Abstract
Berberine is known to possess a wide variety of pharmacological activities, including pro-apoptotic activity. However, its molecular targets are not elucidated at present. NAG-1 and ATF3 are induced by several dietary compounds associated with pro-apoptotic activity. Berberine induces cell growth arrest, apoptosis, NAG-1, and ATF3 in human colorectal cancer cells. ATF3 induction by berberine is mediated in a p53-dependent manner, whereas NAG-1 induction by berberine is mediated by multiple signaling pathways. Our results suggest that berberine facilitates apoptosis and that NAG-1 and ATF3 expression plays an important role in berberine-induced apoptosis.
Keywords: Berberine, NAG-1, ATF3, Apoptosis, p53
1. Introduction
Colorectal cancer is the third leading cause of cancer death in the United States, with the annual incidence approximately equal among men and women. The optimal method for early detection remains unknown, and patient compliance with screening recommendations remains poor. This has led to the development of complementary strategies, such as chemoprevention, to reduce morbidity and mortality from colorectal cancer [1]. Cyclooxygenase (COX) inhibitors have been used as a chemopreventive compound in colorectal cancer [2, 3]. However, it has been recently revealed that COX-2 inhibitors are cardio-toxic in clinical trials, and it appears that research in other chemopreventive strategies may be required for colon cancer. Alternatively, non-toxic botanicals may be one option for the management of colorectal cancers.
Berberine, a natural isoquinoline alkaloid, has been found in many clinically important plants including Coptis chinensis (Coptis or goldenthread), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry), and Coscinium fenestratum [4, 5]. Berberine possesses a wide range of biochemical and pharmacological activities including anti-diarrheal, anti-arrhythmic and anti-tumor activities [6-8]. The most promising actions of berberine are its inhibition of cell growth and induction of apoptosis in several human cancer cells [9-11], but the mechanisms behind these actions need to be elucidated.
Nonsteroidal anti-inflammatory drug (NSAID) activated gene (NAG-1) was identified in our laboratory by PCR- based subtractive hybridization as a pro-apoptotic and anti- tumorigenic protein induced by indomethacin [12]. The human NAG-1 cDNA encodes a secreted protein with homology to members of the TGF-β superfamily and has been previously identified as macrophage inhibitory cytokine-1 (MIC-1), placental transformation growth factor-β (PTGFB), prostate derived factor (PDF), growth differentiation factor 15 (GDF-15), and placental bone morphogenetic protein (PLAB) [3]. NAG-1 overexpression in transgenic mice results in the reduction of tumor formation that was induced by the carcinogen azoxymethane and induced by genetic mutation of the APC tumor suppressor gene [13]. These data support the evidence that NAG-1 is linked to apoptosis and that the reduced expression of NAG-1 may enhance tumorigenesis. Interestingly, NAG-1 induction was seen not only by the treatment with NSAIDs, but also by other anti-tumorigenic compounds found in the diet, such as resveratrol [14], genistein [15], green tea catechins [16], and indole-3-carbinol [17]. While some dietary compounds, including resveratrol and genistein, induce NAG-1 expression through p53 tumor suppressor protein, NSAIDs, epicatechin gallate, and indole-3-carbinol induce NAG-1 in a p53-independent manner. Thus, several pathways are involved in phytochemical-induced NAG-1 expression.
Similarly, ATF3 is a pro-apoptotic protein and many anti-tumorigenic compounds including phyto chemicals induce this protein at the transcriptional level. ATF3 is a transcriptional factor; forms heterodimers with other transcription factors, and promotes the expression of a number of genes involved in apoptosis pathways. Indeed, we and others have shown that ATF3 expression ectopically results in enhancing apoptosis in HCT-116 human colorectal cancer cells [18], and that tetracycline-inducible over expression of ATF3 suppresses cell growth [19]. ATF3 has been postulated to be a tumor suppressor gene because it coordinates the expression of genes that may be linked to cancer [20]. Moreover, the expression of ATF3 was repressed in human colorectal tumors compared to normal adjacent tissue [21]. Interestingly, both ATF3 and NAG-1 expression is induced by the green tea epicatechin gallate or indole-3-carbinol treatment; supporting the concept that ATF3 and NAG-1 expression has a role in phyto chemical-induced apoptosis [16, 17].
Although NAG-1 and ATF3 have been well studied in colorectal cancer as anti-tumorigenic proteins, the expression and regulation of NAG-1 and ATF3 by berberine has not been determined. In this report, we show that berberine inhibits cell growth, induces apoptosis, and induces the expression of two pro-apoptotic genes, NAG-1 and ATF-3. We have also shown that berberine induces ATF3 expression through the p53 tumor suppressor protein, whereas berberine increases NAG-1 expression by the PKC, ERK, and GSK-3β pathways.
2. Materials and methods
2.1 Cell cultures, reagents and plasmids
Cell lines were purchased from ATCC (Rockville, MD). Human colorectal carcinoma HCT-116 and HT-29 cells were maintained in McCoy's 5A medium. SW480 (colorectal carcinoma cell) and A549 (lung carcinoma cell) cells were cultured in RPMI 1640. LoVo (colorectal carcinoma cell), NCI-H292 (lung carcinoma cell) and PC-3 (prostate carcinoma cell) were cultured in Ham's F12K medium. Caco-2 (colorectal carcinoma cell) and T98G (brain carcinoma cell) were maintained in DMEM and EMEM media, respectively. All media were supplemented with 10% fetal bovine serum (Cellgro, Herndon, VA), penicillin and streptomycin (10 mg/ml). HCT-116p53−/− cells were generously provided by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD) and cultured in McCoy's 5A medium. Berberine chloride (Ber) and glutathione (GSH) were purchased from Sigma (St Louis, MO). The NAG-1 antibody was previously described [12]. ATF3, actin and cyclin D1 antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The p53, phospho-p44/42 MAP Kinase (ERK 1/2) and phospho -p38 MAP Kinase antibodies were obtained from Cell Signaling Technology Inc. (Beverly, MA) and p27 antibody was purchased from NeoMakers (Fremont, CA). For the deletion clones of the ATF3 promoter, pATF3 −1850/+34 was used [18] and serially deleted using the Erase-a-Base System (Promega, Madison, WI). The NAG-1 luciferase reporter vectors were previously described [22]. All chemicals were purchased from Fisher Scientific, unless otherwise specified.
2.2 Cell proliferation analysis
The effect of berberine on cell proliferation in HCT-116 and SW480 cells was investigated using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI). The cells were seeded at the concentration of 1000 cells/well for HCT-116 and 2000 cells/well for SW480 in 96-well tissue culture plates in six replicates. The cells were then treated with 1, 10, and 50 μM of berberine in the presence of serum. At 1, 2, and 4 days after treatment, 20 μl of CellTiter96 Aqueous One solution was added to each well, and the plate was incubated for 1 h at 37°C. Absorbance at 490 nm was recorded in an enzyme-linked immunosorbent assay (ELISA) plate reader (Bio-Tek Instruments, Winooski, VT).
2.3 DNA cell cycle analysis
The DNA content for the sub G0 population was determined by flow cytometry. The cells were plated at 6 ×105 cells/well in 6-well plates, incubated for 16 h, and then treated with 10 and 50 μM of berberine in the presence of serum. The cells (attached and floating cells) were then harvested, washed with PBS, fixed by the slow addition of 1ml cold 70% ethanol, and stored at −20 °C. The fixed cells were pelleted, washed with 50%, and 30% ethanol, followed by PBS, and stained with 1 ml of 20 mg/ml propidium iodide containing 1 mg/ml RNase in PBS for 30 min. Twenty thousand cells were examined by flow cytometry, using a Becton Dickinson Fluorescence-activated cell sorter (FACS) equipped with CellQuest software, by gating on an area versus width dot plot to exclude cell debris and cell aggregates. Apoptosis was measured by the level of sub-diploid DNA contained in cells following treatment with compounds using Cell Quest software.
2.4 Western blot analysis
Cells were grown to 60-80% confluence in 6-cm plates followed by 24 h treatment of various concentrations of berberine or DMSO as vehicle control in the absence of serum. Total cell lysates were isolated using RIPA buffer (1 ×PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with protease inhibitors (1 mM PMSF, 5 μg/ml aprotinin and 5 μg/ml leupeptin) and phosphatase inhibitors (1 mM Na3VO4 and 1 mM NaF). The protein concentration was determined by the BCA protein assay (Pierce, Rockford, IL) using BSA as the standard. Thirty micrograms of protein were separated by SDS-PAGE and transferred for 1 h onto a nitrocellulose membrane (Schleicher & Schuell, NH). The blots were blocked for 1 h with 5% skim milk in TBS/Tween 0.05% (TBS-T), and probed with a specific primary antiserum in Tris buffered saline (TBS) containing 0.05% Tween-20 (TBS-T) and 5% non-fat dry milk at 4°C overnight. After washing with TBS-T, the blots were treated with horseradish peroxidase-conjugated secondary antibody for 1 h and washed several times. Proteins were detected by the enhanced chemiluminescence system (Amersham, IL).
2.5 Plasmid transient transfections
Transient transfections were performed using Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Briefly, HCT-116 cells were plated in 12-well plates at the concentration of 2×105 cells/well. After growth for 18 h, plasmid mixtures containing 0.5 μg of the reporter gene and 0.05 μg of the pRL-null vector were transfected for 5 h. The transfected cells were then incubated with 50 μM of berberine for 24 h. The cells were harvested in 1X luciferase lysis buffer, and luciferase activity was normalized to the pRL-null luciferase activity using a dual luciferase assay kit (Promega, Madison, WI).
2.6 Protein kinase inhibitor studies
Cells at 60-80 % confluence were treated with DMSO, PD98059(40 μM, ERK1/2 inhibitor) , AKT inhibitor (10 μM), MG132 (10 μM, NF-κB inhibitor) , RO31-8220 (1, 2.5 and 5 μM, PKC inhibitor), Rottlerin (0.5, 1 and 5 μM, PKC inhibitor), Go6983 (10, 50 and 100 nM, PKC inhibitor), AR-A014418 (30 μM, GSK-3β inhibitor), AG490 (50 μM, JAK-2 inhibitor), or SP600125 (30 μM, JNK inhibitor) in serum-free medium. After 30 min, either DMSO or 50 μM berberine was added directly to the media. Cells were harvested and analyzed by Western blot after 24 h.
2.7. SiRNA transfection and Caspase 3/7 activity
HCT-116 cells were seeded at 2×105 cells / well in 6-well plate and allowed to grow for 24 h in complete media. The cells were then transfected with either shRNAs for control and NAG-1 [23], or siRNA for control and ATF3 [18] using lipofectamine 2000 and TransIT-TKO (Mirus Bio Corporation, Madison, WI), respectively. The transfected cells were treated with either DMSO or 50 μM of berberine in serum free media for 24 h, and the cells were then harvested using RIPA buffer. Caspase activity was measured using Caspase 3/7 Luminescent Assay Kit (Promega, Madison, WI) according to the manufacture's protocol. Luminescence was measured using the microplate reader (BioTek Instruments, Winooski, Vermont).
3. Results
3.1. Berberine inhibits cell proliferation and induces apoptosis in colorectal cancer cells
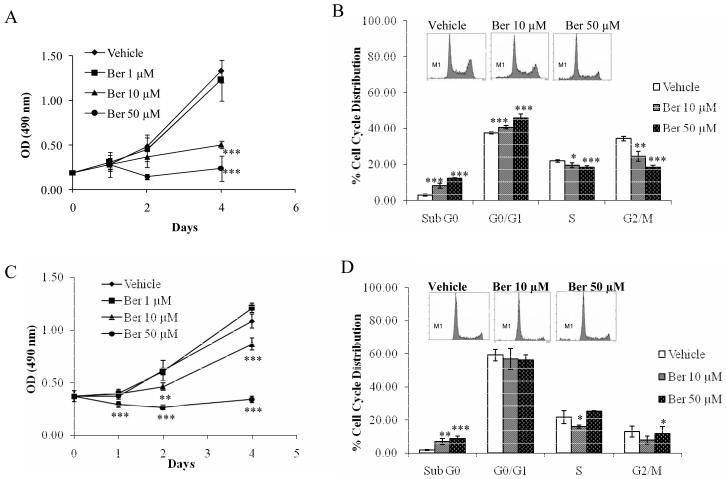
We first investigated the anti-proliferative effects of berberine on human colorectal carcinoma cells, HCT-116 and SW480. The cells were treated with 1, 10, and 50 μM berberine for 1, 2, and 4 days. The treatment of berberine in HCT-116 cells resulted in a significant reduction in cell proliferation at 10 μM (P<0.001) and 50 μM (P<0.001) after 4 days and 2 days treatment, respectively (Fig. 1A). Similar effects were obtained in SW480 cells (Fig. 1C). However, SW480 cells responded more quickly than HCT-116 cells, with the reduction in viability seen at 24 h. These data suggest that berberine has a cytotoxic effect on colorectal cancer cells, both in p53 wild type cells (HCT-116) and p53-mutated cells (SW480). Based on this preliminary data in which we observed the strong growth inhibitory effect of berberine in HCT-116 and SW480 cells, we selected doses of 10 and 50 μM to determine the possible inhibitory effect of berberine on cell cycle progression and apoptosis. As shown in Fig. 1B, treatment of HCT-116 cells with berberine resulted in a significant enrichment in the number of cells in the Sub G0 phase, which represents the apoptotic cells, at both concentrations used: 10 μM (8.20%, P < 0.005) and 50 μM (12.23%, P < 0.005), compared to that of vehicle (2.97%). The cells also showed G1 cell cycle arrest at 10 μM (40.73%, P < 0.005) and 50 μM (46.03%, P < 0.005), compared to vehicle treatment (37.57%). Simultaneously, we observed the reduction of the S and G2/M phases in a dose dependent manner, suggesting that berberine induces apoptosis and G0/G1 phase cell cycle arrest in HCT-116 cells. Similar results were obtained on apoptosis induction by berberine, but we did not observe G1 cell cycle arrest in SW480 cells (Fig. 1D).
Fig. 1. Effect of berberine on cell growth and apoptosis in HCT-116 and SW480 cells.
HCT-116 (A) and SW480 (C) cells were treated with vehicle or various concentrations of berberine for 4 days. Cell growth was measured using the CellTiter96 Aqueous One Solution Cell Proliferation Assay. Values are expressed as mean ±SD of six replicates. **P< 0.01, ***P< 0.001 versus vehicle-treated cells. Flow cytometric analysis of vehicle or berberine-treated HCT-116 (B) and SW480 (D) cells were analyzed. Cells were plated at 6 ×105 cells / well in six-well plates, incubated with or without 10 or 50 μM of berberine for 48 h and analyzed for cell cycle progression as described in Materials and Methods. Representative cell cycle profiles are shown in the box. M1 indicates sub G0 cell population and values are expressed as mean ±SD of three replicates. *P< 0.05, ** P< 0.01, P*** P< 0.001 versus vehicle-treated cells.
3.2. Berberine induces NAG-1 and ATF3 protein expression in several cancer cells
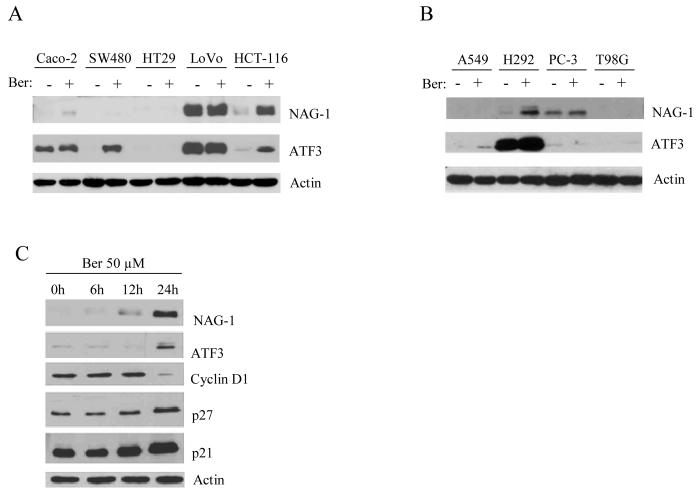
To investigate the effects of berberine on NAG-1 and ATF3 protein expression in different colorectal and other cancer cells, several cancer cells were treated with 50 μM of berberine for 24 h. As shown in Fig. 2A, berberine increases NAG-1 expression in HCT-116 and CaCo-2 cells, whereas ATF3 induction was seen in HCT-116 and SW480 cells. Moreover, we also explored the NAG-1 and ATF3 expression in non-colorectal cancer cells (Fig. 2B). In the presence of 50 μM of berberine, NAG-1 expression was increased in NCI-H292 lung cancer cells, whereas ATF3 induction was observed in A549 lung cancer cells. These data suggest that berberine affects NAG-1 and ATF3 induction in a cell-type specific manner. Since HCT-116 cells are the only cells showing both NAG-1 and ATF3 induction by berberine, we used these cells for further experiments. To investigate the involvement of berberine on the expression of other genes, we next assessed the effect of berberine on the expression of proteins regulating cell cycle. The treatment of HCT-116 cells with 50 μM of berberine for 0, 6, 12 and 24 h resulted in a time-dependent increase in NAG-1 expression. Similar effects were observed in protein levels of ATF3 and the cyclin inhibitors p21 and p27. In contrast, a strong reduction of cyclin D1 was observed after berberine treatment for 24 h (Fig. 2C). In addition, other apoptotic related genes including BAX and Bcl-2 were measured and we found that BAX were increased by berberine treatment, whereas no Bcl-2 expression was detected in HCT-116 cells (data not shown). These results indicate that berberine not only increases NAG-1 and ATF3, but also affects cell cycle regulators, including p21, p27, and cyclin D1.
Fig. 2. Western analysis of NAG-1 and ATF3 expressions in different cancer cells treated with berberine.
(A) Different colorectal cancer cells- Caco-2, SW480, HT29, LoVo, and HCT-116-were treated with or without 50 μM of berberine for 24 h. Thirty micrograms of total protein were subjected to 14 % SDS-PAGE and antibodies for NAG-1, ATF3 or Actin (Santa Cruz) were applied as described in the Materials and Methods section. (B) Different cancer cell lines including A549 (lung), NCI-H292 (lung), PC-3 (prostate), and T98G (brain) were treated with or without 50 μM of berberine for 24 h. Ber, Berberine. (C) HCT-116 cells were treated with 50 μM of berberine at various time points (0, 6, 12, 24 h). The cells were harvested and subjected to Western analysis using NAG-1, ATF3, cyclin D1, p27, p21, and Actin antibodies. Actin was used for normalization.
3.3 Pro-oxidative activity of berberine is not involved in NAG-1 and ATF3 expression
We investigated whether the effect of berberine on protein expression is mediated through the production of reactive oxygen species (ROS) in the culture system, since some phytochemicals including berberine are known to produce ROS in culture media[24-26]. The cells were pretreated with anti-oxidant glutathione (GSH) for 1 h prior to treatment with berberine. As shown in Fig. 3, treatment of berberine in the presence of GSH did not affect NAG-1 and ATF3 expression, indicating that induction of NAG-1 and ATF3 by berberine is not mediated via the pro-oxidative activity of berberine.
Fig. 3. Pro-oxidant property of berberine is not associated with berberine-induced NAG-1 and ATF-3 in HCT-116 cells.
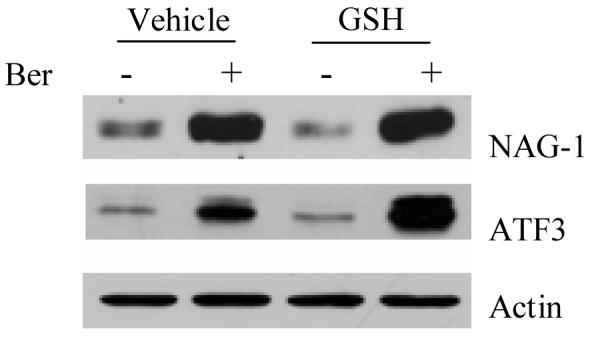
The cells were treated with vehicle and 5 μM of glutathione (GSH) for 1 h prior to incubation with 50 μM of berberine for 24 h. Western analysis was carried out using NAG-1, ATF3, and Actin antibodies.
3.4 Berberine affects p53-dependent transcriptional activity followed by the induction of ATF3 expressions in HCT-116 cells
Because many phytochemicals increase the p53 tumor suppressor protein in human colorectal cancer cells, we next investigated whether berberine activated p53 in HCT-116 cells at the transcriptional level. Two reporter systems were used in this study: pMdm-2 construct contains one p53 binding site, whereas 2X RGC-luc construct contains two p53 binding sites linked to a thymidine kinase (TK) minimal promoter [27, 28]. As shown in Fig. 4A, berberine increased luciferase activity in both p53 responding promoters. Since both NAG-1 and ATF3 contain the p53 binding site in their promoter, we examined whether berberine induced NAG-1 and ATF3 via a p53-dependent pathway. HCT-116WT cells and HCT-116p53−/− cells were treated with berberine for 24 h at various concentrations. As shown in Fig. 4B, NAG-1 and ATF3 were dramatically increased in a dose-dependent manner in HCT-116 WT cells. However, only NAG-1 induction was observed in HCT-116p53−/− cells, whereas ATF3 was not induced in HCT-116p53−/− cells. These data indicate that ATF3 induction by berberine is dependent on p53 activation, and NAG-1 induction by berberine is not mediated by p53 protein expression. Furthermore, NAG-1 and ATF3 promoter activity was examined in the presence of berberine. The promoter constructs of NAG-1 and ATF3, with and without p53 binding sites, were obtained and transfected into HCT-116WT cells. As shown in Fig. 4C, the promoter activity of the ATF3 construct without the p53 binding site was significantly decreased, compared to that with the p53 binding site (P< 0.01). However, both NAG-1 constructs showed an increase of luciferase activity (Fig. 4D), suggesting that berberine induces ATF3 expression at least in part in a p53-dependent manner, whereas berberine induces NAG-1 expression in a p53-independent manner.
Fig. 4. Effect of berberine on ATF-3 expressions through a p53-dependent pathway.
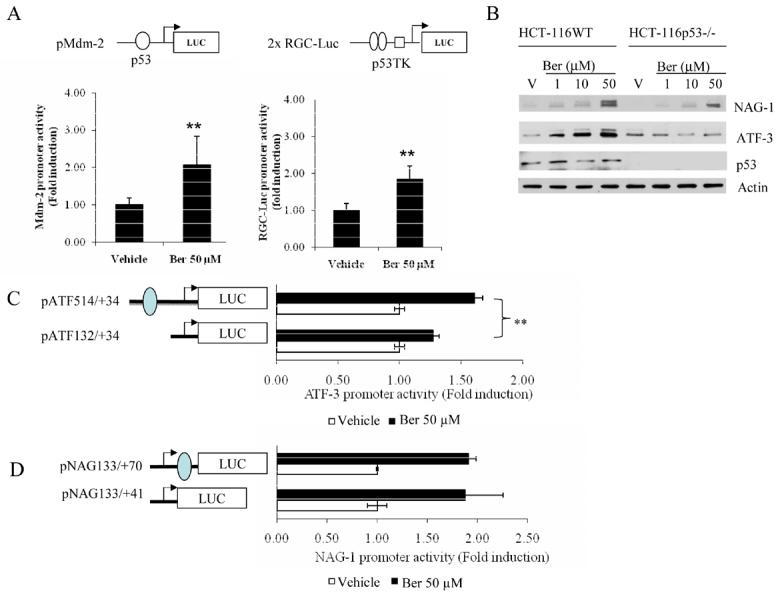
(A) Berberine increased p53-dependent transcriptional activity in HCT-116 cells. The pMdm-2-Luc or 2XRGC-Luc constructs (top panel) were transiently transfected into HCT-116 cells. After 24 h transfection, the cells were treated with either vehicle or berberine for an additional 24 h and harvested to measure luciferase activity. The values obtained from vehicle-treated cells were defined as 1.0. Each value represents mean ± SD from three independent transfections. (B) Western analysis of HCT-116WT and HCT-116p53−/− cells treated with berberine. The HCT-116 cells, both p53 wild-type and p53 null, were treated with berberine 50 μM for 24 h. Western analysis for NAG-1, ATF3, p53 and actin expressions were obtained as described in Materials and Methods. (C) The constructs, pATF 514/+34 and pATF132/+34 (0.5 μg each) and pRL-null (0.05μg) were transiently transfected into HCT-116 cells. The cells were treated with 50 μM of berberine for 24 h and luciferase activity was measured. Each value represents mean ± SD from three independent transfections. The closed oval indicates the p53 binding site. (D) The constructs, pNAG133/+70 and pNAG133/+41 (0.5 μg each), and pRL-null (0.05 μg) were transiently transfected into HCT-116 cells. The cells were treated with 50 μM of berberine for 24 h and luciferase activity was measured. Each value represents mean ± SD from three independent transfections. The closed oval indicates the p53 binding site.
3.5 Effect of kinase inhibitors on NAG-1 expression
To explore the molecular mechanism involved in berberine-induced NAG-1 expression, we examined several signaling pathways that are affected by berberine. We screened several kinase-specific inhibitors at the concentration that does not deviate from their selectivity. HCT-116 cells were pretreated with vehicle or kinase inhibitor for 30 min prior to incubation with 50 μM of berberine. As shown in Fig. 5A, the berberine-induced NAG-1 expression was strongly suppressed in the presence of PD98059 (ERK 1/2 inhibitor), RO31-8220 (PKC inhibitor), and AR-A014418 (GSK-3β inhibitor). Collectively, these results suggest the roles of the ERK1/2, PKC, and GSK-3β protein kinase pathways in berberine-induced NAG-1 expression.
Fig. 5. Effects of kinase inhibitors on berberine-induced NAG-1 expression in HCT-116 cells.
(A) HCT-116 cells were treated with DMSO, PD98059 (40 μM), AKT inhibitor (10 μM), MG132 (10 μM), RO31-8220 (2.5 μM), AR-A014418 (30 μM), AG490 (50 μM) or SP600125 (30 μM), for 30 min followed by treatment with 50 μM of berberine for an additional 24 h in the absence of serum. The cell lysates were harvested, and 30 μg of total protein were subjected to Western blot analysis as described in Materials and Methods. Equal amounts of loading proteins were estimated by Actin probing. (B) HCT-116 cells were treated with DMSO, RO31-8220 (1, 2.5 and 5 μM), Rottlerin (0.5, 1 and 5 μM) and Go6983 (10, 50 and 100 nM) in serum-free media. After 30 min, either DMSO or 50 μM of berberine was added directly to the media. After 24 h, cells were both harvested and analyzed by Western blot, as described in Materials and Methods. (C) HCT-116 cells were serum starved for 24 h and treated with 50 μM of berberine. At the indicated times, the cell lysates were harvested to perform Western blot analysis for p-ERK1/2, total ERK1/2, and NAG-1, and results were normamlized with Actin. (D) The pNAG133/+41 luciferase construct (0.5 μg) was transiently transfected with 0.05 μg of pRL-null vector into HCT-116 cells using lipofectamine. The cells were pretreated for 30 min in serum free media with DMSO, PD98059 (40 μM), AKT inhibitor (10 μM), MG132 (10 μM), RO31-8220 (5 μM), AR-A014418 (30 μM), AG490 (50 μM) or SP600125 (30 μM), and 50 μM of berberine was added in all wells except vehicle. After 24 h, luciferase activity was measured. The y axis shows fold increase in relative luciferase units (RLU) compared to RLU of vehicle-treated cells. Each value represents mean ± SD from three independent transfections. The student t- test was used to determine significance in the NAG-1 promoter activity in the presence of berberine, and the RLU of all the different inhibitor treated samples were compared with that of the berberine-only treated sample.
We next examined the specific isoforms of PKC involved in mediating berberine-induced NAG-1 expression, since RO31-8220 inhibits PKC activity in a nonspecific manner. HCT-116 cells were treated with RO31-8220 (1, 2.5 and 5 μM), Rottlerin (0.5, 1 and 5 μM) and Go6983 (10, 50 and 100 nM) with berberine 50 μM. Interestingly, treatment with RO31-8220, an inhibitor of PKCα, β1, β2, γ and ε, dose-dependently decreased the induction of NAG-1 by berberine. However, Rottlerin (a specific inhibitor of PKCδ), and Go6983 (inhibitor of PKCα, β, γ, ζ and δ) did not affect berberine-induced NAG-1 expression (Fig. 5B). These data indicate that PKCε may be involved in berberine-induced NAG-1 expression. Furthermore, our investigation into the effect of berberine on ERK1/2 activity in HCT-116 cells showed that berberine altered the phosphorylation of ERK1/2 (Fig. 5C). The alteration of phospho- ERK1/2 was highly associated with NAG-1 expression. In contrast, the expression of total ERK1/2 was not altered by berberine. Finally, we investigated NAG-1 promoter activity in the presence of different kinase inhibitors. The construct pNAG 133/+41 was transfected into HCT-116 cells and treated with kinase inhibitors prior to the incubation of berberine. As shown in Fig. 5D, most of the data is consistent with the Western analysis shown in Fig. 5A, with the exception of AG490 treatment. Taken together, our results demonstrated that berberine affects PKC, GSK-3β, and ERK activity, followed by the induction of NAG-1 expression in HCT-116 cells.
3.6. NAG-1 and ATF3 are involved in berberine-induced apoptosis
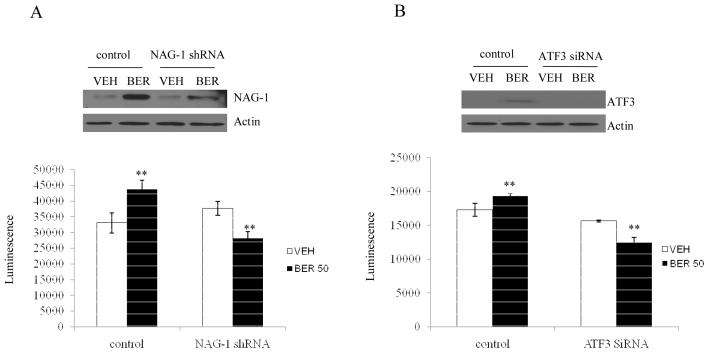
To explore whether NAG-1 and ATF3 expression contribute to berberine-induced apoptosis, we performed knock-down of endogenous NAG-1 or ATF3 gene by RNA interference, and analyzed apoptosis-related caspase 3/7 activity. HCT-116 cells were transfected with shRNA for NAG-1 and siRNA for ATF3. As shown in Fig, 6A and B, transfection with NAG-1 shRNA clearly suppressed berberine-induced NAG-1 expression (Fig. 6A, top panel). Treatment of control shRNA-transfected cells with berberine increased caspase activity, whereas treatment of NAG-1 shRNA-transfected cells with berberine decreased caspase activity, compared to vehicle treatment. These results suggest the link between NAG-1 expression and berberine-induced apoptosis. Likewise, ATF3 siRNA was also transfected into HCT-116 cells and caspase activity was performed. As shown in Fig. 6B, ATF3 expression was suppressed in the presence of ATF3 siRNA (top panel), and caspase activity was dramatically decreased in the ATF3 siRNA transfected cells, compared to control siRNA transfected cells. These results indicate that NAG-1 and ATF3 expression contributes at least in part to berberine-induced apoptosis in HCT-116 cells.
Fig. 6. NAG-1 and ATF3 in part mediate berberine-induced apoptosis.
(A) HCT-116 cells were transfected with the shRNA of NAG-1 and treated with either DMSO (VEH) or 50 μM berberine (BER) for 24 h. Cell lysates were isolated and were subjected to Western analysis using NAG-1 and Actin antibodies. The same cell lysates were used to determine caspase activity using caspase 3/7 Glo reagent and luminescence was recorded as described in Materials and Methods. ** indicates p<0.05 when compared to vehicle treatment using student t-test. (B) HCT-116 cells were transfected with control siRNA and ATF3 siRNA. After 24 hr treatment with either DMSO (VEH) or berberine (BER), cell lysates were subject to Western analysis and caspase activity assay as described above. ** indicates p<0.05 when compared to vehicle treatment using student t-test.
Discussion
Natural products have been a continuous source of novel compounds for the treatment of numerous diseases, with natural products and their synthetic derivatives comprising over 60% of the approved anticancer drug candidates developed between 1981 and 2002[29]. Because the occurrence and mortality rate of colorectal cancer is high, it is important in terms of public health and economics to prevent cancer by reducing carcinogenesis using cancer chemopreventive drugs, especially for the patients who have genetic predisposition to colon cancer or those particularly susceptible to environmental causes of neoplasia. Thus, it is necessary to screen and evaluate natural products systemically for the identification of molecular targets as a first step in finding active compounds and subsequently exploring their mechanism of action.
In the present study, we have shown that berberine, a naturally occurring isoquinoline alkaloid, significantly inhibits cell growth and induces apoptosis in HCT-116 and SW480 cells. Along with these effects, berberine also induces several apoptotic and cell cycle related genes. Further studies were performed to determine the effect of berberine on NAG-1 and ATF3 expression in colorectal cancer cells (Fig. 2A) as well as in other cancer cells (Fig. 2B). It is well established that NAG-1 and ATF3 induces apoptosis in colorectal cancer cells [13, 30, 31] and many investigators, including our lab, have reported that many anti-tumorigenic compounds increase NAG-1 and ATF3 expression [16, 17, 32]. This indicates that NAG-1 and ATF3 may be important molecular target proteins of chemopreventive compounds.
There have been reports that NAG-1 and ATF3 are p53 target genes that mediate p53-dependent apoptosis [14, 33]. Indeed, berberine increases p53 activity as assessed by a reporter system (Fig. 4A). However, p53 dependency may not fully account for increased NAG-1 expression because NAG-1 induction was observed in p53 knock-out cells (Fig. 4B). These results were partially confirmed by reporter assay using NAG-1 promoter constructs containing p53 binding site (Fig. 4D). In contrast, the p53 binding site in the ATF3 promoter contributes significantly to the berberine-induced ATF3 expression.
The berberine responsible element may be located within −133 bp of the NAG-1 promoter (Fig. 4D). Several internal deletion clones within this region were generated and luciferase activity was measured in the presence of berberine, but no obvious cis-acting elements in this region were observed, suggesting that berberine may affect multiple trans-acting elements (data not shown). Alternatively, we decided to investigate the up-stream region of the signaling pathway that affects berberine-induced NAG-1 expression, and further molecular mechanism studies revealed that berberine affects several signaling pathways. In this study, we screened several signaling pathways using kinase inhibitors. Our data suggest that berberine-induced NAG-1 expression may involve the PKC, ERK, and GSK-3β pathway (Fig. 5). Involvement of PKCδ and PKCε in NAG-1 regulation has been previously reported. PKCδ is the upstream kinase for NAG-1 expression in prostate cancer cells [34], and PKCε plays a role in gingerol-induced NAG-1 expression [35]. Berberine may enhance PKCε activity, resulting in the NAG-1 induction (Fig. 5B). Likewise, berberine also activated ERK activity by phosphorylation (Fig. 5C), followed by the induction of NAG-1. The activation of the ERK pathway has been reported in PPARγ ligand-induced NAG-1 expression [36]. We have also found that berberine weakly binds to the ligand binding domain of PPARγ, as assessed by a reporter gene experiment (data not shown). Thus, berberine may act in a similar way as PPARγ ligands to promote ERK activation. However, further experiments are clearly needed to elucidate berberine's effect on ERK activation.
It has been shown that some anti-oxidant compounds, including tea polyphenols and flavonoids, exhibit anti-tumorigenic activity via pro-oxidant properties [37, 38]. Since polyphenol compounds may exhibit both anti-oxidant and pro-oxidant activities [39, 40], we determined whether NAG-1 and ATF3 induction by berberine was mediated by its pro-oxidative activity. We found that berberine did not affect NAG-1 and ATF3 expression through its pro-oxidant property. Although further studies are required, our data clearly show that pro-oxidative activity of berberine does not contribute to the berberine-induced NAG-1 and ATF3 expression.
In conclusion, the current study provides evidence for the anti-tumorigenic activity of berberine. The p53-dependent pathway may be involved in berberine-induced ATF3 expression. However, p53-independent pathways may mediate berberine-induced NAG-1expression. Furthermore, we identified that ERK, PKC, and GSK-3β pathways play a pivotal role in berberine-induced NAG-1 expression.
Acknowledgements
The authors thank Nichelle C. Whitlock and Misty Bailey for their critical review. This work was in part supported by a grant from the National Institutes of Health (R21CA 109423) and by the Center of Excellence from the University of Tennessee. Financial support for R. Piyanuch was provided by the Royal Golden Jubilee Ph.D. Program (PHD/0235/2544), Thailand.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brenner DMGADE. Chemoprevention of colon cancer: Current status and future prospects. Cancer and Metastasis Reviews. 2002;21:323–348. doi: 10.1023/a:1021271229476. [DOI] [PubMed] [Google Scholar]
- 2.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 3.Baek SJ, Eling TE. Changes in gene expression contribute to cancer prevention by COX inhibitors. Prog Lipid Res. 2006;45:1–16. doi: 10.1016/j.plipres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Timothy C BN, Gregory S, Kelly Nd. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Alternative Medicine Review. 1997;2:94–102. [Google Scholar]
- 5.Rojsanga P, Gritsanapan W, Suntornsuk L. Determination of berberine content in the stem extracts of Coscinium fenestratum by TLC densitometry. Med Princ Pract. 2006;15:373–378. doi: 10.1159/000094272. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Takase H, Abe K, Saito Y, Suzuki A. Pharmacological studies on antidiarrheal effects of a preparation containing berberine and geranii herba. Nippon Yakurigaku Zasshi. 1993;101:169–175. doi: 10.1254/fpj.101.3_169. [DOI] [PubMed] [Google Scholar]
- 7.Huang WM, Wu ZD, Gan YQ. Effects of berberine on ischemic ventricular arrhythmia. Zhonghua Xin Xue Guan Bing Za Zhi. 1989;17:300–301, 319. [PubMed] [Google Scholar]
- 8.Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition of activator protein 1 activity by berberine in human hepatoma cells. Planta Med. 1999;65:381–383. doi: 10.1055/s-2006-960795. [DOI] [PubMed] [Google Scholar]
- 9.Lin JP, Yang JS, Lee JH, Hsieh WT, Chung JG. Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J Gastroenterol. 2006;12:21–28. doi: 10.3748/wjg.v12.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis. 2006;27:2018–2027. doi: 10.1093/carcin/bgl043. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JM, Kuo HC, Tseng TH, Liu JY, Chu CY. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch Toxicol. 2006;80:62–73. doi: 10.1007/s00204-005-0014-8. [DOI] [PubMed] [Google Scholar]
- 12.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–908. [PubMed] [Google Scholar]
- 13.Baek SJ, Okazaki R, Lee SH, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425–434. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 15.Wilson LC, Baek SJ, Call A, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene (NAG-1) is induced by genistein through the expression of p53 in colorectal cancer cells. Int J Cancer. 2003;105:747–753. doi: 10.1002/ijc.11173. [DOI] [PubMed] [Google Scholar]
- 16.Baek SJ, Kim JS, Jackson FR, Eling TE, Mcentee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25:2425–2432. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Kim JS, Yamaguchi K, Eling TE, Baek SJ. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun. 2005;328:63–69. doi: 10.1016/j.bbrc.2004.12.138. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi K, Lee SH, Kim JS, Wimalasena J, Kitajima S, Baek SJ. Activating transcription factor 3 and early growth response 1 are the novel targets of LY294002 in a phosphatidylinositol 3-kinase-independent pathway. Cancer Res. 2006;66:2376–2384. doi: 10.1158/0008-5472.CAN-05-1987. [DOI] [PubMed] [Google Scholar]
- 19.Fan F, Jin S, Amundson SA, et al. ATF3 induction following DNA damage is regulated by distinct signaling pathways and over-expression of ATF3 protein suppresses cells growth. Oncogene. 2002;21:7488–7496. doi: 10.1038/sj.onc.1205896. [DOI] [PubMed] [Google Scholar]
- 20.Yan C, Lu D, Hai T, Boyd DD. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. Embo J. 2005;24:2425–2435. doi: 10.1038/sj.emboj.7600712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottone FG, Jr., Martinez JM, Collins JB, Afshari CA, Eling TE. Gene modulation by the cyclooxygenase inhibitor, sulindac sulfide, in human colorectal carcinoma cells: possible link to apoptosis. J Biol Chem. 2003;278:25790–25801. doi: 10.1074/jbc.M301002200. [DOI] [PubMed] [Google Scholar]
- 22.Baek SJ, Horowitz JM, Eling TE. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J Biol Chem. 2001;276:33384–33392. doi: 10.1074/jbc.M101814200. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Lee SH, Eling TE, Baek SJ. A novel peroxisome proliferator-activated receptor gamma ligand, MCC-555, induces apoptosis via posttranscriptional regulation of NAG-1 in colorectal cancer cells. Mol Cancer Ther. 2006;5:1352–1361. doi: 10.1158/1535-7163.MCT-05-0528. [DOI] [PubMed] [Google Scholar]
- 24.Hsu WH, Hsieh YS, Kuo HC, et al. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch Toxicol. 2007 doi: 10.1007/s00204-006-0169-y. In Press. [DOI] [PubMed] [Google Scholar]
- 25.Hsuuw YD, Chan WH. Epigallocatechin gallate dose-dependently induces apoptosis or necrosis in human MCF-7 cells. Ann N Y Acad Sci. 2007;1095:428–440. doi: 10.1196/annals.1397.046. [DOI] [PubMed] [Google Scholar]
- 26.Feng R, Ni HM, Wang SY, et al. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J Biol Chem. 2007;282:13468–13476. doi: 10.1074/jbc.M610616200. [DOI] [PubMed] [Google Scholar]
- 27.Zauberman A, Flusberg D, Haupt Y, Barak Y, Oren M. A functional p53-responsive intronic promoter is contained within the human mdm2 gene. Nucleic Acids Res. 1995;23:2584–2592. doi: 10.1093/nar/23.14.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maki K, Mitani K, Yamagata T, et al. Transcriptional inhibition of p53 by the MLL/MEN chimeric protein found in myeloid leukemia. Blood. 1999;93:3216–3224. [PubMed] [Google Scholar]
- 29.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 30.Lu D, Chen J, Hai T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem J. 2007;401:559–567. doi: 10.1042/BJ20061081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4:233–241. [PubMed] [Google Scholar]
- 32.Lee SH, Yamaguchi K, Kim JS, et al. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis. 2006;27:972–981. doi: 10.1093/carcin/bgi268. [DOI] [PubMed] [Google Scholar]
- 33.Kannan K, Amariglio N, Rechavi G, et al. DNA microarrays identification of primary and secondary target genes regulated by p53. Oncogene. 2001;20:2225–2234. doi: 10.1038/sj.onc.1204319. [DOI] [PubMed] [Google Scholar]
- 34.Shim M, Eling TE. Protein kinase C-dependent regulation of NAG-1/placental bone morphogenic protein/MIC-1 expression in LNCaP prostate carcinoma cells. J Biol Chem. 2005;280:18636–18642. doi: 10.1074/jbc.M414613200. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Cekanova M, Baek SJ. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 2007 doi: 10.1002/mc.20374. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baek SJ, Kim JS, Nixon JB, Diaugustine RP, Eling TE. Expression of NAG-1, a transforming growth factor-beta superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem. 2004;279:6883–6892. doi: 10.1074/jbc.M305295200. [DOI] [PubMed] [Google Scholar]
- 37.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro. 2004;18:555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Dabaghi-Barbosa P, Mariante Rocha A, Franco Da Cruz Lima A, et al. Hispidulin: antioxidant properties and effect on mitochondrial energy metabolism. Free Radic Res. 2005;39:1305–1315. doi: 10.1080/13561820500177659. [DOI] [PubMed] [Google Scholar]
- 39.Shirwaikar A, Shirwaikar A, Rajendran K, Punitha IS. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull. 2006;29:1906–1910. doi: 10.1248/bpb.29.1906. [DOI] [PubMed] [Google Scholar]
- 40.Jantova S, Cipak L, Letasiova S. Berberine induces apoptosis through a mitochondrial/caspase pathway in human promonocytic U937 cells. Toxicol In Vitro. 2007;21:25–31. doi: 10.1016/j.tiv.2006.07.015. [DOI] [PubMed] [Google Scholar]