Abstract
We sought to determine whether patients with hematologic malignancies treated by nonmyeloablative hematopoietic cell transplantation (HCT) at a single institution between December 1997 and June 2006 had worse outcomes with grafts from unrelated donors (n=184) as compared to HLA-identical related donors (n=221). The nonmyeloablative preparative regimen consisted of 2 Gy total body irradiation with (78%) or without (22%) fludarabine, and postgrafting mycophenolate mofetil and cyclosporine. After adjusting for HCT-comorbidity index, relapse risk, patient age, stem cell source, preparative regimen, prior CMV infection and sex-mismatch of donor and recipient in multivariate analysis, we found no statistically significant differences between unrelated and related HCT recipients in risks of non-relapse mortality (hazard ratio [HR], 0.98; 95% confidence interval [CI], 0.6-1.6; p=0.94), relapse (HR, 1.04; 95% CI, 0.7-1.5; p=0.82), or overall mortality (HR, 0.99; 95% CI, 0.7-1.4; p=0.94). Overall rates of severe acute and extensive chronic GVHD were also not significantly different between the two groups. We conclude that within the limitations of a retrospective study, these results indicate that candidates for nonmyeloablative HCT without suitable related donors may expect similar outcomes with grafts from unrelated donors.
Introduction
Most patients with hematologic malignancies who might benefit from treatment by allogeneic HCT lack HLA-identical related donors [1]. For these patients, unrelated volunteers (URD) have been used as donors with increasing frequency. Historically, HCT from URDs after conditioning with myeloablative preparative regimens has been associated with increased risks of non-relapse mortality (NRM) and consequently decreased overall survival (OS) as compared to results with HLA-identical related donors (MRD) [2-6]. These differences have been attributed primarily to the greater degree of genetic disparity between unrelated donor/recipient pairs as compared to related pairs. With the advent of high-resolution HLA-typing and the resulting improvement in matching between unrelated donors and their recipients, outcomes following URD transplantation have improved [7-10]. This improvement has been ascribed to decreased rates of graft-versus-host disease (GVHD) and accelerated immune reconstitution [11]. Even with high-resolution HLA-typing, unrelated donor/recipient pairs may have greater disparity for numerous minor histocompatibility antigens as compared to related pairs, which may contribute to the persistently higher rates of GVHD and consequently NRM after HCT from URDs as compared to MRDs [12].
In nonmyeloablative HCT, graft-versus-tumor (GVT) effects have replaced high-dose cytotoxic therapy as the conceptual basis for treating underlying malignancies [13-17]. The use of potent pre- and postgrafting immunosuppression has allowed a major reduction in pretransplant cytotoxic therapy without compromising engraftment of hematopoietic donor cells. The use of nonmyeloablative conditioning avoids major regimen-related toxicities, making it possible to treat older and medically infirm patients who are at a high risk of complications after treatment with conventional transplant regimens [13,16,18,19]. The immunobiology of nonmyeloablative HCT differs from that of myeloablative HCT in several important aspects. Compared to myeloablative HCT, nonmyeloablative HCT is associated with (i) decreased release of inflammatory cytokines due to limited tissue damage during administration of the conditioning regimen [20-24], (ii) a transient and potentially tolerogenic state of mixed donor/host chimerism [25,26], and (iii) use of novel regimens for immunosuppression after HCT [13,14,27,28]. These differences might account for lower rates of severe GVHD after unrelated HCT with nonmyeloablative conditioning as compared to myeloablative conditioning [29-31].
In this retrospective study we asked whether outcomes among patients who had HCT with nonmyeloablative conditioning also differed according to donor type. After adjusting for factors known to influence outcome after allografting in multivariate analysis, we found that use of URDs did not appear to increase either non-relapse mortality or overall mortality after HCT with nonmyeloablative conditioning.
Patients and methods
Eligibility criteria
Patients who had nonmyeloablative HCT from HLA-identical siblings or unrelated donors for treatment of hematologic malignancies at the Fred Hutchinson Cancer Research Center between December 1997 and June 2006 were screened for study eligibility. Patients had signed forms approved by the Institutional Review Board documenting informed consent to participate in the clinical trials and to allow the use of protected health information for research. To be included in the analysis, all related donors were HLA-matched siblings by family study [8]. Sequence-specific oligonucleotide hybridization and/or sequencing-based typing methods were used to define exons 2 and 3 of HLA-A, B, and C alleles and exon 2 of DRB1 and DQB1 alleles in all donor-recipient pairs. Unrelated pairs were defined as matched if donors and recipients had identical probe hybridization patterns. The 82 DQB1*03 and 06-positive donor-recipient pairs with identical exon 2 oligonucleotide probe patterns potentially representing two different DQB1*0302, 0303, 0602, or 0603 alleles, one frequent and the other extremely rare, were considered matched. The analysis included 405 patients with follow-up as of January 2007. One hundred and eighty-four patients (45%) had unrelated donors and 221 (55%) had related donors.
Preparative regimens, sources of hematopoietic cells and supportive care
Patients received low-dose total body irradiation (TBI; 2 Gy) alone (22%) or in combination with fludarabine (30 mg/m2 body surface area/day, for 3 consecutive days) (78%) [13,27]. Most recipients (98%) were given G-CSF-mobilized peripheral blood mononuclear cells (G-PBMC); 2% were given bone marrow grafts. Antimicrobial and cytomegalovirus (CMV) prophylaxis, and blood product support were administered as described [30].
Prophylaxis against GVHD
Postgrafting immunosuppression included mycophenolate mofetil (MMF) and cyclosporine (CSP) as described previously [13,27]. Fifty (27%) unrelated recipients received MMF 15 mg/kg every 12 hours and 134 (73%) received MMF 15 mg/kg every 8 hours [32]. Grading and treatment of GVHD were done as previously described [30].
Categorization of patients according to their predicted risks of NRM and recurrent malignancy
Outcomes with unrelated versus related grafts were also compared in subgroups of patients according to their predicted risks of NRM and recurrent malignancy after HCT. Patients were grouped according to (i) HCT-Comorbidity Indices (CI) assigned at the time of transplantation (scores: “0”, “1-2”, and “≥ 3”), which serve as strong predictors for NRM [18,33,34], and (ii) their estimated rates of recurrent malignancy per year [35]. In brief, the risks of recurrent malignancy were categorized as follows: “Low” (relapse-rate per patient year, 0.0-0.25): chronic lymphocytic leukemia (CLL) in remission, multiple myeloma (MM) in remission, high-grade non-Hodgkin lymphoma (NHL) in remission, low-grade NHL or mantle cell lymphoma regardless of remission status, acute lymphoblastic leukemia (ALL) in first remission, Waldenström's macroglobulinemia and myelofibrosis. “Intermediate” (relapse-rate per patient year, 0.26-0.50): CLL and MM without remission, acute myeloid leukemia (AML) in remission, chronic myeloid leukemia (CML) in first chronic phase, and early-stage myelodysplastic syndromes (MDS). “High” (relapse-rate per patient year, >0.50): High-grade NHL without remission, Hodgkin disease, ALL beyond first remission, AML without remission, CML beyond first chronic phase, and secondary or advanced MDS.
Statistical analysis
Survival curves were estimated by the Kaplan-Meier method. Cumulative incidence curves were estimated according to methods previously described [36]. Hazard ratios for each endpoint were estimated from Cox regression models, with NRM and relapse treated as competing risks for analysis of these endpoints. Multivariate analyses were adjusted for relapse risk category, HCT-CI, patient age, patient/donor sex-mismatch and prior CMV infection, and stem cell source. All patients with unrelated donors received a conditioning regimen of fludarabine and low-dose TBI, whereas some patients with related donors received low-dose TBI alone. Therefore, analyses were also adjusted for the absence of fludarabine in the conditioning regimen. Modifying effects of comorbidity and relapse risk were evaluated via interaction terms for donor relation with HCT-CI (“0” versus “1-2” versus ≥ 3) or relapse risk (“low” versus “intermediate” versus “high”). All p-values are based on Wald statistics derived from hazard ratio analyses, and are 2-sided. Adjusted survival curves were estimated based on methods derived from Makuch et al. [37]. Briefly, the adjusted survival curve for the group of patients with unrelated donors represents a model-based estimate of survival for a group of patients having the baseline hazard function estimated for patients with unrelated donors, but with the covariate characteristics of the group of patients with related donors. These estimates were derived from Cox regression models stratified on donor relation, with other adjustment factors incorporated as covariates. Curves were estimated for each set of covariates from the related donor group, and then averaged to yield the adjusted survival curve.
Results
Patient characteristics
Details regarding patient characteristics are listed in Table 1. The median patient ages were 54.5 (range, 20-72) years for patients with related donors and 55.9 (range, 5-75) years for those with unrelated donors. The distributions across HCT-CI and relapse risk categories were similar in the two groups. Among URD transplants, 13% of donor/recipient pairs were mismatched for a single HLA-A, -B or −C allele. While all patients with unrelated donors were prepared with 2 Gy TBI and fludarabine, 40% of those with related donors were prepared with TBI alone. The median follow-up times among surviving patients transplanted from MRD and URD were 36.6 (range, 3.3-98.7) months and 28.1 (range, 2.6-71.8) months, respectively.
Table 1.
Characteristics of transplant patients
| MRD
(n=221) |
URD
(n=184) |
|||
|---|---|---|---|---|
| Patient age, years | ||||
| Median (range) | 54.5 (20.4-72.7) | 55.9 (5.1-74.6) | ||
| < 50, n (%) | 76 (34) | 60 (33) | ||
| ≥ 50, n (%) | 145 (66) | 124 (67) | ||
|
| ||||
| Diagnosis, n (%) | ||||
| ALL | 2 (1) | 13 (7) | ||
| AML | 29 (13) | 50 (27) | ||
| MDS | 26 (12) | 22 (12) | ||
| CML | 6 (3) | 11 (6) | ||
| MM | 61 (28) | 19 (10) | ||
| NHL | 46 (21) | 33 (18) | ||
| HD | 18 (8) | 16 (9) | ||
| CLL | 27 (12) | 17 (9) | ||
| Other | 6 (3) | 3 (2) | ||
|
| ||||
| Relapse risk category*, n (%) | ||||
| Low | 46 (21) | 42 (23) | ||
| Intermediate | 111 (50) | 83 (45) | ||
| High | 64 (29) | 59 (32) | ||
|
| ||||
| HCT-CI** scores, n (%) | ||||
| 0 | 65 (29) | 40 (22) | ||
| 1/2 | 69 (31) | 57 (31) | ||
| 3+ | 87 (39) | 87 (47) | ||
|
| ||||
| Prior HCT, n (%) | ||||
| Autologous | 39 (18) | 64 (35) | ||
| Allogeneic | 5 (2) | 5 (3) | ||
|
| ||||
| Preparative regimen, n (%) | ||||
| TBI 2 Gy | 89 (40) | 0 | ||
| TBI 2 Gy + FLU | 132 (60) | 184 (100) | ||
|
| ||||
| Stem cell source, n (%) | ||||
| PBSC | 221 (100) | 176 (96) | ||
| BM | 0 | 8 (4) | ||
|
| ||||
| Donor/ recipient single allele-mismatch at HLA-A, -B or -C, n (%) | ||||
| No | 221 (100) | 160 (87) | ||
| Yes | 0 | 24 (13) | ||
|
| ||||
| Donor/ recipient sex-mismatch, n (%) | ||||
| No | 105 (48) | 103 (56) | ||
| Yes | 116 (52) | 81 (44) | ||
|
| ||||
| Patient CMV-serostatus, n (%) | ||||
| Negative | 96 (43) | 84 (46) | ||
| Positive | 125 (57) | 100 (54) | ||
ALL denotes acute lymphocytic leukemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndromes; CML, chronic myeloid leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease; CLL, chronic lymphocytic leukemia; HCT, hematopoietic cell transplantation; CI, comorbidity-index; FLU, fludarabine; TBI, total body irradiation; PBSC, peripheral blood stem cells; BM, bone marrow; CMV, cytomegalovirus.
“Low-risk”: CLL in remission, MM in remission, high-grade NHL in remission, low-grade NHL or mantle cell lymphoma regardless of remission status, ALL in first remission, Waldenström's macroglobulinemia and myelofibrosis.
“Intermediate-risk”: CLL and MM without remission, AML in remission, CML in first chronic phase, early-stage MDS.
“High-risk”: High-grade NHL without remission, HD, ALL > first remission, AML without remission, CML > first chronic phase, secondary or advanced MDS [35].
HCT-CI: hematopoietic cell transplantation-specific comorbidity index [34].
HCT-CI and relapse risk categories
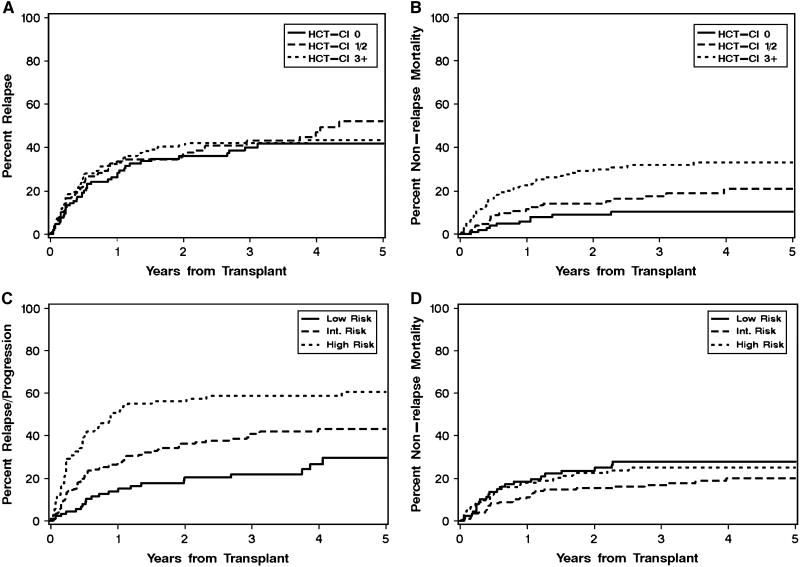
HCT-CI categories separated patients into groups with different risks of NRM (9%, 14% and 29% at 2 years for HCT-CI scores of “0”, “1-2” and “≥ 3”, respectively) while the risk of recurrent malignancy did not correlate with HCT-CI (36%, 38% and 41% at 2 years for “low”, “intermediate” and “high” risk, respectively) (Table 2; Figure 2 A and B). Conversely, the relapse risk categories separated patients into groups with different risks of recurrent malignancy (20%, 36% and 56% at 2 years for “low”, “intermediate” and “high” risk, respectively) while the risk of NRM did not correlate with these categories (24%, 15% and 23% at 2 years for HCT-CI scores of “0”, “1-2” and “≥ 3”, respectively) (Table 2; Figure 2 C and D). Therefore, these risk categorizations were applied in the analysis of outcomes with unrelated versus related HCT.
Table 2.
Multivariate analysis of transplant outcomes among 405 patients following nonmyeloablative HCT
| N | Overall mortality | Non-relapse mortality | Relapse / progression | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Donor: | |||||||
| MRD | 221 | 1.0 | 0.95 | 1.0 | 0.93 | 1.0 | 0.60 |
| URD | 184 | 1.01 (0.7-1.4) | 0.98 (0.6-1.6) | 1.10 (0.8-1.6) | |||
| Comorbidity category: [34] | |||||||
| HCT-CI 0 | 105 | 1.0 | <0.0001 | 1.0 | <0.0001 | 1.0 | 0.52 |
| HCT-CI 1-2 | 126 | 1.61 (1.0-2.5) | 1.89 (0.9-3.9) | 1.20 (0.8-1.8) | |||
| HCT-CI ≥3 | 174 | 2.65 (1.8-4.0) | 3.93 (2.0-7.7) | 1.25 (0.8-1.9) | |||
| Relapse risk category: [35] | |||||||
| Low | 88 | 1.0 | <0.0001 | 1.0 | 0.05 | 1.0 | <0.0001 |
| Intermediate | 194 | 1.31 (0.9-2.0) | 0.76 (0.4-1.3) | 1.82 (1.1-2.9) | |||
| High | 123 | 2.94 (1.9-4.5) | 1.46 (0.8-2.5) | 3.80 (2.3-6.2) | |||
| Age at transplant: | |||||||
| <50 | 136 | 1.0 | 0.04 | 1.0 | 0.06 | 1.0 | 0.83 |
| ≥50 | 239 | 1.38 (1.0-1.9) | 1.61 (1.0-2.7) | 1.04 (0.7-1.4) | |||
| Sex mismatch: | |||||||
| No | 208 | 1.0 | 0.02 | 1.0 | 0.03 | 1.0 | 0.10 |
| Yes | 197 | 1.39 (1.0-1.8) | 1.64 (1.1-2.5) | 1.29 (1.0-1.8) | |||
| Stem cell source: | |||||||
| PBSC | 397 | 1.0 | 0.53 | 1.0 | 0.96 | 1.0 | 0.28 |
| BM | 8 | 1.36 (0.5-3.4) | 0.96 (0.2-4.1) | 1.86 (0.7-5.2) | |||
| Patient CMV status: | |||||||
| Neg | 180 | 1.0 | 0.16 | 1.0 | 0.02 | 1.0 | 0.29 |
| Pos | 225 | 1.23 (0.9-1.6) | 1.71 (1.1-2.7) | 1.19 (0.9-1.6) | |||
| Fludarabine in preparative regimen: | |||||||
| Yes | 316 | 1.0 | 0.20 | 1.0 | 0.64 | 1.0 | 0.95 |
| No | 89 | 0.76 (0.5-1.2) | 0.86 (0.4-1.6) | 0.99 (0.6-1.5) | |||
HR denotes hazard ratio; CI, confidence interval; MRD, HLA-identical sibling donor; URD, HLA-matched unrelated donor; HCT-CI, hematopoietic cell transplantation comorbidity index; PBSC, peripheral blood stem cells; BM, bone marrow; CMV, cytomegalovirus.
Figure 2. Cumulative incidence of recurrent malignancy and non-relapse mortality (NRM) according to HCT-comorbidity index (CI) and relapse risk categories.
The combined groups of patients with related and unrelated donors were categorized according to the presence of pretransplant comorbidities (HCT-CI: 0, 1-2, and ≥ 3) [18] (A, B) and the predicted risk of recurrent malignancy (low, intermediate, and high) [35] (C, D). The cumulative incidence rates of recurrent malignancy (A, C) and NRM (B, D) are shown for respective subgroups of patients.
Non-relapse mortality
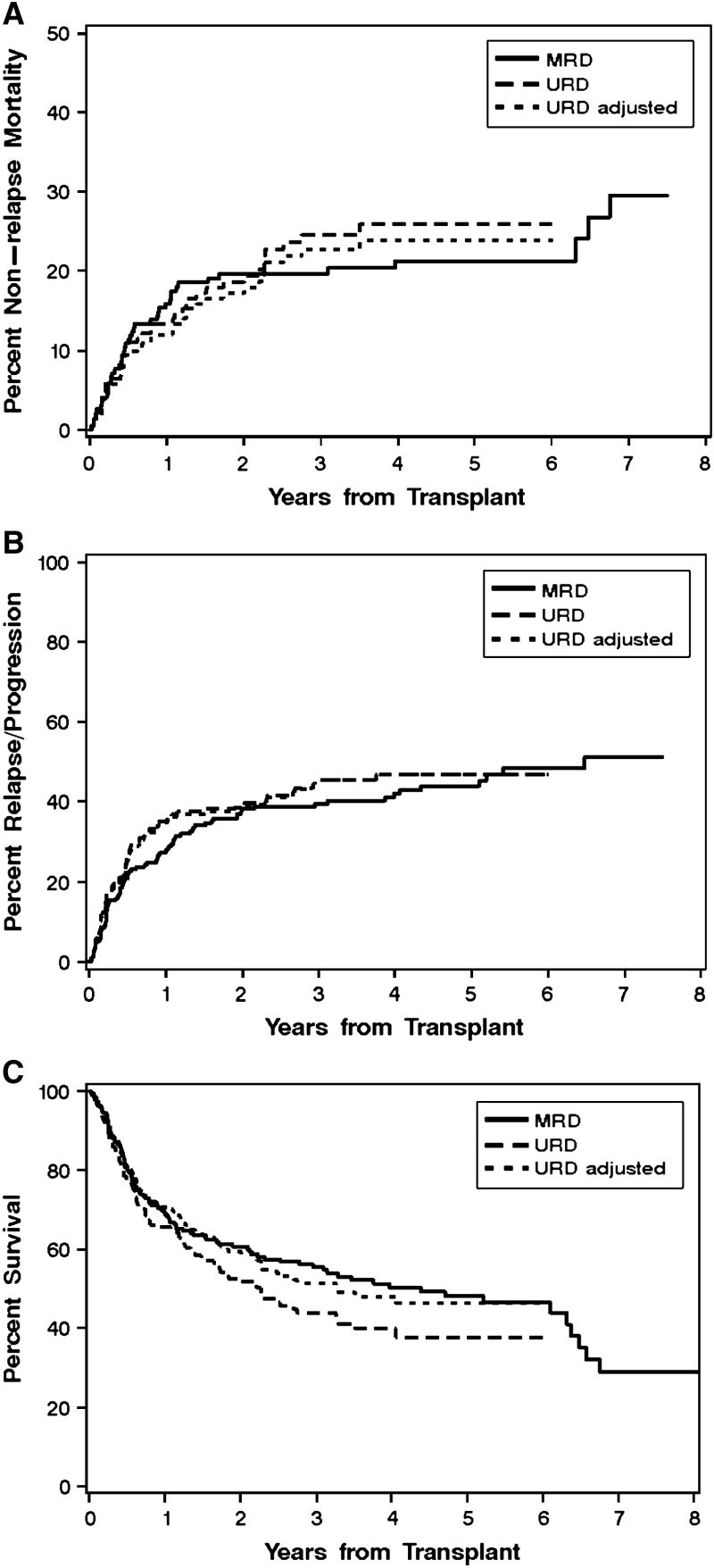
The hazard of NRM for patients with unrelated donors versus related donors showed no statistically significant difference in univariate analysis (HR 1.22: 95% CI, 0.8-1.9; p=0.36) (Figure 1A and Table 3). This conclusion remained unchanged after adjusting for HCT-CI, relapse risk category, use of fludarabine in the preparative regimen, patients age, stem cell source, prior CMV infection and sex mismatch of the donor and recipient in multivariate analysis (HR 0.98: 95% CI, 0.6-1.6; p=0.94) (Figure 1A and Table 3). The hazard of NRM for patients with unrelated donors versus related donors also showed no statistically significant difference across HCT-CI subgroups (Table 3), although the statistical power of this analysis was limited by the smaller number of patients in these subgroups.
Figure 1. Non-relapse mortality, relapse or progression, and overall survival according to donor type.
Cumulative incidence of non-relapse mortality (A) and relapse or progression (B), and Kaplan-Meier survival estimates (C) among patients with HLA-identical sibling donors (“MRD”, n=221) compared to those with HLA-matched unrelated donors (“URD”, n=184) (p=0.08). The third curve in each panel (“URD adjusted”) shows the projected survival with HLA-matched unrelated donors after adjusting for HCT-comorbidity index, relapse risk category, patient age, stem cell source, prior cytomegalovirus infection and donor/recipient sex-mismatch.
Table 3.
Outcomes after transplantation from HLA-matched unrelated donors as compared to HLA-identical sibling donors*
| MRD
N |
URD
N |
Overall mortality | Non-relapse mortality | Relapse / progression | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Univariate | 221 | 184 | 1.29 (1.0-1.7) | 0.08 | 1.22 (0.8-1.9) | 0.36 | 1.17 (0.9-1.6) | 0.32 |
| Multivariate* | 221 | 184 | 1.01 (0.7-1.4) | 0.95 | 0.98 (0.6-1.6) | 0.93 | 1.10 (0.8-1.6) | 0.60 |
| Comorbidity category: [34] | ||||||||
| HCT-CI 0 | 65 | 40 | 1.07 (0.5-2.3) | trend**
0.42 |
1.23 (0.3-4.4) | trend**
0.66 |
||
| HCT-CI 1-2 | 69 | 157 | 1.28 (0.7-2.2) | 1.03 (0.4-2.5) | ||||
| HCT-CI ≥3 | 87 | 87 | 0.87 (0.6-1.3) | 0.92 (0.5-1.7) | ||||
| Relapse risk category: [35] | ||||||||
| Low | 46 | 42 | 1.22 (0.6-2.5) | trend**
0.47 |
0.49 (0.2-1.2) | trend**
0.007 |
||
| Intermediate | 111 | 83 | 1.07 (0.7-1.7) | 0.85 (0.5-1.4) | ||||
| High | 64 | 59 | 0.91 (0.6-1.4) | 1.76 (1.1-2.9) | ||||
| Patient age: | ||||||||
| <50 years | 76 | 60 | 1.10 (0.6-1.9) | 0.70 | 0.65 (0.3-1.6) | 0.29 | 1.70 (1.0-3.0) | 0.06 |
| ≥50 years | 145 | 124 | 0.98 (0.7-1.4) | 1.11 (0.7-1.9) | 0.89 (0.6-1.4) | |||
Adjusted for patient age, HCT-CI, relapse risk category, use of fludarabine in the preparative regimen, donor/recipient sex-mismatch, stem cell source, prior cytomegalovirus-infection.
Test for trend across comorbidity or relapse risk groups in the relative difference between URD and MRD outcomes.
HR denotes hazard ratio; CI, confidence interval; MRD, HLA-identical sibling donor; URD, HLA-matched unrelated donor; HCT-CI, hematopoietic cell transplantation comorbidity index. A hazard ratio of >1.0 indicates a more favorable outcome with related donors.
Recurrent malignancy
The hazard of recurrent malignancy for patients with unrelated donors versus related donors showed no statistically significant difference in univariate analysis (HR 1.17: 95% CI, 0.9-1.6; p=0.32) or multivariate analyses (HR 1.10: 95% CI, 0.8-1.6; p=0.60) (Figure 1B and Table 3). There were also no statistically significant differences in the hazards of relapse between recipients with unrelated versus related donors across subgroups with different risks of recurrent malignancy (Table 3). Again, the statistical power of this analysis was limited by the smaller number of patients in these subgroups
Overall mortality
The hazard of overall mortality for patients with unrelated donors versus related donors showed no statistically significant difference in univariate analysis (HR 1.29: 95% CI, 1.0-1.7; p=0.08) or multivariate analyses (HR 1.01: 95% CI, 0.7-1.4; p=0.95) (Figure 1C and Table 3). There were also no statistically significant differences in the hazards of overall mortality between recipients with unrelated versus related donors across subgroups with different HCT-CI scores or risks of recurrent malignancy (Table 3). Additional adjustment for presence of single allele-mismatches at HLA class I between URD donors and recipients did not change the results (not shown).
Graft-versus-host disease
Table 4 shows the distribution of patients with acute and chronic GVHD according to donor type. Even though patients with unrelated donors had a higher incidence of grade II acute GVHD than those with related donors (59% versus 37%, p<0.0001), the overall incidence of grades III-IV acute GVHD was not different between the two groups (15% versus 15%). The overall incidence of chronic GVHD requiring systemic immunosuppressive therapy was 67% after unrelated HCT and 68% after related HCT (p=0.55). In Cox regression analysis, the adjusted hazard of grades II–IV acute GVHD was higher among recipients of unrelated compared to those of related grafts (HR 1.93: 95% CI, 1.5-2.5; p<0.0001), but the risks of developing grades III–IV acute GVHD (HR 1.03: 95% CI, 0.8-1.4; p=0.84) and chronic GVHD requiring systemic immunosuppressive therapy (HR 1.21: 95% CI, 0.9-1.6; p=0.15) were similar among the two groups.
Table 4.
Incidence of acute and chronic graft-versus-host disease according to donor type
| GVHD | MRD
(N=221) |
URD
(N=184) |
||
|---|---|---|---|---|
| N | % | N | % | |
| Acute | ||||
| 0 | 86 | 39 | 38 | 21 |
| I | 19 | 9 | 10 | 5 |
| II | 82 | 37 | 109 | 59 |
| III | 24 | 11 | 23 | 13 |
| IV | 8 | 4 | 4 | 2 |
| III + IV | 22 | 15 | 27 | 15 |
| Chronic* | 221 | 68 | 184 | 67 |
GVHD denotes graft-versus-host disease; MRD, HLA-identical sibling donor; URD, HLA-matched unrelated donor.
Requiring systemic immunosuppressive treatment.
Discussion
Results of this retrospective analysis showed that compared to nonmyeloablative HCT for hematologic malignancies from HLA-identical sibling donors, transplantation from URD did not increase the risks of NRM and overall mortality. In addition to factors known to influence outcome after allogeneic HCT, including patient age, stem cell source, type of preparative regimen, prior CMV infection, and sex-mismatch of the donor and recipient, our overall analysis was also adjusted for HCT-CI [18,33,34], a powerful predictor of NRM, and for relapse risk categories [35]. Two different systems were used to categorize patients according to their predicted risks of NRM [34] and recurrent malignancy [35] because, in contrast to the experience with myeloablative HCT, a single categorization system equally predictive for both outcomes could not be defined for nonmyeloablative HCT.
Decades of experience with allogeneic myeloablative HCT has shown that transplantation from URD is associated with a greater risk of overall mortality than HCT from MRD [12,38-41]. The net-detrimental effect associated with unrelated grafts is largely mediated by an increased risk of GVHD, and the consequently increased risk of NRM, which is typically not outweighed by more potent immunological effects of unrelated grafts against malignant cells. With improved HLA-typing technology and better matching between unrelated donors and their recipients, however, outcomes following URD transplantation have substantially improved [7] and, at least for certain patient groups, may have approached those observed with MRDs [42-46].
The similar risks of NRM and overall survival with unrelated and related donors following nonmyeloablative conditioning found in our analysis could reflect the similar risks of developing grades III/IV acute GVHD (HR 1.03; p=0.84). We speculate that despite the greater genetic disparity between unrelated compared to related donor/recipient pairs, decreased tissue damage and decreased release of inflammatory cytokines, transient mixed donor/host chimerism, or differences in the pharmacologic immunosuppressive regimen associated with nonmyeloablative HCT might have diminished the activation and clonal expansion of cells that cause clinical GVHD. This conclusion was supported by results of earlier studies showing that the onset of GVHD occurred later and the incidence was lower after HCT with nonmyeloablative conditioning as compared to myeloablative regimens [29-31].
The findings observed with our study population might not apply to other populations treated with possibly more toxic nonmyeloablative conditioning regimens. It is conceivable that more severe gastrointestinal tissue damage or differences in postgrafting immunosuppression associated with other nonmyeloablative preparative regimens might translate into differences in NRM that could affect overall survival.
Only 30% of patients with hematologic malignancies who might benefit from treatment by HCT have HLA-identical sibling donors. For older patients, in particular for those above the age of 60 years, the availability of suitable sibling donors is further limited by the concordant increased age of their siblings. Even though older patients are typically ineligible for myeloablative HCT, they can frequently be considered for a nonmyeloablative transplant approach. In this context, our findings of comparable outcomes with related and unrelated donors for patients prepared with our nonmyeloablative regimen are important because they suggest that in the absence of suitable related donors, well-matched unrelated donors may offer a very reasonable alternative that does not appear to be associated with a detrimental outcome.
Retrospective designs have many limitations, including the possibility of selection bias. In this study, unrelated donors were used only when a matched related donor was not available, and baseline characteristics of the two cohorts were similar. Nonetheless, other types of bias could have been present, but one would ordinarily expect any such bias to have an unfavorable effect on outcomes among patients with unrelated donors. Despite the absence of statistically significant differences between outcomes for patients with unrelated versus related donors in the overall study population, it is possible that further studies could identify specific subgroups where unrelated grafts are disadvantageous
In summary, except for an increased risk of mild acute GVHD, outcomes after HCT with nonmyeloablative conditioning appear to be similar with HLA-matched unrelated and related donors. We conclude that the lack of a suitable related donor should not pose an obstacle to consideration of HCT with nonmyeloablative conditioning for patients with hematological malignancies.
Acknowledgments
We would like to thank the research nurses Mary Hinds and John Sedgwick, and the staff of the Long-Term Follow-Up Program for their invaluable assistance with data collection. We thank Helen Crawford and Bonnie Larson for assistance with preparation of the manuscript. We acknowledge the excellent care provided to patients and families by the inpatient and outpatient physicians, physician's assistants, nursing teams and support staff at the Fred Hutchinson Cancer Research Center and at the University of Washington Medical Center.
Supported in part by grants CA78902, CA18029, CA15704, HL36444, DK064715, and K99-HL088021 from the NIH, DHHS, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hurley CK, Fernandez VM, Setterholm M. Maximizing optimal hematopoietic stem cell donor selection from registries of unrelated adult volunteers. Tissue Antigens. 2003;61:415–424. doi: 10.1034/j.1399-0039.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- 2.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765–771. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 3.Beatty PG, Hansen JA, Anasetti C, et al. Marrow transplantation from unrelated HLA-matched volunteer donors. Transplant Proc. 1989;21:2993–2994. [PubMed] [Google Scholar]
- 4.Ash RC, Casper JT, Chitambar CR, et al. Successful allogeneic transplantation of T-cell-depleted bone marrow from closely HLA-matched unrelated donors. N Engl J Med. 1990;322:485–494. doi: 10.1056/NEJM199002223220801. [DOI] [PubMed] [Google Scholar]
- 5.Marks DI, Cullis JO, Ward KN, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia using sibling and volunteer unrelated donors. A comparison of complications in the first 2 years. Ann Intern Med. 1993;119:207–214. doi: 10.7326/0003-4819-119-3-199308010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Schiller G, Feig SA, Territo M, et al. Treatment of advanced acute leukaemia with allogeneic bone marrow transplantation from unrelated donors. Br J Haematol. 1994;88:72–78. doi: 10.1111/j.1365-2141.1994.tb04979.x. [DOI] [PubMed] [Google Scholar]
- 7.Nademanee A, Schmidt GM, Parker P, et al. The outcome of matched unrelated donor bone marrow transplantation in patients with hematologic malignancies using molecular typing for donor selection and graft-versus-host disease prophylaxis regimen of cyclosporine, methotrexate, and prednisone. Blood. 1995;86:1228–1234. [PubMed] [Google Scholar]
- 8.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 9.Petersdorf EW, Longton GM, Anasetti C, et al. Association of HLA-C disparity with graft failure after marrow transplantation from unrelated donors. Blood. 1997;89:1818–1823. [PubMed] [Google Scholar]
- 10.Petersdorf EW, Longton GM, Anasetti C, et al. The significance of HLA-DRB1 matching on clinical outcome after HLA-A, B, DR identical unrelated donor marrow transplantation. Blood. 1995;86:1606–1613. [PubMed] [Google Scholar]
- 11.Speiser DE, Tiercy JM, Rufer N, et al. High resolution HLA matching associated with decreased mortality after unrelated bone marrow transplantation. Blood. 1996;87:4455–4462. [PubMed] [Google Scholar]
- 12.Horowitz MM, Loberiza FR, Bredeson CN, Rizzo JD, Nugent ML. Transplant registries: guiding clinical decisions and improving outcomes. Oncology. 2001;15:649–659. [PubMed] [Google Scholar]
- 13.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 14.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 15.Sandmaier BM, Maloney DG, Gooley T, et al. Nonmyeloablative hematopoietic stem cell transplants (HSCT) from HLA-matched related donors for patients with hematologic malignancies: clinical results of a TBI-based conditioning regimen. Blood. 2001;98(Part 1):742a–743a. #3093 [abstr.] [Google Scholar]
- 16.Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 17.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 18.Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplant comorbidities. Blood. 2004;104:961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 19.Chakraverty R, Peggs K, Chopra R, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantion by using a nonmyeloablative conditioning regimen. Blood. 2002;99:1071–1078. doi: 10.1182/blood.v99.3.1071. [DOI] [PubMed] [Google Scholar]
- 20.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 21.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. Review. [PubMed] [Google Scholar]
- 22.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor- alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor α during graft-versus-host disease. J Exp Med. 1992;175:405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 25.Colson YL, Lange J, Fowler K, Ildstad ST. Mechanism for cotolerance in nonlethally conditioned mixed chimeras: negative selection of the Vβ T-cell receptor repertoire by both host and donor bone marrow-derived cells. Blood. 1996;88:4601–4610. [PubMed] [Google Scholar]
- 26.Manilay JO, Pearson DA, Sergio JJ, Swenson KG, Sykes M. Intrathymic deletion of alloreactive T cells in mixed bone marrow chimeras prepared with a nonmyeloablative conditioning regimen. Transplantation. 1998;66:96–102. doi: 10.1097/00007890-199807150-00015. [DOI] [PubMed] [Google Scholar]
- 27.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 28.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–3241. [PubMed] [Google Scholar]
- 29.Mielcarek M, Burroughs L, Leisenring W, et al. Prognostic relevance of “early-onset” graft-versus-host disease following nonmyeloablative hematopoietic cell transplantation. Br J Haematol. 2005;129:381–391. doi: 10.1111/j.1365-2141.2005.05458.x. [DOI] [PubMed] [Google Scholar]
- 30.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 31.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10:178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Maris MB, Sandmaier BM, Storer BE, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Diaconescu R, Flowers CR, Storer B, et al. Morbidity and mortality with nonmyeloablative compared to myeloablative conditioning before hematopoietic cell transplantation from HLA matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 34.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk among patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007 June; doi: 10.1182/blood-2007-03-078592. published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 37.Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35:437–443. doi: 10.1016/0021-9681(82)90058-3. [DOI] [PubMed] [Google Scholar]
- 38.Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 39.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Ann Intern Med. 2006;144:407–414. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 40.Petersdorf EW, Anasetti C, Martin PJ, et al. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104:2976–2980. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 41.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 42.Moore J, Nivison-Smith I, Goh K, et al. Equivalent survival for sibling and unrelated donor allogeneic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2007;13:601–607. doi: 10.1016/j.bbmt.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 43.Hansen JA, Gooley TA, Martin PJ, et al. Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia. N Engl J Med. 1998;338:962–968. doi: 10.1056/NEJM199804023381405. [DOI] [PubMed] [Google Scholar]
- 44.Ottinger HD, Ferencik S, Beelen DW, et al. Hematopoietic stem cell transplantation: contrasting the outcome of transplantations from HLA-identical siblings, partially HLA-mismatched related donors, and HLA-matched unrelated donors. Blood. 2003;102:1131–1137. doi: 10.1182/blood-2002-09-2866. [DOI] [PubMed] [Google Scholar]
- 45.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 46.Kiehl MG, Kraut L, Schwerdtfeger R, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. 2004;22:2816–2825. doi: 10.1200/JCO.2004.07.130. [DOI] [PubMed] [Google Scholar]