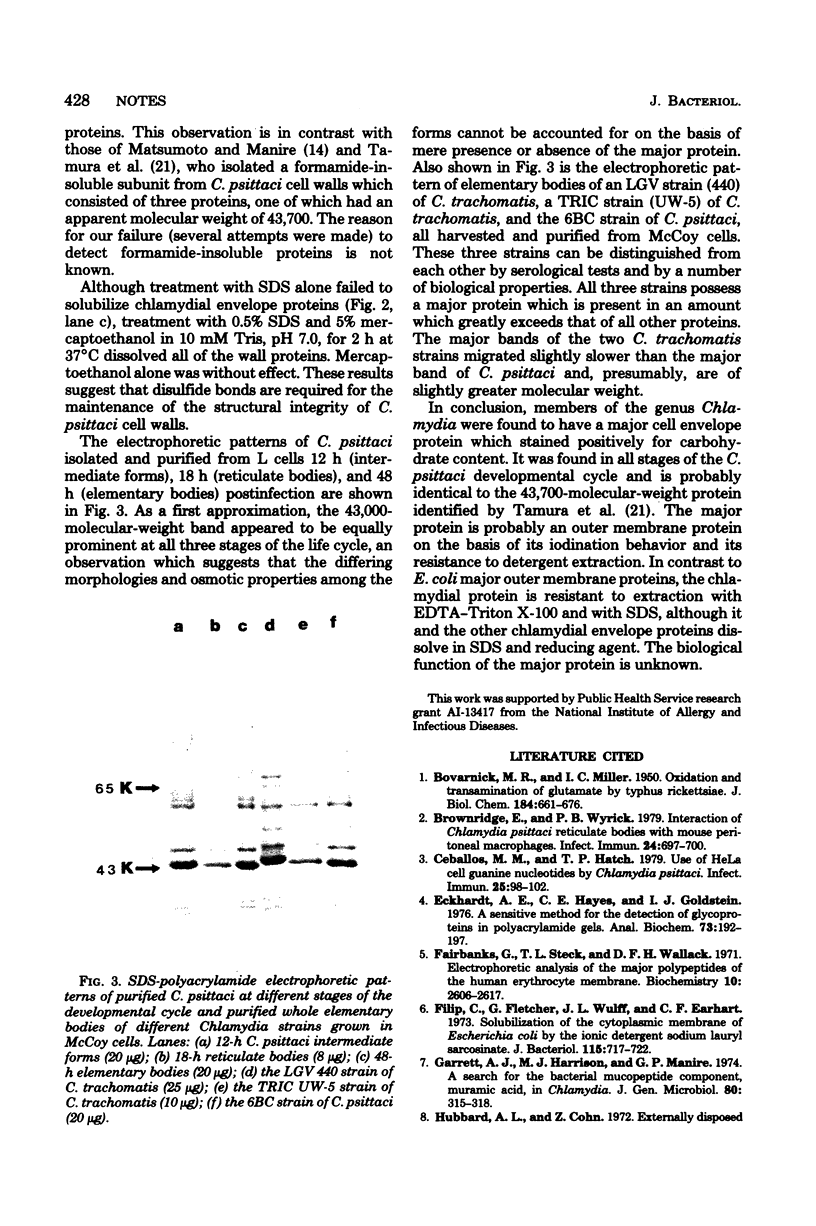
A major cell envelope protein of Chlamydia psittaci with a molecular weight of approximately 43,000 was identified and partially characterized. It was present at all stages of the C. psittaci developmental cycle. A major protein with a similar molecular weight was also observed in two Chlamydia trachomatis strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C. Oxidation and transamination of glutamate by typhus rickettsiae. J Biol Chem. 1950 Jun;184(2):661–676. [PubMed] [Google Scholar]
- Brownridge E., Wyrick P. B. Interaction of Chlamydia psittaci reticulate bodies with mouse peritoneal macrophages. Infect Immun. 1979 Jun;24(3):697–700. doi: 10.1128/iai.24.3.697-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos M. M., Hatch T. P. Use of HeLa cell guanine nucleotides by Chlamydia psittaci. Infect Immun. 1979 Jul;25(1):98–102. doi: 10.1128/iai.25.1.98-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt A. E., Hayes C. E., Goldstein I. J. A sensitive fluorescent method for the detection of glycoproteins in polyacrylamide gels. Anal Biochem. 1976 May 21;73(1):192–197. doi: 10.1016/0003-2697(76)90154-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A. J., Harrison M. J., Manire G. P. A search for the bacterial mucopeptide component, muramic acid, in Chlamydia. J Gen Microbiol. 1974 Jan;80(1):315–318. doi: 10.1099/00221287-80-1-315. [DOI] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C., Nicolas G., Eb F., Lefebvre J. F., Orfila J. Modifications of the envelope of Chlamydia psittaci during its developmental cycle: freeze-fracture study of complementary replicas. J Bacteriol. 1980 Feb;141(2):868–875. doi: 10.1128/jb.141.2.868-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron Microscopic Observations on the Fine Structure of Cell Walls of Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1332–1337. doi: 10.1128/jb.104.3.1332-1337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T., Manire G. P. Protein-carbohydrate-lipid complex isolated from the cell envelopes of Chlamydia psittaci in alkaline buffer and ethylenediaminetetraacetate. J Bacteriol. 1976 Jan;125(1):308–316. doi: 10.1128/jb.125.1.308-316.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V. Effect of hot formamide on Gram-positive and Gram-negative cell walls. Experientia. 1965 Sep 15;21(9):502–503. doi: 10.1007/BF02138954. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Higashi N. Purification and chemical composition of reticulate bodies of the meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2003–2008. doi: 10.1128/jb.93.6.2003-2008.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Manire G. P., Higashi N. Electron microscopic observations on the structure of the envelopes of mature elementary bodies and developmental reticulate forms of Chlamydia psittaci. J Bacteriol. 1971 Jan;105(1):355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Tanaka A., Manire G. P. Separation of the polypeptides of Chlamydia and its cell walls by polyacrylamide gel electrophoresis. J Bacteriol. 1974 Apr;118(1):139–143. doi: 10.1128/jb.118.1.139-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]