Abstract
The common marmoset (Callithrix jacchus) is a New World primate species that is highly susceptible to fatal infections caused by various strains of bacteria. We present here a first step in the molecular characterization of the common marmoset’s Mhc class II genes by nucleotide sequence analysis of the polymorphic exon 2 segments. For this study, genetic material was obtained from animals bred in captivity as well as in the wild. The results demonstrate that the common marmoset has, like other primates, apparently functional Mhc-DR and -DQ regions, but the Mhc-DP region has been inactivated. At the -DR and -DQ loci, only a limited number of lineages were detected. On the basis of the number of alleles found, the -DQA and -B loci appear to be oligomorphic, whereas only a moderate degree of polymorphism was observed for two of three Mhc-DRB loci. The contact residues in the peptide-binding site of the Caja-DRB1*03 lineage members are highly conserved, whereas the -DRB*W16 lineage members show more divergence in that respect. The latter locus encodes five oligomorphic lineages whose members are not observed in any other primate species studied, suggesting rapid evolution, as illustrated by frequent exchange of polymorphic motifs. All common marmosets tested were found to share one monomorphic type of Caja-DRB*W12 allele probably encoded by a separate locus. Common marmosets apparently lack haplotype polymorphism because the number of Caja-DRB loci present per haplotype appears to be constant. Despite this, however, an unexpectedly high number of allelic combinations are observed at the haplotypic level, suggesting that Caja-DRB alleles are exchanged frequently between chromosomes by recombination, promoting an optimal distribution of limited Mhc polymorphisms among individuals of a given population. This peculiar genetic make up, in combination with the limited variability of the major histocompatability complex class II repertoire, may contribute to the common marmoset’s susceptibility to particular bacterial infections.
The Mhc is a cluster of loci coding for polymorphic glycoproteins that provide the context for the recognition of antigens by T lymphocytes and is thought to be present in most, if not all, vertebrate species (1). There are two major types of gene products, named major histocompatability complex (MHC) class I and II molecules. The classical MHC class I molecules are expressed on virtually all nucleated cells and present peptides from intracellular origin to CD8-positive T cells. Such peptides usually originate from intracellular parasites and viruses, and recognition may result in the lysis of the infected target cell. The MHC class II molecules are heterodimeric structures showing restricted tissue distribution. MHC class II molecules present peptides of extracellular origin to T cells of the helper phenotype. Activation of these cells often results in cytokine release leading to a variety of effects, such as antibody production.
In humans, the class I region contains at least 18 highly related genes, which include those encoding the highly polymorphic transplantation antigens (HLA-A, -B, and -C) and the oligomorphic nonclassical HLA-E, -F, -G, -H, and -J genes (2). Some of the latter show differential tissue distribution, whereas others are pseudogenes. The human class II region is arranged into the HLA-DP, -DQ, and -DR regions, each containing at least one pair of A and B genes encoding the α and β polypeptide chains (3). Most nucleotide sequence variability is confined to exon 2 of the Mhc-DPB, -DQA, -DQB, and -DRB genes. Recent studies have demonstrated that humans, apes, and Old World monkeys (Catarrhini) share numerous Mhc class I and II loci (3–9). Such studies also have illustrated that many Mhc class I and II lineages predate the speciation of the contemporary living primate species (10–14). In particular, some of the Mhc class II lineages are extremely stable and may be >35 million-years-old. Mhc class I lineages, on the other hand, seem to evolve much more rapidly (15–16).
Numerous duplications and condensations took place during the evolution of the MHC region, and most species possess Mhc genes, which display extensive degrees of polymorphism. There are, however, exceptions. In cotton-top tamarins (Saguinus oedipus), low variability was reported for the Mhc class I region, as evidenced by sequencing and immunoprecipitation studies (17–19). The Mhc class II region of this species exhibits abundant polymorphism, and at least 50 alleles have been described thus far (19–20). The cheetah (Acinonyx jubatus) and two species of mole-rat (Heterocephalus glaber and Spalax leucodon) are thought to have condensed Mhc repertoires. Unfortunately, detailed nucleotide sequence analysis of the Mhc genes of these species is lacking (21–23). The Syrian hamster was reported to have a monomorphic Mhc class I region, but this claim may be the result of a sampling error (24–25).
The Callitrichidae family of New World monkeys (Platyrrhini) comprises marmosets and tamarins, which are used in biomedical research as models for several human diseases, e.g., multiple sclerosis (26). Nonidentical twins are born as natural bone-marrow chimeras because of a sharing of the placental circulation. Their apparently increased susceptibility to several viral, bacterial, protozoan, and helminth agents has been documented (27). In some cases, such as incidence of ulcerative colitis, Epstein–Barr virus, Herpes virus saimiri, and tamarinus infections, there is compelling evidence for a more resistant status of the common marmoset as compared with the cotton-top tamarin. The increased susceptibility of the cotton-top tamarin to viral infections, in particular, may be due to a severely condensed Mhc class I repertoire (17–19). On the other hand, common marmosets seem to be more susceptible than tamarins to fatal infections caused by bacteria such as Klebsiella, Bordetella, Clostridium, and Shigella (27). For that reason, we wished to investigate the variability of the Mhc class II region of the common marmoset. In this context, genetic variability is defined as the number of allelic lineages and alleles per locus, the number of Mhc class II genes present per haplotype, and allelic combinations seen at the haplotypic level also were included in this analysis.
MATERIALS AND METHODS
Animals.
The Biomedical Primate Research Centre houses a common marmoset colony of a≈100 pedigreed individuals. New animals were introduced on several occasions to maintain the outbred character of the colony. For this study, 25 monkeys, eight of them consisting of four pairs of twins, were selected. They are recorded descendants of individuals from two former Dutch colonies, one Italian colony, and at least one German colony. Hence, the present selection of animals is considered to reflect a representative sample. To investigate the possibility of inbreeding or potential founder effects, four common marmosets from a Brazilian colony (Primatology Nucleus, Universidade Federal de Rio Grande do Norte, Brazil) were examined. Two of these animals were born in the wild.
DNA Isolation and PCR.
B lymphoblastoid cell lines were established from 2-ml blood samples by transformation with a cotton-top tamarin Epstein–Barr virus (B95–8) -producing cell line. These cells were used for genomic DNA isolation as described previously (28). In the case of individuals for which the B cell lines were lacking, DNA isolation was conducted on tissue samples such as frozen spleen and lymph nodes, whereas for the Brazilian animals, 2 ml of peripheral blood was used. Exon 2 of the Caja class II genes was amplified by the PCR methodology (29). Negative controls lacking DNA were part of all experiments, and whenever necessary a positive control was included by using samples of rhesus macaque DNA. The primers and protocols used for amplification of the Mhc class II A and B genes were reported previously (19–20, 30–35).
Typing for Caja-DPB and -DPA-like PCR Products.
DPB and DPA probes, spanning highly conserved regions in the DPB (Caja-DPB-5′-YAACAGCCAGAAGGAC-3′) and DPA (Caja-DPA-5′-CTGGAGGAGTTTGGCCGAGCC-3′) sequences of all primate species studied so far, were used to detect amplified PCR products. One to three microliters of each sample was bound to a nylon membrane (Hybond-N, version 2.0, Amersham) and subsequently crosslinked (Bio-Rad GS Gene Linker UV Chamber) at 250 mJ. Prehybridization took place for 30 min at 58°C (Hybridization Oven, New Brunswick Scientific) in 5 ml of 3 M tetramethylammonium chloride, 5 mM EDTA, 50 mM Tris (pH 7.5), and 1% SDS (TMACl) with denatured herring sperm (1 mg/5 ml). For hybridization, 1 pmol of each oligo was added per milliliter of TMACl, for at least 1 hr, at 60°C (-DPA) and 50°C (-DPB). After hybridization, each filter was washed 20 min at 61°C (-DPA) and 51°C (-DPB) in TMACl. Filters were washed afterward twice for 5 min in 1*standard saline phosphate (SSPE) and 0.1% SDS and incubated for 10 min with 1 μg Streptavidin Horseradish Peroxidase Conjugate (GIBCO/BRL) per milliliter of washing buffer. Washing was done as before, and then the membranes were incubated for 5 min in blockbuffer (50 ml of Triton X-100/60 g urea/5.8 g NaCl/10 g dextransulfate, together 1,000 ml, pH 7.3). After further washing, nucleic acids were detected by incubation with ECL detection reagents (Amersham) for 1 min at room temperature; the blots were placed in a cassette with a sheet of autoradiography film for 10 min.
Nucleotide Sequencing.
PCR-amplified DNA was prepared for sequencing, as described (28). In brief, a digestion with appropriate endonuclease restriction enzymes was carried out at 37°C, for 2 hr: PstI and BamHI for GH26/GH27, GH98/GH99, and DB01/DB03; SalI and XbaI for 5′-DQB SalI/3′-DQB XbaI, and 5′-DPB SalI/3′-DPB XbaI; and SalI and BamHI for Tu215/Tu216 (all enzymes from GIBCO/BRL). The PCR fragments were then cloned into bacteriophage M13 derivatives mp18 and mp19 (vectors from Boehringer Mannheim GmbH) and sequenced by using the Sequenase Version 2.0 DNA Sequencing Kit (Amersham). The reported alleles represent at least three identical clones that were obtained after independent amplifications or in different animals.
Sequence Specific Oligonucleotide Typing for Caja-DQA1 and -DQB1 Alleles.
After analyzing the Caja-DQ locus sequences, we developed an oligotyping method to assay the presence or absence of the known Caja-DQ alleles. The biotinylated oligos used were: for Caja-DQA1*0101, 5′-YCTCGCTGTGTCAAAACACAAC-3′; Caja-DQA1*2501, 5′-YATCGCTACGATGAAACCCGGC-3′; Caja-DQB1*2201, 5′-YCCGCTTGTGACCCGATTCATCTAT-3′; Caja-DQB1*2301, 5′-YTTTAAGGGTTTCTGCTAC-3′; Caja-DQB1*2302, 5′-YTTTAAGTTTCTCTGCTAC-3′; Caja-DQB2*0101, 5′-YCGGGCGGTGACCGAG-3′; and Caja-DQB2*0102, 5′-CGGGCGATGACCGAG-3′. The technical procedure was the same as described in the section “Typing for DPB and DPA-like PCR products.” Hybridization and washing temperatures were, respectively, 58–59°C (-DQA1, -DQB1*2201), 50–51°C (-DQB1*2301), 48–49°C (-DQB1*2302), 49–50°C (-DQB2*0101), and 45–46°C (-DQB2*0102).
Nomenclature.
Marmoset alleles that are similar to human equivalents are depicted by identical lineage numbers. For instance, the Caja-DRB1*03 and HLA-DRB1*03 alleles group into the same lineage. The last two digits are arbitrary and reflect the order in which the alleles were found. The marmoset lineages for which no apparent human equivalent has been identified are designated by a workshop number, such as Caja-DRB*W12. This also indicates that the physical location has not been mapped.
RESULTS AND DISCUSSION
Loci and Lineages
Caja-DQA and -DQB.
After PCR amplification, only two orthologues of HLA-DQA1 were identified in our population of marmosets. Phylogenetic analyses demonstrated that both Caja-DQA1 alleles cluster into separate lineages and are very similar to sequences obtained from an undefined species of marmoset (36). The Caja-DQA1*0101 allele clusters together with equivalents from Old and New World monkeys, apes, and humans: hence, the Mhc-DQA1*01 lineage appears to be at least 58 millions of years old. The Caja-DQA1*2501 allele groups into a younger lineage that seems to be restricted to New World monkeys. The inferred amino acid sequences of Caja-DQA1*0101 and -DQA1*2501 alleles are depicted in Fig. 1a. Only three Caja-DQB1 alleles were detected in our colony. The Caja-DQB1*2201, -DQB1*2301, and -DQB1*2302 (Fig. 1b) alleles group into two lineages earlier observed in cotton-top tamarins (19, 32). However, two alleles were discovered that encode the same Caja-DQB1*2201 amino acid sequence, and differ only for one synonymous substitution at position 168 (CCG→CCC).
Figure 1.

Alignment of the Caja-DQA- (1a), -DQB- (1b) and -DRB- (1c) deduced amino acid sequences given in the one-letter code. A dash or an asterisk indicates identity with the consensus or deletion of an amino acid, respectively. Unreported sequences are indicated by an N. Colored boxes highlight motifs shared between the lineages of the -DRB1*03 and DRB*W16 loci. The Caja-DRB*W1201 sequence in this report replaces the one reported by Trtková (20). †The Caja-DQB1*2201 amino acid sequence is encoded by two different nucleotide sequences that differ only for one synonymous substitution at position 168 (CCG→CCC).
No homologue of the HLA-DQA2 exon 2 gene segment was recovered, as is in agreement with data obtained from studies on other New World monkeys (19, 33, 35). The two alleles retrieved from the common marmoset DQB2 locus were named Caja-DQB2*0101 and -DQB2*0102 (Fig. 1b) and share a high degree of similarity with their human equivalents. Although Mhc-DQB2 is considered to be a pseudogene locus, the present results underline its high degree of conservation and antiquity.
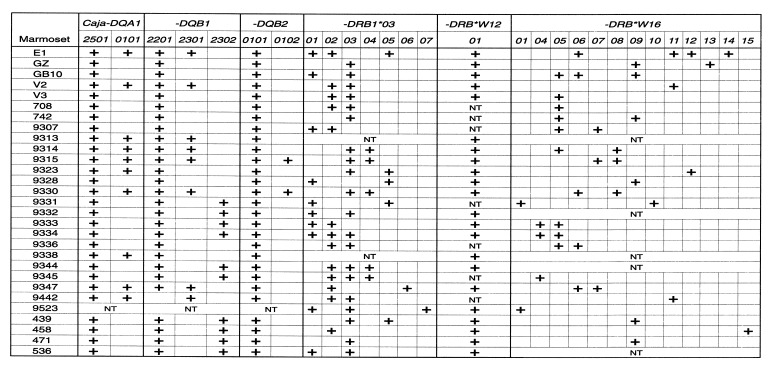
On the basis of the nucleotide sequence information, a sequence-specific oligotyping method was developed that allowed us to type all animals for their DQ loci (Fig. 2). As can be seen, some animals, like V3 and 742, appear to be homozygous for particular Caja-DQ entities. All animals were found to be Caja-DQA1*2501 positive whereas only animal 9442 lacked the Caja-DQB1*2201 allele. Segregation data suggest that the Caja-DQA1*2501 allele is preferentially linked to the -DQB1*2201 and -DQB1*2302 alleles, whereas the Caja-DQA1*0101 allele is linked to -DQB1*2301 (Fig. 2). Exceptions to the rule exist as is evidenced by animals 9323, 9338, and 9442. It should be stressed, however, that the detection of only two Caja-DQA1, three -DQB1, and two (nonfunctional) -DQB2 alleles in common marmosets is in contrast to the observations concerning abundant polymorphism in populations of other primate species, such as humans, chimpanzees, and rhesus macaques (2–6). An overview of these data has been provided in Table 1.
Figure 2.
Distribution of alleles at the Caja-DQA1, -DQB1, -DQB2, -DRB1*03, and DRB*W16 loci in 29 common marmosets. Pairs 9314/9315, 9331/9332, 9333/9334, and 9344/9345 are twins. Animals 439, 458, 471, and 536 are from the Brazilian colony; 439 and 536 were born in the wild. NT, nontested. In the case of -DRB, the alleles have been detected by nucleotide sequence analysis after PCR amplification. This means that some -DRB alleles may not have been amplified and that their presence was not detected. This phenomenon explains the heterogeneity in the number of -DRB alleles as depicted in the last two columns of this table. The presence of Caja-DRB*W1201 also was confirmed by denaturing gradient gel electrophoresis.
Table 1.
Distribution of number of alleles at some Mhc-DRB, -DQA, -DQB, -DPA, and -DPB loci/lineages among different populations of primate species
| Mhc loci/lineages | HLA | Patr | Mamu | Saoe | Caja |
|---|---|---|---|---|---|
| DRB1*01/10 | 5 | 1 | 7 | — | — |
| DRB1*02 | 17 | 11 | — | — | — |
| DRB1*03† | 133 | 10 | 17 | 5 | 7 |
| DRB1*04 | 26 | — | 6 | — | — |
| DRB1*07 | 1 | 2 | 1 | — | — |
| DRB1*09 | 1 | — | — | — | — |
| DRB3 | 11 | 16 | 5 | 16 | — |
| DRB4 | 9 | 7 | 1 | — | — |
| DRB5 | 12 | 15 | 5 | 1 | — |
| DRB6 | 3 | 15 | 14 | — | — |
| DRB7 | 2 | 1 | — | — | — |
| DRB11 | — | — | — | 10 | — |
| DRB*W1–7 | — | — | 29 | — | — |
| DRB*W8 | — | 1 | — | — | — |
| DRB*W9 | — | 5 | — | — | — |
| DRB*W12 | — | — | — | 4 | 1 |
| DRB*W16 | — | — | — | — | 13 |
| DRB*W22 | — | — | — | 9 | — |
| DQA1 | 18 | 5 | 15 | 2 | 2 |
| DQB1 | 31 | 12 | 28 | 2 | 3 |
| DPB1 | 77 | 28 | 15 | 1‡ | — |
| DPA1 | 10 | 3 | 1 | NT | — |
The data were taken from ref. 44 and the nonhuman primate MHC database (NHPDB) that is maintained at the Biomedical Primate Research Centre. HLA, human; Patr, common chimpanzee; Mamu, rhesus macaque; Saoe, cotton-top tamarin; and Caja, common marmoset.
This lineage includes the HLA-DRB1 genes encoded by HLA-DR3, -DR5, DR6, and DR8 positive individuals.
Only one individual studied (33).
Caja-DP.
Despite the fact that several sets of primers were tested in combination with different PCR programs, we failed to clone exon 2 of the Caja-DPB1 gene. Although PCR products were obtained occasionally, screening with several conserved primate-specific Mhc-DPB1 probes excluded the presence of relevant exon 2 nucleotide sequences. In contrast, successful amplification was obtained in cotton-top tamarins, even with the primers designed for HLA-DPB genes (33). Subsequent Southern blot experiments with conserved Mhc-DPB1 exon 2 sequences as probes failed to prove the existence of bona fide Caja-DPB1 genes. We therefore conclude that the Caja-DPB1 gene, if present, has at least a defunct exon 2 gene segment and is probably inactivated. Studies are underway to determine which genetic defect(s) affect the Caja-DPB1 gene. Regarding Caja-DPA1, only extremely low amounts of exon 2 sequences were amplified, as was evidenced by screening with the relevant probe (data not shown). The same primers, however, worked very efficiently in amplifying Mhc-DPA1 exon 2 sequences derived from other New World monkey species (34). The detection of low amounts of the Caja-DPA1 PCR product again suggests that the Caja-DP region may have an altered appearance in common marmosets. In all higher primate species studied thus far, three distinct functional Mhc class II regions have been detected: namely -DR, -DQ, and -DP. The common marmoset is the first example of a higher primate species in which the equivalents of HLA-DR and -DQ seem to represent the main types of functional MHC class II molecules.
Caja-DRB.
In excess of 500 clones from >30 individuals were sampled. This resulted in the description of 21 DRB sequences (Fig. 1c), confirming two of six previously reported, designated Caja-DRB1*0301 and -DRB*W1601 (20). In contrast, the existence of the Caja-DRB11*0101, -DRB*W1201, -DRB*W1602, and -DRB*W1603 alleles was not confirmed (20). To clarify this issue, samples from the same animals as used in the study by Trtková and coworkers or marmosets related in blood line were (re)analyzed with different primer sets. Again, no evidence was found for the existence of the four above-mentioned alleles, which may represent in vitro PCR artifacts. Consequently, these alleles were not listed in the alignment, except for DRB*W1201, which is a new allele, encoded by a different locus, replacing the one previously reported (Fig. 1c).
In the case of a heterozygous marmoset, one would expect to find a maximum of two alleles per locus. Marmosets are born as natural bone-marrow chimeras and therefore may share blood cells with their nonidentical twin(s). Consequently, some animals have in their blood circulation cells possessing DRB genes that were not inherited in a Mendelian fashion. This situation is evidently applicable to the three Caja-DRB1*03 and four -DRB*W16 lineage members detected in animal E1 (Fig. 2). All marmosets tested were found to share the Caja-DRB*W1201 allele. On the basis of segregation studies and nucleotide sequence comparisons, it seems likely that the Caja-DRB*W1201 allele is controlled by a separate locus. Detailed contig mapping studies are needed to confirm this assumption, which, if true, indicates that the common marmoset is the first known example of a species that possesses a monomorphic DR locus that is present in all individuals.
With regard to the level of polymorphism, only 7 Caja-DRB1*03 and 13 -DRB*W16 alleles were detected. In one of the four Brazilian animals, Caja-DRB*W1615 was found, which appears to be absent in the individuals from the Biomedical Primate Research Centre population. This illustrates that the Biomedical Primate Research Centre captive bred population does not differ significantly from other common marmoset populations. To obtain these results, different sets of primers were used. This was done to minimize the chance that not all DRB loci were amplified. Our results contrast the observations done on the MHC Class II repertoire for another New World monkey species, the cotton-top tamarin (Table 1). The question is whether the number of Caja-DRB alleles detected indicates a low, moderate, or high degree of polymorphism. Thus far, only a few populations of primate species, such as humans, common chimpanzees, rhesus macaques, and cotton-top tamarins, have been tested thoroughly for Mhc-DRB loci diversity at the nucleotide sequence level. Compared with these species the common marmoset displays a low or moderate degree of polymorphism at the DRB loci (Table 1).
Convergence, Divergence, and Conservation
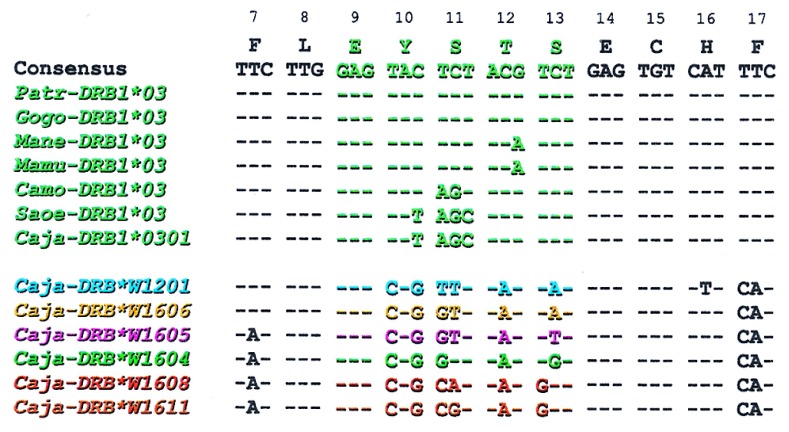
The seven Caja-DRB1*03 alleles share the EYSTS amino acid motif at positions 9–13, characteristic of the lineage. The importance of Mhc-DRB1*03 is highlighted by the observation that the lineage is evolutionarily stable and its members have been detected in many species and play an important role in mounting immune response against mycobacteria (37). Alternatively, if a certain motif is functionally of relevance, it may be generated de novo by convergent evolution as appears to be the case for the HLA-DRB1*03-like lineages in New World monkeys. First of all, in the amino acid motif EYSTS, the serine residue at position 11 can use two different codons. The Catarrhini use the TCT codon, whereas the Platyrrhini make use of codons AGT and AGC (Fig. 3). The EYSTS motif is present also in DRB allomorphs of bovids, equids, and murids, where again differential codon usage indicates convergent evolution (38–40). Secondly, all the platyrrhine Mhc-DRB sequences described thus far have a characteristic motif at the 3′ end of exon 2, which suggests a common ancestry. Finally, analysis of intron 2, exon 3, and the 3′-untranslated region of DRB sequences does not reveal an orthologous relationship of New World monkey DRB genes to any of the known catarrhine DRB genes (41). Thus the New World monkey DRB genes seem to originate from a different ancestor than the progenitor of the catarrhine DRB genes.
Figure 3.
Nucleotide sequences of the EYSTS amino acid motif as encoded in different primate species, of the infra-orders Catarrhini (Patr, common chimpanzee; Gogo, gorilla; Mamu, rhesus macaque; and Mane, pig-tailed macaque) and Platyrrhini (Camo, capuchin monkey; Saoe, cotton-top tamarin; and Caja, common marmoset). In addition, the different DNA motifs that are observed at the Caja-DRB*W12 and -DRB*W16 loci are shown. The motifs are depicted in color code and correspond with the alleles listed in Fig. 1c and the phylogenetic tree in Fig. 4.
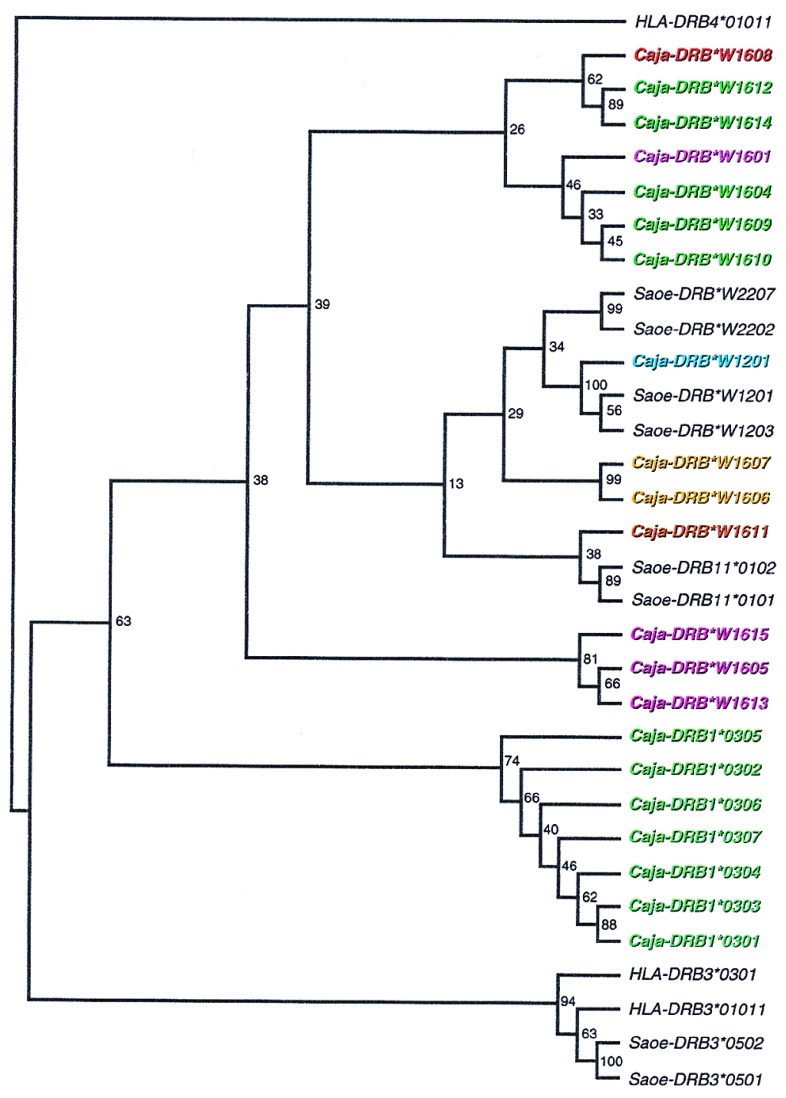
In the case of the Caja-DRB1*03 lineage, the contact residues in the β pleated sheets of the peptide-binding site are highly conserved. In this respect, more variation is seen in the Caja-DRB*W16 lineage, in which five different motifs are present in the first β-pleated sheet section (Figs. 1c and 3). Nevertheless, all the alleles assigned to the Caja-DRB*W16 lineage share an identical DNA motif at codons 15–25, reflecting their common ancestry. Most of the variation seen at the codons for the contact residues within the first β-pleated sheet of the Caja-DRB*W16 lineage can be explained by the occurrence of simple point mutations (Fig. 3). This indicates that positive selection for diversity has been acting on the Caja-DRB*W16 sequences. Many Caja-DRB*W16 lineage members share patchwork motifs that apparently have been exchanged between alleles, probably as a result of recombination-like events as indicated by colored boxes (Fig. 1c). Some of these motifs are even shared between two different DRB loci. This concerted action of point mutations in combination with frequent recombination promotes fast evolution of the Caja-DRB*W16 locus. Similar findings concerning rapid evolution have been reported for the Mhc-B (15–16, 42) and -DP (31, 43) loci in primates. Phylogenetic analyses demonstrate that the alleles at the Caja-DRB*W16 locus cluster into different lineages, each with a remarkably low number of alleles (Fig. 4). The observation that those lineages defined by the various peptide-binding site motifs as depicted in the same color code in Figs. 3 and 4 are arranged in different branches of the phylogenetic tree confirms that recombination-like events must have occurred.
Figure 4.
Phylogenetic tree constructed according to the Neighbor-joining method showing the evolutionary relationships between DRB alleles from New World monkeys. Bootstrap values are indicated. The names of the Caja-DRB alleles are depicted in colors. These correspond to the color code used in Figs. 1c and 3.
Haplotypes
The primate Mhc-DR region contains one monomorphic A gene, whereas the number of B genes varies between haplotypes and species. As a consequence, the primate DRB region displays haplotype polymorphism (45–46). Although rare, DR regions or even extended regions can be shared in a similar fashion between species, as has been documented for humans and some ape species (3–4). All common marmosets seem to share a monomorphic Caja-DRB*W12 locus, a locus controlling the more or less conserved Caja-DRB1*03 alleles and one locus encoding the highly divergent DRB*W16 lineage members (Fig. 2). This suggests that all common marmosets tested possess DR regions that harbor only three DRB loci and lack haplotype polymorphism. Although pedigree data are available, a wide variety of allelic combinations, especially at the DR loci, prohibits the definition of haplotypes (Fig. 2). At this stage, the possibility cannot be excluded that different Caja-DRB genes are located on distinct chromosomes. On the other hand, alleles encoded in a cis configuration may be exchanged frequently between chromosomes by recombination, possibly enhanced due to a high degree of similarity between the loci. This may reflect an optimal strategy to spread the limited number of allelic polymorphisms at the population level in common marmosets.
Chimerism and Disease Susceptibility
In theory, condensation of MHC variability may have been caused by inbreeding, a founder effect, by selective pressures such as diseases or by a combination of these factors. The sampling procedure excludes the possibility that the observed condensed Caja class II repertoire resulted from inbreeding. The chimeric status of the Callitrichidae is thought to have an impact on the condensed Mhc class I repertoire (17, 18), but this is not the case for the class II region in cotton-top tamarins, which displays abundant polymorphism (Table 1). Despite the limited MHC class II variation seen at the population level, some chimeric marmosets (E1) express up to eight different types of MHC-DR molecules (Fig. 2). Such a situation must have an impact on the selection of T cells in the thymus. If, due to the presence of multiple MHC molecules, too many T cells are deleted, an individual may become susceptible to infectious diseases. At some stage, a delicate balance should be reached between the number of MHC molecules present in a individual and the capacity to respond adequately to foreign pathogens. Common marmosets live in relatively small topographic pockets and have adapted to their environment. When taken out of their original habitat, they may, under stressful situations, encounter new pathogens with which they have not learned to cope. That common marmosets are especially prone to die from particular bacterial and helminthic diseases (27) is in line with the present observation that this species possesses limited MHC class II variability. Moreover, all common marmosets tested were found to share the monomorphic Caja-DRB*W1201 allele. In the marmoset’s natural habitat, the wide distribution of this monomorphic DR molecule was probably positively selected because it controlled an important protective function. When common marmoset populations encounter new pathogens, however, such as particular bacterial infections, the presence of a monomorphic immune response gene may turn to their profound disadvantage, especially when such a locus controls a susceptibility trait. The result may be the decimation of a given population of individuals, or in the most severe case, the extermination of a species.
Acknowledgments
During the course of this study, Susana G. Antunes died tragically on July 1, 1997. To the memory of Sue, whom we cherished for her personal and intellectual qualities, the coauthors lovingly dedicate this manuscript. The authors would like to thank Henk van Westbroek for preparation of the figures, Donna Devine for editing, and Dr. Maria de Sousa, Dr. Jon J. van Rood, Dr. Bert ‘t Hart, and Dr. Jim Kaufman for support and helpful comments. This study was financially supported by the MACROPA Foundation, the European Union human, capital and mobility Grant CHGE-CT94-0071 (DG 12 COMA), and Junta Nacional de Investigacio Cientifica e Technológica (Grants PRAXIS XXI BM/2375/94 and PRAXIS XXI BD/9588/96).
ABBREVIATION
- MHC
major histocompatability complex
Footnotes
References
- 1. Kaufman J, Skjoedt K, Salomonsen J. Immunol Rev. 1990;113:83–117. doi: 10.1111/j.1600-065x.1990.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 2.Parham P, Ohta T. Science. 1996;272:67–74. doi: 10.1126/science.272.5258.67. [DOI] [PubMed] [Google Scholar]
- 3.Klein J, Satta Y, O’hUigin C, Takahata N. Annu Rev Immunol. 1993;11:269–295. doi: 10.1146/annurev.iy.11.040193.001413. [DOI] [PubMed] [Google Scholar]
- 4.Bontrop R E, Otting N, Slierendregt B L, Lanchbury J S. Immunol Rev. 1995;143:33–62. doi: 10.1111/j.1600-065x.1995.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein J, O’hUigin C. Immunol Rev. 1995;143:89–111. doi: 10.1111/j.1600-065x.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 6.Watkins D. Crit Rev Immunol. 1995;15:1–29. doi: 10.1615/critrevimmunol.v15.i1.10. [DOI] [PubMed] [Google Scholar]
- 7.Lawlor D A, Warren E, Ward F E, Parham P. Immunol Rev. 1990;113:147–185. doi: 10.1111/j.1600-065x.1990.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 8.Klein J. Hum Immunol. 1987;19:155–162. doi: 10.1016/0198-8859(87)90066-8. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa F, Gunther E, Klein J. Nature (London) 1988;355:265–267. doi: 10.1038/335265a0. [DOI] [PubMed] [Google Scholar]
- 10.Lawlor D A, Ward F E, Ennis P E, Jackson A P, Parham P. Nature (London) 1988;335:268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- 11.Gyllensten U, Erlich H A. Proc Natl Acad Sci USA. 1989;86:9986–9990. doi: 10.1073/pnas.86.24.9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyllensten U, Lashkari D, Erlich H A. Proc Natl Acad Sci USA. 1990;87:1835–1839. doi: 10.1073/pnas.87.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otting N, Kenter M, van Weeren P, Jonker M, Bontrop R. J Immunol. 1992;149:461–469. [PubMed] [Google Scholar]
- 14.Bergström T, Gyllensten U. Immunol Rev. 1995;143:13–31. doi: 10.1111/j.1600-065x.1995.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 15.Watkins D I, McAdams S N, Liu X, Strang C, Milford E L, Levine C G, Garber T L, Dogon A L, Lord C I, Ghim S H, et al. Nature (London) 1992;357:329–333. doi: 10.1038/357329a0. [DOI] [PubMed] [Google Scholar]
- 16.Belich M P, Madrigal A, Hildebrand W H, Zemmour J, Williams R C, Luz R, Petzl-Erler M L, Parham P. Nature (London) 1992;357:326–329. doi: 10.1038/357326a0. [DOI] [PubMed] [Google Scholar]
- 17.Watkins D I, Hodi F S, Letvin N L. Proc Natl Acad Sci USA. 1988;85:7714–7718. doi: 10.1073/pnas.85.20.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins D I, Chen Z W, Hughes A L, Evans M G, Tedder T F, Letvin N L. Nature (London) 1990;346:60–63. doi: 10.1038/346060a0. [DOI] [PubMed] [Google Scholar]
- 19.Gyllensten U, Bergström T, Josefsson A, Sundvall M, Savage A, Blumer E S, Giraldo L H, Soto L H, Watkins D I. Immunogenetics. 1994;40:167–176. doi: 10.1007/BF00167076. [DOI] [PubMed] [Google Scholar]
- 20.Trtková K, Kupfermann H, Grahovac B, Mayer W E, O’hUigin C, Tichy H, Bontrop R, Klein J. Immunogenetics. 1993;38:210–222. doi: 10.1007/BF00211521. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien S J, Roelke M E, Marker L, Newman A, Winkler C A, Meltzer D, Colly L, Evermann J F, Bush M, Wildt D E. Science. 1985;227:1428–1434. doi: 10.1126/science.2983425. [DOI] [PubMed] [Google Scholar]
- 22.Nizetic D, Stevanovic M, Soldatovic B, Savic I, Crkvenjakov R. Immunogenetics. 1988;28:91–98. doi: 10.1007/BF00346156. [DOI] [PubMed] [Google Scholar]
- 23.Faulkes C G, Abbot D H, Mellor A L. J Zool. 1990;221:87–91. [Google Scholar]
- 24.Darde A G, Streilein J W. Immunogenetics. 1984;20:603–622. doi: 10.1007/BF00430319. [DOI] [PubMed] [Google Scholar]
- 25.Watkins D I, Chen Z W, Hughes A L, Lagos A, Lewis A M, Shadduck J A, Letvin N L. J Immunol. 1990;145:3483–3490. [PubMed] [Google Scholar]
- 26.Massacesi L, Genain C P, Lee-Parritz D, Letvin N L, Canfield D, Hauser S L. Ann Neurol. 1995;37:519–530. doi: 10.1002/ana.410370415. [DOI] [PubMed] [Google Scholar]
- 27.Potkay S. J Med Primatol. 1992;21:189–236. [PubMed] [Google Scholar]
- 28.Kenter M, Otting N, Anholts J, Jonker M, Schipper R, Bontrop R E. Immunogenetics. 1992;37:1–11. doi: 10.1007/BF00223539. [DOI] [PubMed] [Google Scholar]
- 29.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 30.Slierendregt B L, Otting N, van Besouw N, Jonker M, Bontrop R E. J Immunol. 1994;152:2298–2307. [PubMed] [Google Scholar]
- 31.Slierendregt B L, Otting N, Kenter M, Bontrop R E. Immunogenetics. 1995;41:29–37. doi: 10.1007/BF00188429. [DOI] [PubMed] [Google Scholar]
- 32.Slierendregt B L, Otting N, Jonker M, Bontrop R E. Tissue Antigens. 1993;41:178–185. doi: 10.1111/j.1399-0039.1993.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 33.Bidwell J L, Lu P, Wang Y, Zhou K, Clay T M, Bontrop R E. Eur J Immunogenetics. 1994;21:67–77. doi: 10.1111/j.1744-313x.1994.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 34.Otting N, Bontrop R E. Hum Immunol. 1995;42:184–187. doi: 10.1016/0198-8859(94)00095-8. [DOI] [PubMed] [Google Scholar]
- 35.Kenter M, Otting N, Anholts J, Leunissen J, Jonker M, Bontrop R E. Immunogenetics. 1992;36:71–78. doi: 10.1007/BF00215282. [DOI] [PubMed] [Google Scholar]
- 36.Gyllensten U, Erlich H. Proc Natl Acad Sci USA. 1989;86:9986–9990. doi: 10.1073/pnas.86.24.9986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geluk A, Elferink D G, Slierendregt B L, van Meijgaarden K E, de Vries R R P, Ottenhoff T H M, Bontrop R E. J Exp Med. 1993;177:979–987. doi: 10.1084/jem.177.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustafsson K, Germana S, Hirsch F, Pratt K, LeGuern C, Sachs D H. Proc Natl Acad Sci USA. 1990;87:9798–9802. doi: 10.1073/pnas.87.24.9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafsson K, Andersson L. Immunogenetics. 1994;39:355–358. doi: 10.1007/BF00189233. [DOI] [PubMed] [Google Scholar]
- 40.Titus-Trachtenberg E A, Rickards O, De Stefano G F, Erlich H A. Am J Hum Genet. 1994;55:160–167. [PMC free article] [PubMed] [Google Scholar]
- 41.Satta Y, Mayer W E, Klein J. Hum Immunol. 1996;51:1–12. doi: 10.1016/s0198-8859(96)00155-3. [DOI] [PubMed] [Google Scholar]
- 42.McAdam S N, Boyson J E, Liu X, Garber T L, Hughes A L, Bontrop R E, Watkins D I. Proc Natl Acad Sci USA. 1994;91:5893–5897. doi: 10.1073/pnas.91.13.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gyllensten U, Bergström T, Josefsson A, Sundvall M, Erlich H A. Tissue Antigens. 1996;47:212–221. doi: 10.1111/j.1399-0039.1996.tb02543.x. [DOI] [PubMed] [Google Scholar]
- 44.Bodmer J G, Marsh S G, Albert E D, Bodmer W F, Bontrop R E, Charron D, Dupont B, Erlich H A, Fauchet R, Mach B, et al. Tissue Antigens. 1997;49:297–321. doi: 10.1111/j.1399-0039.1997.tb02759.x. [DOI] [PubMed] [Google Scholar]
- 45.Slierendregt B L, Otting N, van Besouw N, Jonker M, Bontrop R E. J Immunol. 1994;154:2298–2307. [PubMed] [Google Scholar]
- 46.Kasahara M, Klein D, Vincek V, Sarapata D E, Klein J. Genomics. 1992;14:340–349. doi: 10.1016/s0888-7543(05)80224-1. [DOI] [PubMed] [Google Scholar]