Abstract
Purpose
Preventing adolescents from smoking and becoming addicted to nicotine is an important public health issue. New research on the genetics of susceptibility to nicotine addition is emerging and may someday help identify adolescents at high risk. Over time, genetic counseling and testing for nicotine addiction susceptibility may become incorporated into tobacco control practice, and providers in primary care settings are likely to be at the forefront of these services. As such, it is important to understand the attitudes and practices of adolescent medicine providers toward tobacco control and genetic testing to better anticipate their needs and interests and prepare for the future. This study describes adolescent medicine providers’ interest, and correlates of their interest, in genetic counseling and testing for nicotine addiction susceptibility among their adolescent patients--a test which is not yet clinically available.
Methods
Adolescent medicine providers attending a national scientific conference (N = 232) completed a survey about their patient tobacco control and other screening behaviors, perceptions of their patients’ attitudes and beliefs toward tobacco control, and their own attitudes and beliefs about smoking and genetics.
Results
Providers who engaged in more regular tobacco screening behaviors with their adolescent patients (Odds Ratio [OR] = 4.07, 95% Confidence Interval [CI] = 2.20, 7.751, p = .00) and those who were more optimistic that biobehavioral research would lead to significant improvements in adolescent smoking prevention and treatment (OR = 2.47, 95% CI = 1.40, 4.37, p = .00), were more interested in counseling and testing.
Conclusions
Someday, adolescent wellness visits may present an opportunity to offer genetic counseling and testing for nicotine addiction susceptibility. Implementation at the provider level may depend on tobacco screening behavior and research optimism. Educating providers about safe and effective adolescent tobacco control strategies incorporating genetics will be essential.
Keywords: Adolescent smoking, genetics, continuing medical education
Introduction
Cigarette smoking and nicotine addiction are significant public health problems. Twenty percent of the adult population in the U.S. smokes cigarettes [1] and 50% of adult smokers die prematurely from medical conditions associated with smoking [2]. An important approach to address the problem of smoking is to prevent initiation of the habit, especially during adolescence [3]. Adolescents smoke at alarming rates, and more than 2,000 youth start smoking each day [4,5]. Unfortunately, smoking prevention and cessation interventions among teenagers have had only mild success, have not been studied extensively, and have been inconsistently implemented in public health contexts [6].
Smoking initiation and nicotine addiction are caused by a complex mix of psychosocial influences and physiological processes [3,7]. In adolescents, nicotine addiction may be characterized as diminished autonomy over smoking behavior, as well as a physiological and psychological need to smoke, including smoking-related sensations and situation-specific perceptions [8]. Symptoms of nicotine addition develop rapidly in adolescents, often after the onset of intermittent smoking, without regard to specific minimum nicotine doses or duration [9].
The role of genetics is recognized as an increasingly important determinant of nicotine addiction susceptibility. Genetic variants in the dopaminergic and serotonergic pathways (e.g., DAT, DRD2, 5-HTT) and genes associated with nicotine metabolism (e.g., CYP2A6, CYP2D6) appear to influence nicotine addiction [10]. Two important studies published recently directly address the influence of dopamine genes on adolescents’ and young adults’ smoking. Timberlake and colleagues conducted a family study of the relationship between smoking behavior and normal variations in DAT and DRD2 genes in a nationally-representative sample of 2,448 young adults [11]. They reported that DAT’s 9-repeat allele was inversely associated with smoking, thus suggesting a protective effect of this allele by altering dopamine neurotransmission and mitigating against smoking behavior. A second paper by Audrain-McGovern and colleagues examined the impact of DAT and DRD2 on adolescents’ smoking progression over time. Among adolescents who had ever smoked, higher levels of smoking progression were observed nearly twofold with each additional DRD2 A1 allele; this was most pronounced among adolescents with substantial depression symptoms [12]. A related study of genetic and environmental effects on adolescent smoking progression found physical activity (a protective factor) may moderate the influence of genes on smoking [13]. A third study noted the effect of genes that regulate nicotine metabolism (CYP2A6) on the rate which adolescents progress to nicotine dependence [14].
This raises the possibility that genetic effects, when present, are likely to be complex and not easily interpreted. These effects also are relatively small compared to other well-known social and behavioral risk factors [15-17], raising questions about the benefits of genetic information in the prevention and treatment of cigarette smoking [18]. However, as the contribution of genetics to smoking behavior becomes better defined, it may be possible to integrate genetic testing into the prevention and management of smoking, including adolescent smoking prevention and cessation efforts. Such information could identify youth who may be more vulnerable to start smoking, more rapidly progress to nicotine addiction, and/or benefit from genetically-targeted pharmacotherapies [19,20].
Although genetic testing for susceptibility to nicotine addiction is not yet validated, preliminary studies among adolescents and young adults suggest they are likely to be receptive to such testing [19]. However, it is unclear whether adolescent medicine providers have the interest in, time, or expertise to utilize such tests (see Shields et al., 2005 for related discussion) [21]. One study suggested pediatricians would support routine genetic testing in children to confirm a diagnosis of conditions like cystic fibrosis or Duchenne muscular dystrophy, but would be less likely to utilize a test for inherited susceptibility to type 1 diabetes [22]. Nevertheless, it is becoming more accepted for providers to screen adolescents for common medical conditions that have genetic determinants, but which present with complications in adulthood (e.g., hypercholesterolemia and type 2 diabetes). Even though genotype-phenotype relationships may be ambiguous, receptivity to genetic testing for adolescent medical conditions and behaviors may increase over time.
Within the adolescent population, the outpatient medical setting provides an opportune venue for adolescent medicine providers to assess and counsel youngsters about various behaviors and health habits, such as smoking [3,23]. There are several guidelines/recommendations for screening teenagers. For example, the Guidelines for Adolescent Preventive Services (GAPS) [23] is a series of general wellness screening recommendations endorsed by the American Medical Association. The U.S. Public Health Service “5 A’s Model” is a tool for assessing and treating tobacco use among adults and youth [24]. The 5 A’s Model involves Asking about tobacco use; Advising experimenters/smokers to stop smoking; Assessing smokers’ willingness to make a quit attempt; Assisting in the stop attempt; and Arranging follow-up [24].
The American Academy of Pediatrics further endorses the critical role pediatricians play in adolescent tobacco control [4]. However, studies among adolescents and their physicians demonstrate that physician adherence to recommended guidelines is suboptimal [25-27]. Given the potential for the diffusion of genetic technology into tobacco control in medical contexts [28], it is important to understand current attitudes and practices of adolescent medicine providers toward tobacco control and genetic testing. As such, the primary purpose of this research is to describe adolescent medicine providers’ interest, and correlates of their interest, in genetic counseling and testing for nicotine addiction susceptibility among their patients.
MATERIALS AND METHODS
Survey Development
The survey development team consisted of all authors, with backgrounds and expertise in: (a) clinical pediatrics and adolescent medicine, (b) youth tobacco control, and (c) public health genetic counseling and testing. Survey content was guided by biobehavioral models of tobacco use [29] and research literature on genes and smoking behavior [10]. Survey items were drafted by team members, and subsequently reviewed by an outside panel of adolescent medicine providers for feedback. The survey instrument was then revised prior to implementation.
Survey Content
The survey contained 4 main sections: (1) respondent demographics and information about their clinical practice, (2) screening behaviors (including tobacco control) with their patients, (3) perceptions of their patients’ attitudes and beliefs toward tobacco control, and (4) respondents’ attitudes and beliefs about smoking and genetics.
Independent Variables
Demographics and Clinical Practice Information
Respondent age, gender, race, professional affiliation and training, years in independent practice, practice setting and geographic location, and hours spent in clinical practice were obtained. Additionally, patient volume, age ranges, gender, race, and insurance status were collected.
Screening Behaviors
General wellness
Survey respondents rated on a 5-point Likert scale (1 = Never, 5 = Always) how often they engaged in general wellness screening for 8 conditions noted by the GAPS [23], all of which are noted in the Bright Futures program also [30]. This included eating disorders, sexual activity, alcohol and other drug use, tobacco use, physical abuse, school performance, depression, and suicidality. Responses to these items were summed to create an overall GAPS general wellness screening score that could range from 8 to 40 (M = 36.3, SD = 16.6; Cronbach’s coefficient α = .88), with higher scores indicating more consistent screening.
At-risk
Using the same Likert scale, respondents rated how often they ordered 4 screening tests for adolescents recommended by GAPS [23]. The screening tests were for high cholesterol, tuberculosis, sexually transmitted diseases, and HIV. An overall GAPS at-risk screening score was computed in the same manner described above (M = 16.5, SD = 3.6; Cronbach’s coefficient α = .88). Scores could range from 4 to 20 and higher scores indicated more consistent screening.
Biomarker
Respondents were also asked how often (1 = Never, 5 = Always) they ordered or recommended 4 types of biomarker tests: chromosome analyses, biochemical or laboratory tests for asymptomatic high risk patients (e.g., cholesterol testing in patients with family histories of heart disease), genetic testing for childhood chronic diseases (e.g., cystic fibrosis), and genetic testing for adult-onset diseases (e.g., hereditary breast cancer). The summed total of these items provided an overall biomarker screening score (M = 11.1, SD = 3.1; Cronbach’s coefficient α = .77), possibly ranged from 4 to 20, and higher scores indicated more consistent screening.
Tobacco use
For tobacco use screening, respondents were asked how often (1 = Never, 5 = Always) they utilized each of the U.S. Public Health Services’ 5 A’s (Ask, Advise, Assess, Assist, Arrange) for comprehensively addressing adolescent tobacco use in their practice. With respect to Assistance, education and counseling techniques were inquired about separately from pharmacotherapy techniques. The overall tobacco use screening score was created by adding up responses to each item (M = 23.4, SD = 3.9; Cronbach’s coefficient α = .78). It had a possible range of 6 to 30 and higher scores indicated more consistent screening.
Attitudes and Beliefs
Perceived adolescent interest in tobacco control
Using a 5-point Likert scale (1 = Never, 5 = Always), respondents were asked how often they believed their adolescent patients were interested in knowing about ways to prevent smoking, smoking’s addictiveness, and their risks for smoking-related diseases and cancer. The summed total of these 3 items created a perceived adolescent interest in tobacco control score, with a range of 3 to 15 (M = 7.7, SD = 2.0; Cronbach’s coefficient α = .74) and higher scores indicated greater perceived interest.
Variation in smoking behavior due to genetic and nongenetic factors
Respondents indicated how much individual variation in smoking behavior (in percent) they believed was determined by genetic factors (M = 22.1, SD = 16.0) and by social, environmental, or other (i.e., nongenetic) factors (M = 77.9, SD = 16.2) [21].
Research optimism
A multipart question was used to measure how optimistic respondents were that biobehavioral research would lead to significant improvements in adolescent smoking prevention and treatment (2 items), and that genetic research would yield new insights into the prediction of the development of and treatment for smoking and other complex traits (2 items). The sum of these Likert items (1 = Very Much So, 5 = Not at All) served as an overall research optimism score (M = 9.6, SD = 4.4; Cronbach’s coefficient α = .71)--with a range of 4 to 16 and higher scores indicating greater optimism [21].
Genetic counseling barriers
Respondents indicated if they believed that (a) time, (b) service reimbursement, (c) education and training, and (d) practice setting were potential barriers to counseling patients about genetic testing related to smoking behavior, prevention, and cessation. Respondents could endorse (Yes = 1, No = 0) none, some, or all possible barriers. Barrier responses were then added together to create an overall counseling barriers score with a range of 0 to 4, where higher scores indicated greater potential barriers (M = 1.8, SD = 1.0).
Dependent Variable
Counseling Interest
Respondents were asked how interested they were in counseling their patients about smoking prevention and cessation, genetic testing, and genetic testing related to smoking behavior, prevention, and cessation. These 3 items were rated on a 4-point Likert scale (1 = Not at All, 4 = Very Much So) and, when summed together, had a range of 3 to 12 (M = 7.4, SD = 1.7; Cronbach’s coefficient α = .71). Higher scores reflected adolescent medicine providers’ greater interest in genetic counseling and testing for nicotine addiction susceptibility among their adolescent patients.
Survey Administration and Data Collection
The survey was distributed in March 2005 at the annual scientific conference of the Society for Adolescent Medicine (SAM). SAM is a professional organization dedicated to promoting adolescent health and well-being [31]. SAM membership is multidisciplinary, including the fields of medicine, nursing, psychology, public health, social work, nutrition, education, and law. The society has approximately 1,300 members, 64% of whom are females, and about 80% of whom are physicians (personal communications, SAM central office, October 11, 2005 and February 8, 2006).
At the conference, research assistants in the registration area asked conference attendants (identifiable via SAM conference badge) to voluntarily complete an anonymous and confidential 10 minute survey regarding adolescent medicine providers’ tobacco control practices. Upon returning a completed survey on the premises, respondents were offered a $5 gift certificate to a media store to acknowledge their time and participation. The study protocol was reviewed and approved by the sponsoring institution’s Institutional Review Board.
Data Analysis
All data were entered into Microsoft Access, 100% verified, and analyzed in SPSS version 13.0. For multipart survey questions and questions with several items, summary scores were created. The summary score/scale creation process followed steps outlined in Nunally and Bernstein (1994) [32] and adolescent health research examples from Sieving and colleagues (2001) [33] to determine each scale’s reliability by computing its internal consistency reliability (Cronbach’s coefficient α). Next, descriptive statistics (M, SD, median, range, frequency) were used to summarize each item and continuous variable summary score. To ease their interpretation in bivariate and multivariate analyses, summary scores were then dichotomized via a median-split procedure (i.e., divided at their median) to create groups of respondents who were either high or low on each scale (≥ median = “High”, < median = “Low”). The bivariate association of each independent variable with counseling interest (dependent variable) was then computed using χ2 tests. Finally, variables significantly (p < .05) associated with counseling interest at the bivariate level were entered into a multivariate logistic regression model. This model generated odds ratios and 95% confidence intervals on characteristics of individuals with high interest in genetic counseling and testing for nicotine addiction susceptibility for adolescent patients.
RESULTS
Conference Attendance and Survey Response Rate
Based on conference advance registration statistics (personal communication, SAM central office, October 11, 2005), the majority of conference attendants were physicians (68%, 451/661). Nurses (6%, 41/661), other allied health professionals (e.g., psychologists, social workers, physician assistants, health educators, dietitians) (13%, 89/661), and trainees (12%; 80/661) also attended. Among the 661 advance registrants, 561 (85%) were from the U.S. and were relatively evenly distributed throughout the 4 major regions of the country: 27% (154/561) Northeast, 17% South (95/561), 26% Midwest 145/561, and 30% West (167/561).
A total of 420 surveys were distributed over 3 days of the conference. Of the 420 surveys distributed, 235 (56%) were completed and returned. Of the 235 completed and returned surveys, 232 (99%) were determined to be valid with fewer than 10% of items missing due to nonresponse.
Provider and Practice Demographics
As shown in Table 1, the majority of survey respondents were Caucasian females in their early forties. Respondents were primarily physicians (most specialized in adolescent medicine) who lacked formal training in clinical genetics and had been practicing independently for 10+ years. Those practicing in academic settings comprised the bulk of survey respondents, with relatively even representation from all 4 major regions of the U.S. In comparing the conference’s advance registration statistics for attendant’s professional affiliation and U.S. region of practice to those of survey respondents, the data suggest adequate sampling of conference attendees.
Table 1.
Provider and Practice Demographics (N = 232)
| n (%) | M (SD) | |
|---|---|---|
| Age, in years | 42.3 (9.8) | |
| Gender | ||
| Female | 157 (68) | |
| Male | 73 (32) | |
| Race | ||
| Caucasian or White | 168 (72) | |
| African American or Black | 23 (10) | |
| Asian or Pacific Islander | 21 (9) | |
| Hispanic or Latino | 15 (7) | |
| Native American or American Indian | 1 (<1) | |
| Professional affiliation | ||
| Physician (adolescent medicine, general pediatrics, family, or other) | 137 (62) | |
| Trainee | 57 (26) | |
| Nurse | 21 (9) | |
| Allied health | 7 (3) | |
| Formal training in clinical genetics | ||
| Yes | 33 (14) | |
| Years in independent practice | 10.1 (11.5) | |
| Practice setting | ||
| Academic | 170 (73) | |
| Community or health department | 40 (17) | |
| HMO | 5 (2) | |
| Private practice | 18 (8) | |
| US region of practice | ||
| Northeast | 69 (30) | |
| South | 32 (14) | |
| Midwest | 54 (23) | |
| West | 48 (21) | |
| Hours spent in clinical practice per week | 25.3 (12.9) | |
| Patients seen per week | 43.2 (37.7) | |
| Patient age, in % in years | ||
| ≤ 11 | 14.3 (23.1) | |
| 12 to 17 | 55.1 (26.2) | |
| 18 to 21 | 22.7 (17.5) | |
| ≥22 | 7.7 (14.5) | |
| Patient female gender, in % | 65.1 (16.9) | |
| Patient race, in % | ||
| Caucasian or White | 34.0 (26.7) | |
| African American or Black | 36.3 (28.2) | |
| Hispanic or Latino | 19.9 (22.5) | |
| Asian or Pacific Islander | 5.7 (8.5) | |
| Native American or American Indian | 3.5 (15.4) | |
| Patient insurance status, in % | ||
| Medicaid | 49.5 (29.3) | |
| Uninsured | 17.8 (22.1) |
Note. N’s are variable due to sporadic non-response.
With respect to clinical practice, most respondents spent more than one-half their time delivering care to 40+ patients per week. The majority of these patients were adolescents (≥ age 11), most were female, Caucasian or African American, and were either recipients of public insurance or were uninsured.
Interest in Genetic Counseling and Testing for Nicotine Addiction Susceptibility
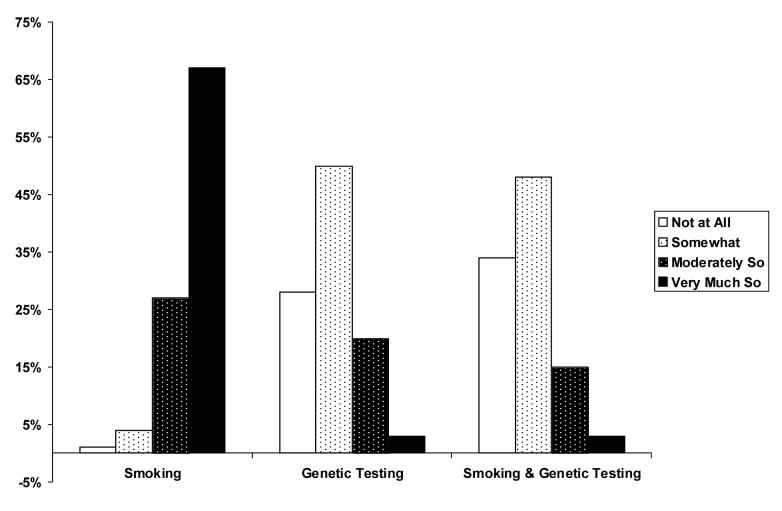
Nearly 95% of providers were moderately or very interested in counseling their patients about smoking prevention and cessation only. With respect to genetic testing only and genetic testing related to smoking behavior, prevention, and cessation only, the proportions of providers who were moderately or very interested were less (22% and 18%, respectively) (see Figure 1).
Figure 1.
Counseling Interest
Screening Behavior
Screening adolescents for general wellness issues was consistently high. Based upon responses of “frequently” and “always”, 73% consistently screened for eating disorders, 97% for sexual activity, 97% for alcohol and other drug use, 95% for tobacco use, 85% for physical abuse, 92% for school performance, 91% for depression, and 85% for suicidality. So too was screening adolescents at-risk for high cholesterol (78%), tuberculosis (69%), sexually transmitted diseases (90%), and HIV (77%).
With regard to other diagnostic or predictive tests, chromosome analyses were frequently or always order by 40% of respondents, biochemical or laboratory tests for asymptomatic high risk patients by 67%, genetic testing for childhood chronic diseases by 21%, and genetic testing for adult-onset diseases by 4%.
When screening for tobacco use was broken down into its component parts, variability in respondent behavior was evidenced. Respondents were more likely to frequently or always Ask (96%), Advise (91%), and Assess (84%) their patients, then they were to Assist via education and counseling (75%), Assist via pharmacotherapy (27%), and Arrange (32%). Specifically, 16% of respondents consistently (frequently or always) performed all As, 64% consistently performed the first 3 As only, and 20% did not consistently perform the first or last 3 As, χ2 (1) = 8.4, p = .004.
Bivariate Associations with Counseling Interest
Bivariate associations with counseling interest are shown in Table 2. No demographic and clinical practice information variables collected were associated with providers’ interest in genetic counseling and testing for nicotine addiction susceptibility.
Table 2.
Bivariate Associations with High Counseling Interest
| n (%) | χ2 (df) = p | |
|---|---|---|
| Demographic and Clinical Practice Information | ||
| Age, in years | ||
| ≥41 | 63 (54) | |
| <41 | 51 (48) | 0.86 (1) = ns |
| Gender | ||
| Male | 34 (47) | |
| Female | 82 (53) | 0.80 (1) = ns |
| Race | ||
| Caucasian or White | 83 (49) | |
| Other | 31 (53) | 0.28 (1) = ns |
| Professional affiliation | ||
| Physician | 73 (54) | |
| Other | 40 (47) | 1.08 (1) = ns |
| Formal training in clinical genetics | ||
| Yes | 18 (55) | |
| No | 96 (48) | 0.26 (1) = ns |
| Years in independent practice | ||
| ≥6 | 63 (54) | 0.41 (1) = ns |
| <6 | 52 (50) | |
| Practice setting | ||
| Academic | 86 (51) | |
| Other | 30 (49) | 0.05 (1) = ns |
| Hours spent in clinical practice per week | ||
| ≥20 | 80 (51) | |
| <20 | 32 (49) | .08 (1) = ns |
| Patients seen per week | ||
| ≥30 | 70 (54) | |
| <30 | 37 (46) | 1.27 (1) = ns |
| Screening Behaviors | ||
| GAPS wellness screening | ||
| Low | 39 (42) | |
| High | 77 (57) | 5.09 (1) = .02 |
| GAPS at-risk screening | ||
| Low | 43 (43) | |
| High | 73 (56) | 3.64 (1) = .06 |
| Biomarker screening | ||
| Low | 40 (46) | |
| High | 76 (54) | 1.35 (1) = ns |
| Tobacco use screening | ||
| Low | 24 (28) | |
| High | 92 (64) | 29.22 (1) = .00 |
| Attitudes and Beliefs | ||
| Adolescent perceived interest in tobacco control | ||
| Low | 52 (47) | |
| High | 64 (54) | 1.25 (1) = ns |
| Variation in smoking due to genes | ||
| Low | 41 (45) | |
| High | 71 (55) | 2.18 (1) = ns |
| Variation in smoking not due to genes | ||
| Low | 47 (59) | |
| High | 67 (46) | 3.42 (1) = .06 |
| Research optimism | ||
| Low | 39 (36) | |
| High | 77 (63) | 15.66 (1) = .00 |
| Genetic counseling barriers | ||
| Low | 63 (52) | |
| High | 50 (48) | 0.36 (1) = ns |
Among screening behaviors, more frequent general wellness screening and tobacco use screening were positively associated with greater counseling interest.
Among providers’ attitudes and beliefs, only research optimism was associated with counseling interest, such that providers who were more optimistic expressed greater interest in counseling.
Multivariate Associations with Counseling Interest
The multivariate model of providers’ interest in counseling included general wellness and tobacco use screening behaviors and their optimistic belief in biobehavioral and genetic research. The results (see table 3) indicate that relative to providers who engaged in fewer tobacco screening behaviors, those who engaged in more of these screening behaviors were 4 times more likely to have an interest in counseling. Additionally, relative to providers who were less optimistic that biobehavioral and genetic research would lead to significant breakthroughs in smoking prevention and treatment, those who were more optimistic were nearly 2.5 times more likely to be interested in counseling. In the multivariate model, general wellness screening was not associated with counseling interest.
Table 3.
Multivariate Model of Counseling Interest
| Variable | Level | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|---|
| GAPS wellness screening | Low* | |||
| High | 1.19 | 0.66, 2.17 | ns | |
| Tobacco use screening | Low* | |||
| High | 4.07 | 2.20, 7.51 | .00 | |
| Research optimism | Low* | |||
| High | 2.47 | 1.40, 4.37 | .00 |
Denotes referent group.
DISCUSSION
Genetic discoveries in tobacco control are emerging, but still have a substantial way to go before they are utilized in clinical settings. Within this context, the purpose of this paper was to examine two questions. First, providers’ receptiveness to applying genomic medicine to their tobacco control practices (i.e., the possibility of assessing adolescent patients for susceptibility to nicotine addiction based on new genetic markers). Second, the correlates of interest among providers more prepared for (and, therefore, possibly “early adopters” of) the demands of genetic counseling and testing adolescents for nicotine addiction susceptibility.
It is important to note that reliable and valid genetic tests for nicotine addiction susceptibility are not yet available and these results should be interpreted in this light. By exploring these issues now, prior to the availability of widespread testing, it is possible to better anticipate the professional community’s needs and interests, to better prepare ourselves to meet those needs, and to help ensure an appropriate translation of genomic science in tobacco control to the clinical and public health spheres. To date, only one published study has examined the attitude of primary care physicians in the United States toward a new test for tailored smoking cessation in adults [21]. This study reported that the description of the new test as “genetic” versus one assessing a “serum protein” resulted in an 11% decrease in hypothetical utilization or adoption of such a test in patients. As noted by the study authors, the decline in test utilization could have a significant public health impact in that potentially many smokers would not be offered a test that could help them in their attempt to stop smoking. Based upon this and other information, the present study was interested in making a preliminary determination of the potential utilization of a genetic test for nicotine addiction susceptibility and treatment by adolescent medicine providers.
The study sample was highly compliant with screening guidelines for adolescent preventive health services. For example, with respect to implementation of general wellness screening behaviors specified in GAPS and Bright Futures, respondents reported they were likely to screen their adolescent patients for the 8 parameters 90% of the time, and 82% of the time for high-risk conditions such as cholesterolemia, tuberculosis, and sexually transmitted diseases. These findings are generally higher than those reported elsewhere [34], and paralleled patterns observed among adult primary care providers [35,36]. Study respondents were more evenly divided in terms of how often they ordered biomarker tests and genetic testing.
Consistent with findings from other studies [25-27], providers were more likely to assess smoking habits and advise their adolescent patients about quitting than they were to actively intervene by prescribing medication or arranging follow-up. Nevertheless, the vast majority of respondents were moderately or very interested in educating and counseling their patients about smoking prevention and cessation, but less than one-quarter of the sample was interested in using genetic testing to achieve this goal.
The above finding is not surprising given that the sample of providers was not strongly convinced about the contribution of genetics to variations in smoking behavior. However, a majority of the sample was optimistic that biobehavioral and genetic research would lead to improvements in prediction and treatment of smoking-related behaviors. It is significant that interest in counseling about smoking prevention and cessation was highly associated with current utilization of screening behaviors and optimism about future research breakthroughs. This suggests early adopters of genetic testing for nicotine addiction susceptibility and treatment are those who already devote time to preventive health issues. As these providers were all attendees at a scientific conference, one might anticipate their interest in promoting overall adolescent health and well-being to be elevated, interest in health promotion to be higher, and appreciation for research to be somewhat pronounced.
The study results presently indicates low levels of enthusiasm in nicotine addiction susceptibility counseling among adolescent medicine providers attending a conference; however, a subgroup of potential early adopters also appears to exist. Related scientific advances deserving further attention in tobacco control include vaccines against nicotine--which block nicotine’s effect in the brain [37]. Someday, these vaccines may be available to adolescents to prevent nicotine dependence [38], and may be targeted for use in adolescents deemed at high risk based on family history information and nicotine addiction susceptibility genetic test results. Adolescent medicine providers may be on the frontlines of parents’ inquires about these forthcoming products, and may be among the earliest adopters of these products [18]. Of course, any developments in these areas should be assessed carefully by parents, adolescents, or adolescent medicine providers.
There are notable study limitations that must be considered. First, the sample size was small and the survey response rate was under 60%-- phenomena that are not uncommon for surveys administered at professional conferences. The sample was also not representative of all adolescent medicine providers: many providers are not members of SAM or attend its annual conference. Further, not all attendees were Society members. There are several significant sources of self-selection bias that may be at work here and limit the applicability of our results to outside contexts. Many providers attending the SAM annual conference were also specialists in adolescent medicine and practicing in academic settings. It would be interesting to determine if and how responses in general pediatric practitioners might compare with these findings, if differences emerge based on primary practice setting or other background characteristics, or how a physician-only sample might respond. The present study cannot address these issues. In addition, the mean number of years of practice in the sample was 10 years, and the sample included providers in training. It has been demonstrated that knowledge of genetics is greater among more recent medical school graduates and that this may be associated with early adoption of genetic tests [39]. Finally, given the data collection method employed the survey was as brief as possible. Using different, varied, or expanded terminology to describe genetic tests or smoking behavior may have produced different results [21].
In conclusion, wellness visits within the primary care setting provide an opportune time to screen for and discuss preventive health behaviors among adolescent patients. The results of the study suggest that although most adolescent medicine providers who participated in this research do not have a high interest in genetic counseling and testing for nicotine addiction susceptibility within their patient population, a subgroup exists that does. Because such testing has not yet been validated, there is a window of time that exists presently to educate providers about genetic concepts and principles of genetic screening, and how these complement traditional approaches to public health. There are also other clinical considerations that must be addressed prior to any implementation of genetic tests for smoking [21,40]. Some of the barriers to offering tests in the future may be mitigated as primary care providers and genetics professionals work closely together to determine the circumstances under which it might be appropriate to refer for comprehensive pre- and post-test education and develop detailed follow-up plans for behavioral and medical monitoring.
Acknowledgments
Funding/Support: This study was supported by grant K07CA91831 (K.P. Tercyak) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Schroeder SA. Tobacco still is oral health enemy number one. J Am Dent Assoc. 2006;137:144–148. doi: 10.14219/jada.archive.2006.0126. [DOI] [PubMed] [Google Scholar]
- (2).Edwards R. The problem of tobacco smoking. BMJ. 2004;328:217–219. doi: 10.1136/bmj.328.7433.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Vickers KS, Thomas JL, Patten CA, et al. Prevention of tobacco use in adolescents: review of current findings and implications for healthcare providers. Curr Opin Pediatr. 2002;14:708–712. doi: 10.1097/00008480-200212000-00012. [DOI] [PubMed] [Google Scholar]
- (4).Committee on Substance Abuse American Academy of Pediatrics: Tobacco’s toll: implications for the pediatrician. Pediatrics. 2001;107:794–798. [PubMed] [Google Scholar]
- (5).Kann L, Kinchen SA, Williams BI, et al. Youth risk behavior surveillance--United States, 1997. MMWR CDC Surveill Summ. 1998;47:1–89. [PubMed] [Google Scholar]
- (6).Sussman S, Lichtman K, Ritt A, et al. Effects of thirty-four adolescent tobacco use cessation and prevention trials on regular users of tobacco products. Subst Use Misuse. 1999;34:1469–1503. doi: 10.3109/10826089909039411. [DOI] [PubMed] [Google Scholar]
- (7).Henningfield JE, Jude NR. Prevention of nicotine addiction: neuropsychopharmacological issues. Nicotine Tob Res. 1999;1(Suppl 1):S41–S48. doi: 10.1080/14622299050011581. [DOI] [PubMed] [Google Scholar]
- (8).Wellman RJ, DiFranza JR, Pbert L, et al. A comparison of the psychometric properties of the hooked on nicotine checklist and the modified Fagerstrom tolerance questionnaire. Addict Behav. 2006;31:486–495. doi: 10.1016/j.addbeh.2005.05.031. [DOI] [PubMed] [Google Scholar]
- (9).DiFranza JR, Savageau JA, Rigotti NA, et al. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control. 2002;11:228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lerman C, Berrettini W. Elucidating the role of genetic factors in smoking behavior and nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2003;118:48–54. doi: 10.1002/ajmg.b.10003. [DOI] [PubMed] [Google Scholar]
- (11).Timberlake DS, Haberstick BC, Lessem JM, et al. An association between the DAT1 polymorphism and smoking behavior in young adults from the national longitudinal study of adolescent health. Health Psychol. 2006;25:190–197. doi: 10.1037/0278-6133.25.2.190. [DOI] [PubMed] [Google Scholar]
- (12).Audrain-McGovern J, Lerman C, Wileyto EP, et al. Interacting effects of genetic predisposition and depression on adolescent smoking progression. Am J Psychiatry. 2004;161:1224–1230. doi: 10.1176/appi.ajp.161.7.1224. [DOI] [PubMed] [Google Scholar]
- (13).Audrain-McGovern J, Rodriguez D, Wileyto EP, et al. Effect of team sport participation on genetic predisposition to adolescent smoking progression. Arch Gen Psychiatry. 2006;63:433–441. doi: 10.1001/archpsyc.63.4.433. [DOI] [PubMed] [Google Scholar]
- (14).Audrain-McGovern J, Al Koudsi N, Rodriguez D, et al. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–e274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- (15).Harrell JS, Bangdiwala SI, Deng S, et al. Smoking initiation in youth: the roles of gender, race, socioeconomics, and developmental status. J Adolesc Health. 1998;23:271–279. doi: 10.1016/s1054-139x(98)00078-0. [DOI] [PubMed] [Google Scholar]
- (16).Siqueira L, Diab M, Bodian C, et al. Adolescents becoming smokers: the roles of stress and coping methods. J Adolesc Health. 2000;27:399–408. doi: 10.1016/s1054-139x(00)00167-1. [DOI] [PubMed] [Google Scholar]
- (17).van den Bree MB, Whitmer MD, Pickworth WB. Predictors of smoking development in a population-based sample of adolescents: a prospective study. J Adolesc Health. 2004;35:172–181. doi: 10.1016/j.jadohealth.2003.09.021. [DOI] [PubMed] [Google Scholar]
- (18).Hall WD. Will nicotine genetics and a nicotine vaccine prevent cigarette smoking and smoking-related diseases? PLoS Med. 2005;2:e266. doi: 10.1371/journal.pmed.0020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Tercyak KP, Peshkin BN, Wine LA, et al. Interest of adolescents in genetic testing for nicotine addiction susceptibility. Prev Med. 2006;42:60–65. doi: 10.1016/j.ypmed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- (20).Wilfond BS, Geller G, Lerman C, et al. Ethical issues in conducting behavioral genetics research: the case of smoking prevention trials among adolescents. J Health Care Law Policy. 2002;6:73–88. [PubMed] [Google Scholar]
- (21).Shields AE, Blumenthal D, Weiss KB, et al. Barriers to translating emerging genetic research on smoking into clinical practice. Perspectives of primary care physicians. J Gen Intern Med. 2005;20:131–138. doi: 10.1111/j.1525-1497.2005.30429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Acharya K, Ackerman PD, Ross LF. Pediatricians’ attitudes toward expanding newborn screening. Pediatrics. 2005;116:e476–e484. doi: 10.1542/peds.2005-0453. [DOI] [PubMed] [Google Scholar]
- (23).Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; Baltimore, MD: 1994. [Google Scholar]
- (24).US Public Health Service A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. JAMA. 2000;283:3244–3254. [PubMed] [Google Scholar]
- (25).Kaplan CP, Perez-Stable EJ, Fuentes-Afflick E, et al. Smoking cessation counseling with young patients: the practices of family physicians and pediatricians. Arch Pediatr Adolesc Med. 2004;158:83–90. doi: 10.1001/archpedi.158.1.83. [DOI] [PubMed] [Google Scholar]
- (26).Shelley D, Cantrell J, Faulkner D, et al. Physician and dentist tobacco use counseling and adolescent smoking behavior: results from the 2000 National Youth Tobacco Survey. Pediatrics. 2005;115:719–725. doi: 10.1542/peds.2004-0873. [DOI] [PubMed] [Google Scholar]
- (27).Thorndike AN, Ferris TG, Stafford RS, et al. Rates of U.S. physicians counseling adolescents about smoking. J Natl Cancer Inst. 1999;91:1857–1862. doi: 10.1093/jnci/91.21.1857. [DOI] [PubMed] [Google Scholar]
- (28).Lerman C, Patterson F, Berrettini W. Treating tobacco dependence: state of the science and new directions. J Clin Oncol. 2005;23:311–323. doi: 10.1200/JCO.2005.04.058. [DOI] [PubMed] [Google Scholar]
- (29).Hiatt RA, Rimer BK. A new strategy for cancer control research. Cancer Epidemiol Biomarkers Prev. 1999;8:957–964. [PubMed] [Google Scholar]
- (30).Ferguson SL. Every child deserves Bright Futures. J Pediatr Nurs. 1999;14:123–124. doi: 10.1016/S0882-5963(99)80046-3. [DOI] [PubMed] [Google Scholar]
- (31).Society for Adolescent Medicine The Older Adolescent/Young Adult; SBM 2005 annual meeting, March 30-April 2; The Century Plaza Hotel & Spa, Century City, CA. [Google Scholar]
- (32).Nunnally JC, Bernstein IH. Psychometric Theory. 3rd ed. McGraw-Hill; New York: 1994. [Google Scholar]
- (33).Sieving RE, Beuhring T, Resnick MD, et al. Development of adolescent self-report measures from the National Longitudinal Study of Adolescent Health. J Adolesc Health. 2001;28:73–81. doi: 10.1016/s1054-139x(00)00155-5. [DOI] [PubMed] [Google Scholar]
- (34).Halpern-Felsher BL, Ozer EM, Millstein SG, et al. Preventive services in a health maintenance organization: how well do pediatricians screen and educate adolescent patients? Arch Pediatr Adolesc Med. 2000;154:173–179. doi: 10.1001/archpedi.154.2.173. [DOI] [PubMed] [Google Scholar]
- (35).Anderson LM, May DS. Has the use of cervical, breast, and colorectal cancer screening increased in the United States? Am J Public Health. 1995;85:840–842. doi: 10.2105/ajph.85.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Yarnall KS, Pollak KI, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Pentel PR, Malin DH, Ennifar S, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65:191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- (38).Vocci FJ, Chiang CN. Vaccines against nicotine: how effective are they likely to be in preventing smoking? CNS Drugs. 2001;15:505–514. doi: 10.2165/00023210-200115070-00001. [DOI] [PubMed] [Google Scholar]
- (39).Hofman KJ, Tambor ES, Chase GA, Geller G, Faden RR, Holtzman NA. Physicians’ knowledge of genetics and genetic tests. Acad Med. 1993;68:625–632. doi: 10.1097/00001888-199308000-00013. [DOI] [PubMed] [Google Scholar]
- (40).Shields A, Lerman C, Sullivan P. Translating emerging research on the genetics of smoking into clinical practice: ethical and social considerations. Nicotine Tob Res. 2004;6:675–688. doi: 10.1080/14622200410001734058. [DOI] [PubMed] [Google Scholar]