Abstract
Emotions have been conceptualized as representations of bodily responses to a stimulus that critically involves the autonomic nervous system (ANS). An association between amygdala activation and ANS activity has been shown in adults. However, to date, no studies have demonstrated this association in adolescents. Examining the interaction between the ANS and amygdala in healthy adolescents may provide information about age-related changes in the association between amygdala activation and ANS measures. Therefore, the aim of this study was to examine the relationship between amygdala activation and heart rate in normal adolescents. Eighteen 12- to 17-year old adolescents participated. Heart rate data was collected during functional magnetic resonance imaging while subjects performed a facial expression matching task that reliably activates the amygdala. Adolescents showed significant amygdala activation for all facial expressions relative to the shape-matching, control task. Moreover, the degree of activation in the right amygdala for Fearful faces was significantly correlated with heart rate (Spearman’s rho = 0.55, p = 0.018, two-tailed). This study shows that amygdala activity is related to heart rate in healthy adolescents. Thus, similar to adults, adolescents show a coupling between processing emotional events and adjusting the ANS accordingly. Furthermore, this study confirms previous adolescent studies showing amygdala activation to Fearful, Angry, and Happy faces. Finally, the results of the present study lay the foundation for future research to investigate whether adolescents with mood or anxiety disorders show an altered coupling between processing emotionally salient events and ANS activity.
INTRODUCTION
The James-Lange theory of emotion posits that an emotion is a perceived central representation of bodily responses to a stimulus [13, 17]. As part of this theory, emotional feelings are dependent on physiological bodily responses that are generated automatically by the autonomic nervous system (ANS). The amygdala is an important part of the limbic system that is well positioned to control basic autonomic arousal processes. Through the hypothalamus and brainstem circuits, the amygdala innervates the autonomic networks and produces visceral signs of emotional arousal---e.g., changes in heart rate [18].
Neuroimaging studies suggest that examination of the amygdala may be of particular significance in psychiatric illnesses. Functional MRI studies in adults have shown abnormal amygdala activity in depression [31], schizophrenia [29], bipolar disorder [40], posttraumatic stress disorder [32], and autism [26]. In pediatric populations, fMRI studies have demonstrated abnormal amygdala activity in depression [35], anxiety disorders [35], bipolar disorder [27], autism [8], and conduct disorder [33]. Furthermore, structural MRI studies of the amygdala have found abnormalities in patients with depression [28], dissociative identity disorder [36], and autism [14].
The amygdala appears to play an especially important role in normal and abnormal adolescent behavior. In the triadic model of adolescent development, the authors propose that perturbations in the neural development of the components of this system (e.g., amygdala) may contribute to the expression of adolescent psychopathology [5]. For example, the authors suggest that abnormal maturation of the amygdala during adolescence may lead to greater vulnerability to psychiatric disorders such as depression and anxiety. The authors cite both functional [35] and structural [28] MRI studies of the amygdala in pediatric depression and anxiety to support their theory.
Given the amygdala’s influence on the ANS and its role in emotion, a logical step would be to pursue the simultaneous recording of physiological and functional neuroimaging data. To examine concomitant changes in emotional arousal, skin conductance was recorded with fMRI in a study of the amygdala’s response to facial signals of fear in normal adults [37]. The researchers observed that mean skin conductance level was positively correlated with right amygdala activity. By using different pressor challenges to elevate blood pressure, two fMRI studies in normal adults found that the pressor challenges elicited significant regional fMRI signal changes in the amygdala [11, 12]. To address the question of central control of heart rate in emotions, parallel measurement of heart rate changes and changes in activation as indexed by fMRI was performed on normal adults [15]. This study found that the amygdala was an integral part of the central circuit controlling heart rate in negative affect (e.g., fear).
Although rare, two published human depth electrode studies exist that are pertinent. In two similar studies, the authors recorded electrocardiogram activity together with single cell activity from the amygdala in epileptic patients undergoing chronic depth electrode monitoring [6, 7]. The authors discovered that changes in heart rate correlated with the firing of neurons in the human amygdala.
Other fMRI studies in normal adults have found additional brain regions that are activated in association with the ANS. In one study where brain activity and heart rate were simultaneously measured, the authors found that the level of activity in the amygdala, insula, anterior cingulate, and brainstem predicted subjects’ heart rate responses to the presentation of emotional facial expressions [4]. By examining neural activity related to modulation of skin conductance level, another fMRI study reported that activity within the insular cortices, anterior cingulate, striate and extrastriate cortices, thalamus, and hypothalamus reflected the rate of change in electrodermal activity [23]. Although studies combining fMRI and physiological measurements have been done in adults, to our knowledge, no such studies have been published in the pediatric population.
The purpose of this study was to use blood oxygenation level dependent fMRI (BOLD-fMRI) in combination with the simultaneous acquisition of physiological data to examine the relationship between amygdala activation and heart rate in normal adolescents. To this end, we used an emotional face paradigm that has been shown in fMRI studies to robustly activate the amygdala [25] and well-established methods of heart rate data analysis [9, 34]. We hypothesized that the adolescent amygdala would be activated in response to the perception of Fearful, Angry, and Happy faces relative to the control condition. Furthermore, based upon the human depth electrode studies [6, 7] and fMRI findings [4], we hypothesized that an increased amygdala fMRI BOLD signal due to the perception of Fearful, Angry, and Happy faces compared to the control condition would be correlated with an increased heart rate in adolescent subjects.
METHODS
Subjects
This study was approved by the University of California at San Diego (UCSD)-Children’s Hospital and Health Center (CHHC) Institutional Review Board and conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinki). All subjects provided written assent, and their parent/legal guardians provided written informed consent to participate.
Eighteen healthy, right-handed adolescent subjects (15 females and 3 males; ages 13 to 17 years; mean age 16.17 ± 1.20 years) were recruited from all regions of San Diego.
Each participant was administered: (1) Computerized Diagnostic Interview Schedule for Children version 4.0 [30] and the Diagnostic Predictive Scale (DPS) [19] to assess for the presence of any Axis I diagnoses, (2) Standard Snellen Eye Chart, (3) Ishihara Color Plates Test (8 plate, 2005 edition), and (4) Edinburgh Handedness Inventory [24]. Furthermore, each participant completed the following self-administered questionnaires: (1) demographics questionnaire, and (2) medical and developmental history form. Exclusion criteria for this study were: (1) any current or lifetime DSM-IV Axis I psychiatric disorder, (2) color blindness, (3) less than 20/40 corrected vision, (4) history of a serious medical, developmental, or neurological disorder, (5) history of loss of consciousness greater than 2 minutes, (6) left handedness, (7) MRI contraindications (ferrometallic implants, braces, pregnancy or claustrophobia), and (8) inability to fully comprehend and cooperate with study procedures.
Experimental Task
All participants were trained to perform the emotional face task prior to fMRI scanning. During the scan, each participant was shown a modified [25] version of the task by Hariri et al. [10]. For each trial, a target face was presented at the top of the screen and two probe faces were presented at the bottom. The participants were asked to match the target face with one of the two probe faces that had the same emotional expression by pressing the left or right button on a Current Designs box. Each block contained six consecutive, 5-second trials where the target was a Fearful, Happy, or Angry face. The control task consisted of 5-second trials of either tall or wide circles or ovals in a similar configuration to the facial expression task. Analogous to the facial expression task, participants were told to match the shape of the target to one of the two probes in the control task. There were a total of twelve blocks: three blocks for each of the Fearful, Happy, and Angry faces, and three blocks of the control task. Blocks were separated by a 10-second fixation cross and a two-second instruction period. In addition, a brief fixation was added to the start and end of the task to make a total task time of 512 seconds.
Image Acquisition
Images were acquired on a 3-T GE scanner (General Electric, Milwaukee, WI) with Twin Speed gradients using a GE 8-channel head coil. Each session consisted of a three-plane scout scan (10 s), a high-resolution anatomical scan, a series of T2*-weighted echo-planar imaging (EPI) scans to measure the blood oxygen-level dependent (BOLD) response, and EPI-based field maps to correct for susceptibility induced geometric distortions. Functional scans covering the entire brain were acquired parallel to the anterior and posterior commissure (T2*-weighted EPI, TR = 2000 ms, TE = 32 ms, FOV = 23 cm, 64 × 64 matrix, thirty 2.6 mm oblique slices with a 1.4 mm gap, 256 repetitions). During the same experimental session, a T1-weighted image with an inversion time of TI = 450 ms to null the CSF (FSPGR, TR = 8.0 ms, TE = 3.1 ms, flip angle = 12°, FOV = 25 cm, matrix = 256 × 256, 0.98 × 0.98 × 1.0 mm3 voxels) was collected in the sagittal plane for anatomical reference. These sequences were optimized for the amygdala.
Statistical Analysis of Imaging Data
All functional and structural image processing and analyses were conducted with the Analysis of Functional NeuroImages (AFNI) software [3]. To minimize motion artifact, an AFNI 3D-coregistration algorithm (3DVolreg) was used to realign all echoplanar images to the scan located closest to the 128th (middle) acquired scan that showed the least amount of head movement. Based upon visual inspection of the data, time points with isolated head movements not corrected by the coregistration algorithm were removed from statistical analysis. Three motion parameters (roll, pitch, yaw) were used as nuisance regressors to account for motion artifacts. The Angry, Fearful, Happy, and Shape (circle/oval) condition served as the four orthogonal regressors of interest. A modified gamma variate function was convolved with these four regressors to account for the dispersion brain response and delay of the BOLD-fMRI signal due to hemodynamics. Other regressors modeled residual motion in the baseline and linear trends as wells as in the roll, pitch, and yaw directions. The AFNI program, 3dDeconvolve, determined the estimated voxel-wise response amplitude. To account for individual variations in the anatomical landmarks, a Gaussian filter with a full-width-half-maximum of 4 mm was applied to the voxel-wise percent signal change data.
After smoothing, imaging data for each subject was normalized to stereotaxic Talairach coordinates. Using masks defined by the Talairach demon atlas [16], an a priori analysis of regions of interest (ROIs) was performed on the bilateral amygdala [1]. For these ROIs, it was found through computer simulations that a voxel-wise a-priori probability of 0.05 would result in a corrected cluster-wise activation probability of 0.05 if a minimum volume of 128 μl and two connected voxels was used for the amygdala. The ROIs were superimposed on each subject’s voxel-wise percent signal change brain image. Activations located inside these ROIs that met the voxel and volume connection criteria were then extracted and used for further analysis.
Physiological Monitoring
Physiological data were acquired using an In Vivo Magnitude TM 3150 MRI patient monitor. A pulse oximeter was placed on the subject’s left index finger to record heart rate (R-R interval) data. Physiological data were sampled at 40 samples per second using a multi-channel data acquisition board. Scanner TTL pulse data (10 ms duration, 5 V pulse per slice acquisition) were recorded at 1 kHz. The TTL pulse data were used to synchronize the physiological data to the acquired images. Artifacts were removed by editing out data points (beats) that deviated by more than three standard deviations from the beats that immediately preceded or followed the discrepant beat. Data were then interpolated from the surrounding beats. Subjects were excluded if 5% or more of beats were edited. The review and calculation of heart rate variability (HRV) was accomplished using a program developed at the University of Miami, Behavioral Medicine Research Center [9]. Fast Fourier Transformations (FFT) were performed on the R-R interval data to obtain power values for HRV in the high (0.4–0.15 Hz) frequency (HF) and low (0.15–0.04 Hz) frequency (LF) spectra. These ranges are based on previously established standards defined in the special report on HRV by the European Society of Cardiology and North American Society of Pacing and Electrophysiology [34]. Following the FFT of the data, the peak LF and HF power values were identified. The ratio of the peak LF divided by peak HF values (LF/HF) were used as an index of heart rate and correlated with the percent signal change in the bilateral amygdala during performance of the modified Hariri task. The peak LF/HF was selected due to the literature supporting its use as an accurate reflection of sympathovagal balance [22]: a measure of the relative contributions of the sympathetic and parasympathetic nervous system which is proportional to heart rate [2].
Statistical Analyses of Behavioral and Physiological Data
All behavioral and correlational statistical analyses were carried out with SPSS 14.0. Correlational analyses examined the relationship between peak LF/HF data and the total percent signal change for each of the different facial emotions (Fearful, Angry, Happy) in the left and right amygdala. Furthermore, the same correlational analyses were performed using a control region (the fusiform gyrus) to investigate whether any significant findings might be due to overall blood flow changes rather than being specific to the amygdala.
RESULTS
Behavioral Data
All 18 subjects performed the task accurately matching the probe face to the target face with great precision (mean ± SD, 97.6 % ± 0.04 %). The adolescents’ response time was significantly affected by face type (F(2,16) = 57.8, p < 0.001, η2 = 0.88). Employing the Bonferroni post-hoc test, significant differences were found between the Fearful and Happy faces (p < 0.001), Fearful and Angry faces (p < 0.001), and Angry and Happy faces (p < 0.001). Mean response time (ms) ± SD (ms): Fearful: 1681 ms ± 340 ms; Angry: 1471 ms ± 312 ms; Happy: 1301 ms ± 261 ms.
Amygdala Activation
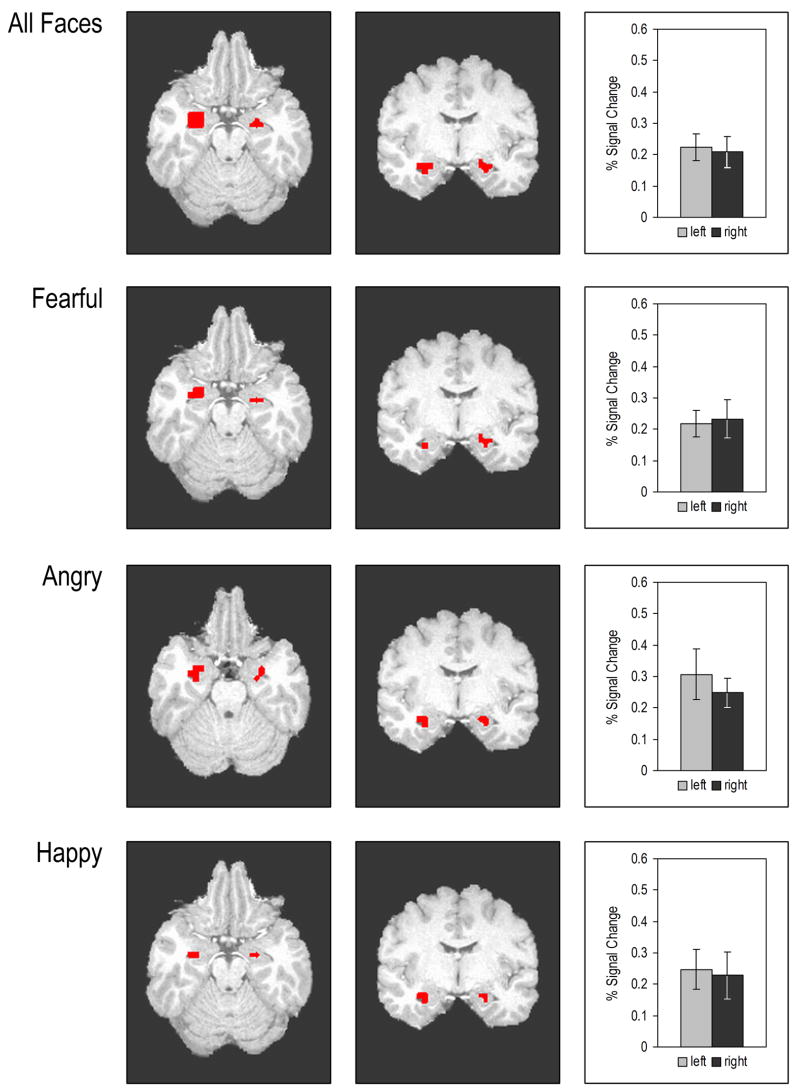
Adolescents showed significant bilateral amygdala activation to Fearful, Angry, and Happy faces relative to the shape-matching, control condition. For brain images and coordinates of amygdala activation, see Figure 1 and Table 1 in Supplementary material.
Figure 1.
Axial and Coronal views showing significant amygdala activation to All Faces, Fearful, Angry, and Happy vs. Shape conditions. The All Faces brain images and bar graphs represent the sum total of the amygdala activation to Fearful + Angry + Happy Faces compared to the control Shape condition. Bar graphs show percent signal changes in the left and right amygdala. Error bars indicate Standard Erro Error.
Table 1.
Talairach Coordinates for Amygdala Activation
| Contrast | Volume (μL) | Left/Right | x | Y | z | T-score |
|---|---|---|---|---|---|---|
| All Faces | 1024 | R | 26 | −5 | −16 | 4.25 |
| All Faces | 384 | L | −21 | −8 | −16 | 5.35 |
| Fearful | 768 | R | 26 | −4 | −16 | 3.85 |
| Fearful | 320 | L | −21 | −9 | −16 | 5.11 |
| Angry | 1088 | R | 26 | −4 | −18 | 5.28 |
| Angry | 320 | L | −20 | −7 | −-17 | 3.79 |
| Happy | 192 | R | 27 | −9 | −17 | 3.01 |
| Happy | 192 | L | −21 | −9 | −17 | 3.94 |
Correlation of Heart Rate with Amygdala Activation
The correlation between the peak LF/HF and right amygdala percent signal change for the Fearful faces condition in the 18 adolescents was statistically significant (Spearman’s rho = 0.55, p = 0.018, two-tailed). For scatterplot, see Figure 2. The correlations between the peak LF/HF and percent signal change in the right amygdala for Angry (Spearman’s rho = 0.19, p = 0.45) and Happy (Spearman’s rho = 0.24, p = 0.33) faces were not significant. The correlations between peak LF/HF and percent signal change in the left amygdala for Fearful (Spearman’s rho = 0.23, p = 0.36), Angry (Spearman’s rho = −0.61, p = 0.81), and Happy (Spearman’s rho = −0.18, p = 0.47) faces were not significant. No significant correlations were found for the control region: (1) right fusiform: Fearful (Spearman’s rho = 0.13, p = 0.62), Angry (Spearman’s rho = −0.14, p = 0.57), Happy (Spearman’s rho = −0.27, p = 0.29); (2) left fusiform: Fearful (Spearman’s rho = −0.16, p = 0.53), Angry (Spearman’s rho = −0.14, p = 0.58), Happy (Spearman’s rho = −0.29, p = 0.24).
Figure 2.
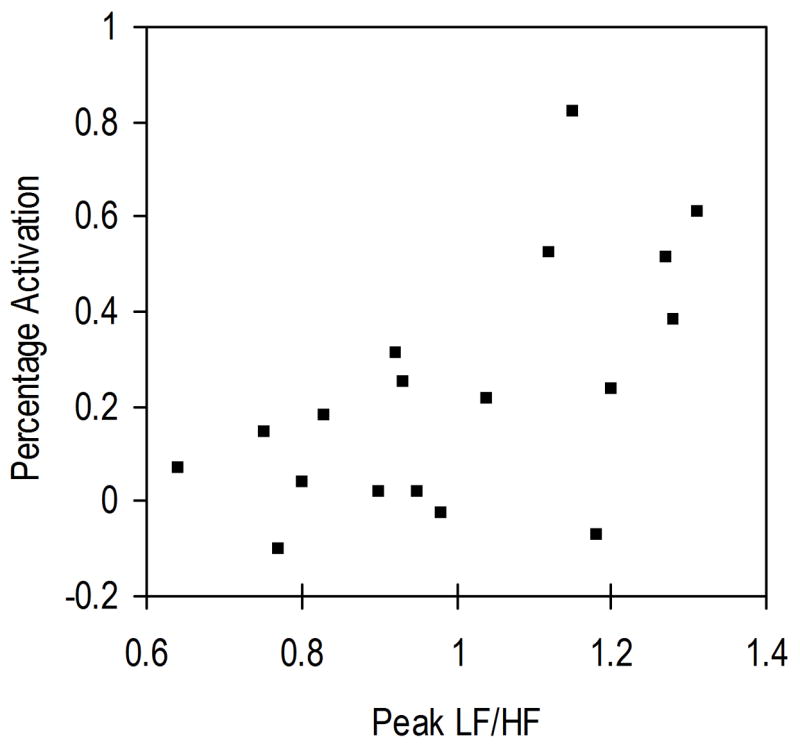
Scatterplot of the percent signal change in the right amygdala and the peak LF/HF values for Fearful faces compared to the control (shape matching) condition.
DISCUSSION
The present study is the first one to investigate the relationship between amygdala activation and heart rate in adolescents. There were two main results. First, we found significant increases in amygdala activation in response to the perception of faces with happy, angry or fearful affect relative to the control condition. These findings are consistent with other fMRI studies in adults [38] and adolescents [20, 21, 39]. Second, using well-established methods of heart rate data analysis [9, 34], we found a significant positive correlation between the percent signal change in the right amygdala and peak LF/HF. Taken together, our results support the idea that, as with adults, the adolescent amygdala processes emotionally salient events, and it is coupled to the functional status of the ANS.
Our findings are consistent with those of previous depth electrode [6, 7] and functional neuroimaging studies in adults. The functional neuroimaging of adults have found concomitant increases in amygdalar activation and measures of autonomic arousal such as skin conductance [37], blood pressure [11, 12] and heart rate [4, 15].
Our finding of a positive correlation between amygdala activation and peak LF/HF supports the notion that greater amygdala activation results in an increase in heart rate in normal adolescents. The James-Lange theory states that emotional feelings (e.g., fear) are dependent on bodily responses (e.g., quickening heartbeat) which are automatically generated by the ANS. Thus, our finding of the coupling between the degree of amygdala activation and heart rate is consistent with the James-Lange theory.
Within the framework of the triadic model of adolescent development [5], our finding of a significant correlation between amygdala activation and peak LF/HF suggests that the examination of the interaction between emotion and physiology may be interesting in depressed or anxious adolescents. The amygdala, nucleus accumbens, and medial/ventral prefrontal cortices are presented as the key neural systems in the triadic model. In their paper, the authors suggest that developmental perturbations in any of these key neural systems may result in psychopathology. At the time of publication of their model, only structural and functional MRI studies of the amygdala in the pediatric population had been done. No studies examining the correlation between amygdala activation and ANS activity in the pediatric population had been published. Our study establishes a normative baseline in adolescents against which future studies of different psychiatric populations (e.g., depressed or anxious adolescents) may be compared.
In conclusion, our findings contribute to the field of neuroscience in at least three areas. First, our results show that amygdala activity is related to heart rate in normal adolescents. Hence, as with adults, healthy adolescents demonstrate a coupling between processing emotional stimuli and adjusting the ANS accordingly. Our results are consistent with the James-Lange theory of emotion. Second, our finding of significant increases in amygdala activation in response to the perception of faces with happy, angry or fearful affect relative to the control condition provides additional support for a broader role for the adolescent amygdala beyond simply responding to Fearful faces. Of note, our results separately replicate the initial finding of adolescent amygdalar activation to positive facial expressions [39]. Finally, our study establishes a normative baseline in adolescents against which future studies of different psychiatric populations (e.g., depressed or anxious adolescents) may be compared.
Supplementary Material
Acknowledgments
We would like to thank Dr. Rebecca Theilmann, Dr. Scott Roesch, Dr. Don Slymen, David Fadale, Daniel Miller, Michael Kim, and Jing Wu. This work was supported by grants from NIMH (K23MH70791), NARSAD, and Klingenstein Foundations to TTY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arce E, Miller DA, Feinstein JS, Stein MB, Paulus MP. Lorazepam dose-dependently decreases risk-taking related activation in limbic areas. Psychopharmacology (Berl) 2006;189:105–116. doi: 10.1007/s00213-006-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bootsma M, Swenne CA, Van Bolhuis HH, Chang PC, Cats VM, Bruschke AV. Heart rate and heart rate variability as indexes of sympathovagal balance. Am J Physiol. 1994;266:H1565–1571. doi: 10.1152/ajpheart.1994.266.4.H1565. [DOI] [PubMed] [Google Scholar]
- 3.Cox RW. Software for analysis and visualization of functional magnetic neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 4.Critchley HD, Rotshtein P, Nagai Y, O’Doherty J, Mathias CJ, Dolan RJ. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. Neuroimage. 2005;24:751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frysinger RC, Harper RM. Cardiac and respiratory correlations with unit discharge in epileptic human temporal lobe. Epilepsia. 1990;31:162–171. doi: 10.1111/j.1528-1167.1990.tb06301.x. [DOI] [PubMed] [Google Scholar]
- 7.Frysinger RC, Harper RM. Cardiac and respiratory correlations with unit discharge in human amygdala and hippocampus. Electroencephalogr Clin Neurophysiol. 1989;72:463–470. doi: 10.1016/0013-4694(89)90222-8. [DOI] [PubMed] [Google Scholar]
- 8.Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, Volkmar FR, Schultz RT. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43:373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Han K, Nagel JH, Hurwitz BE. Decomposition of heart rate variability by adaptive filtering for estimation of cardiac vagal tone. In: Nagel JH, Smith WM, Nagel JH, Smith WM, editors. Bioelectric phenomena, electrocardiography, electromagnetic interactions, neuromuscular systems. IEEE Publishing Services; New York: 1991. pp. 660–661. [Google Scholar]
- 10.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 11.Harper RM, Bandler R, Spriggs D, Alger JR. Lateralized and widespread brain activation during transient blood pressure elevation revealed by magnetic resonance imaging. J Comp Neurol. 2000;417:195–204. doi: 10.1002/(sici)1096-9861(20000207)417:2<195::aid-cne5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Harper RM, Gozal D, Bandler R, Spriggs D, Lee J, Alger J. Regional brain activation in humans during respiratory and blood pressure challenges. Clin Exp Pharmacol Physiol. 1998;25:483–486. doi: 10.1111/j.1440-1681.1998.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 13.James W. Physical Basis of Emotion. Psychol Rev. 1894;1:516–529. doi: 10.1037/0033-295x.101.2.205. [DOI] [PubMed] [Google Scholar]
- 14.Juranek J, Filipek PA, Berenji GR, Modahl C, Osann K, Spence MA. Association between amygdala volume and anxiety level: magnetic resonance imaging (MRI) study in autistic children. J Child Neurol. 2006;21:1051–1058. doi: 10.1177/7010.2006.00237. [DOI] [PubMed] [Google Scholar]
- 15.Kuniecki M, Urbanik A, Sobiecka B, Kozub J, Binder M. Central control of heart rate changes during visual affective processing as revealed by fMRI. Acta Neurobiol Exp (Wars) 2003;63:39–48. doi: 10.55782/ane-2003-1453. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey LH, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange C. The Emotions. Williams & Wilkins; Baltimore, Maryland: 1922. [Google Scholar]
- 18.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Lucas C, Zhang H, Mroczek D. The DISC predictive scales: efficiently predicting DISC diagnoses. 44th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Toronto. 1997. [Google Scholar]
- 20.McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, Charney DS, Ernst M, Pine DS. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 22.Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994;90:1826–1831. doi: 10.1161/01.cir.90.4.1826. [DOI] [PubMed] [Google Scholar]
- 23.Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a "default mode" of brain function. Neuroimage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 25.Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- 26.Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 27.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, Muller-Gartner HW. Differential amygdala activation in schizophrenia during sadness. Schizophr Res. 1998;34:133–142. doi: 10.1016/s0920-9964(98)00085-1. [DOI] [PubMed] [Google Scholar]
- 30.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 32.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 33.Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Task, Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 35.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 36.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. Am J Psychiatry. 2006;163:630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E, Bryant RA. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage. 2005;28:618–626. doi: 10.1016/j.neuroimage.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 38.Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, Gotlib IH, Reiss AL. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13:1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- 39.Yang TT, Menon V, Reid AJ, Gotlib IH, Reiss AL. Amygdalar activation associated with happy facial expressions in adolescents: a 3-T functional MRI study. J Am Acad Child Adolesc Psychiatry. 2003;42:979–985. doi: 10.1097/01.CHI.0000046886.27264.BA. [DOI] [PubMed] [Google Scholar]
- 40.Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.