Abstract
Spinal cord neurons can support a simple form of instrumental learning. In this paradigm, rats completely transected at the second thoracic vertebra learn to minimize shock exposure by maintaining a hindlimb in a flexed position. Prior exposure to uncontrollable shock (shock independent of leg position) disrupts this learning. This learning deficit lasts for at least 24 hours and depends on the NMDA receptor. Intrathecal application of an opioid antagonist blocks the expression, but not the induction, of the learning deficit. A comparison of selective opioid antagonists implicated the kappa opioid receptor. The present experiments further explore how opioids affect spinal instrumental learning using selective opioid agonists. Male Sprague Dawley rats were given an intrathecal injection (30 nmol) of a kappa-1 (U69593), a kappa-2 (GR89696), a mu (DAMGO), or a delta opioid receptor agonist (DPDPE) 10 minutes prior to instrumental testing. Only the kappa-2 opioid receptor agonist GR89696 inhibited acquisition (Experiment 1). GR89696 inhibited learning in a dose dependent fashion (Experiment 2), but had no effect on instrumental performance in previously trained subjects (Experiment 3). Pretreatment with an opioid antagonist (naltrexone) blocked the GR89696-induced learning deficit (Experiment 4). Administration of GR89696 did not produce a lasting impairment (Experiment 5) and a moderate dose of GR89696 (6 nmol) reduced the adverse consequences of uncontrollable nociceptive stimulation (Experiment 6). The results suggest that a kappa-2 opioid agonist inhibits neural modifications within the spinal cord.
Keywords: GR89696, Opioids, Plasticity, Instrumental Learning, Spinal Cord
1. Introduction
Recovery after a spinal cord injury is influenced by events that impact neural activity below the injury (Edgerton, Tillakartne, Bigbee, de Leon, & Roy, 2004; Hodgson, Roy, de Leon, Dobkin, & Edgerton, 1994; Wernig, Muller, Nanassy, & Cagol, 1995). Evidence suggests that regular, response-contingent, training can foster recovery (Edgerton et al., 2004; Hook & Grau, in press), whereas exposure to uncontrollable stimulations has an adverse effect that impedes recovery (Grau, Washburn, Hook, Ferguson, Crown, Garcia, Bolding, & Miranda, 2004). To examine how controllable versus uncontrollable stimulation affects spinal circuits (for a recent review, see Grau, Crown, Ferguson, Washburn, Hook, & Miranda, 2006), we isolate the lower (lumbo-sacral) spinal cord from the brain by means of a mid-thoracic transection in the rat. When transected (spinalized) rats are given shock to the tibialis anterior muscle of one hind leg whenever the leg is extended (controllable shock), they exhibit a progressive increase in flexion duration that minimizes net shock exposure (Grau, Barstow, & Joynes, 1998). Evidence indicates that this learning depends on the relationship between leg position (the response) and shock onset (the reinforcer) and reflects a simple form of instrumental conditioning. Interestingly, when shock is applied independent of leg position (uncontrollable shock), rats not only fail to learn, they exhibit a learning deficit that blocks subsequent instrumental learning for up to 48 hrs (Crown, Joynes, Ferguson, & Grau, 2002; Grau et al., 1998).
Further work has verified that instrumental learning depends on neurons within the lumbo-sacral (L4-S2) spinal cord and involves a form of NMDA receptor (NMDAR) mediated plasticity (Joynes, Janjua, & Grau, 2004; Liu, Crown, Miranda, & Grau, 2005). Prior exposure to uncontrollable stimulation appears to inhibit this learning through an opioid-mediated process. Supporting this, we have shown that pretreatment with the opioid antagonist naltrexone blocks the deficit in a dose-dependent fashion (Joynes & Grau, 2004). When exposure to uncontrollable stimulation and instrumental testing were separated by 24 hrs, we found that administration of naltrexone prior to testing restored the capacity for learning. However, naltrexone had no effect when it was given a day earlier, prior to the period of uncontrollable stimulation. Thus, the opioid antagonist blocked the expression of the deficit, but not its induction. Recognizing that naltrexone could act at the mu, delta, or kappa opioid receptor, we compared the relative impact of selective opioid antagonists. Only a kappa antagonist (nor-BNI) blocked the deficit. This pattern of results suggests that exposure to uncontrollable shock produces a lasting modification (a form of memory) that inhibits learning through a kappa opioid-mediated process. This fits well with our earlier observation that instrumental learning depends on a form of NMDAR-mediated plasticity (Joynes et al., 2004), because other physiological effects that depend on this form of plasticity (e.g., long-term potentiation [LTP]) are inhibited by kappa opioids (Caudle, Chavkin, & Dubner, 1994; Caudle, Mannes, & Iadarola, 1997; Terman, Drake, Simmons, Milner, & Chavkin,, 2000; Terman, Wagner, & Chavkin, 1994).
Because opioids can inhibit pain (nociceptive) signals within the dorsal horn of the spinal cord, we posited that the learning deficit might be the result of nociceptive inhibition (antinociception). Contrary to this hypothesis, we found that intermittent shock treatments that induce a learning deficit do not produce antinociception (Crown, Joynes, Ferguson, & Grau, 2002). Moreover, exposure to a different shock regimen (a long, continuous, shock) that produces a robust antinociception, as measured by tail-withdrawal from radiant heat (the tail-flick test), does not inhibit instrumental learning. Further analysis revealed that, rather than inhibiting nociceptive reactivity, exposure to intermittent/uncontrollable stimulation has a sensitizing effect that enhanced responsiveness to mechanical stimulation (Ferguson, Crown, & Grau, 2006). This phenomenon, known as allodynia, is regularly observed in response to peripheral inflammation and has been linked to the development of neuropathic pain (Coderre, 1993; Dickenson, 1996; Willis, Sluka, Rees, & Westlund, 1996; Willis, 2001).
Inflammatory agents (e.g., administration of capsaicin into one hind paw) are thought to enhance mechanical reactivity through an NMDAR-dependent increase in neural excitability within the spinal cord, a phenomenon known as central sensitization (Ji, Kohno, Moore, & Woolf, 2003; Willis, 2001; Willis, et al., 1996). We hypothesized that uncontrollable shock might disrupt learning because it induces a similar state. Supporting this, we showed that peripheral inflammation inhibits spinal learning (Ferguson et al., 2006) and, like the deficit observed after uncontrollable shock, this effect was reversed by the opioid antagonist naltrexone (Hook, Huie, & Grau, submitted). Further, like central sensitization, the induction of the learning deficit can be blocked by pretreatment with an NMDAR antagonist (Ferguson et al., 2006). Interestingly, the link to inflammation and neuropathic pain again implicates kappa opioids, for inflammation induces a lasting increase in the endogenous kappa opioid dynorphin (Wagner, Terman, & Chavkin, 1993; Wang et al., 2001) and microinjection of a kappa agonist into the spinal cord (an intrathecal [i.t.] injection) inhibits behavior signs of neuropathic pain (Eliav, Herzberg, & Caudle, 1999; Ho, Mannes, Dubner, & Caudle, 1997).
The data reviewed above suggest that a kappa-opioid mediated process modulates spinal learning. Our prior work (Joynes & Grau, 2004) relied on selective opioid antagonists, demonstrating that an opioid ligand was essential (necessary) to the expression of the learning deficit. In the present paper, we substitute an opioid agonist for intermittent shock to examine whether a kappa opioid is sufficient to inhibit instrumental learning. We show that an opioid agonist (GR89696) that acts at the kappa-2 receptor inhibits learning in a dose-dependent fashion (Experiments 1 and 2). Further, GR89696 had no effect on instrumental performance (Experiment 3) and its effect on learning was naltrexone-reversible (Experiment 4). The effect of GR89696 waned within 24 hrs (Experiment 5) and drug treatment blunted the consequences of uncontrollable stimulation (Experiment 6). As a result of these findings, we suggest that the up-regulation of kappa opioid activity may serve an adaptive function designed to limit NMDAR-dependent plasticity within the spinal cord.
2. General Method
2.1. Subjects
All protocols were approved by the Animal Care and Use committee at Texas A&M University. Male Sprague-Dawley rats obtained from Harlan (Houston, TX) served as subjects. Animals were approximately 100–120 days old and weighed between 360 and 460 g. Subjects were maintained on a 12-hr light-dark schedule and housed individually. Food and water was available ad libitum, and behavioral testing was performed during the light portion of the cycle.
2.2. Surgery
Subjects were anesthetized with pentobarbital (50 mg/kg, i.p.), and the area surrounding the shoulders was shaved and sterilized with iodine. An anterior-posterior incision, approximately 1.5 cm long, was made over the 2nd thoracic vertebra (T2). The tissue immediately anterior to T2 was then cleared, and the exposed spinal cord was transected using cauterization. The resulting space was filled with Gelfoam (Harvard Apparatus, Holliston, MA), and a cannula consisting of 25 cm of polyethylene tubing (PE-10, VWR International, Bristol, CT) fitted with a stainless steel wire (0.09 mm diameter) (Small Parts Inc., Miami Lakes, FL) was inserted into the subarachanoid space on the dorsal surface of the cord. The cannula was inserted 9 cm down the vertebral column, and the exposed end of the tubing was secured externally to the skin with cyanoacrylate, as described by Yaksh and Rudy (1976). The wound caudal to the exposed tubing was closed with Michel Clips (Fine Science Tools, Foster City, CA) and the stainless steel wire was carefully removed.
Rats were injected with 0.9% saline (2.5 ml, i.p.) immediately following surgery and the hindlimbs were shaved and secured in a natural flexed position with a piece of porous tape (Ortholetic 1.3 cm width) wrapped once around the body and legs to prevent muscular damage due to unnatural extension during recovery. Subjects were allowed to recover in a temperature-controlled room (26.7°C) with food and water available ad libitum. In addition, subjects received daily injections of saline (2.5 ml, i.p.) to prevent dehydration. Bladder expression took place twice a day and immediately before any behavioral procedures were conducted. At the end of testing, animals were euthanized with pentobarbital (100 mg/kg).
Transections were confirmed by a) visually inspecting the cord during surgery, b) observing behavior after recovery to ensure complete paralysis below the forelimbs and no vocalization when exposed to leg shock, and c) examining the cord post mortem in a randomly selected subset of subjects.
2.3. Uncontrollable Shock Treatment
Shock treatment occurred while subjects were loosely restrained in Plexiglas tubes as previously described in Crown et al., 2002. Intermittent constant current shock (1.5 mA) was applied through electrodes taped to the tail. Shocked rats received 80-ms tailshocks on a variable time schedule with a mean of 2 seconds (range = 0.2–3.8 s) for six minutes. This shock regimen elicited little or no leg movement. Unshocked controls were placed in the restraining tubes, had the electrodes attached, but did not receive shock.
2.4. Instrumental Learning Testing Procedure
Testing for instrumental learning occurred as previously described (Grau et al., 1998). Briefly, on the day of testing, a wire electrode was inserted through the skin over the distal portion of the tibialis anterior (1.5 cm from the plantar surface of the foot) and one lead from the generator was attached to this wire. A contact electrode was secured to the foot between the second and third digits with a piece of porous tape. The shock generator was set to deliver a 0.4 mA shock, and the proximal portion of the tibialis anterior (approximately 1.7 cm proximal to the wire electrode) was probed with a 2.5-cm stainless steel pin attached to a shock lead to find a robust flexion response. The pin was then inserted 0.4 cm into the muscle. A strain gauge was utilized to verify that a single, intense (1.6 mA, 0.3 s), test shock elicited at least a 0.8 N flexion force. Next, shock intensity was set at a level that elicited a flexion force of 0.4 N. In each experiment, half the subjects in each group were tested on the left leg and half were tested on the right leg.
To minimize lateral leg movements, a 20 cm piece of porous tape was wrapped around the leg and was attached to a bar extending across the apparatus directly under the front panel of the restraining tube. The tape was adjusted so that it was taut enough to slightly extend the knee. Finally, three short (0.15 s) shock pulses were applied, and the level of the salt solution was adjusted so that the tip of the contact electrode (attached to the rat’s foot) was submerged 4.0 mm below the surface. A rat’s capacity to perform the instrumental response was then tested with exposure to 30 min of controllable shock. Whenever the rat’s leg fell below the level of the salt solution, the electrodes delivered a shock to the tibialis anterior muscle causing flexion of the hind limb. Performance was measured over time in 30 1-min time bins.
2.5 Behavioral Measures
Time in solution, response number and response duration were used to assess learning and performance (See Grau et al., 1998). A computer monitoring leg position recorded the time in solution, which refers to the amount of time the contact electrode stayed in the salt solution during the 1-min time bin. The computer also monitored the amount of times the contact electrode left the salt solution (response number). Response number was increased by 1 each time the hind limb was raised and the electrode left the solution. Response duration refers to how long the subject maintained the hind limb in a flexed position during the 1-min time bin and was derived from time in solution and response number using the following equation: Response Durationi = (60 s − time in solutioni)/(Response Numberi + 1) where i is the current time bin.
2.6. Statistical Analysis
The effects of experimental treatment over time were analyzed using a repeated measures analysis of variance (ANOVA). Group differences were further evaluated using Duncan’s New Multiple Range post hoc tests. Differences were considered significant at p values < 0.05.
3. Experiment 1
A comparison of selective mu, delta, and kappa opioid receptor antagonists showed that only a kappa opioid receptor antagonists given immediately before testing eliminated the behavioral deficit induced by uncontrollable shock (Joynes & Grau, 2004). If the deficit results from activation of kappa receptors, then an agonist for this receptor should substitute for uncontrollable shock and produce the behavioral deficit. Furthermore, equal molar concentrations of a delta or mu opioid receptor agonist should have no effect.
The kappa opioid receptor is thought to exist in at least two forms, kappa-1 and kappa-2, both of which are present within the spinal cord (Caudle et al., 1994, 1997; Zukin, Eghbali, Olive, Unterwald, & Tempel, 1988). While the study by Joynes and Grau (2004) implicated the kappa receptor, it did not differentiate between receptor subtypes. We addressed this issue by employing two distinct kappa opioid agonists, one selective for the kappa-1 receptor (U69593) and another selective for the kappa-2 receptor (GR89696). Experiment 1A examined whether these drugs affected instrumental learning. Experiment 1B assessed the impact of a mu agonist (DAMGO) and Experiment 1C tested a delta agonist (DPDPE). Experiment 1D examined whether a higher molar concentration of DAMGO or DPDPE affected learning. In all cases, the drugs were administered intrathecally (i.t.) at equal molar concentrations and subjects were tested in our instrumental learning paradigm for 30 min.
3.1. Procedure
Table 1 outlines the experimental design used in all experiments. All subjects in Experiment 1 were tested 24 hrs after surgery. After the wire electrode was implanted, subjects were placed in the test apparatus and the cannula was threaded through an airhole on the side of the Plexiglas tube used to restrain the subject. A baseline flexion force was taken before subjects received any pharmacological agents and shock intensity was adjusted to yield a flexion response of 0.4 N. Subjects in Experiments 1A–1C received 30 nmol of the opioid agonist dissolved in 10 μl of saline, or an equal volume of saline, intrathecally followed by a 10 μl saline flush. Subjects in Experiment 1D received 60 nmol of DAMGO or DPDPE in 10 μl of saline, or the vehicle alone, followed by a 10 μl saline flush. Drugs were obtained from Sigma (St. Louis, MO). Flexion force was retested 10 minutes later. Subjects were then tested with 30 minutes of controllable shock. An equal number (n=8) of subjects were used in each experimental condition with the exception of Experiment 1C, which employed 9 subjects per cell.
Table 1.
Experimental designs
| Exp. | n | Procedure |
|---|---|---|
| 1A | 8 | Vehicle, U69593 (30 nmol), or GR89696 (30 nmol) → 10 min → Test with controllable shock |
| 1B | 8 | Vehicle or DAMGO (30 nmol) → 10 min → Test with controllable shock |
| 1C | 9 | Vehicle or DPDPE (30 nmol) → 10 min → Test with controllable shock |
| 1D | 8 | Vehicle or DAMGO (60 nmol) or DPDPE (60 nmol) → 10 min → Test with controllable shock |
| 2 | 6 | Vehicle or GR89696 (1.2, 6, or 30 nmol) → 10 min → Test with controllable shock |
| 3 | 6 | Train with controllable shock (30 min) → Vehicle or GR89696 (30 nmol; @ min 25) → Test with controllable shock (30 min)
or no training (30 min) → Vehicle or GR89696 (30 nmol; @ min 25) → Test with controllable shock (30 min) |
| 4 | 10 | Vehicle or naltrexone (? dose) → Vehicle or GR89696 (30 nmol) → 10 min → Test with controllable shock |
| 5 | 8 | Vehicle or GR89696 (6, or 30 nmol) → 24 hrs → Test with controllable shock |
| 6 | 8 | Vehicle or GR89696 (6 nmol) → 10 min → Uncontrollable shock or nothing → 24 hrs → Test with controllable shock |
3.2. Results
3.2.1. Impact of kappa agonists
To examine whether drug treatment affected baseline behavioral reactivity, the shock intensity required to produce a 0.4 N change in flexion force both before and after drug administration were analyzed. The mean shock intensity needed to elicit a 0.4 N change in flexion force before drug treatment ranged from 0.51 (± 0.05) to 0.58 (± 0.06) mA and after drug administration ranged from 0.51 (± 0.06) to 0.66 (± 0.07) mA. In no case did these group differences approach statistical significance, all Fs < 1.42, p > .05.
The effect of U69593 and GR89696 (30 nmol) on our primary measure of learning, response duration, is depicted in Figure 1A. As in prior studies, saline-treated animals exhibited an increase in response durations over time. This learning was blocked by GR89696, whereas U69593 had no effect. A repeated measures ANOVA on response duration revealed significant main effects of drug, F(2, 21) = 4.68, p < .05, and time, F(29, 609) = 3.06, p < .01. A significant Drug X Time interaction was also found, F(58, 609) = 1.76, p < .01. Post hoc comparisons of the group means (Figure 1B) revealed that the GR89696-treated group differed from the other groups, p < .05. No other group differences were significant, p > .05.
Figure 1.
The impact of U69593 and GR89696 administered 10 minutes prior to instrumental testing on response duration (A) and response number (C) over time. The right panels (B & D) represent group means (±SE) for response duration and response number, respectively.
Figures 1C and 1D summarize the impact of U69593 and GR89696 on response number. There was no evidence that GR89696 disrupted the performance of the target response. Indeed, if anything, the drug-induced disruption in learning led to a higher rate of responding. However, these group differences were not statistically significant, all Fs < 3.18, p > .05.
3.2.2. Impact of a mu agonist
The mean shock intensity before DAMGO administration ranged from 0.53 (± 0.06) to 0.56 (± 0.06) mA and after drug treatment ranged from 0.57 (± 0.06) to 0.61 (± 0.07) mA. These group differences were not significant, all Fs < 1.0, p > .05.
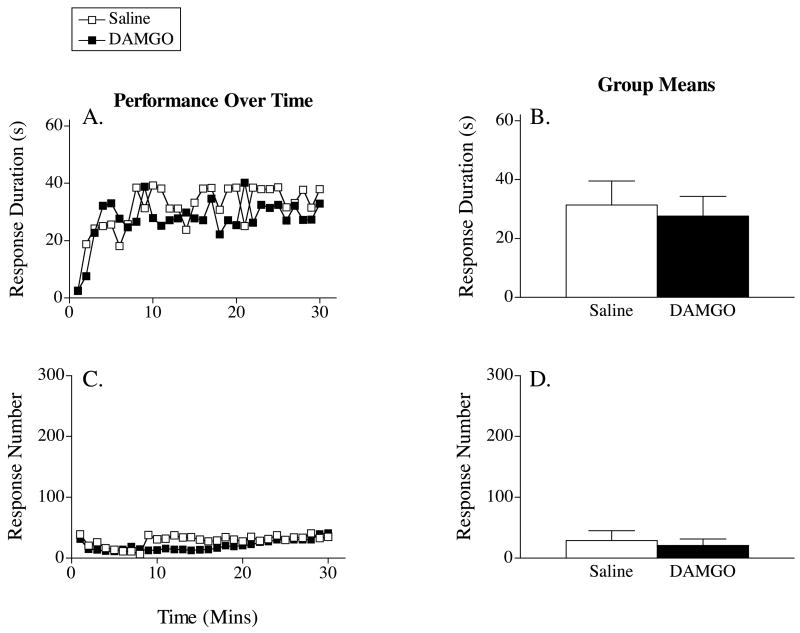
Figure 2A depicts the effect of DAMGO (30 nmol) on response duration across time. Saline-treated animals exhibited an increase in response durations over the 30-minute testing period. DAMGO had no impact on this learning. An ANOVA revealed a main effect of time, F(29, 406) = 2.66, p < .001. Neither the main effect of drug nor the Drug X Time interaction approached significance, both Fs < 1.0, p > .05.
Figure 2.
Response duration (A) and number of responses (C) made by subjects that received DAMGO 10 minutes prior to instrumental testing across time. The right panels (B & D) display the group means (±SE) for response duration and response number, respectively.
Figures 2C and 2D show the effect of DAMGO on response number. As saline-treated subjects learned the instrumental response, they showed a progressive decrease in the number of responses made over the thirty-minute testing period. DAMGO-treated subjects displayed a similar pattern of results. An ANOVA revealed a significant main effect of time, F(29, 406) = 1.93, p < .01. Neither the main effect of drug, nor the Drug X Time interaction, reached significance, both Fs < 1.0, p > .05.
3.2.3. Impact of a delta agonist
The mean shock intensity needed to induce a 0.4 N change in flexion force before DPDPE treatment ranged from 0.64 (± 0.09) to 0.67 (± 0.07) and after treatment ranged from 0.67 (± 0.06) to 0.69 (± 0.09). Again, these group differences were not significant, all Fs < 1.0, p > .05.
The effect of DPDPE (30 nmol) on response duration is depicted in Figure 3A. Subjects that received saline prior to testing exhibited an increase in response duration over the testing period. DPDPE treatment had no effect on this learning. An ANOVA yielded a significant main effect of time, F(29, 464) = 3.42, p <. 001. Neither the main effect of drug, nor the Drug X Time interaction, reached significance, both Fs < 1.0, p > .05.
Figure 3.
The effect of DPDPE on response duration (A) and response number (C) in subjects tested 10 minutes after drug treatment. The group means (±SE) for response duration and response number are presented in the right panels (B & D), respectively.
The effect of DPDPE on response number is depicted in Figure 3C. Saline-treated subjects displayed a decrease in response number as they acquired the instrumental response. Although DPDPE-treated animals exhibited a higher level of responding across the test period, neither the main effect of drug treatment, nor its interaction with time, were significant, both Fs < 1.56, p > .05. Only the main effect of time reached significance, F(29, 464) = 1.96, p < .01.
3.2.4. Impact of a higher molar concentration
Administration of DAMGO or DPDPE at a higher molar concentration (60 nmol) did not affect the shock intensity needed to elicit a 0.4 N response. Mean shock intensities ranged from 0.51 (±0.04) to 0.58 (±0.05) and these differences were not significant, F(2, 21) < 1.0, p > .05.
Neither drug affected instrumental learning. Mean response durations collapsed across the 30 min of testing were 18.0 (±7.2), 16.0 (±5.8), 24.8 (±7.1) s for the saline, DAMGO and DPDPE treated groups, respectively. As usual, an ANOVA confirmed that training produced an increase in response duration across time, F(29, 609)=4.59, p < .0001. Neither the main effect of drug treatment, nor its interaction with time, approached statistical significance, both Fs < 1.0, p > .05.
3.3. Discussion
In summary, Experiment 1A showed that pretreatment with the selective kappa-2 opioid receptor agonist, GR89696, disrupted learning. The kappa-1 opioid agonist U69593 had no effect. Experiments 1B–1D showed that neither a mu (DAMGO) nor a delta (DPDPE) opioid receptor agonist affected instrumental learning. These results complement our earlier finding that a kappa opioid antagonist blocks the expression of the learning deficit (Joynes & Grau, 2004). Together, the data suggest that a kappa opioid plays an essential role (Joynes & Grau, 2004) and that the adverse consequences of uncontrollable shock treatment can be emulated through the administration of a selective kappa opioid agonist (Experiment 1A).
The effect of GR89696 on instrumental learning was quite robust. An ANOVA comparing the GR89696 treated group from Experiment 1A to the vehicle controls revealed that this manipulation yielded an effect size of 0.68 and a Cohen’s d of 1.86. Given the magnitude of this effect, and a power of 0.8, just 6 subjects per cell should be needed to resolve an effect of GR89696 treatment. This n was used in Experiments 2 and 3. Importantly, these experiments included independent replications of our basic effect—that pretreatment with GR89696 immediately before instrumental testing impairs learning. Experiments 4–6 further explore the effect of GR89696 and used larger n’s (8/cell), which yielded increased power (0.93).
4. Experiment 2
Experiment 1 found that only the selective kappa-2 opioid receptor agonist, GR89696, disrupted instrumental learning. Experiment 2 establishes the effective dose by assessing the impact of GR89696 across three molar concentrations.
4.1. Procedure
Twenty-four hours after spinal transection, subjects received intrathecal administration of 10 μl of GR89696 at one of four doses (0, 1.2, 6, or 30 nmol) followed by a 10 μl saline flush (n = 6). The impact of drug treatment on instrumental learning was tested 10 min later.
4.2. Results
As in Experiment 1A, GR89696 had no impact on the shock intensity needed to produce a 0.4 N flexion force. Group means after drug treatment ranged from 0.38 (± 0.05) to 0.47 (± 0.03) mA and were not significantly different, F(3, 20) < 1.0, p > .05.
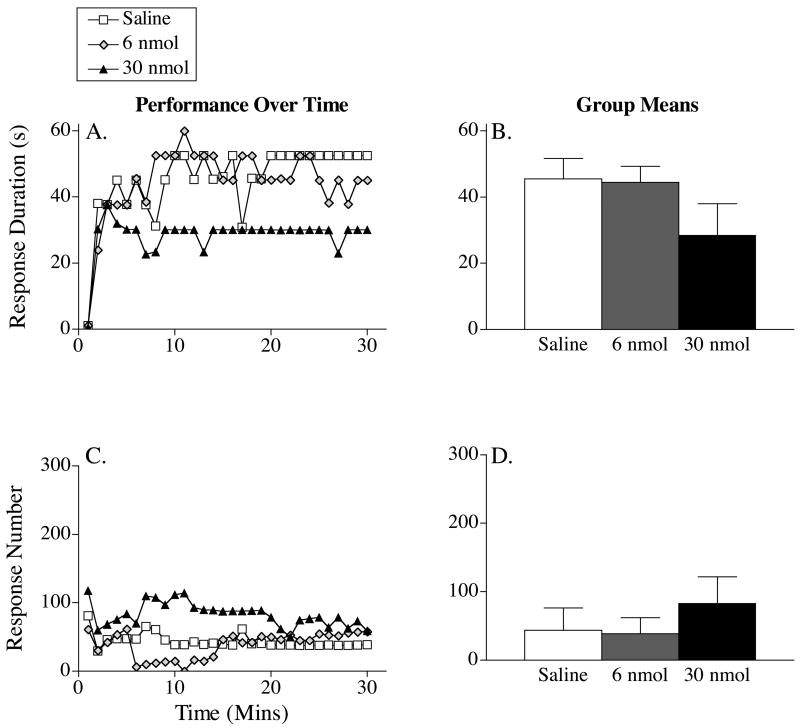
The effect of GR89696 on response duration is depicted in Figure 4A. Subjects that received saline exhibited an increase in response duration over the 30-minute testing period. GR89696 disrupted this learning in a dose-dependent fashion. A repeated measures ANOVA revealed significant main effects of dose and time, Fs > 3.37, p < .05. The Time X Dose interaction failed to reach significance, F(87, 580) = 1.08, p > .05. Trend analysis of mean performance (Figure 4B) revealed a significant linear effect of dose, p < .05. Neither the quadratic or cubic component was significant, p > .05.
Figure 4.
The impact of three doses of GR89696 on response duration (A) and response number (C) across time in subjects tested 10 minutes following drug delivery. The right panels (B & D) portray the group means (±SE) for response duration and response number, respectively.
Figure 4C illustrates the impact of GR89696 on response number. As vehicle-treated subjects learned the instrumental response, they showed a progressive decrease in the number of responses made over the 30-minute testing period. GR89696 dose-dependently prevented the acquisition of the flexion response, resulting in a steady increase in the number of responses made. An ANOVA revealed a significant main effect of dose, F(3, 580) = 4.20, p < .05. Neither the main effect of time, nor its interaction with dose, was significant, Fs < 1.02, p > .05. Trend analysis of the group means (Figure 4D) revealed a linear effect of dose, p < .05. Neither the quadratic or cubic component was significant, p > .05.
4.3. Discussion
As expected, the kappa-2 agonist GR89696 impaired learning in a dose dependent fashion and did so without disrupting the performance of a flexion response.
5. Experiment 3
Experiments 1 and 2 showed that pretreatment with the kappa-2 agonist disrupts spinal learning. Elsewhere, we have shown that pretreatment with an NMDA antagonist (APV or MK-801) also prevents learning (Ferguson et al., 2006; Joynes et al., 2004). Because an NMDA antagonist affects the maintenance of other forms of spinal plasticity (Ma & Woolf, 1995; Woolf & Thompson, 1991), we evaluated whether administration of APV affects instrumental performance after the response has been acquired. To do this, we doubled the duration of the test session (to 60 min) and administered APV by means of an i.t. cannula after instrumental performance had stabilized, at 25 min into the session. Application of APV caused the learned response to deteriorate, suggesting that the NMDAR plays an important role in both acquisition and maintenance of the learned response. Experiment 3 used the same methodology to evaluate whether GR89696 affects the maintenance/performance of the instrumental response.
5.1. Procedure
All subjects were set-up for instrumental testing 24 hours after surgery. Half the subjects then received controllable shock to one hind leg for 60 min. After 26 min of training, subjects were given an intrathecal injection (30 nmol) of GR89696 or saline while they remained undisturbed in the test apparatus. The injection took place over the course of 4 min and instrumental testing continued for an additional 30 min. To verify that GR89696 was effective when given prior to training, two control groups remained unshocked for the first 30 min of the session. These subjects were given GR89696 (30 nmol) or saline starting at min 26. Four min later, they were tested for 30 min with controllable leg shock. The 2 (pretraining or nothing) × 2 (drug treatment) factorial design used 24 subjects (n = 6).
5.2. Results
The mean (± SEM) shock intensity needed to elicit a 0.4 N flexion response ranged from 0.42 (± 0.02) to 0.45 (± 0.03) across groups. These differences were not significant, all Fs < 1.0, p > .05.
An analysis of instrumental performance during the first 30 min of testing (Figure 5A) confirmed that the pretrained groups exhibited similar levels of instrumental learning/performance prior to drug treatment, F(1, 10) < 1.0, p > .05. During the second 30 min of testing, the pretrained vehicle treated rats maintained a high level of instrumental performance. Administration of GR89696 had no effect in pretrained rats, but blocked learning when subjects were trained after the drug was given. An ANOVA revealed significant main effects of drug and treatment time and a significant Drug X Treatment Time interaction, Fs > 5.06, p < .05. Post hoc comparisons of the group means (Figure 5B) showed that subjects given GR89696 prior to instrumental testing differed from all other groups, p < .05. No other group differences were significant, p > .05.
Figure 5.
The impact of GR89696 on response duration (A) and response number (C) across time when given 30 minutes after the acquisition of instrumental learning. The right panels (B & D) represent the group means (±SE) for response duration and response number, respectively.
The impact of GR898696 on response number is shown in Figure 5C. Subjects that received drug treatment after they had already acquired the instrumental response (30 min into testing) continued to maintain the hindlimb in a flexed position and showed very few responses. Rats that were not pretrained before GR89696 was administered failed to learn, and consequently, exhibited a high rate of responding. An ANOVA of the data from the last 30 min of testing revealed a significant effect of drug treatment, time, and a Drug X Time interaction, all Fs > 5.56, p < .05. Post hoc comparisons of the group means (Figure 5D) showed that subjects given GR89696 prior to instrumental testing differed from all other groups, p < .05. No other group differences were significant, p > .05.
5.3. Discussion
As in Experiments 1 and 2, pretreatment with the kappa-2 agonist GR89696 disrupted the acquisition of the instrumental response. The drug had no effect when it was given after the instrumental response was acquired, suggesting that a kappa agonist does not influence the maintenance of learning in this paradigm. It appears that the NMDAR-dependent mechanisms that underlie instrumental learning can be inhibited during acquisition, but once the behavioral modification is acquired, the process is relatively less sensitive to this form of opioid-mediated inhibition.
Vehicle treated controls in the present experiment appeared to acquire the instrumental task faster than the vehicle treated controls in Experiments 1–2. Our impression is that the performance of the untreated controls varies across studies, in part, because our training mission requires introducing new students to the surgical and behavioral procedures. Not surprisingly, this introduces some variability in the test performance across experiments. Within an experiment, we hold surgeon(s) and the individual(s) setting up the subjects for behavioral testing constant and make sure that, if multiple individuals are involved in a particular phase, that their relative contribution is counter-balanced across groups. In addition, two extra safe-guards are employed. First, care is taken to assure that those performing the surgeries and behavioral testing are blind to the subject’s group assignment. Second, our experimental designs routinely include independent replications of our basic effect. Thus, in Experiments 2 and 3 we verified that pretreatment with GR89696 impairs instrumental learning. The fact drug treatment yielded similar results across 3 experiments, conducted at different times and involving different sets of researchers, suggests that this is a reliable effect.
The results of Experiment 3 also speak to some alternative interpretations of our results. In some cases, kappa opioids can disrupt NMDAR-mediated plasticity by direct interaction with the NMDAR (Lai, Ossipov, Vanderah, Malan, & Porreca, 2001). This effect has been linked to a nonopioid (naloxone/naltrexone-insensitive) effect of dynorphin, tied to that portion of the peptide (2–17) that lacks the N-terminal component needed to engage opioid receptors (Baski & Faden, 1992; Faden, 1996; Faden & Jabcobs, 1984; Long, Petras, Mobley, & Holaday, 1988; Vanderah et al., 1996). If GR89696 emulated this nonopioid effect, we would expect it to have an action that parallels the effect of an NMDAR antagonist, degrading maintenance as well as the acquisition of the instrumental response. The fact an NMDAR antagonist and the kappa opioid yield a different pattern of results suggest that they impact learning in different ways.
The intrathecal application of kappa opioids has been reported to produce motor paralysis (Faden & Jacobs, 1984; Stevens & Yaksh, 1986), an effect that would obviously compromise instrumental responding. Again, this outcome appears tied to a nonopioid effect. If GR89696 disrupted instrumental learning because it inhibited the capacity to perform the instrumental response, pretrained rats given the drug should have exhibited a deterioration in performance. No such deterioration was observed, which suggests that GR89696 affects learning, not performance.
The current experiments were motivated, in part, by the observation that GR89696 attenuates behavioral signs of neuropathic pain (Eliav et al., 1999; Ho et al., 1997). Interestingly, this inhibitory effect was observed at a lower dose (6 nmol) than that used in the present study. This implies a quantitative difference exists between NMDAR-mediated instrumental learning and central sensitization. Specifically, that the maintenance of central sensitization must require a higher level of sustained Ca++ influx and, therefore, is more sensitive to kappa-2 opioid inhibition.
6. Experiment 4
As noted above, administration of a kappa opioid can have nonopioid (naltrexone-insensitive) consequences that could influence learning and/or performance of the instrumental response. We tried to avoid this complexity by using an agent that selectively binds to the kappa-2 opioid receptor. Nonetheless, it remains possible that the effects reported in Experiments 1–3 are due to an action at a nonopioid site. To evaluate this possibility, we examined whether the GR89696-induced inhibition of learning is blocked by pretreatment with the opioid antagonist naltrexone.
6.1. Procedure
Twenty-four hours after surgery, half the subjects received an intrathecal injection of naltrexone at a dose (7 μg/μl) known to block the expression of the learning deficit (Joynes & Grau, 2004). The drug, which was dissolved in 1 μl dH20, was administered over a period of 2 min and was followed by a 20 μl flush. The remaining subjects received the vehicle alone. All subjects (n=10) received an intrathecal injection (10 μl) of GR89696 at a dose of 30 nmol fifteen minutes later followed by a 10 μl saline flush. Ten minutes later, subjects were then tested for 30 min using the instrumental learning paradigm described previously.
6.2. Results
Drug treatment did not affect the shock intensity required to elicit a 0.4 N flexion response, F(1, 18) = 2.29, p > .05. Treatment with GR89696 alone undermined instrumental learning and this effect was blocked by pretreatment with naltrexone (Figure 6). An ANOVA confirmed that the main effects of drug treatment and time were significant, Fs > 3.53, p < .05. Although subjects that failed to learn exhibited more responses, neither the main effect of drug treatment, nor its interaction with time, reached significance, both Fs < 3.33, p > .05.
Figure 6.
The effect of GR89696 treatment on response duration (panels A & B) and response number (C & D) across time in subjects pretreated with naltrexone (black squares) or nothing (white squares). The group means (± SE) for response duration and response number are given in the right panels (B & D).
6.3. Discussion
As observed in Experiments 1–3, GR89696 inhibited instrumental learning. This effect was blocked by pretreatment with the opioid antagonist naltrexone, which implies that GR89696 affects learning through its interaction with an opioid receptor.
7. Experiment 5
In experiments 1–4 we found that the kappa-2 opioid receptor agonist GR89696 impaired learning when subjects were tested at least 10 minutes after drug treatment. Experiment 5 examined whether drug treatment has a long-term effect. Because the long-term effects of shock treatment develop independent of whether opioid receptors are blocked (Joynes & Grau, 2004), we did not expect GR89696 to have a long-term effect. It is possible, though, that GR89696 has an unanticipated consequence that produces a lasting, perhaps permanent, disruption in spinal learning. To evaluate this possibility, subjects were given either a moderate (6 nmol) or high (30 nmol) dose of GR89696 and tested 24 hrs later.
7.1. Procedure
Twenty-four hours after surgery, subjects were brought to the instrumental testing room and received intrathecal delivery of GR89696 (10 μl) at one of three doses (0, 6, or 30 nmol) followed by a 10 μl saline flush (n = 8). The cannula was removed ten minutes later, and all animals were returned to the recovery room, where they remained until instrumental testing 24 hours later. The next day, rats were tested for 30 min in the instrumental learning paradigm.
7.2. Results
The shock intensity needed to elicit a 0.4 N response ranged from 0.50 (± 0.03) to 0.60 (± 0.04) mA across groups. These group differences were not significant, F(2, 21) = 2.10, p > .05.
Figure 7A depicts the impact of GR89696 on response duration when subjects were tested 24 hours after drug delivery. Saline-treated rats exhibited an increase in response duration over the 30-min of testing. GR89696 had no long-term effect on learning at any of the doses tested. An ANOVA revealed only a significant main effect of time, F(29, 609) = 5.53, p < .05. Neither the main effect of drug treatment, nor its interaction with time, approached statistical significance, both Fs < 1.59, p > .05.
Figure 7.
The effect of GR89696 on response duration (panels A & B) and response number (panels D & E) across time in subjects tested 24 after drug treatment. The group means (±SE) for response duration and response number appear in the right panels (B & D), respectively.
All subjects exhibited a progressive decrease in the rate of responding as they acquired the instrumental response. (Figure 7C–D). The main effects of time and drug treatment, as well as their interaction, did not approach statistical significance, all Fs < 1.0, p > .05.
7.3. Discussion
While both 6 and 30 nmol of GR89696 inhibited learning when subjects were tested 10 min after drug administration (Experiments 1–4), the kappa agonist had no effect when instrumental testing was delayed for 24 hrs. The latter suggests that GR89696 only impairs learning when the drug is pharmacologically active and that GR89696 treatment (within the dose range tested) does not have a lasting adverse effect on instrumental learning.
8. Experiment 6
Experiment 5 showed that GR89696 does not substitute for shock treatment to produce a lasting inhibition of learning. Coupled with our prior work (Joynes & Grau, 2004), it is clear that the neurobiological mechanisms that underlie the induction of a learning deficit by uncontrollable shock do not depend on opioid activity. In fact, kappa opioid activity could be antagonistic to the induction of the learning deficit. As noted earlier, the induction of learning deficit depends on a form of NMDAR-mediated plasticity (Ferguson et al., 2006). Because GR89696 inhibits NMDAR-dependent synaptic currents, administration of the kappa-2 agonist could inhibit the induction of the learning deficit. Experiment 6 examined this issue by testing whether rats given GR89696 prior to uncontrollable shock exhibit less of a learning deficit when tested 24 hrs later.
8.1. Procedure
Twenty-four hours following surgery, subjects received intrathecal administration of either saline or GR89696 (6 nmol) followed by uncontrollable tailshock or nothing, yielding a 2 (saline or GR89696) X 2 (shock or nothing) design (n = 8). We used an intermediate dose of GR89696 because this dose inhibited instrumental learning (Experiment 2) and had no apparent effect on learning when subjects were tested 24 hrs after drug treatment (Experiment 5). Ten minutes after drug treatment, the cannula was removed, and the animals were placed into opaque Plexiglas tubes (as described above). Three pieces of porous tape were extended across the tube to loosely restrain the subject, and an electrode coated with ECG gel was secured approximately 6 cm behind the base of the tail with a piece of porous tape. Rats then received 6 minutes of uncontrollable tailshock (1.5 mA, intermittent) or an equal amount of tube restraint (no shock). After shock treatment, subjects were returned to the recovery room until they were tested for instrumental learning 24 hours later.
8.2. Results
Mean (± SEM) shock intensity ranged from 0.40 (± 0.05) to 0.45 (± 0.02) mA. These group differences were not statistically significant, all Fs < 0.69, p > .05.
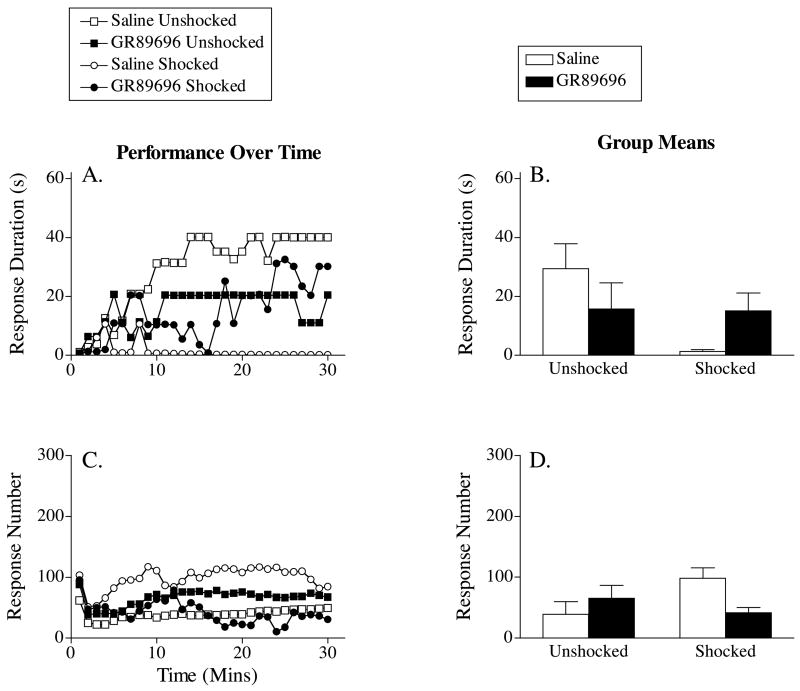
When tested with controllable shock, both unshocked groups exhibited a progressive increase in response duration (Figure 8A). Rats that received saline prior to uncontrollable shock failed to learn. This learning deficit was attenuated by pretreatment with GR89696. A two-way, repeated measures ANOVA revealed a significant main effect of time, F(29, 580) = 4.03, p < .05, and a significant Drug X Shock X Time three-way interaction, F(29, 580) = 2.62, p < .05. To further characterize this interaction, additional analyses were performed to compare the effect of GR89696 on instrumental learning in shocked and unshocked subjects. In shocked rats, drug treatment significantly improved performance over time, F(29, 290) = 1.82, p < .05. Drug treatment did not have a significant effect in the unshocked subjects, F(29, 290) = 1.42, p > .05.
Figure 8.
The impact of GR89696 administered prior to uncontrollable shock on response duration (A) and response number (C) across time. Subjects were tested 24 hours after shock exposure. The right panels (B & D) represent the group means (±SE) for response duration and response number, respectively. US=unshocked; Shk=shocked.
Figure 8C illustrates the impact of GR89696 and shock treatment on response number. Saline-treated subjects that received uncontrollable shock prior to testing displayed a higher rate of responding than any other group as they failed to acquire the instrumental response. The impact of shock treatment was attenuated by GR89696. An ANOVA uncovered a significant Drug X Shock interaction, F(1, 20) = 4.70, p < .05. No other significant effects were found, p > .05.
8.3. Discussion
As predicted, administration of GR89696 prior to uncontrollable shock reduced the long-term effect of shock treatment. Clearly, kappa opioid activity is not required for the induction of the learning deficit (Joynes & Grau, 2004) and, instead, opposes its induction. What integrates our findings is the observation that both instrumental learning and the induction of the learning deficit depend on a form of NMDAR-mediated plasticity. Inhibiting the NMDAR by pretreating subjects with either a NMDAR antagonist, or a kappa-2 opioid, disrupts both learning and the induction of the deficit. The kappa-2 agonist does not, however, simply emulate the effect of a NMDAR antagonist, for only the latter impacts performance of the instrumental response (cf Experiment 3 and Joynes et al. [2004]). As discussed below, we believe that this dissociation arises because the opioid-mediated inhibition of the NMDAR reduces, but does not block (antagonize), NMDAR-dependent Ca++ flow (Caudle et al., 1994, 1997).
9. General Discussion
We found that the kappa-2 opioid agonist GR89696 inhibited instrumental learning in a dose dependent manner (Experiments 1 and 2). Equal molar concentrations of a kappa-1 (U69593), mu (DAMGO), or delta (DPDPE) receptor agonist had no effect and DAMGO and DPDPE remained ineffective when given at a higher concentration (Experiment 1). Administration of GR89696 did not affect the maintenance of a previously acquired instrumental response (Experiment 3) and the drug’s effect was blocked by pretreatment with the opioid antagonist naltrexone (Experiment 4). GR89696 did not have a lasting effect on instrumental learning (Experiment 5) and it attenuated the induction of the learning deficit produced by exposure to uncontrollable shock (Experiment 6).
The present results complement earlier work implicating the kappa opioid receptor in the behavioral deficit observed after uncontrollable shock (Joynes & Grau, 2004). Those studies showed that administration of an opioid antagonist blocks the expression of the learning deficit and implicated the kappa opioid receptor. The current study showed that administration of a kappa agonist is sufficient to inhibit instrumental learning. However, just as the release of an opioid does not appear necessary for the induction of the deficit, administration of a kappa opioid agonist was not sufficient to produce a lasting impairment—the kappa-2 opioid only inhibited learning while it was pharmacologically active. Taken together, the results suggest that the expression of the learning deficit depends on the release of a kappa opioid and that this effect is coupled to a mechanism that effectively maintains the memory for the earlier shock episode.
9.1 Kappa Opioid Pharmacology
Relatively little is known regarding the physiological function of the kappa-2 receptor, which was defined operationally using the opioid bremazocine. Kappa-2 opioid receptor binding refers to the opioid binding that remains when mu, delta, and kappa-1 opioid receptors have been blocked. Research has shown that kappa-1 and kappa-2 opioids have distinct physiological effects. Kappa-1 agonists appear to act presynaptically to inhibit glutamate release, whereas kappa-2 agonists are thought to function post-synaptically to inhibit NMDAR synaptic currents (Caudle et al., 1994, 1997; Caudle, Finegold, Mannes, & Iadarola, 1998; Wagner, Caudle, & Chavkin, 1992; Wagner et al., 1993). At the level of the spinal cord, only the kappa-2 agonist reduces behavioral signs of neuropathic pain without producing antinociception (Eliav et al., 1999; Ho et al., 1997). Interestingly, within the spinal cord, the ratio of kappa-2 to kappa-1 receptors is roughly 10 to 1 (Caudle et al., 1998). Because just one kappa opioid receptor has been cloned, researchers have suggested that the kappa-1/kappa-2 distinction could reflect a post-translational modification, differences in how the kappa receptor interacts with other surface-bound proteins, and/or alternative affinity states (Butelman, Ko, Traynor, Vivian, Kreek, & Woods, 2001; Rusovici, Negus, Mello, & Bidlack, 2004). These alternative hypotheses suggest the interesting possibility that there may be some malleability in receptor function based on a switch between the kappa-1/kappa-2 states. For example, a shift from the kappa-1 to kappa-2 state could generally down-regulate plasticity by fostering an opioid-induced inhibition of NMDAR-mediated plasticity.
Elucidating the physiological role of kappa-1 and kappa-2 receptors has been impeded, in part, by the lack of selective antagonists. Both naltrexone and naloxone share a high affinity for the mu receptor, but also bind to delta and kappa receptors. Nor-BNI, which has been traditionally used to explore the role of kappa opioid receptors, has a higher affinity for the kappa-1 receptor but also acts as an antagonist at the kappa-2 receptor at higher molar concentrations (Rusovici et al., 2004). This cross-reactivity presumably explains why Joynes & Grau (2004) found that the learning deficit was reversed by a moderately high dose of naltrexone or nor-BNI, but not equal molar concentrations of a selective mu or delta antagonist. Here, as in past studies (e.g., Eliav et al., 1999; Ho et al., 1997), coupling our behavioral effects to kappa opioid subtypes has required evaluation of opioid agonists that exhibit more selective binding profiles.
More generally, the specificity of our physiological claims must be tempered by the relative selectivity of our opioid manipulations. In the present paper, neither a mu nor delta agonist impaired instrumental learning, and this remained true even when the drug dose was doubled. It remains possible, however, that further increasing drug dosage could yield an impairment, but the interpretation of this effect would be complicated by an increase in non-specific binding. It is for this reason that we examined both the impact of selective agonists (Experiments 1–6) and antagonists (Joynes & Grau, 2004a). Taken together, the data suggest that the long-term consequences of uncontrollable stimulation on spinal plasticity depend on a ligand that acts at the kappa opioid receptor. This claim does not preclude the possibility that other opioid systems, perhaps regulated by descending pathways, may modulate neural plasticity within spinal cord.
9.2 Impact on NMDAR-Mediated Plasticity
An especially interesting feature of kappa-2 opioids is that they inhibit NMDAR-mediated synaptic plasticity (Caudle et al., 1994, 1997; Ho et al., 1997). Given this, we anticipated that GR89696 would inhibit instrumental learning (Experiments 1 and 2). GR89696 does not, however, simply mimic the effect of an NMDAR antagonist. First, the inhibition of NMDAR-mediated learning was naltrexone-reversible (Experiment 4), implicating the opioid receptor. Second, in contrast to the NMDAR antagonist APV (Joynes et al., 2004), GR89696 did not affect the maintenance of instrumental responding (Experiment 3). The fact instrumental performance was not disrupted is important because it helps to discount an interpretation of our results in terms of a kappa-opioid-induced motor impairment. However, the finding also raises some questions. Following others (Ma & Woolf, 1995), we have suggested that APV may impact the maintenance of instrumental learning because the prolonged neural excitation that drives the response may depend on NMDAR-mediated synaptic currents. If GR89696 indirectly (through the kappa-2 receptor) blocked NMDAR currents, then it too should have disrupted the maintenance of the learned response. The fact it did not suggests that it has a more subtle effect, potentially related to its modulatory action; GR89696 does not block NMDAR current (Ca++) flow but instead, reduces it (Caudle et al., 1994, 1997; Eliav et al., 1999). The remaining Ca++ flow may be sufficient to maintain instrumental performance, but insufficient to enable new synaptic modifications. Both LTP and long-term depression (LTD) depend on an influx of Ca++. As a result, inhibiting Ca++ flow would impede both incremental (LTP) and decremental (LTD) synaptic alterations. These observations suggests an interesting hypothesis regarding kappa-2 opioid function: that kappa-2 opioid activity effectively “locks” the system in its current state, reducing plastic potential so that new perturbances have relatively little impact.
Our hypothesis is consistent with the notion that a kappa opioid (dynorphin) may be released in response to inflammation/injury and serve to limit further increases in neural excitability (Caudle & Mannes, 2000). What is different is that we propose that kappa opioids may also block synaptic alterations that would have the opposite effect, freezing the network in its current state. This would help to explain other aspects of our data. For example, instrumental training can reverse the learning deficit, but only if its conducted in the presence of an opioid antagonist (Crown & Grau, 2001)—for the behavioral therapy to be effective, the opioid lock must be removed. The notion kappa opioids help maintain a form of physiological status quo also helps to explain what otherwise appeared somewhat paradoxical—that administration of a kappa agonist attenuates the induction of the learning deficit as well as instrumental learning. The former is anticipated because the kappa opioid would act to inhibit the NMDAR-dependent modifications that underlie the induction of the learning deficit. By helping to hold the system in its current state, pretreatment with the kappa-2 agonist has a protective effect that works to maintain the capacity for instrumental learning.
Some support for our hypothesis can also be derived from the literature on neuropathic pain (Caudle & Mannes, 2000). Of particular interest, a genetic deletion of the prodynorphin gene does not block the development of neuropathic pain in mice (Sharifi, Diehl, Yaswen, & Brennan, 2001; Wang et al., 2001). The manipulation does, however, cause the behavioral signs of neuropathic pain to wane much more rapidly. It appears that, in the absence of increased kappa-2 opioid activity, the inflammation induced modification is not preserved. This experiment further suggests that dynorphin is not required to observe allodynia. At the same time, it must be acknowledged that a low dose of dynorphin can produce allodynia, but this effect is nonopioid (naloxone-insensitive) in nature (Laughlin, Vanderah, Lashbrook, Nichols, Ossipov, Porreca, & Wilcox, 1997; Vanderah et al., 1996). The fact dynorphin has multiple effects is important because it suggests that our hypothesis regarding kappa-2 opioid function is not necessarily at odds with other data suggesting a link between dynorphin and allodynia (Lai et al., 2001; Malen, Ossipov, Gardell, Ibrahim, Bian, Lai, & Porreca, 2000)—dynorphin may both lock-in the current neural state (by means of the kappa-2 opioid receptor) and contribute to the enhancement of mechanical reactivity (at a nonopioid site). As noted by others (Caudle & Mannes, 2000; Lai et al., 2001), the fact dynorphin acts at multiple sites complicates the analysis of its function. A more tractable scientific question may be the resolution of particular dynorphin-mediated events using agonists/antagonists that target a particular receptor subtype.
9.3 Relation to Learning and Memory
Researchers studying the behavioral correlates of neural plasticity often draw a distinction between learning and memory. Learning refers to the processes involved in producing a neural modification while memory is coupled to the maintenance of this modification over time. Our work suggests that kappa-2 opioids are not needed for learning. Rather, if anything, kappa-2 opioids impede learning, an effect we have suggested might function as kind of physiological lock that helps maintain the system in its current state. Notice, though, that a process that helps preserve a physiological state in the face of new environmental stimulation is not equivalent to a memory; a modification that has yet to be identified must be responsible for engaging the kappa-2 opioid lock. This could reflect either a process that drives increased release of the endogenous ligand (dynorphin) or a modification in receptor function (e.g., a switch in the affinity state from kappa-1 to kappa-2). In the former case, the effective memory is pre-release; in the latter, the effective memory is post-release. Interestingly, in intact subjects, some physiological consequences of uncontrollable stimulation appear to be maintained by a post-release modification in opioid function (Grau, Hyson, Maier, Madden, & Barchas, 1981). Further work is needed to determine whether the same is true within the spinal cord.
The high distribution of kappa-2 opioid receptors within lamina I and II of the dorsal horn (Gouarderes, Audigier, & Cros, 1982; Iadarola, Brady, Draisci, & Dubner, 1988) suggest that GR89696 impacts plasticity at an early (sensory) stage of processing. In addition, the drug may directly, or indirectly, impact motor neurons in deeper lamina. However, in either case, it is clear that GR89696 does not generally inhibit nociceptive signals or motor reactivity for we and others have found no evidence of a drug-induced antinociception or motor impairment (Eliav et al., 1999; Ho et al., 1997). Similarly, shock schedules that inhibit learning appear to enhance, rather than diminish, sensory/motor reactivity (Ferguson et al., 2006). These observations are consistent with our claim that kappa-2 opioid activity may have more to do with the suppression of plasticity than the general regulation of neural excitability. These observations have implications for how we interpret the nature of the learning deficit. Elsewhere, we suggested that it might reflect a diffuse saturation of neural excitability (Ferguson et al., 2006). This was based on the presumed link to central sensitization; in other neural neural systems, a diffuse increase in neural excitability can saturate the system and impede selective synapatic modifications (Moser, Krobert, Moser, & Morris, 1998; Moser & Moser, 1999). Our data implicating the kappa-2 receptor suggest an alternative interpretation of the learning deficit—that it reflects a dampening of plastic potential. A number of observations would appear to favor the latter interpretation. First, other manipulations that dampen neural excitability (e.g., pretreatment with the GABA agonist muscimol) inhibit instrumental learning (Ferguson, Washburn, Crown, & Grau, 2003). Conversely, a manipulation that should foster neural excitability (administration of the GABA-A antagonist bicuculline) restores the capacity for learning in previously shocked animals. Further, evidence suggests that instrumental training counters the adverse effects of uncontrollable stimulation by encouraging the up-regulation of brain-derived neurotrophin factor (BDNF), a neurotrophin that generally enhances neural excitability and enables neural plasticity (Gómez-Pinilla, Huie, Ying, Ferguson, Crown, Baumbauer, Edgerton, & Grau, in press).
A kappa-2 opioid mediated suppression of spinal cord plasticity could subserve an important physiological function in intact animals, helping to preserve acquired motor commands and impeding the development of mal-adaptive processes (e.g., neuropathic pain). In intact animals, spinal motor programs can be modified, but this requires extensive training (Wolpaw & Carp, 1990). We have shown that descending serotonergic fibers engage an intraspinal process that normally inhibits the maladaptive consequences of uncontrollable stimulation (Crown & Grau, 2005). This inhibition of spinal learning may be linked to intraspinal kappa-2 opioid activity. Further research is needed to evaluate this possibility and to explore whether kappa-2 opioids have a similar effect in other neural systems. Interestingly, microinjecting the kappa agonist dynorphin into the hippocampus impairs learning in the Morris water maze (Sandin, Nylander, Georgieva, Schott, Ogren, & Terenius, 1998). Conversely, microinjecting an opioid antagonist into the hippocampus, or genetically deleting the kappa opioid receptor, enhances learning in this task (Gallagher, King, & Young, 1983; Jamot, Matthes, Simonin, Kieffer, & Roder, 2003). However, it remains unclear whether these effects are tied to the kappa-1 or kappa-2 opioid receptor.
9.4 Clinical Implications
We have shown that a kappa-2 opioid agonist inhibits both instrumental learning and the long-term effect of uncontrollable stimulation. We have hypothesized that kappa-2 opioids may have this effect because they generally impede plastic modifications within the spinal cord. This suggests that the clinical usefulness of a kappa-2 agonist will depend on whether or not allowing synaptic modifications would yield an adaptive or maladaptive consequence. In the presence of peripheral inflammation or injury that lead to potentially damaging levels of neural excitation, and the development of neuropathic pain, moderate doses of a kappa-2 agonist could have a beneficial effect (for evidence GR89696 has a protective effect in models of cerebral ischemia, see Birch, Rogers, Hayes, Hayward, Tyers, Scopes, Naylor, & Judd, 1991). In other cases, patients may undergo a behavioral therapy designed to enhance a form instrumental performance. For example, some receive functional electrical stimulation (FES) designed to elicit a particular motor response. FES could yield greater gains, and long-term benefit, if the behavioral regimen involves controllable stimulation (Hook & Grau, in press) and is coupled with a pharmacological blockade of opioid activity to promote adaptive plasticity within the spinal cord.
Acknowledgments
This work was funded by the National Institute of Neurological Disorders and Stroke Grant NS41548. The authors would like to thank Drs. Adam R. Ferguson and Michelle A. Hook, Russell Huie, Christine Petrich, and Cynthia Lin for their comments on an earlier version of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakshi R, Ni RX, Faden AI. N-methyl-D-aspartate (NMDA) and opioid receptors mediate dynorphin-induced spinal cord injury: behavioral and histological studies. Brain Research. 1992;580:255–264. doi: 10.1016/0006-8993(92)90952-6. [DOI] [PubMed] [Google Scholar]
- Birch PJ, Rogers H, Hayes AG, Hayward NJ, Tyers MB, Scopes DI, Naylor A, Judd DB. Neuroprotective actions of GR89696, a highly potent and selective kappa-opioid receptor agonist. British Journal of Pharmacology. 1991;103:1819–1823. doi: 10.1111/j.1476-5381.1991.tb09869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Ko MCH, Traynor JR, Vivian JA, Kreek M-J, Woods JH. GR89,696: A potent K-opioid agonist with subtype selectivity in rhesus monkeys. Journal of Pharmaoclogy and Experimental Therapeutics. 2001;298:1049–1059. [PubMed] [Google Scholar]
- Caudle RM, Chavkin C, Dubner R. Kappa 2 opioid receptors inhibit NMDA receptor-mediated synaptic currents in guinea pig CA3 pyramidal cells. Journal of Neuroscience. 1994;14:5580–5589. doi: 10.1523/JNEUROSCI.14-09-05580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle RM, Finegold AA, Mannes AJ, Tobias MD, Kenshalo DR, Jr, Iadarola MJ. Spinal kappa1 and kappa2 opioid binding sites in rats, guinea pigs, monkeys and humans. Neuroreport. 1998;9:2523–2525. doi: 10.1097/00001756-199808030-00018. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Mannes AJ. Dynorphin: friend or foe? Pain. 2000;87:235–239. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Mannes AJ, Iadarola MJ. GR89,696 is a kappa-2 opioid receptor agonist and a kappa-1 opioid receptor antagonist in the guinea pig hippocampus. Journal of Pharmacology and Experimental Therapeutics. 1997;283:1342–1349. [PubMed] [Google Scholar]
- Coderre TJ. The role of excitatory amino acid receptors and intracellular messengers in persistent nociception after tissue injury in rats. Molecular Neurobiology. 1993;7:229–246. doi: 10.1007/BF02769177. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Preserving and restoring behavioral potential within the spinal cord using an instrumental training paradigm. Journal of Neurophysiology. 2001;86:845–855. doi: 10.1152/jn.2001.86.2.845. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Evidence that descending systems protect behavioral plasticity against the disruptive effect of nociceptive stimulation. Experimental Neurology. 2005;196:164–176. doi: 10.1016/j.expneurol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Crown ED, Joynes RL, Ferguson AR, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behavioral Neuroscience. 2002;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Dickenson AH. Balances between excitatory and inhibitory events in the spinal cord and chronic pain. Progress in Brain Research. 1996;110:225–231. doi: 10.1016/s0079-6123(08)62577-7. [DOI] [PubMed] [Google Scholar]
- Eliav E, Herzberg U, Caudle RM. The kappa opioid agonist GR89,696 blocks hyperalgesia and allodynia in rat models of peripheral neuritis and neuropathy. Pain. 1999;79:255–264. doi: 10.1016/s0304-3959(98)00177-8. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakartne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annual Review of Neuroscience. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Faden AI. Neurotoxic versus neuroprotective actions of endogenous opioid peptides: implications for treatment of CNS injury. NIDA Research Monograph. 1996;163:318–330. [PubMed] [Google Scholar]
- Faden AI, Jacobs TP. Dynorphin-related peptides cause motor dysfunction in the rat through a non-opiate action. British Journal of Pharmacology. 1984;81:271–276. doi: 10.1111/j.1476-5381.1984.tb10074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Washburn SN, Crown ER, Grau JW. GABA-A receptor activation is involved in non-contingent shock inhibition of instrumental conditioning in spinal rat. Behavioral Neuroscience. 2003;117:799–812. doi: 10.1037/0735-7044.117.4.799. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Gallagher M, King RA, Young NB. Opiate antagonists improve spatial memory. Science. 1983;221:975–976. doi: 10.1126/science.6879198. [DOI] [PubMed] [Google Scholar]
- Gouarderes C, Audigier Y, Cros J. Benzomorphan binding sites in rat lumbo-sacral spinal cord. European Journal of Pharmacology. 1982;78:483–486. doi: 10.1016/0014-2999(82)90494-0. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Huie JR, Ying Z, Ferguson A, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and learning: Evidence that instrumental training promotes learning within the spinal Cord by up-regulating BDNF expression. Neuroscience. doi: 10.1016/j.neuroscience.2007.05.051. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behavioral Neuroscience. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: Underlying mechanisms and implications for recovery after injury. Behavioral and Cognitive Neuroscience Reviews. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Hyson RL, Maier SF, Madden J, Barchas JD. Long-term stress-induced analgesia and activation of the opiate system. Science. 1981;213:1409–1411. doi: 10.1126/science.7268445. [DOI] [PubMed] [Google Scholar]
- Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC. Uncontrollable nociceptive stimulation undermines recovery after spinal cord injury. Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Ho J, Mannes AJ, Dubner R, Caudle RM. Putative kappa-2 opioid agonists are antihyperalgesic in a rat model of inflammation. Journal of Pharmacology and Experimental Therapeutics. 1997;281:136–141. [PubMed] [Google Scholar]
- Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Medicine and Science in Sports and Exercise. 1994;26:1491–1497. [PubMed] [Google Scholar]
- Hook MA, Grau JW. An animal model of functional electrical stimulation: Evidence that the central nervous system modulates the consequences of training. Spinal Cord. doi: 10.1038/sj.sc.3102096. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. doi: 10.1037/0735-7044.122.1.233. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- Jamot L, Matthes HWD, Simonin F, Kieffer BL, Roder JC. Differential involvment of the mu and kappa opioid receptors in spatial learning. Genes, Brain and Behavior. 2003;2:80–92. doi: 10.1034/j.1601-183x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends in Neurosciences. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Grau JW. Instrumental learning within the spinal cord: III. Prior exposure to noncontingent shock induces a behavioral deficit that is blocked by an opioid antagonist. Neurobiology of Learning and Memory. 2004;82:35–51. doi: 10.1016/j.nlm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Janjua K, Grau JW. Instrumental learning within the spinal cord: VI. The NMDA receptor antagonist, AP5, disrupts acquisition and maintenance of an acquired flexion response. Behavioural Brain Research. 2004;154:431–438. doi: 10.1016/j.bbr.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Lai J, Ossipov MH, Vanderah TW, Malan TP, Jr, Porreca F. Neuropathic pain: the paradox of dynorphin. Molecular Interventions. 2001;1:160–167. [PubMed] [Google Scholar]
- Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL. Spinally administered dynorphin A produces a long-lasting allodynia: Involvement of NMDA but not opioid receptors. Pain. 1997;7:253–260. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Liu GT, Crown ED, Miranda RC, Grau JW. Instrumental learning within the rat spinal cord: Localization of the essential neural circuit. Behavioral Neuroscience. 2005;119:538–547. doi: 10.1037/0735-7044.119.2.538. [DOI] [PubMed] [Google Scholar]
- Long JB, Petras JM, Mobley WC, Holaday JW. Neurological dysfunction after intrathecal injection of dynorphin A(1-13) in the rat. II. Nonopioid mechanism mediate loss of motor, sensory and autonomic function. Journal of Pharmacology and Experimental Therapeutics. 1988;88:1167–1174. [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Noxious stimuli induce an N-methyl-D-aspartate receptor-dependent hypersensitivity of the flexion withdrawal reflex to touch: implications for the treatment of mechanical allodynia. Pain. 1995;61:383–390. doi: 10.1016/0304-3959(94)00195-K. [DOI] [PubMed] [Google Scholar]
- Malen TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injurred rats. Pain. 2000;86:185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB. Is learning blocked by saturation of synaptic weights in the hippocampus? Neuroscience Biobehavioral reviews. 1999;23:661–672. doi: 10.1016/s0149-7634(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Rusovici DE, Negus SS, Mello NK, Bidlack JM. K-Opioid receptors are differentially labeled by arylacetamides and benzomorphans. European Journal of Pharmacology. 2004;485:119–125. doi: 10.1016/j.ejphar.2003.11.078. [DOI] [PubMed] [Google Scholar]
- Sandin J, Nylander I, Georgieva J, Schott PA, Ogren SO, Terenius L. Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience. 1998;85:375–382. doi: 10.1016/s0306-4522(97)00605-2. [DOI] [PubMed] [Google Scholar]
- Sharifi N, Diehl N, Yaswen L, Brennan MB. Generation of dynorphin knockout mice. Molecular Brain Research. 2001;86:70–75. doi: 10.1016/s0169-328x(00)00264-3. [DOI] [PubMed] [Google Scholar]
- Stevens CW, Yaksh TL. Dynorphin A and related peptides administered intrathecally in the rat: A search for putative kappa opiate receptor activity. Journal of Pharmacology and Experimental Therapeutics. 1986;238:833–838. [PubMed] [Google Scholar]
- Terman GW, Drake CT, Simmons ML, Milner TA, Chavkin C. Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. Journal of Neuroscience. 2000;20(43):79–4388. doi: 10.1523/JNEUROSCI.20-12-04379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman GW, Wagner JJ, Chavkin C. Kappa opioids inhibit induction of long-term potentiation in the dentate gyrus of the guinea pig hippocampus. Journal of Neuroscience. 1994;14:4740–4747. doi: 10.1523/JNEUROSCI.14-08-04740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malen TP, Porreca F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: Blockade by MK-801 but not naloxone. Pain. 1996;68:275–281. doi: 10.1016/s0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Caudle RM, Chavkin C. Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hippocampus. Journal of Neuroscience. 1992;12:132–141. doi: 10.1523/JNEUROSCI.12-01-00132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. Journal of Neuroscience. 2001;21:279–286. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. European Journal of Neuroscience. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Willis WD. Mechanisms of central sensitization of nociceptive dorsal horn neurons. In: Patterson MM, Grau JW, editors. Spinal cord plasticity: Alterations in reflex function. Kluwer Academic Publishers; 2001. [Google Scholar]
- Willis WD, Sluka KA, Rees H, Westlund KN. Cooperative mechanisms of neurotransmitter action in central nervous sensitization. Progress in Brain Research. 1996;110:151–166. doi: 10.1016/s0079-6123(08)62572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Carp JS. Memory traces in spinal cord. Trends in Neuroscience. 1990;13:137–142. doi: 10.1016/0166-2236(90)90005-u. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation: implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiology and Behavior. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain kappa opioid receptors: evidence for kappa 1 and kappa 2 opioid receptors. Proceedings of the National Academy of Sciences U S A. 1988;85:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]