Abstract
The spatial distribution of axonal projections descending from rat field CA1 to thalamus and hypothalamus was analyzed previously with the PHAL method (Cenquizca and Swanson, 2006). The same experimental material was used here to define the topography of field CA1 association projections to other cerebral cortical areas. First, the results confirm and extend known intrahippocampal formation inputs to dentate gyrus, subiculum, presubiculum, parasubiculum, and entorhinal area, which are arranged generally along the formation’s transverse axis and dominated by the subicular projection—by far the densest established by field CA1 anywhere in the brain. And second, field CA1 innervates a virtually complete ring of extrahippocampal formation cortex via three routes. A dorsal pathway from the dorsal third of field CA1 innervates moderately the retrosplenial area; a moderately strong ventral pathway from the ventral two-thirds of field CA1 passing through the longitudinal association bundle sends offshoots to visual, auditory, somatosensory, gustatory, main and accessory olfactory, and visceral areas—as well as the basolateral amygdalar complex and the agranular insular and orbital areas; and a cortical-subcortical-cortical pathway through the fornix from the whole longitudinal extent of field CA1 innervates rather strongly a rostral region that includes the tenia tecta along with the anterior cingulate, prelimbic, infralimbic, and orbital areas. The functional consequences of long-term potentiation in field CA1 projection neurons remain to be explored.
Keywords: accessory olfactory bulb, agranular insular cortex, amygdala, anterior cingulate area entorhinal area, infralimbic area, olfactory cortex, orbital cortex, prefrontal cortex, retrosplenial area, subiculum
1. INTRODUCTION
The hippocampal formation is a prominent region of the adult cerebral cortical mantle that is usually described as an infolding of the medial temporal lobe. Based on a modern understanding of how the trisynaptic or intrahippocampal circuit is organized structurally and functionally, it is convenient (Blackstad, 1956; Swanson et al., 1987; Swanson, 2004) to define the hippocampal formation as consisting of two major divisions: hippocampal region (with dentate gyrus and Ammon’s horn, and their thin extensions, the fasciola cinerea and then indusium griseum), and retrohippocampal region (with subiculum, presubiculum/postsubiculum, parasubiculum, and finally entorhinal area—the traditional starting point of the trisynaptic circuit). Topologically, the hippocampal formation displays a unique cortical architecture: each of its areas has a longitudinal axis, overall forming an 8-membered palisade beginning at the mantle’s embryologically medial edge with the dentate gyrus, followed sequentially by Ammon’s horn (with its successive fields CA3, CA2, and CA1), subiculum, presubiculum and postsubiculum, parasubiculum, and entorhinal area (see Swanson et al., 1978; Petrovich et al., 2001). Superimposed on this macrostructure, the intrahippocampal circuit—which actually interconnects all 8 cortical areas—is arranged along the transverse axis of each area, and thus the hippocampal formation as a whole (Anderson et al., 1971; Swanson et al., 1987). In addition, the laminar (radial) organization of the hippocampal region is as simple as any mammalian cerebral cortical area, with a single layer of projection neurons in the dentate gyrus (granular layer) and Ammon’s horn (pyramidal layer).
Partly because of this relatively simple structural arrangement, the organization of intrinsic and extrinsic hippocampal formation axonal connections has been clarified to a greater extent than any other set of functionally interrelated cortical areas (see Cajal, 1909-1911; Lorente de Nó, 1934; Blackstad, 1956; Nauta, 1956; Raisman et al., 1966; Hjorth-Simonsen, 1973; Meibach and Siegel, 1977; Swanson and Cowan, 1977a,b; Swanson et al., 1978, 1981, 1987; Ino et al., 1987, 2001; van Groen and Wyss, 1990a,b; Lim et al., 1997a,b; Witter and Amaral, 2004). However, the exact relationship between this mass of neural network information and the proposed functions of the hippocampal formation—which are among the most complex ascribed to the cerebral hemispheres and range from spatial navigation (Gothard et al., 1996) and learning (Moser et al., 1993; Bunsey and Eichenbaum 1996; Duva et al., 1997), to short-term episodic or declarative memory (Paulesu et al., 1993; Zola-Morgan and Squire, 1993; Nakamura and Kubota, 1996; Vnek and Rothblat, 1996; Reber and Squire, 1998; Rolls, 2000; Eichenbaum, 2001), to the elaboration of a cognitive map of the animal’s environment and experience (O’Keefe and Nadel, 1978)—is far from clear. At least part of the conundrum is that organizing principles of hippocampal formation axonal outputs to other parts of the brain, in one sense defining its function, are in need of clarification and revision (Amaral and Witter, 1995; Vanderwolf, 2001).
We have begun to address this issue by reexamining at high spatial resolution the extrinsic projections of field CA1 in the rat with the sensitive anterograde tracer PHAL. The organization of pathways to, and terminal fields within, the hypothalamus and thalamus—which are considerably more widely distributed than previously appreciated—has already been described (Cenquizca and Swanson, 2006), and here we analyze projections to the rest of the cortical mantle. Inputs to the cerebral nuclei (basal ganglia) will be dealt with in the next paper of the series.
2. RESULTS
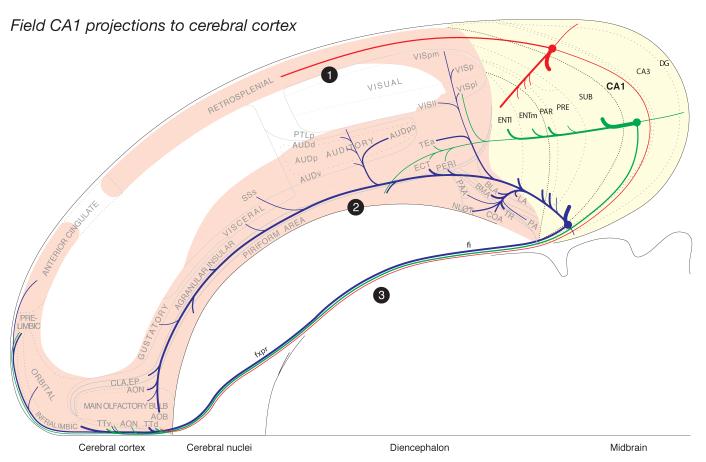
Overall, axons of field CA1 pyramidal neurons descend to the deep white matter (alveus) and bifurcate, with branches extending rostrally through the fornix system and caudally toward the retrohippocampal region of the hippocampal formation where they contribute to the intrahippocampal circuit (Fig. 2). Some axons in the precommissural fornix traverse the septal region of the cerebral nuclei to reenter the cerebral cortex in frontal regions, although axons from the dorsal (septal) end of field CA1 extend no farther rostrally than the dorsal tenia tecta. Outside the hippocampal formation, caudally directed axons follow two general routes. The dorsal third of field CA1 sends axons dorsally and rostrally to the retrosplenial area of the cingulate region, whereas the ventral two-thirds of field CA1 projects ventrally and rostrally through the longitudinal association bundle all the way to the olfactory bulb. These are predominantly intracortical association projections, although relatively sparse commissural additions are noted.
Fig. 2.
Summary diagram to indicate the general organization of projections from dorsal (red), intermediate (green), and ventral (blue) levels of field CA1 to the rest of the hippocampal formation (shaded yellow), and then the rest of the cortical mantle (shaded light red). Note three general projections to the rest of the cortex: dorsal (1) to the retrosplenial area and caudal end of the anterior cingulate area, ventral (2) through the longitudinal association bundle, and rostral (3) via a cortical-subcortical-cortical pathway involving the fornix system. Projections to the diencephalon were described previously (Cenquizca and Swanson, 2006), and those to the cerebral nuclei will be described in the next contribution to this reevaluation of field CA1 efferents. The relative size of each pathway is roughly proportional to the thickness of the line representing it. The flatmap is based on Swanson (2004).
2.1. Projections from the dorsal third of field CA1
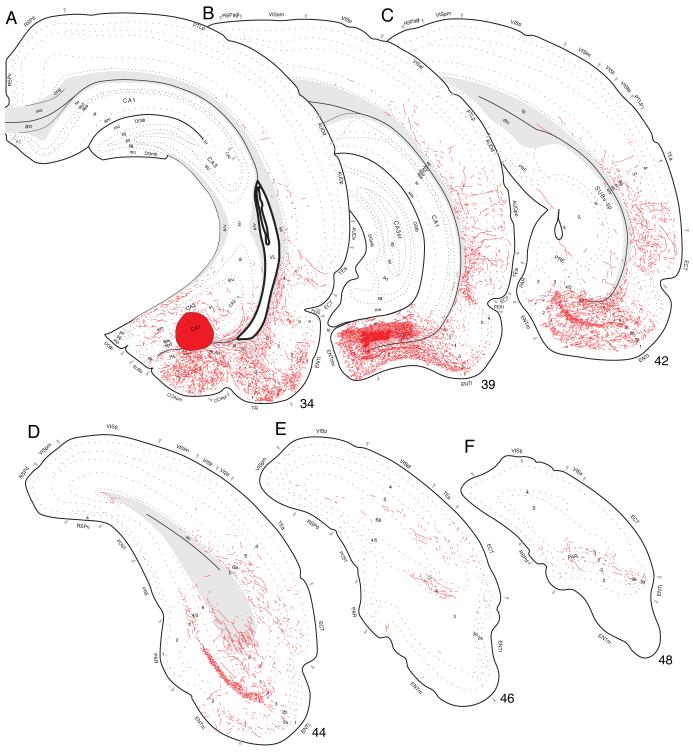
Cortical projections labeled in Experiment HIPPO4 are representative of those centered in the dorsal or septal third of field CA1; note that the injection site in Experiment HIPPO4 is also centered near the midpoint of field CA1’s transverse axis at this dorsoventral level of the longitudinal axis (Figs. 1 and 3). With one exception to be noted, no unambiguous topography was detected in projections labeled by the various dorsal field CA1 injections. PHAL-immunoreactive fibers innervate regions of field CA1 immediately surrounding the injection site (Fig. 4A) both transversely and longitudinally. They are most obvious in the stratum oriens, and are more dispersed in the stratum radiatum and pyramidal cell layer. A few labeled axons also cross the hippocampal fissure to innervate lightly the molecular and polymorph layers of the dentate gyrus.
Fig. 1.
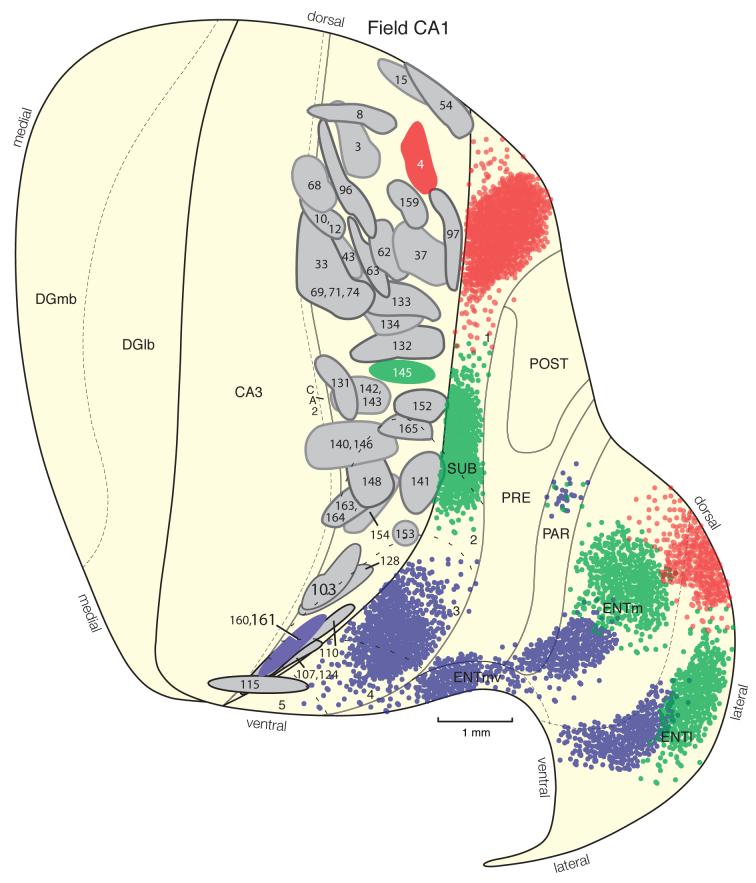
Distribution of injection sites centered in hippocampal field CA1. Relative locations of PHAL injections from 62 experiments, plotted onto an unfolded map of the rat hippocampal formation (adapted from Petrovich et al., 2001). Three colored injection sites illustrate prototypical experiments with injection sites centered in the dorsal or septal third (red, Experiment HIPPO4), intermediate or middle levels (green, Experiment HIPPO145), and ventral or temporal pole (blue, Experiments HIPPO160 and HIPPO161) of field CA1. Note especially the inverted V shape of the field CA1 to entorhinal area projection when viewed on the flatmap.
Fig. 3.
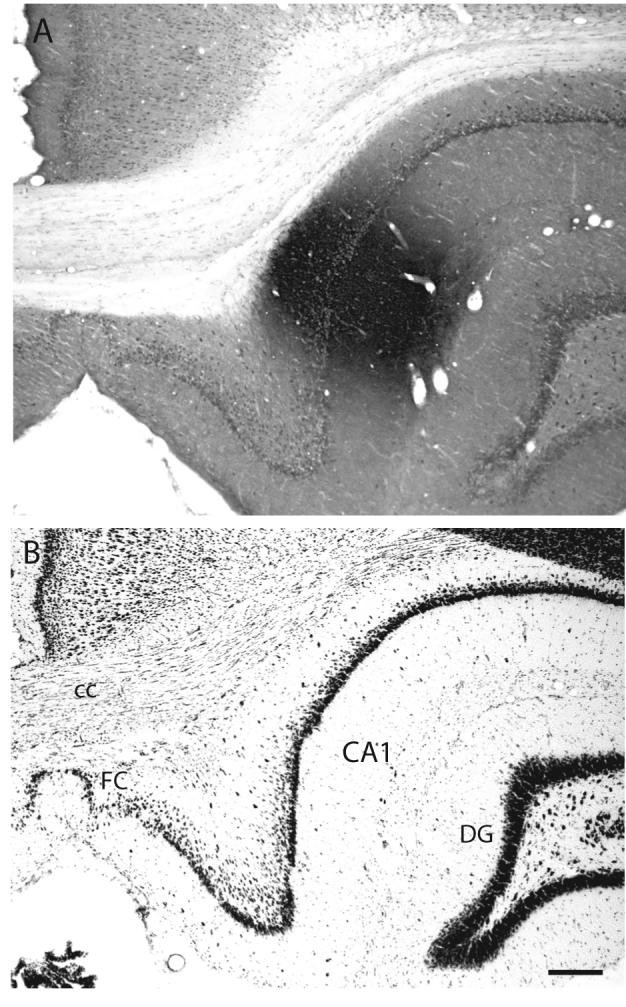
A: Brightfield photomicrograph illustrating the appearance and location of the PHAL injection site in Experiment HIPPO4. B: Brightfield photomicrograph of caudally adjacent thionin-stained transverse histological section. The injection site in this experiment is centered in the dorsal third of field CA1, about midway along the transverse axis at this level (see Figs. 1 and 4A). Scale = 200 μm.
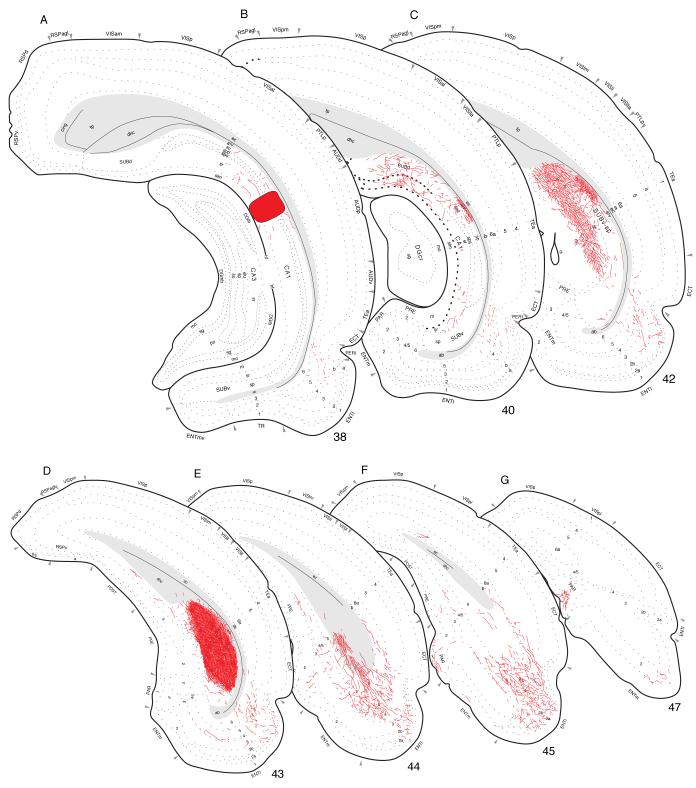
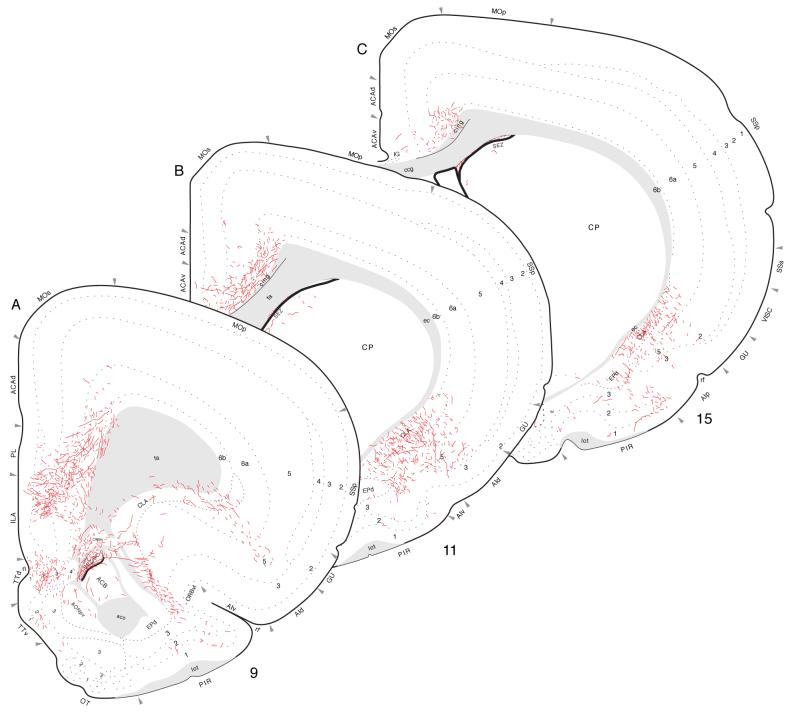
Fig. 4.
Projections from dorsal (septal) field CA1 to parts of the hippocampal formation and retrosplenial area of the cerebral cortex. PHAL-labeled axons were plotted on a series of standard or reference drawings of the rat brain derived from an atlas (Swanson, 2004). Atlas Level (A) shows injection site location in Experiment HIPPO4 (black area; also see Figs. 1 and 3). Arranged from rostral (A) to caudal (B); the number in the lower left corner of each drawing refers to the corresponding rostrocaudal level of the atlas.
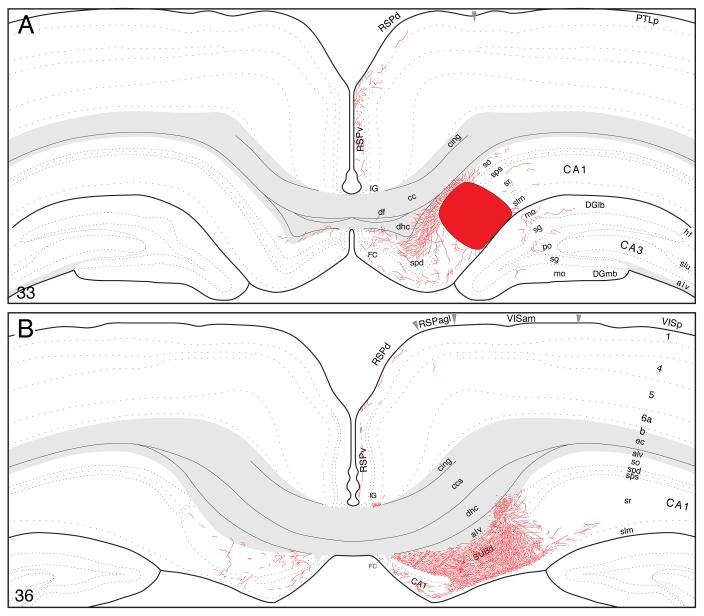
On reaching the subiculum, fibers extend radially from the alveus to generate an exceptionally dense plexus in dorsal (septal) levels of this cortical field (Figs. 4B and 5A). The terminal field covers the entire depth of the pyramidal layer and adjacent deep zone of the molecular layer. It extends a considerable distance along the longitudinal (dorsoventral) axis of the subiculum (at least twice the length of the injection site; Fig. 1) and it is easily the densest of any generated by field CA1. As this terminal field in the subiculum begins to diminish, a very light projection spreads to what appears to be deeper layers of the adjacent presubiculum.
Fig. 5.
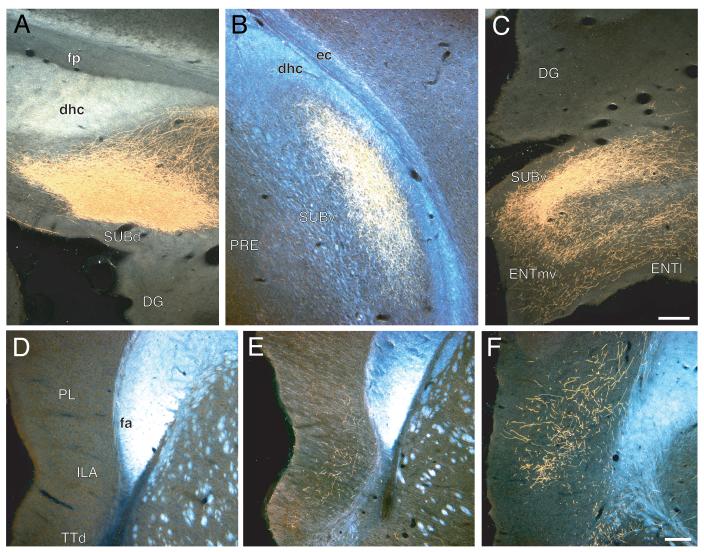
A-C: Darkfield photomicrographs showing the distribution of PHAL-labeled axons within the dorsal (A), intermediate or middle (B), and ventral (C) subiculum following injections centered in dorsal (Experiment HIPPO33, roughly at the level of Fig. 4B, from a different experiment), intermediate or middle (Experiment HIPPO145, roughly level of Fig. 8D), and ventral (Experiment HIPPO161, roughly level of Fig. 10B) regions of field CA1, respectively. Scale = 200 μm. D-F: Darkfield photomicrographs showing the distribution of PHAL-labeled axons within the medial prefrontal cortical region following injections centered in dorsal (D; Experiment HIPPO33), intermediate or middle (E; Experiment HIPPO145, roughly level of Fig. 9D), and ventral (F; Experiment HIPPO161, roughly level of Fig. 12A) levels of field CA1. Scale = 200 μm.
A transverse topography in projections from field CA1 to the subiculum was observed. Field CA1 PHAL injections centered midway between the borders with field CA3 and the subiculum (for example, experiment HIPPO4, Fig. 1) did not label a terminal field extending across the entire transverse width of the subiculum—there was an unlabeled gap near the subicular border with the postsubiculum (and more ventrally, the presubiculum). Similarly, field CA1 PHAL injections centered near the border with field CA3 (for example, experiment HIPPO131, Fig. 1) labels a transverse band in the subiculum that extends from its border with the postsubiculum/presubiculum but does not reach the border with field CA3. And finally, field CA1 injections adjacent to the subiculum (for example, HIPPO97, Fig. 1) label a transverse band in the subiculum that begins at the border with field CA1 but does not extend as far toward the border with the postsubiculum/presubiculum as the terminal field generated by the intermediate injection HIPPO4. This unusual topography of the transverse field CA1-subicular projection was illustrated very clearly, and much more precisely, by tracing the axon of individually filled CA1 neurons (Tamamaki and Nojyo, 1990). Apparently the topography is blurred when contiguous neuron populations are labeled, as here.
Labeled axons extend caudally from the subiculum through the region of the dorsal hippocampal commissure (Fig. 6). A dense terminal field is generated in layers 4 and 5 of lateral (or dorsolateral) regions of the lateral entorhinal area (Fig. 6A-C), whereas a light projection is observed in layer 6, along with a few scattered fibers in layers 2 and 3. Only a light projection is observed to layer 6 of the medial entorhinal area—in a dorsal region adjacent to the lateral entorhinal area (Fig. 1)—along with scattered fibers in layers 3 and 6 of the adjacent parasubiculum. In addition to these ipsilateral projections, labeled axons course through the ventral hippocampal commissure to provide a sparse input to contralateral field CA1 and a slightly denser input to the contralateral subiculum (Fig. 4B).
Fig. 6.
Projections from dorsal field CA1 to caudal regions of the cerebral cortex (Experiment HIPPO4). PHAL-labeled axons were plotted as described for Figure 4.
Finally, labeled fibers bend dorsally around the splenium of the corpus callosum and then extend rostrally to provide a moderate input to the dorsal and ventral parts of the retrosplenial area (Fig. 4A,B). Importantly, labeled axons are virtually restricted to layer 1, although a few isolated fibers are observed in layers 2-4. This projection extends to the rostral end of the retrosplenial area and even appears to enter caudal regions of the anterior cingulate area, although less densely. Labeled projections associated with frontal regions of the cerebral cortex are limited to a sparse innervation of the dorsal tenia tecta, as mentioned above (see Fig. 2).
2.2. Projections from intermediate levels of field CA1
The projection pattern labeled in Experiment HIPPO145 is typical of that from injections involving intermediate dorsoventral levels of field CA1 (Figs. 1, 2 and 7). It will be described in detail.
Fig. 7.
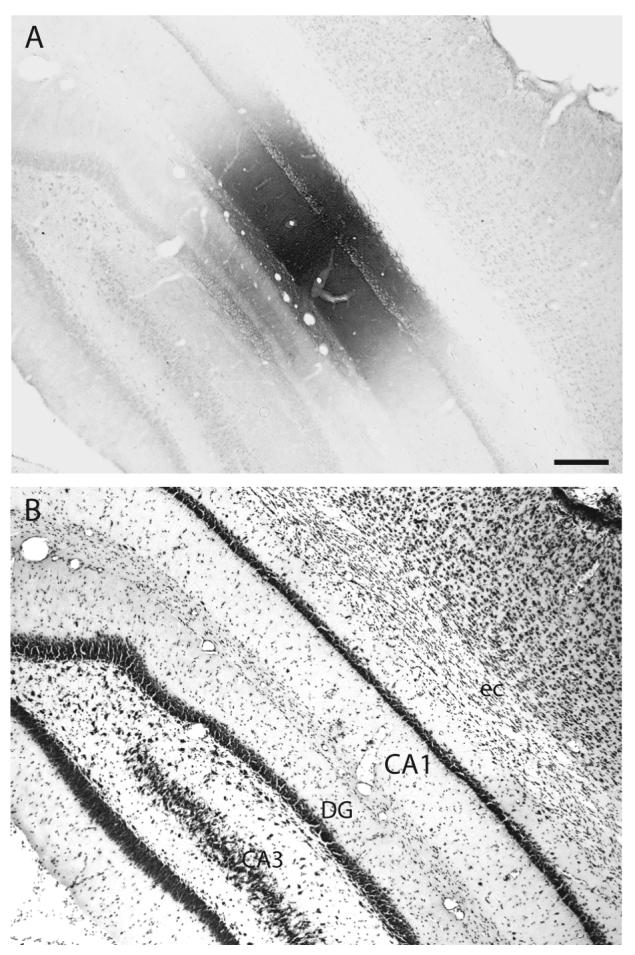
A: Brightfield photomicrograph illustrating the appearance and location of the PHAL injection site in Experiment HIPPO145 (also see Figs. 1 and 8A). B: Brightfield photomicrograph of the caudally adjacent thionin-stained transverse histological section. The injection site in this experiment is centered intermediately along the dorsoventral or longitudinal axis of field CA1 (see Fig. 1). Scale = 200 μm.
2,2,1, Intrahippocampal projections
At the level of the injection site, PHAL-labeled axons innervate adjacent regions within the field (Fig. 8A), notably in the stratum oriens and stratum radiatum and the pyramidal cell layer. A few labeled axons cross the hippocampal fissure to innervate the molecular layer of the dentate gyrus, and at this level a few axons also extend medially to innervate sparsely layer 1 in far lateral regions of the ventral retrosplenial area-indicating that virtually all field CA1 inputs to the retrosplenial area arise from more dorsal longitudinal levels.
Fig. 8.
Projections from the middle or intermediate dorsoventral region of field CA1 to caudal regions of the cerebral cortex, plotted as described for Figure 4. The injection site for this experiment (HIPPO145; see Figs. 1 and 7) is indicated by the black filled region in panel A.
Many axons enter the alveus and then extend caudally. As with more dorsal levels of field CA1, intermediate levels generate by far their densest terminal plexus (with abundant terminal boutons) in the adjacent subiculum (Figs. 5B and 8C,D). This plexus extends through the entire thickness of the pyramidal layer and the adjacent deep zone of the molecular layer, and it lies within the intermediate dorsoventral third of the subiculum (Fig. 1). As the subicular terminal field begins to end, a very light projection spreads to deep layers of the presubiculum (Fig. 8E,F). The field CA1-subiculum topography described above (along the transverse axis) could also be detected at these levels, but it becomes progressively more difficult to observe as field CA1 progressively narrows toward the ventral tip of its longitudinal axis (Fig. 1).
This projection extends caudally through the region of the dorsal hippocampal commissure, and a dense terminal field is then generated in layers 4 and 5 of the lateral entorhinal area (Fig. 8E,F). These results now begin to suggest an interesting topographic ordering of the field CA1 to entorhinal projection. Whereas projections from dorsal field CA1 are densest in lateral regions of the lateral and medial entorhinal areas, projections from intermediate (in the longitudinal dimension) field CA1 are densest in middle (in the transverse dimension) regions of the lateral and medial entorhinal areas (Fig. 1). In concert with this, projections to the medial entorhinal area from intermediate field CA1 appear to be considerably denser than the relatively light input from dorsal field CA1. Evidence for this topographic trend is strengthened by the observation that the injection site in Experiment HIPPO4 (dorsal field CA1) is considerably larger than that in Experiment HIPPO145 (intermediate field CA1), and thus presumably labels many more pyramidal (projection) neurons.
A moderately dense terminal field is observed in layer 6 of both the medial and lateral entorhinal area, and in layer 2 of the lateral entorhinal area—the latter being another clear difference between intermediate and dorsal regions of field CA1. In fact, a moderate to dense terminal field is established across layers 2-5, and superficial layer 6, of the lateral entorhinal area (Fig. 8E). Labeled axons from intermediate field CA1 also extend rostrally in the lateral entorhinal area and provide a light to moderate innervation of layer 2, and layers 4 and 5, with scattered fibers across all layers except layer 1. This labeling diminishes rostrally in lateral (dorsolateral) regions of the lateral entorhinal area, just ventral to the perirhinal area (Fig. 8A).
Curiously, a dense cluster of labeled fibers is observed in layer 1 of the parasubiculum, in the “pocket” formed by the infolding of layer 2 (Fig. 8F,G), along with scattered fibers in layers 2-6. In contrast to dorsal field CA1, no contralateral projection was observed in this or other experiments involving intermediate field CA1.
2.2.2. Rostrally directed projections through the fimbria-fornix
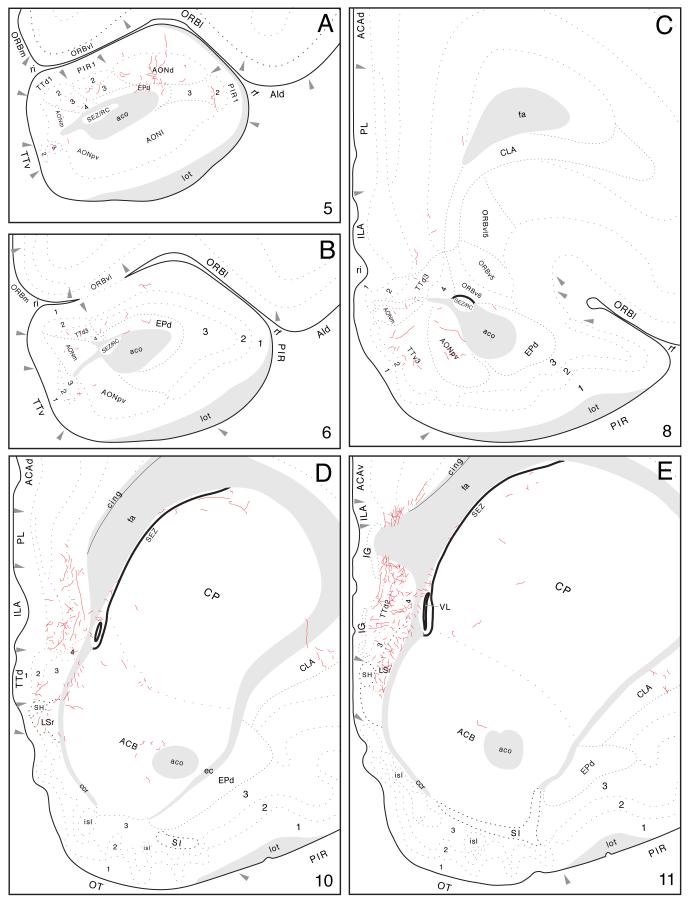
From the precommissural fornix in the lateral septal nucleus, rostrally directed labeled axons generate a moderately dense input to the dorsal tenia tecta, where most terminal boutons are concentrated in layer 2, and a few labeled axons are also observed in the contralateral tenia tecta. Some fibers in the tenia tecta then extend dorsally near the genu of the corpus callosum to innervate moderately the infralimbic area (layers 2-6), lightly the prelimbic area (deeper layers), and very lightly the anterior cingulate area (Figs. 5E and 9D,E). The latter terminal field extends caudally until about the level of the crossing of the anterior commissure.
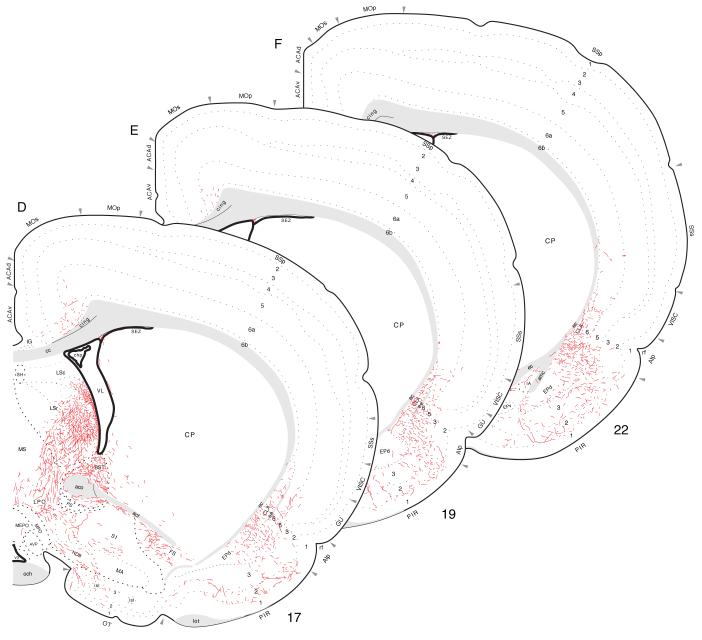
Fig. 9.
Projections from mid-dorsoventral regions of field CA1 to prefrontal and olfactory regions of the cerebral cortex, from Experiment HIPPO145 and plotted as described for Figure 4.
Other labeled axons emerging from the precommissural fornix extend ventrally to innervate the anterior olfactory nucleus (Fig. 9A-C). A light input is centered in the dorsal and posteroventral parts, and even more scattered fibers are observed in the medial part—and in layers 2 and 3 of the ventral tenia tecta.
2.2.3. Rostral projections through the longitudinal association bundle
This projection is much more substantial from ventral field CA1, and its distribution will be described more fully below. Suffice it to say that scattered axons extend rostrally from the lateral entorhinal area to end in all layers of the piriform area and dorsal endopiriform nucleus, as well as layer 6 of the perirhinal area, ventral temporal association areas, and posterolateral visual area (Figs. 2 and 9F).
2.3 Projections from ventral field CA1
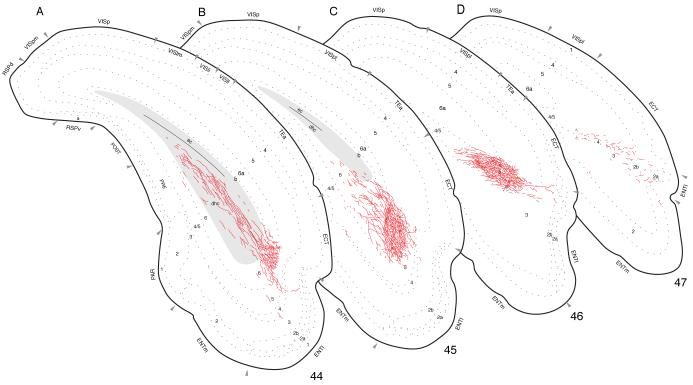
Ventral regions of field CA1 have by far the most diverse cortical projections, directed both caudally and rostrally (Fig. 2). Experiment HIPPO161 was chosen for illustration because it displays the projection pattern typical of experiments centered in ventral field CA1 (Fig. 10A; for photographs of the injection site see Fig. 4 in Cenquizca and Swanson, 2006). The rather large injection site lies in the ventral tip of field CA1 and whereas the edges of the injection site spread minimally to involve immediately adjacent regions of the amygdala and field CA3, all PHAL-labeled neurons detected with the present methods are restricted to field CA1.
Fig. 10.
Projections from ventral field CA1 to caudal regions of the cerebral cortex, from Experiment HIPPO161 and plotted as described for Figure 4.
2.3.1 Intrahippocampal projections
At the injection site level, PHAL-labeled axons innervate adjacent regions of ventral field CA1. Such fibers are observed in the stratum oriens and stratum radiatum, and in the pyramidal cell layer, and they extend a short distance along the longitudinal axis of field CA1. In addition, sparse fibers innervate ventral field CA3 (stratum oriens and stratum radiatum) along the border with stratum lucidum (Fig. 10A). A few isolated axons also cross the hippocampal fissure to end in the molecular layer of the dentate gyrus.
Abundant labeled axons enter the alveus and then extend caudally toward the subiculum where they generate a dense plexus in the ventral subiculum (Fig. 1)—across the entire thickness of the pyramidal layer and adjacent deep zone of the molecular layer, with a few axons extending through the most superficial zone of the molecular layer (Figs. 5C and 10B). Interestingly, whereas the terminal field in ventral subiculum is easily the most dense of any generated by ventral field CA1, it is clearly less dense than inputs from dorsal field CA1 to dorsal subiculum. This difference is not correlated with injection site size. For example, the injection site described here for ventral field CA1 (Experiment HIPPO161) is considerably larger than the one used (Experiment HIPPO4) to describe dorsal field CA1 projections (see Fig. 1).
From the subiculum, labeled axons extend medially across the angular bundle to innervate densely ventral (longitudinally) and medial (transversely) regions of the medial entorhinal area—in a terminal field that spans all but layer 1 of this cortical area. Medial regions of the lateral entorhinal area are also densely innervated (Fig. 10B-D), following the general topographic organization displayed by more dorsal regions of field CA1 (Fig. 1). Interestingly, this projection is distributed heavily through all but layer 1, and is most concentrated in layer 5. Caudally, terminal fibers generate a dense band across layers 4 and 5 in medial regions of the lateral entorhinal area (Figs. 10D and 11B), whereas the input is moderate in layer 2, and sparse in layers 3 and 6. A light projection is also observed in layer 1 of the medial entorhinal area at this level, and some fibers extend rostrally in the lateral entorhinal area to innervate its most rostral tip. A dense cluster of fibers is concentrated in layer 1 of the parasubiculum in a majority (e.g., HIPPO103) (but not all, e.g., HIPPO161) of experiments involving ventral field CA1 injections, and all such injections label a moderate number of fibers in layers 4 and 5 of the parasubiculum, and scattered fibers in layers 2 and 4/5 of limited regions of the presubiculum (Fig. 10D).
Fig. 11.
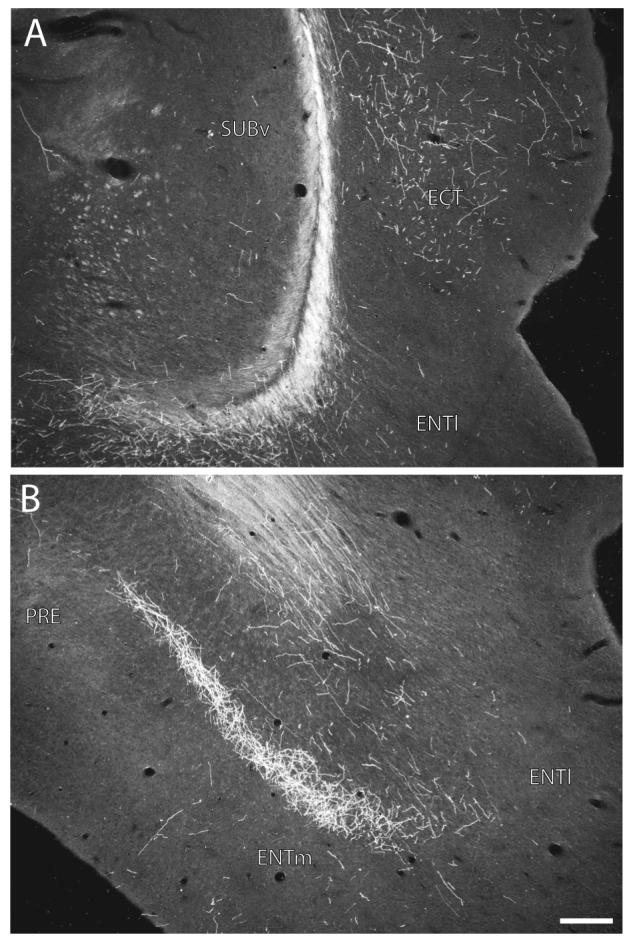
Darkfield photomicrographs showing the distribution of PHAL-labeled axons within the ectorhinal area of the ventral temporal region (A, about level C in Fig. 10) and the entorhinal area of the hippocampal formation (B, about level D in Fig. 10) following an injection centered in the ventral tip of field CA1 (Experiment HIPPO161, see Figs. 1 and 10A). Scale = 200 μm.
2.3.2 Rostrally directed projections through the precommissural fornix
Labeled ventral field CA1 axons projecting to frontal regions of the cerebral cortex extend rostrally through the lateral septal nucleus in the precommissural fornix, during the course of their subcortical detour. On leaving the lateral septal nucleus they first generate an input to the dorsal tenia tecta, which is relatively dense in layer 2 and moderately dense in the others (Figs. 2 and 12A). In the immediate vicinity, a relatively very dense terminal field is generated in the infralimbic area of the medial prefrontal region (Fig. 12A,B). It ramifies across layers 2 through 6 but appears most dense in layer 2. Ventral levels of field CA1, like intermediate levels, project selectively to dorsal regions of the infralimbic area.
Fig. 12.
Projections from ventral field CA1 to cortical regions associated with the piriform area and prefrontal and insular regions, from Experiment HIPPO161 and plotted as described for Figure 4; arranged from rostral (A) to caudal (I).
A dense component of this terminal field spreads into deep layers of the dorsally adjacent prelimbic area, and then some labeled axons bend dorsally around the genu of the corpus callosum to innervate the anterior cingulate area (Fig. 12B-E). A moderate field of terminal boutons is centered in deep layers of both the dorsal and ventral parts of the anterior cingulate area, with a light field of terminal boutons in superficial layers of the ventral part. This fiber system extends caudally as it generates a progressively lighter input. The projection to the dorsal part rapidly decreases in magnitude at the level of the lateral septal nucleus, whereas the projection to the ventral part ends with a light input to deep layers at about the level of the crossing of the anterior commissure. In addition, a moderately dense projection extends from the prelimbic area, and agranular insular area (see below), into ventral and deep layers of the ventrolateral orbital area. A few labeled axons generate a light input to deep layers of the medial orbital area as well (Fig. 12C).
Labeled fibers can be followed ventrally from the region of the dorsal tenia tecta to terminal fields in the anterior olfactory nucleus and ventral tenia tecta. However, they will be described more fully below because they may arrive by way of the longitudinal association bundle and/or precommissural fornix, whereas other olfactory related areas almost certainly receive their predominant input via the longitudinal association bundle. As with PHAL injections centered in intermediate field CA1 levels, there is a light input to the contralateral dorsal tenia tecta, and in experiments with more ventral injections, scattered fibers are also found in the contralateral anterior olfactory nucleus (medial part) and ventral tenia tecta. These contralateral terminals appear to arise from axons that cross the midline in the ventral hippocampal commissure and then course rostrally through the contralateral precommissural fornix in the lateral septal nucleus.
2.3.3. Ventrally directed projections continuing rostrally and dorsally through the longitudinal association bundle
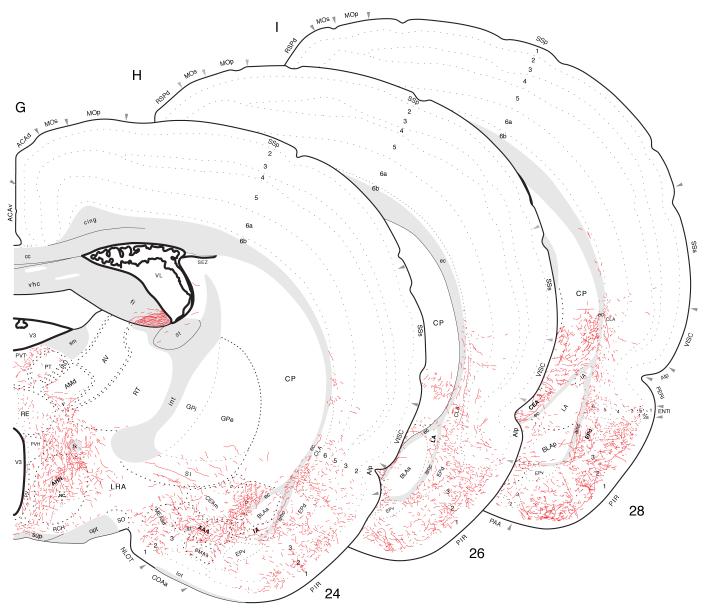
In essence, this is a diffusely organized projection that extends rostrally from the entorhinal area all the way to the olfactory bulb, with dorsal offshoots to the occipital, temporal, parietal, insular, and prefrontal regions (Fig. 2). PHAL-labeled axons appear to course from the injection site in ventral field CA1 through the entorhinal area, and perhaps directly through the fiber tracts and obliterated ventricle separating ventral field CA1 and the caudal amygdalar region (Fig. 10A,B) to generate a dense input to layers 2 and 3 of the posteromedial cortical amygdalar nucleus, a light to moderate input to layers 2 and 3 of the posterolateral cortical nucleus, and a dense input to layers 2 and 3 of the postpiriform transition area (Figs. 10A and 13B).
Fig. 13.
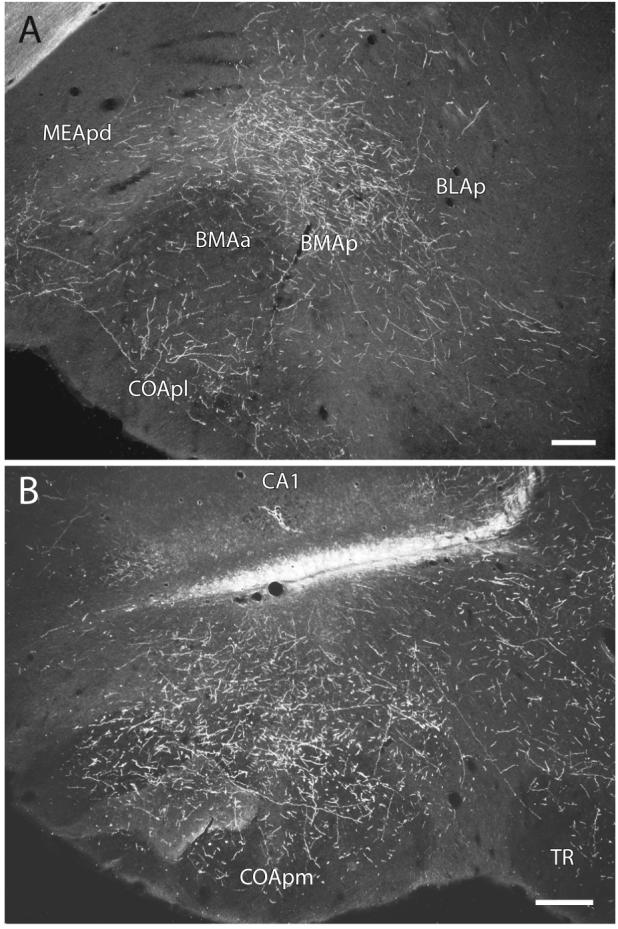
Darkfield photomicrographs showing the distribution of PHAL-labeled axons within the posterior basomedial amygdalar nucleus (A) and the posteromedial cortical amygdalar nucleus (B) following an injection centered in ventral field CA1 (Experiment HIPPO161). Scale = 200 μm.
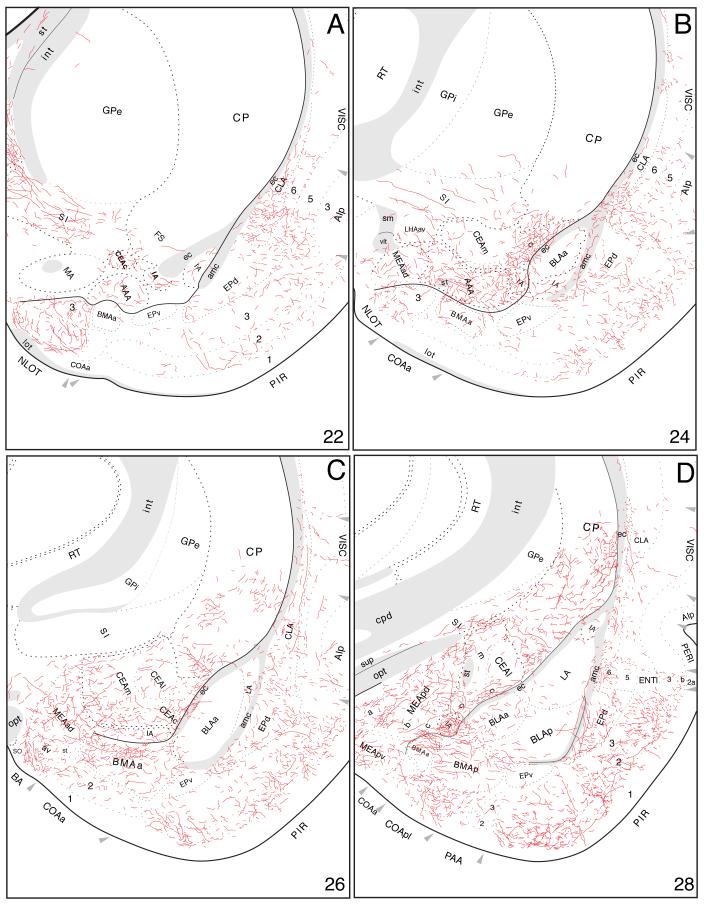
Labeled fibers then extend rostrally to innervate densely the posterior basomedial amygdalar nucleus (Figs. 13A and 14D). Caudomedial regions of the anterior basomedial amygdalar nucleus are also densely innervated, whereas inputs to more rostrolateral regions are relatively light (Fig. 14A-C). Furthermore, medial regions of the anterior basolateral nucleus, and ventrolateral regions of the lateral amygdalar nucleus, are moderately innervated, while the posterior basolateral nucleus is only very lightly innervated (Fig. 14).
Fig. 14.
Projections from ventral field CA1 to the amygdalar region, with an emphasis here on those to the cortical “nuclei” and basolateral complex. The results of Experiment HIPPO161 are plotted here, as described for Figure 4.
The rostral extreme of the amygdalar projection is formed by a curious, circumscribed terminal field in layer 2 of the nucleus of the lateral olfactory tract (Fig. 14A,B). A few labeled axons from ventral field CA1 are observed in the bed nucleus of the accessory olfactory tract, layer 2 of the anterior cortical nucleus, layer 2 of the piriform-amygdalar area, the posterior nucleus, and the ventral endopiriform nucleus (Figs. 12G-I and 14). Projections to the “striatal division” of the amygdalar region (Swanson and Petrovich, 1997), including the central, medial, and intercalated nuclei, and anterior amygdalar area, will be described in the next paper in the series, on inputs to the cerebral nuclei.
Other labeled axons from ventral field CA1 injection sites leave the entorhinal area in a more dorsal orientation to enter and innervate the adjacent perirhinal, ectorhinal, and temporal association areas. They provide a moderate input to layers 5 and 6 of the perirhinal area along its entire rostrocaudal extent (Figs. 12A,B and 14D). Deep layers of the ectorhinal area are also innervated densely, with a light input to layer 2 (Figs. 10A-E and 11A). This projection diminishes caudally with a few axons observed in the same layers and is extended by a moderate input to layers 5 and 6, and a light input to layers 2-4, of ventral temporal association areas (TEa, Fig. 10B).
From the temporal association areas some labeled axons radiate even farther dorsally (Fig. 2). First, there is a moderately dense input to the posterior auditory area (Fig. 10B), and light inputs to deep layers of the primary, dorsal, and ventral auditory areas (Fig. 10A,B). Second, labeled axons continue extending dorsally through deep cortical layers to innervate layer 6 of the posterior association areas of the parietal region (PTLp, Fig. 10A-C). Third, there is a sparse innervation of the primary, laterolateral, posterolateral, and posteromedial visual areas (Fig. 10B-E). And finally, several labeled axons end in layer 6 of the dorsal retrosplenial area (Fig. 10E,F).
From the amygdalar region and overlying temporal association areas, axons from ventral field CA1 extend rostrally in the piriform area and endopiriform nucleus, as well as in the overlying insular region and claustrum. The entire rostrocaudal extent of the piriform area maintains a projection from ventral field CA1, predominantly in layer 2 (Figs. 12 and 14). The projection is dense near the amygdalar region and progressively weakens to a light terminal field rostrally, adjacent to the anterior olfactory nucleus. In parallel with this, the dorsal endopiriform nucleus, sometimes regarded as the deepest layer of the piriform area, is moderately densely innervated at the level of the amygdalar region, and this becomes progressively sparser at more rostral levels.
Caudal regions of the claustrum at the level of the central amygdalar nucleus are moderately to densely innervated, and this terminal field becomes progressively weaker as far as its rostral tip (Fig. 12A-I). In concert, from caudal to rostral, overlying cortical regions are innervated. They include light inputs to deeper layers of the primary visceral and gustatory (caudally) areas, and the supplemental somatosensory area; and moderately dense inputs to the posterior, dorsal, and ventral parts of the agranular insular area (Fig. 12).
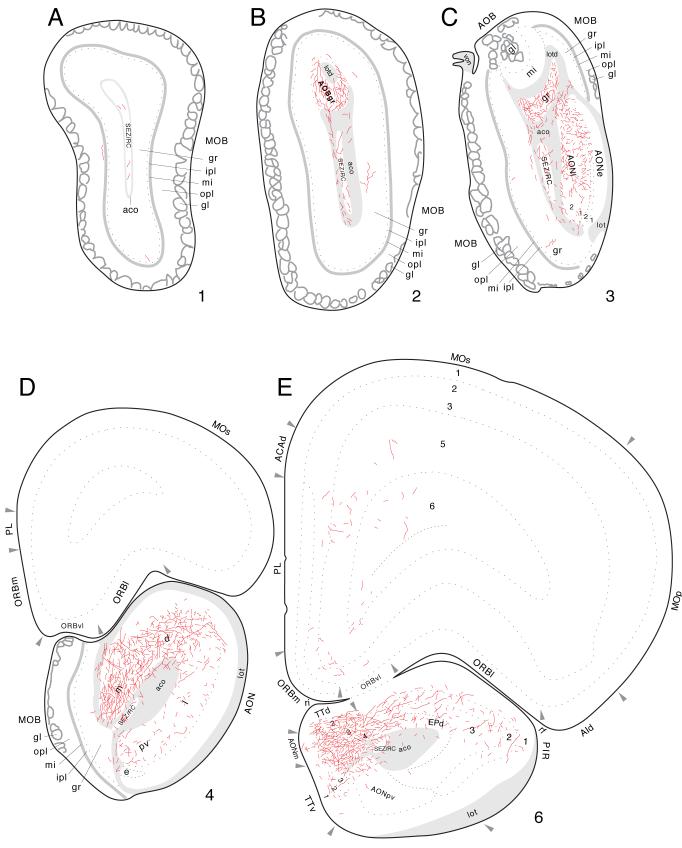
Farther rostrally, the lateral and posteroventral parts of the anterior olfactory nucleus present dispersed labeled axons with terminal boutons, whereas very dense terminal fields are centered in the medial and dorsal parts on this “nucleus”, and more caudally the lateral and posteroventral parts contain dispersed fibers (Figs. 12A, 15D-E, and 16A). At this level, a few labeled axons are also observed in the ventral tenia tecta (Figs. 12A and 15E). In the rostral tip of the ipsilateral anterior olfactory nucleus, a moderately dense terminal field is restricted to the lateral part, with the external part remaining devoid of labeling (Figs. 15C and 16B). Within the olfactory bulb, a dense terminal field is restricted to the granular layer of the accessory bulb (Figs. 15B,C and 16B); scattered fibers are observed in the main olfactory bulb’s granule cell layer (Fig. 15A-D).
Fig. 15.
Projections from ventral field CA1 to regions associated with the olfactory bulb and peduncle, from Experiment HIPPO161 and plotted as described for Figure 4; arranged from rostral (A) to caudal (E).
Fig. 16.
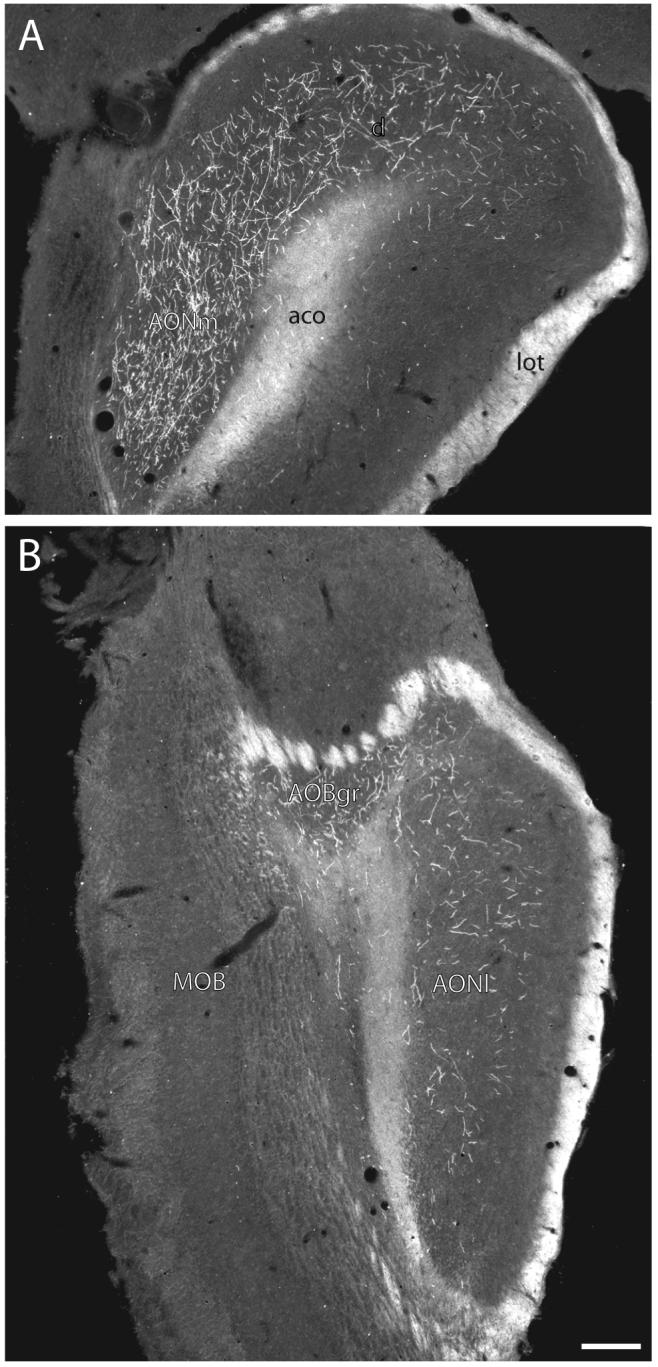
Darkfield photomicrographs showing the distribution of PHAL-labeled axons in the anterior olfactory nucleus (A, about level D in Fig. 15) and accessory olfactory bulb (B, about level C in Fig. 15) following an injection centered in ventral field CA1 (Experiment HIPPO161). Scale = 200 μm.
It is important to reiterate that field CA1 projections through the longitudinal association bundle and precommissural fornix (described above) appear to merge rostrally (Fig. 2). Thus, the method applied here cannot determine unambiguously whether terminal fields in the rostral ends of the piriform area, endopiriform nucleus, agranular insular area, and claustrum—as well as in the ventral tenia tecta, orbital area, and parts of the anterior olfactory nucleus—arrive by one or the other, or both, pathways.
For the sake of completeness, we record that a considerable number of labeled axons from ventral field CA1 are scattered in and around the length of the lateral ventricle’s subependymal zone (Figs. 12 and 15).
3. DISCUSSION
The results provide a detailed reanalysis of extrinsic axonal projections from hippocampal field CA1 to the rest of the cortical mantle in the rat, pathways that appear to arise mainly from pyramidal neurons whose axon descends into the alveus before bifurcating into one branch extending initially into the subiculum of the retrohippocampal region, and another coursing rostrally into the fornix system (Cajal 1909-1911; Lorente de Nó, 1934; Swanson et al., 1981; Altemus et al., 2005). Overall, two features of field CA1 projections are especially relevant in this context. First, precise target areas and innervation density are clearly a function of spatial position (transverse level) along field CA1’s longitudinal axis, as suggested some time ago (Swanson and Cowan, 1977b). And second, the rostrally directed branches of field CA1 pyramidal neurons innervate certain components of the prefrontal and adjacent olfactory cortical regions by taking an unusual cortical-subcortical-cortical route through the precommissural fornix, innervating especially the subcortical lateral septal complex along the way. In contrast, the caudally directed fibers innervate first various cortical areas of the hippocampal formation’s retrohippocampal region. Then axons from the dorsal third of field CA1 course dorsally and rostrally to innervate the retrosplenial area, whereas axons from the ventral two-thirds of field CA1 course ventrally and rostrally through the longitudinal association bundle as far as the olfactory bulb, with offshoots to widespread areas of the temporal, occipital, parietal, insular, and prefrontal regions (Fig. 2).
Considering how extensively differentiated field CA1 cortico-cortical projection patterns now appear to be—involving over 50 areas and recognized areal subdivisions—it is impractical here to discuss the functional significance of our results in anything but general terms. Instead, attention focuses on a comparison with previous anatomical results and on broad functional implications. For perspective, no extrahippocampal formation cortical projections of field CA1 at all had been established as recently as 30 years ago (Swanson and Cowan, 1977b), and the projection pattern reported here is not necessarily surprising for the hippocampal formation: the entorhinal area—the start of the intrahippocampal trisynaptic circuit—projects to the entire cortical mantle, as demonstrated over 20 years ago with PHAL (Swanson and Köhler, 1986). Thus, the cortical projections of field CA1 are a (substantial) subset of those arising in the entorhinal area.
3.1 Intrahippocampal circuitry
Field CA1 projections are often viewed from the narrow perspective of the excitatory trisynaptic or intrahippocampal circuit, which is organized qualitatively along the hippocampal formation’s transverse axis—with an input to field CA1 from field CA3 Schaffer collaterals and a “return” pathway from field CA1 to the entorhinal area that “closes” the circuit (Steward and Scoville, 1976; Amaral and Witter, 1995; Calderazzo et al., 1996; Naber et al., 2001; Seress et al., 2002; van Haeften et al., 2003). However, the present results reemphasize that field CA1’s projection to adjacent subiculum is much more dense (Hjorth-Simonsen 1973; Swanson et al., 1978) than that to entorhinal area, and in fact is by far the most dense terminal field generated by field CA1. It is generally agreed that the field CA1-subiculum projection is lamellar or transversely organized (Hjorth-Simonsen 1973; Swanson et al., 1978; van Groen and Wyss, 1990a); our results indicate that it is more divergent (the subicular terminal field extends significantly farther longitudinally than the field CA1 injection site) than previously elucidated (Fig. 1).
The overall transverse organization of field CA1-entorhinal projections is also known (Beckstead, 1978; Swanson et al., 1978; van Groen and Wyss, 1990a; Amaral and Witter, 1995), and our results provide further refinements (Fig. 1). The dorsal end of field CA1 projects most densely to lateral regions of the lateral and medial entorhinal areas (with the dorsal tip innervating a narrow strip across the topologically dorsal end of the entorhinal area), whereas progressively more ventral regions innervate progressively more medial regions of the lateral and medial entorhinal areas—with the medial entorhinal terminal field becoming progressively more dense from dorsal to ventral along the longitudinal axis. The field CA1-entorhinal projection thus appears to display an inverted V-shape when viewed on a hippocampal formation flatmap (Fig. 1). The qualitatively transverse arrangement of the essential intrahippocampal circuit is strengthened by recent experimental evidence that projections from entorhinal area to field CA1 and subiculum also fit the pattern (Naber et al. 2001).
It is established that the field CA1-entorhinal projection ends in layers 3-5 (see Swanson et al., 1978; van Groen and Wyss, 1990a; Amaral and Witter, 1995). Our PHAL material displayed a dense terminal field in layers 4 and 5 of the entorhinal area, along with light projections to layers 2 and 6 after injections in dorsal levels of field CA1, and moderate projections to layers 2, 3, and 6 from more ventral levels of field CA1.
Suggestive evidence for light projections from dorsal field CA1 to the pre- and parasubiculum was adduced with the autoradiographic method (Swanson and Cowan, 1977b), and earlier PHAL material suggested a projection from ventral field CA1 to the parasubiculum (van Groen and Wyss, 1990a). The present results indicate that all levels of field CA1 project rather sparsely to the presubiculum, and somewhat more heavily to the parasubiculum, with a prominent input to layer 1 of the latter from roughly the ventral two-thirds of field CA1. A substantial projection from the dorsal half of field CA1 to the postsubiculum (van Groen and Wyss, 1990a) was not labeled here. Either this projection arises predominantly in the subiculum (van Groen, 1990a) or from a very limited region of field CA1 not included in our material.
Major intrahippocampal circuit components are unidirectional and transversely organized. However, previous work identified a relatively light, “reverse” GABAergic projection from field CA1 nonpyramidal neurons to the dentate gyrus molecular layer (Sik et al., 1994; Adams et al., 2001; Naber et al., 2001). It is confirmed here with PHAL, along with a light input to the polymorph layer from dorsal field CA1. Furthermore, at least some hippocampal GABAergic neurons project rather far along the transverse and apparently the longitudinal axes, and even to extrahippocampal targets ((Acsady et al., 1996; Freund and Buzsaki, 1996; Katona et al., 1999; Acsady et al., 2000; Gulyas et al., 2003). Long projections along the longitudinal axis of field CA1 were not detected here with the PHAL method, as plotted and interpreted on a flatmap. In contrast, though, a dense halo of terminal labeling surrounding in all directions (transverse and longitudinal) each field CA1 PHAL injection site was obvious, confirming the results of older Golgi (Cajal, 1909-1911, Lorente de Nó, 1934), and more recent intracellular filling (see Knowles & Schwartzkroin ’81; Finch & Babb ’81; Altemus et al., 2005), studies demonstrating rich local axon collateral ramifications from field CA1 pyramidal neurons.
It seems reasonable to begin any consideration of the functional significance of field CA1 axonal projections with the exceptionally massive, topographically ordered projection to the adjacent cortical field, the subiculum. Outside the hippocampal formation, the two best established subicular projections are to the lateral septal complex and hypothalamus, both of which are topographically organized, although there are also significant inputs to the prefrontal and retrosplenial cortical regions, nucleus accumbens, and amygdalar region (see Swanson and Cowan, 1975, 1977b; Meibach and Siegel, 1977; Canteras and Swanson, 1992; Kishi et al., 2000). Furthermore, the lateral septal complex establishes massive bidirectional connections with hypothalamus (see Risold and Swanson, 1996, 1997)—and prefrontal (see Brittain, 1989, Sesack et al., 1989; Hurley et al., 1991; Risold et al. 1997; Petrovich et al., 2001) and amygdalar (see Petrovich et al., 2001) regions both project massively to hypothalamus as well. Thus, neural activity at different levels along the intrahippocampal circuit longitudinal axis differentially influence hypothalamic neural mechanisms via direct and indirect descending projections of field CA1 and the subiculum—projections which, of course, are also influenced by massive projections from field CA3 to field CA1 and to lateral septal complex (see Swanson et al., 1987).
3.2. Dorsal and rostral projection to the retrosplenial area
Dorsal field CA1 generates a moderately dense projection to the molecular layer of the entire rostrocaudal length of the retrosplenial area’s dorsal and ventral parts, a projection that spills over lightly into the caudal end of the anterior cingulate area. At least part of this projection has been reported before in the rat (van Groen and Wyss, 1990a; Wyss and van Groen, 1992), and it is even stronger from the adjacent subiculum in the rat (Vogt and Miller, 1983, van Groen and Wyss, 1992; Wyss and van Groen, 1992) and monkey (Kobayashi and Amaral, 2003). Swanson and Cowan (1977b) reported a similar projection from field CA3, which has not yet been confirmed, and van Groen and Wyss (1990a) showed with retrograde tracers that it arises at least partly in field CA1. The cingulate region’s retrosplenial area has rather widespread projections, but of particular interest here are substantial cortical inputs to anterior cingulate area in rat (Vogt and Miller, 1983; van Groen and Wyss, 1992, 2003; Wyss and van Groen, 1992; Conde et al., 1995; Jones et al., 2005) and monkey (see Parvizi et al., 2006); orbitofrontal regions in rat (van Groen and Wyss, 1992) and monkey (see Morris et al., 1999); occipital visual areas in rat (see Vogt and Miller, 1983; Wyss and van Groen, 1992 review); and back to hippocampal formation in rat (Vogt and Miller, 1983; Wyss and van Groen, 1992; van Groen and Wyss, 2003) and monkey (Morris et al., 1999; Parvizi et al., 2006), along with the perirhinal area (see Wyss and van Groen, 1992; van Groen and Wyss, 2003).
The retrosplenial area’s functional correlates appear to be very complex. Recent evidence indicates that it supports environmental exploration (spatial navigation) in rat (see Harker and Whishaw, 2004) and human (see Spiers and Maguire, 2006). In rat, the dorsal hippocampus is involved preferentially in spatial memory (see Bannerman et al., 2004), making direct projections to retrosplenial area from just the dorsal third of field CA1 particularly interesting. In humans, the retrosplenial area is also implicated in associating emotional content with autobiographical memory (see Steinvorth et al., 2005; Svoboda et al., 2006), particular words (see Maddock, 1999; Maddock et al., 2003), or happy events (Damasio et al., 2000). A broader region of human posteromedial cortex including retrosplenial area is even implicated in self-reflection with emotional content (Raichle and Gusnard, 2005; Vogt and Laureys, 2005).
3.3. Rostral projection through the fornix system
A clear projection from the ventral half of field CA1 to the prefrontal region—including the infralimbic and prelimbic areas—that is even more substantial from the adjacent ventral subiculum—has been known for some time (Swanson, 1981; van Groen and Wyss, 1990b; Verwer et al., 1997; Chiba, 2000; Thierry et al., 2000), and the total projection pattern has been greatly clarified. It is now known that the entire longitudinal extent of field CA1 participates in this unusual cortical-subcortical-cortical projection, which increases progressively in strength from dorsal to ventral, as displayed especially clearly for dorsal tenia tecta (van Groen and Wyss, 1990b; present results). Intermediate levels of field CA1 also project moderately to the infralimbic area, lightly to the prelimbic area, and very lightly to the anterior cingulate area—and this pattern is denser yet from ventral field CA1 (Swanson, 1981; van Groen and Wyss, 1990b; Jay and Witter, 1991; Carr and Sesack, 1996; Verwer et al., 1997; Chiba, 2000; Thierry et al., 2000; Gabbott et al., 2002; present results). The infralimbic area (Brodmann’s area 25) occupies the ventromedial tip of the medial prefrontal region and has bidirectional axonal connections with the dorsal vagal complex and parabrachial nucleus (Ricardo and Koh, 1978; Saper and Loewy, 1980; van der Kooy et. al., 1984; Moga et al., 1990; Saper 2002), as well as massive projections to the hypothalamus (see Brittain, 1989; Hurley et al., 1991).
These terminal fields arriving via the precommissural fornix from the ventral two-thirds of field CA1 are observed to extend ventrally as well, into the ventral tenia tecta, orbital area, and anterior olfactory nucleus. However, these sites may also to receive intermixing axonal inputs from the longitudinal association pathway described next.
3.4. Ventral and rostral projection through the longitudinal association bundle
This loosely organized axon system from the ventral two-thirds of field CA1 becomes progressively more substantial toward the ventral tip of field CA1 and for description can be viewed as having dorsal and ventral components—the former emerging from the lateral entorhinal area and entering the perirhinal area, and the latter coursing more ventrally, initially into the amygdalar region.
3.4.1. Inputs to temporal and parietal association areas
A rather vaguely defined projection from the dorsal half of field CA1 to the perirhinal area was described some time ago in rats (Swanson and Cowan, 1977b; van Groen and Wyss, 1990b). The present results indicate that it arises basically from the ventral two-thirds of field CA1 and becomes progressively stronger toward its ventral tip. This projection extends to include the ectorhinal, temporal association areas, and even part of the posterior parietal association areas in rat (Fig. 2). Except for that from field CA1 to the perirhinal area, these projections were described first in primates (Blatt and Rosene, 1998; Yukie, 2000; Insausti and Munoz, 2001; Zhong et al., 2005).
3.4.2. Inputs to visual and auditory areas
The present results indicate that roughly the ventral half of field CA1 projects lightly to the primary auditory and visual areas, as well as a number of unimodal auditory and visual association areas, as defined in rat. These projections, which extend dorsally from temporal association areas, have apparently not been reported before, although these areas are innervated by the entorhinal area in rat (Swanson and Köhler, 1986), and a projection to field CA1 from a visual area (dorsal TE) in the primate temporal lobe is known (Zhong and Rockland, 2004).
3.4.3. Inputs to the amygdalar region
Projections to parts of the amygdalar region hypothesized (see McDonald, 1992; Swanson and Petrovich, 1997; Swanson, 2000) to arise developmentally from the cortical plate and subplate (the cortical “nuclei” and basolateral complex, respectively) are analyzed here; those to regions hypothesized to arise from the ventricular ridges (medial, central, and intercalated nuclei, and anterior area) are dealt with in a companion paper. Generally, only ventral regions of field CA1 project directly to the amygdalar region, where the densest terminal fields are established in the posteromedial cortical and posterior basomedial nuclei (Fig. 14). The former receives a massive direct input from the accessory olfactory bulb whereas the latter receives a similar input from the main olfactory bulb (Scalia and Winans, 1975; Shipley et al., 1995).
Ventral field CA1 projections were reported to the posteromedial cortical nucleus (Ottersen, 1982; van Groen and Wyss, 1990a) and posterior basomedial nucleus (McDonald, 1998), as well as to certain other amygdalar parts dominated by olfactory inputs including the postpiriform transition area and posterior basolateral nucleus (Ottersen, 1982), and posterior nucleus (Canteras et al., 1992; McDonald, 1998). The anterior basomedial and posterior basolateral amygdalar nuclei receive ventral field CA1 axonal projections, project back to ventral field CA1 (Petrovich et al., 2001), and integrate olfactory and gustatory information (Shi and Cassell, 1998). These results suggest a role in feeding behavior, which has been confirmed for projections from posterior basolateral nucleus to lateral hypothalamic area (Petrovich et al., 2005).
Other olfactory related amygdalar parts identified here as receiving ventral field CA1 input include the rest of the cortical nucleus, piriform-amygdalar area, anterior basomedial nucleus, and nucleus of the lateral olfactory tract. While preparing this work for publication, an independent analysis of rat ventral field CA1 to amygdalar region projections appeared (Kishi et al., 2006). The results of both studies are in good agreement, although their analysis did not extend rostrally to include the level of the nucleus of the lateral olfactory tract.
Two amygdalar cell groups that are more related connectionally with fronto-parieto-insulo-temporal association areas than with the olfactory system, and that do not send descending projections to the bed nuclei of the stria terminalis and hypothalamus, also receive moderate inputs from ventral field CA1—the lateral (Ottersen, 1982; Kishi et al., 2006; present results) and anterior basolateral (Ottersen, 1982, van Groen and Wyss, 1990a; Kishi et al., 2006; present results) nuclei. Their role in conditioned emotional responses is currently under intense investigation (see McGaugh, 2004; Rodrigues et al., 2004; Fanselow and Poulos, 2005; Maren, 2005; Rattiner et al., 2005).
3.4.4. Other olfactory-related projections
Labeled axons from field CA1 preferentially enter the piriform area and its deep endopiriform nucleus rostral and lateral to the amygdalar region, whereas from the overlying perirhinal, ectorhinal, and other temporal association areas they preferentially extend rostrally into the insular region and its deep claustrum. A projection from ventral field CA1 to caudal regions of the piriform area (predominantly layer 2) and dorsal endopiriform nucleus was recently described (Kishi et al., 2006). Here these inputs are shown to extend in a progressively lighter way to the rostral ends of the two cell groups, that there is also a sparse input to the ventral endopiriform nucleus, and that intermediate field CA1 levels contribute lightly to this overall projection.
Ventral field CA1 is also known to innervate the medial and dorsal parts of the anterior olfactory nucleus (van Groen and Wyss, 1990a); here we show that it also innervates the lateral and posteroventral parts, and that intermediate levels of field CA1 contribute to the input. Finally, a ventral field CA1 to main olfactory bulb projection was demonstrated with anterograde and retrograde tracers (van Groen and Wyss, 1990a). The present experiments labeled scattered axons in the granule cell layer, and identified a relatively dense input to the granule cell layer of the accessory olfactory bulb as well. Taken together, our results indicate that field CA1 innervates directly all known cortical areas (Scalia and Winans, 1975; Shipley et al., 1995) receiving direct inputs from the main and accessory olfactory bulbs, as well as the bulbs themselves—which some regard as the primary main and accessory cortical areas (for example, Brodmann, 1909).
3.4.5. Inputs to somatosensory, gustatory, and visceral areas
Previously unreported light inputs were observed from ventral field CA1 to the supplemental somatosensory area, primary gustatory area (at least caudally), and primary visceral sensory-motor area (Figs. 2 and 12). These projections are most dense in layer 6 of all three cortical areas. Similarly, a projection from (ventral) field CA1 to the claustrum, deep to the insular region, does not appear to have been detected before.
3.4.6. Inputs to the prefrontal-orbital-agranular insular region
A previously unreported input to all three recognized parts of the rat agranular insular area from ventral field CA1 was observed here (Figs. 2 and 12). This relatively moderate innervation is densest in layer 6 and then layer 5, and it appears to spread rostrally into deep layers of the orbital area, mostly from the ventral part of the agranular insular area to the ventrolateral and ventral parts of the orbital area, although the medial part of the orbital area receives a relatively sparse input as well. Innervated parts of the orbital area lie adjacent to the infralimbic and prelimbic areas of the medial prefrontal region, which, as discussed above, receive substantial inputs from field CA1 via the precommissural fornix. It is in a region including the orbital and infralimbic areas, and the tenia tecta and medial anterior olfactory nucleus, that rostrally directed axons from field CA1 traveling through the precommissural fornix and longitudinal association bundle converge and distribute differentially in a pattern awaiting experimental dissection.
A projection from field CA1 to the primate orbital area has been known for some time (Morecraft et al., 1992; Barbas and Blatt, 1995; Cavada et al., 2000; Zhong et al., 2006), and the overall projection to the prefrontal region described above has been shown in primates as well (Barbas and Blatt, 1995; Cavada et al., 2000; Insausti and Munoz, 2001; Zhong et al., 2006).
Like the retrosplenial area, the connections and functional relations of the prefrontal-orbital-agranular insular region are exceptionally complex. Broadly speaking, recent evidence suggests that it plays a key role in attention, response selection and decision making, working or episodic memory, and emotional processing, including aspects of reward and reinforcement and accompanying visceral sensory-motor control (see Bechara et al., 2000; Cavada et al., 2000; Sewards and Sewards, 2001; Wall and Messier, 2001; Saper, 2002; Dalley et al., 2004; Deco and Rolls, 2005; Kringelbach, 2005; Mayberg et al., 2005; Oya et al., 2005; Rolls, 2005). In rats, more dorsal parts of this region, in particular the anterior cingulate area, appear preferentially involved in memory for motor responses, which influences response selection, whereas more ventral parts may be involved preferentially in attention and emotional processing (see Fisk and Wyss, 1999; Heidbreder and Groenewegen, 2003; Dalley et al., 2004; Jones et al., 2005). Overall, the prefrontal-orbital-agranular insular region has widespread inputs from sensory and association cortical areas, as well as important descending projections to the ventral striatum and pallidum, hypothalamus, and periaqueductal gray (see An et al., 1998; McDonald, 1998; Freedman et al., 2000; Swanson, 2000; Floyd et al., 2001; Petrovich et al., 2001; Uylings and Groenewegen, 2003; Price, 2005).
3.5. Overview
When viewed on a flatmap, hippocampal field CA1 association projections innervate an unexpectedly extensive peripheral ring of cerebral cortical areas (Fig. 2). Topologically, the ring’s caudal end consists of strong intrahippocampal formation connections arranged perpendicular (transverse) to the entire longitudinal axis of field CA1 and the hippocampal formation as a whole. Three longer projections extend rostrally. The first is a moderately strong dorsal projection to the retrosplenial area of the cingulate region (gyrus in humans) from the dorsal third of field CA1. The second arises from the ventral two-thirds of field CA1 and is an extensive ventral projection through the longitudinal association bundle to visual, auditory, somatosensory, gustatory, olfactory, and visceral cortical areas, as well as to the basolateral amygdalar complex and agranular insular and orbital areas. And the third arises from the longitudinal extent of field CA1. It is a strong cortical-subcortical-cortical projection through the fornix system to the prefrontal, orbital, and olfactory areas that essentially lies rostrally, at or near the rostral ends of the dorsal and ventral projections—in essence forming the rostral end of the peripheral cortical ring.
As reviewed in the Discussion, the functional mechanisms subserved by this cortical ring are exceptionally complex although imaging studies in awake humans point to it involvement in the episodic memory—with associated emotions—that accompanies spatial navigation in foraging or exploring for specific goal objects (see Steinvorth et al., 2005; Spiers and Maguire, 2006; Svoboda et al., 2006). In any event, there is considerable experimental support in rats for differential functional localization along the longitudinal axis of field CA1 (see Bannerman et al., 2004; Pentkowski et al., 2006), and thalamocortical “feedback” projections of the medial hypothalamic control column for goal-oriented or motivated behaviors are confined to the peripheral cortical ring defined by association projections from hippocampal field CA1 (Swanson 2000, see his Fig. 33c).
4. MATERIALS AND METHODS
The materials and methods, brains, and data analysis strategy are the same as described in the first paper of this series (Cenquizca and Swanson, 2006). Experiments were performed according to the NIH Guidelines for the Care and Use of Laboratory Animals, and all protocols were approved by the University of Southern California Institutional Animal Care and Use Committee. The experiments described here were chosen from a collection of over 60 PHAL injections throughout field CA1, each in a different adult male Harlan Sprague-Dawley rat (250-325 g). The overall spatial distribution of each injection site is plotted onto an unfolded map of the hippocampal formation (Fig. 1). The “flatmap” represents the surface of a solid model of the hippocampal formation that was reconstructed from a series of transverse sections (Swanson et al., 1978; Petrovich et al., 2001). A plot of injection sites on the unfolded map allows rapid, qualitative analysis and comparison of their distribution. Parceling of the rat brain, terminology for describing morphologic features of PHAL-labeled axons, and mapping strategies and procedures follow Swanson (2004), unless indicated otherwise.
Acknowledgements
Grant sponsor: National Institutes of Health (NS16686 and MH12765).
ABBREVIATIONS
- AAA
anterior amygdalar area
- ab
angular bundle
- ACAd
anterior cingulate area, dorsal part
- ACAv
anterior cingulate area, ventral part
- ACB
nucleus accumbens
- aco
anterior commissure, olfactory limb
- act
anterior commissure, temporal limb
- AHN
anterior hypothalamic nucleus
- AId
agranular insular area, dorsal part
- AIp
agranular insular area, posterior part
- AIv
agranular insular area, ventral part
- alv
alveus
- amc
amygdalar capsule
- AMd
anteromedial nucleus thalamus, dorsal part
- AOB
accessory olfactory bulb
- AOBgl
accessory olfactory bulb, glomerular cell layer
- AOBgr
accessory olfactory bulb, granule cell layer
- AOBmi
accessory olfactory bulb, mitral cell layer
- AON
anterior olfactory nucleus
- AONd
anterior olfactory nucleus, dorsal part
- AONe
anterior olfactory nucleus, external part
- AONl
anterior olfactory nucleus, lateral part
- AONm
anterior olfactory nucleus, medial part
- AONpv
anterior olfactory nucleus, posteroventral part
- AUD
auditory areas
- AUDd
dorsal auditory areas
- AUDp
primary auditory areas
- AUDpo
posterior auditory area
- AUDv
ventral auditory areas
- AV
anteroventral nucleus thalamus
- AVP
anteroventral preoptic nucleus
- BA
bed nucleus accessory olfactory tract
- BLA
basolateral nucleus amygdala
- BLAa
basolateral nucleus amygdala, anterior part
- BLAp
basolateral nucleus amygdala, posterior part
- BMA
basomedial nucleus amygdala
- BMAa
basomedial nucleus amygdala, anterior part
- BMAp
basomedial nucleus amygdala, posterior part
- BST
bed nuclei of the stria terminalis
- CA1
field CA1, Ammon’s horn
- CA1slm
field CA1, stratum lacunosum-moleculare
- CA1so
field CA1, stratum oriens
- CA1spd
field CA1, pyramidal layer, deep
- CA1sps
field CA1, pyramidal layer, superficial
- CA1sr
field CA1, stratum radiatum
- CA2
field CA2, Ammon’s horn
- CA3
field CA3, Ammon’s horn
- CA3slm
field CA3, stratum lacunosum-moleculare
- CA3slu
field CA3, stratum lucidum
- CA3so
field CA3, stratum oriens
- CA3sp
field CA3, pyramidal layer
- CA3sr
field CA3, stratum radiatum
- cc
corpus callosum
- ccr
corpus callosum, rostrum
- ccs
corpus callosum, splenium
- CEAc
central nucleus amygdala, capsular part
- CEAl
central nucleus amygdala, lateral part
- CEAm
central nucleus amygdala, medial part
- chp
choroid plexus
- cing
cingulum bundle
- CLA
claustrum
- COA
cortical nucleus amygdala
- COAa
cortical nucleus amygdala, anterior part
- COApl
cortical nucleus amygdala, posterior part, lateral zone
- COApm
cortical nucleus amygdala, posterior part, medial zone
- CP
caudoputamen
- cpd
cerebral peduncle
- df
dorsal fornix
- DG
dentate gyrus
- DGcr
dentate gyrus, crest
- DGcr-mo
dentate gyrus, crest-molecular layer
- DGcr-sg
dentate gyrus, crest-granule cell layer
- DGlb
dentate gyrus, lateral blade
- DGlb-mo
dentate gyrus, lateral blade-molecular layer
- DGlb-po
dentate gyrus, lateral blade-polymorph layer
- DGlb-sg
dentate gyrus, lateral blade-granule cell layer
- DGmb
dentate gyrus, medial blade
- DGmb-mo
dentate gyrus, medial blade-molecular layer
- DGmb-po
dentate gyrus, medial blade-polymorph layer
- DGmb-sg
dentate gyrus, medial blade-granule cell layer
- dhc
dorsal hippocampal commissure
- ec
external capsule
- ECT
ectorhinal area
- ee
extreme capsule
- ENTl1-6
entorhinal area, lateral part, layers 1-6
- ENTm1-6
entorhinal area, medial part, layers 1-6
- ENTmv
entorhinal area, medial part, ventral zone
- EP
endopiriform nucleus
- EPd
endopiriform nucleus, dorsal part
- EPv
endopiriform nucleus, ventral part
- fa
corpus callosum, anterior forceps
- fi
fimbria
- FC
fasciola cinerea
- fp
corpus callosum, posterior forceps
- FS
fundus of the striatum
- fx
columns of the fornix
- fxpr
precommissural fornix
- GPe
globus pallidus, external segment
- GPi
globus pallidus, internal segment
- GU
gustatory area
- hf
hippocampal fissure
- IA
intercalated nuclei amygdala
- IAD
interanterodorsal nucleus
- IG
induseum griseum
- ILA
infralimbic cortical area
- int
internal capsule
- isl
islands of Calleja
- LA
lateral amygdalar nucleus
- LHA
lateral hypothalamic area
- LHAav
lateral hypothalamic area, anterior region, ventral zone
- lot
lateral olfactory tract
- lotd
lateral olfactory tract, dorsal limb
- LPO
lateral preoptic area
- LSc
lateral septal nucleus, caudal part
- LSr
lateral septal nucleus, rostral part
- MA
magnocellular preoptic nucleus
- MEAad,av
medial nucleus amygdala, anterodorsal, anteroventral parts
- MEApd-a
medial nucleus amygdala, posterodorsal part, sublayer a
- MEApd-b
medial nucleus amygdala, posterodorsal part, sublayer b
- MEApd-c
medial nucleus amygdala, posterodorsal part, sublayer c
- MEApv
medial nucleus amygdala, posteroventral part
- MEPO
median preoptic nucleus
- MOp
primary motor area
- MOs
secondary motor area
- MOB
main olfactory bulb
- MOBgl
main olfactory bulb, glomerular layer
- MOBgr
main olfactory bulb, granule cell layer
- MOBipl
main olfactory bulb, inner plexiform layer
- MOBmi
main olfactory bulb, mitral layer
- MOBopl
main olfactory bulb, outer plexiform layer
- MPO
medial preoptic area
- MS
medial septal nucleus
- NC
nucleus circularis
- NDB
nucleus of the diagonal band
- NLOT
nucleus of the lateral olfactory tract
- NLOT1
nucleus of the lateral olfactory tract, molecular layer
- NLOT2
nucleus of the lateral olfactory tract, pyramidal layer
- NLOT3
nucleus of the lateral olfactory tract, dorsal cap
- och
optic chiasm
- opt
optic tract
- ORBl
orbital area, lateral part
- ORBm
orbital area, medial part
- ORBv
orbital area, ventral part
- ORBvl
orbital area, ventrolateral part
- OT
olfactory tubercle
- OT1
olfactory tubercle, molecular layer
- OT2
olfactory tubercle, pyramidal layer
- OT3
olfactory tubercle, polymorph layer
- PA
posterior nucleus amygdala
- PAA
piriform-amygdalar area
- PAR
parasubiculum
- PERI
perirhinal area
- PIR
piriform area
- PIR1
piriform area, molecular layer
- PIR2
piriform area, pyramidal layer
- PIR3
piriform area, polymorph layer
- PL
prelimbic cortical area
- POST
postsubiculum
- PRE
presubiculum
- PS
parastrial nucleus
- PT
paratenial nucleus
- PTLp
parietal region, posterior association areas
- PV
periventricular nucleus hypothalamus
- PVH
paraventricular nucleus hypothalamus
- PVT
paraventricular nucleus thalamus
- RC
rhinocele
- RCH
retrochiasmatic area
- RE
nucleus reuniens
- rf
rhinal fissure
- ri
rhinal incisure
- RSPagl
retrosplenial area, lateral agranular part
- RSPd
retrosplenial area, dorsal part
- RSPv
retrosplenial area, ventral part
- RSPv-a
retrosplenial area, ventral part, zone a
- RSPv-b/c
retrosplenial area, ventral part, zone b/c
- RT
reticular nucleus thalamus
- SEZ
subependymal zone
- SH
septohippocampal nucleus
- SI
substantia innominata
- sm
stria medullaris
- SO
supraoptic nucleus
- SSp
primary somatosensory area
- SSs
supplemental somatosensory area
- st
stria terminalis
- SUB
subiculum
- SUBd
subiculum, dorsal part
- SUBv
subiculum, ventral part
- SUBv-m
subiculum, ventral part, molecular layer
- SUBv-sp
subiculum, ventral part, pyramidal layer
- SUBv-sr
subiculum, ventral part, stratum radiatum
- sup
supraoptic commissures
- TEa
temporal association areas
- TR
postpiriform transition area
- TTd1-4
tenia tecta, dorsal part, layers 1-4
- TTv1-3
tenia tecta, ventral part, layers 1-3
- V3
third ventricle
- vhc
ventral hippocampal commissure
- VISal
anterolateral visual area
- VISam
anteromedial visual area
- VISli
intermediolateral visual area
- VISll
laterolateral visual area
- VISlla
anterior laterolateral visual area
- VISlm
mediolateral visual area
- VISp
primary visual area
- VISpl
posterolateral visual area
- VISpm
posteromedial visual area
- VISC
visceral area
- VL
lateral ventricle
- vlt
ventrolateral hypothalamic tract
- von
vomeronasal nerve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acsady L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neurosci. 1996;73:299–315. doi: 10.1016/0306-4522(95)00610-9. [DOI] [PubMed] [Google Scholar]
- Acsady L, Pascual M, Rocamora N, Soriano E, Freund TF. Nerve growth factor but not neurotrophin-3 is synthesized by hippocampal GABAergic neurons that project to the medial septum. Neurosci. 2000;98:23–31. doi: 10.1016/s0306-4522(00)00091-9. [DOI] [PubMed] [Google Scholar]
- Adams CE, Stitzel JA, Collins AC, Freedman R. Alpha7-nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Res. 2001;922:180–190. doi: 10.1016/s0006-8993(01)03115-8. [DOI] [PubMed] [Google Scholar]
- Altemus KL, Lavenex P, Ishizuka N, Amaral DG. Morphological characteristics and electrophysiological properties of CA1 pyramidal neurons in macaque monkeys. Neurosci. 2005;136:741–756. doi: 10.1016/j.neuroscience.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. Academic Press; San Diego: 1995. pp. 443–493. [Google Scholar]
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Anderson P, Bliss TV, Skrede KK. Lamellar organization of hippocampal pathways. Exp Brain Res. 1971;13:571–591. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang W-N, Pothuizen HHJ, Feldon J. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beckstead RM. Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res. 1978;152:249–264. doi: 10.1016/0006-8993(78)90254-8. [DOI] [PubMed] [Google Scholar]
- Blackstad TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Rosene DL. Organization of direct hippocampal efferent projections to the cerebral cortex of the rhesus monkey: projections from CA1, prosubiculum, and subiculum to the temporal lobe. J Comp Neurol. 1998;392:92–114. doi: 10.1002/(sici)1096-9861(19980302)392:1<92::aid-cne7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Brittain DA. The efferent connections of the infralimbic area in the rat. Department of Neurosciences; University of California at San Diego: 1989. PhD thesis. [Google Scholar]
- Brodmann K. Vergleichende localisation der Grosshirnrinde in ihren Prinzipien dargestellt auf grund des zellenbauses. Leipzig, Barth. 1909 [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Cajal SR y. Histologie du système nerveux de l’homme & des vertébrés. Maloine; Madrid: 19091911. [Google Scholar]
- Calderazzo L, Cavalheiro EA, Macchi G, Molinari M, Bentivoglio M. Branched connections to the septum and to the entorhinal cortex from the hippocampus, amygdala, and diencephalon in the rat. Brain Res Bull. 1996;40:245–251. doi: 10.1016/0361-9230(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. The connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. An analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497:101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T. Collateral projection from the amygdalo--hippocampal transition area and CA1 to the hypothalamus and medial prefrontal cortex in the rat. Neurosci Res. 2000;38:373–383. doi: 10.1016/s0168-0102(00)00183-8. [DOI] [PubMed] [Google Scholar]
- Condé F, Maire-Lepoivre E, Audinat E, Crépel F. Afferent connections of the medial frontal cortex of the rat. II. cortical and subcortical afferents. J Comp Neurol. 1995;353:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Deco G, Rolls ET. Attention, short-term memory, and action selection: a unifying theory. Prog Neurobiol. 2005;76:236–256. doi: 10.1016/j.pneurobio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Duva CA, Floresco SB, Wunderlich GR, Lao TL, Pinel JP, Phillips AG. Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behav Neurosci. 1997;111:1184–1196. doi: 10.1037//0735-7044.111.6.1184. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Ann Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Finch DM, Babb TL. Demonstration of caudally directed hippocampal efferents in the rat by intracellular injection of horseradish peroxidase. Brain Res. 1981;214:405–410. doi: 10.1016/0006-8993(81)91203-8. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Wyss JM. Associational projections of the anterior midline cortex in the rat: intracingulate and retrosplenial connections. Brain Res. 1999;825:1–13. doi: 10.1016/s0006-8993(99)01182-8. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gabbott P, Headlam A, Busby S. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (areas 25/32) of the rat. Brain Res. 2002;946:314–322. doi: 10.1016/s0006-8993(02)02487-3. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, McNaughton BL. Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J Neurosci. 1996;16:8027–8040. doi: 10.1523/JNEUROSCI.16-24-08027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Hajos N, Katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. A reaffirmation of the retrosplenial contribution to rodent navigation: reviewing the influences of lesion, strain, and task. Neurosci Biobehav Rev. 2004;28:485–496. doi: 10.1016/j.neubiorev.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A. Some intrinsic connections of the hippocampus in the rat: an experimental analysis. J Comp Neurol. 1973;147:145–161. doi: 10.1002/cne.901470202. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Ino T, Kaneko T, Mizuno N. Projections from the hippocampal and parahippocampal regions to the entorhinal cortex. An anterograde and retrograde tract-tracing study in the cat. Neurosci Res. 2001;39:51–69. doi: 10.1016/s0168-0102(00)00199-1. [DOI] [PubMed] [Google Scholar]
- Ino T, Yasui Y, Itoh K, Nomura S, Akiguchi T, Kameyama M, Mizuno N. Direct projections from Ammon’s horn to the septum in the cat. Exp Brain Res. 1987;68:179–188. doi: 10.1007/BF00255243. [DOI] [PubMed] [Google Scholar]
- Insausti R, Munoz M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis) Eur J Neurosci. 2001;14:435–451. doi: 10.1046/j.0953-816x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neurosci. 2005;133:193–207. doi: 10.1016/j.neuroscience.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Katona I, Acsady L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neurosci. 1999;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Yokota S, Yasui Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol. 2006;496:349–368. doi: 10.1002/cne.20919. [DOI] [PubMed] [Google Scholar]
- Knowles WD, Schwartzkroin PA. Axonal ramifications of hippocampal Ca1 pyramidal cells. J Neurosci. 1981;1:1236–1241. doi: 10.1523/JNEUROSCI.01-11-01236.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lim C, Blume HW, Madsen JR, Saper CB. Connections of the hippocampal formation in humans: I. The mossy fiber pathway. J Comp Neurol. 1997a;385:325–351. doi: 10.1002/(sici)1096-9861(19970901)385:3<325::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lim C, Mufson EJ, Kordower JH, Blume HW, Madsen JR, Saper CB. Connections of the hippocampal formation in humans: II. The endfolial fiber pathway. J Comp Neurol. 1997b;385:352–371. [PubMed] [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;23:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 67–96. [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Ann Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977;124:197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999;11:2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. The primate temporal pole: its putative role in object recognition and memory. Behav Brain Res. 1996;77:53–77. doi: 10.1016/0166-4328(95)00227-8. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. An experimental study of the fornix system in the rat. J Comp Neurol. 1956;104:247–271. doi: 10.1002/cne.901040205. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford University Press; Oxford: 1978. [Google Scholar]
- Oya H, Adolphs R, Kawasaki H, Bechara A, Damasio A, Howard MA., 3rd. Electrophysiological correlates of reward prediction error recorded in the human prefrontal cortex. Proc Natl Acad Sci USA. 2005;102:8351–8356. doi: 10.1073/pnas.0500899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Parvizi J, van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. J Comp Neurol. 2005;493:132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA. Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol. 2005;493:167–176. doi: 10.1002/cne.20752. [DOI] [PubMed] [Google Scholar]
- Raisman G, Cowan WM, Powell TP. An experimental analysis of the efferent projection of the hippocampus. Brain. 1966;89:83–108. doi: 10.1093/brain/89.1.83. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Encapsulation of implicit and explicit memory in sequence learning. J Cogn Neurosci. 1998;10:248–263. doi: 10.1162/089892998562681. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Rodriques SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Memory systems in the brain. Ann Rev Psychol. 2000;51:599–630. doi: 10.1146/annurev.psych.51.1.599. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Emotion explained. Oxford University Press; New York: 2005. [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Ann Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1977;161:31–56. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Seress L, Abraham H, Lin H, Totterdell S. Nitric oxide-containing pyramidal neurons of the subiculum innervate the CA1 area. Exp Brain Res. 2002;147:38–44. doi: 10.1007/s00221-002-1242-2. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sewards TV, Sewards MA. Cortical association areas in the gustatory system. Neurosci Biobehav Rev. 2001;25:395–407. doi: 10.1016/s0149-7634(01)00021-5. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:469–491. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Shipley MT, McLean JH, Ennis M. Olfactory system. In: Paxinos G, editor. The rat nervous system. Academic Press; San Diego: 1995. pp. 899–926. [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsaki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Thoughts, behavior, and brain dynamics during navigation in the real world. Neuroimage. 2006;31:1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Corkin S, Halgren E. Ecphory of autobiographical memories: an fMRI study of recent and remote memory retrieval. Neuroimage. 2005;30:285–298. doi: 10.1016/j.neuroimage.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon’s horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. An atlas with printed and electronic templates for data, models, and schematics. 3rd edition Elsevier Academic Press; Amsterdam: 2004. Brain maps: structure of the rat brain. [Google Scholar]
- Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex not Ammon’s horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. Autoradiographic studies of the development and connections of the septal area in the rat. In: DeFrance JF, editor. The septal nuclei. Plenum Press; New York: 1977a. pp. 37–64. [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977b;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Köhler C. Anatomical evidence for direct projections from the entorhinal area to the entire cortical mantle in the rat. J Neurosci. 1986;6:3010–3023. doi: 10.1523/JNEUROSCI.06-10-03010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Köhler C, Björklund A. The limbic region. I: The septohippocampal system. In: Björklund A, Hökfelt T, Swanson LW, editors. Integrated systems of the CNS, Part I. Elsevier; Amsterdam: 1987. pp. 125–277. [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1997;28:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Cowan WM. Evidence for collateral projections by neurons in Ammon’s horn, the dentate gyrus, and the subiculum: a multiple retrograde labeling study in the rat. J Neurosci. 1981;1:548–559. doi: 10.1523/JNEUROSCI.01-05-00548.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Tamamaki M, Nojyo Y. Disposition of the slab-like modules formed by axon branches originating from single CA1 pyramidal neurons in the rat hippocampus. J Comp Neurol. 1990;291:509–519. doi: 10.1002/cne.902910403. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990a;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990b;518:227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315:200–206. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- van Haeften T, Baks-te-Bulte L, Goede PH, Wouterlood FG, Witter MP. Morphological and numerical analysis of synaptic interactions between neurons in deep and superficial layers of the entorhinal cortex of the rat. Hippocampus. 2003;13:943–952. doi: 10.1002/hipo.10144. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. The hippocampus as an olfacto-motor mechanism: were the classical anatomists right after all? Behav Brain Res. 2001;127:25–47. doi: 10.1016/s0166-4328(01)00354-0. [DOI] [PubMed] [Google Scholar]
- Verwer RW, Meijer RJ, Van Uum HF, Witter MP. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus. 1997;7:397–402. doi: 10.1002/(SICI)1098-1063(1997)7:4<397::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Vnek N, Rothblat LA. The hippocampus and long-term object memory in the rat. J Neurosci. 1996;16:2780–2787. doi: 10.1523/JNEUROSCI.16-08-02780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neuroal network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Miller MW. Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol. 1983;216:192–210. doi: 10.1002/cne.902160207. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. The hippocampal formation--orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res. 2001;127:99–117. doi: 10.1016/s0166-4328(01)00355-2. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. third edition Elsevier; Amsterdam: 2004. pp. 637–704. [Google Scholar]
- Wyss JM, van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus. 1992;2:1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- Yukie M. Connections between the medial temporal cortex and the CA1 subfield of the hippocampal formation in the Japanese monkey (Macaca fuscata) J Comp Neurol. 2000;423:282–298. doi: 10.1002/1096-9861(20000724)423:2<282::aid-cne7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Rockland KS. Connections between the anterior inferotemporal cortex (area TE) and CA1 of the hippocampus in monkey. Exp Brain Res. 2004;155:311–319. doi: 10.1007/s00221-003-1728-6. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Yukie M, Rockland KS. Direct projections from CA1 to the superior temporal sulcus in the monkey, revealed by single axon analysis. Brain Res. 2005;1035:211–214. doi: 10.1016/j.brainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Yukie M, Rockland KS. Distinctive morphology of hippocampal CA1 terminations in orbital and medial frontal cortex in macaque monkeys. Exp Brain Res. 2006;169:549–553. doi: 10.1007/s00221-005-0187-7. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Neuroanatomy of memory. Ann Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]