Abstract
In vitro import assays have shown that the thylakoid twin-arginine translocase (Tat) system transports folded proteins in a unidirectional manner. Here, we expressed a natural substrate, pre-23K, and a 23K presequence–green fluorescent protein (GFP) chimera in vivo in tobacco protoplasts. Both are imported into chloroplasts, targeted to the thylakoids, and processed to the mature size by the lumen-facing processing peptidase. However, the vast majority of mature GFP and about half of the 23K are then returned to the stroma. Mutations in the twin-arginine motif block thylakoid targeting and maturation, confirming an involvement of the Tat apparatus. Mutation of the processing site yields membrane-associated intermediate-size protein in vivo, indicating a delayed reversal of translocation to the stroma and suggesting a longer lived interaction with the Tat machinery. We conclude that, in vivo, the Tat system can reject substrates at a late stage in translocation and on a very large scale, indicating the influence of factors that are absent in reconstitution assays.
Introduction
The twin-arginine translocase (Tat) system transports proteins across the chloroplast thylakoid membrane and the plasma membranes of most bacteria (for review see Robinson and Bolhuis, 2004). It recognizes substrates bearing cleavable signal peptides that usually contain a critical twin-arginine (RR) motif in the NH2-terminal domain (Chaddock et al., 1995; Stanley et al., 2000). Most notably, there is strong evidence from in vitro studies that it is capable of transporting fully folded globular proteins across the thylakoid membrane (Clark and Theg, 1997; Hynds et al., 1998). Studies on the substrate specificity of bacterial Tat systems, particularly in Escherichia coli, support this premise; the primary substrates include periplasmic proteins that bind any of a range of redox cofactors such as FeS or molybdopterin centers (Berks, 1996; Santini et al., 1998; Weiner et al., 1998). Because these cofactors are inserted enzymatically, and only in the cytoplasm, the proteins need to be exported in an active form, and this necessitates translocation in a largely, if not fully, folded form.
Genetic studies in plants and E. coli have shown the importance of three primary tat genes. In plants, these are tha4, hcf106, and cptatC (Voelker and Barkan, 1995; Settles et al., 1997; Walker et al., 1999; Motohashi et al., 2001); the corresponding bacterial genes are tatABC, which form an operon in E. coli and many other Gram-negative bacteria (Bogsch et al., 1998; Sargent et al., 1998; Weiner et al., 1998). Two distinct Tat complexes appear to be involved in the translocation process: an ∼370-kD TatABC complex and a separate homooligomeric TatA complex (Bolhuis et al., 2001; Porcelli et al., 2002; Oates et al., 2005).
The mechanism of the Tat system is still poorly understood, but in vitro cross-linking studies in thylakoids and bacterial vesicles have provided data on the early events in the translocation process. Under binding conditions, substrates bind primarily to the Hcf106 and cpTatC subunits in thylakoids or the corresponding TatB and TatC subunits in E. coli (Cline and Mori, 2001; Alami et al., 2003). These subunits thus probably form the substrate binding site. Other work on the purified E. coli TatABC complex (Bolhuis et al., 2001) has shown these subunits to be present as a tightly linked heterodimeric unit (present as several copies in this large complex). In thylakoids, Tha4 has only been detected in association with Hcf106–cpTatC in the presence of substrate and a proton motive force (Mori and Cline, 2002), and this has led to models in which the binding of substrate to the Hcf106–Tha4 core complex triggers recruitment of a separate Tha4 complex to generate the full, active supercomplex capable of translocation. However, many aspects remain vague, and previous studies have not identified components of the translocation channel. Indeed, it has not even been confirmed that such a channel exists, and other possibilities such as vesicle transport have remained open.
Although genetic studies have been responsible for characterization of the known tat genes and the significance of their gene products, in vitro translocation assays have been the favored means to probe the translocation mechanism. These assays have involved the incubation of intact chloroplasts, isolated thylakoids, or inverted bacterial membrane vesicles with in vitro–synthesized substrates, and the combined data have shown translocation by the thylakoid Tat system to be efficient, reliant on a proton motive force, and invariably unidirectional. However, a study performed on the green alga Chlamydomonas reinhardtii recently suggested a cautionary note because in vivo pulse labeling and analysis of Tat substrates showed that their maturation did not rely on the thylakoidal ΔpH as expected (Finazzi et al., 2003). In this article, we explore the targeting of an authentic Tat substrate and GFP constructs in transfected tobacco protoplasts. This in vivo system offers the advantage of time scales that resemble those of in vitro assays, and we show that the constructs are targeted to the chloroplasts and processed to the mature size. However, although mature-size Tat substrates are exclusively found in the lumen during in vitro import assays, the targeting characteristics of both pre-23K and pre-GFP are notably different in this in vivo system. After processing by the lumen-facing thylakoidal processing peptidase (TPP), the vast majority of mature GFP and much of the mature 23K are returned to the stroma. Translocation within this pathway is thus reversible to a significant extent. Notably, mutations that block cleavage by TPP result in the accumulation of intermediate-size protein that is tightly associated with the thylakoid membrane and largely resistant to proteolysis. We believe this to represent protein that is delayed in the reverse translocation process and probably trapped within the translocation machinery.
Results
GFP chimeras behave as typical Tat substrates in chloroplast import experiments
The primary substrates used in this study were the lumenal 23-kD oxygen-evolving complex subunit (23K), along with mutated variants and GFP fusions. 23K is synthesized in the cytosol as a 33-kD precursor protein (pre-23K) with a presequence that contains two signals in tandem. The first domain specifies translocation across the chloroplast envelope and is removed by a stromal processing peptidase, after which the second signal directs translocation across the thylakoid membrane by the Tat pathway (Mould and Robinson, 1991; Cline et al., 1992). After translocation, the signal is removed by a lumen-facing TPP (Kirwin et al., 1988).
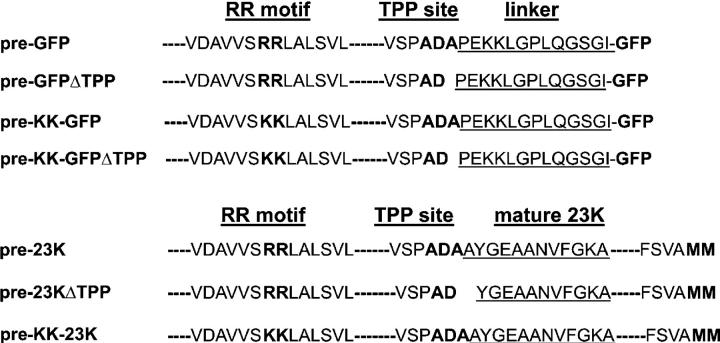
A variety of constructs were prepared for expression in tobacco protoplasts or in vitro import assays, as shown in Fig. 1. The coding sequence for the GFP variant mGFP5 (Siemering et al., 1996) was fused behind the presequence of pea 23K cDNA (Wales et al., 1989), with a linker of 13 amino acids from the cytochrome b6 coding sequence introduced between the 23K presequence and GFP. This construct, termed pre-GFP, was expressed in transfected tobacco protoplasts under the control of the cauliflower mosaic virus 35S promoter. A variant was generated in which the terminal residue of the presequence (Ala 73) was deleted. TPP cleaves after an Ala-Xaa-Ala consensus motif (Shackleton and Robinson, 1991), and the removal of the −1 Ala thus blocks processing; this construct is denoted by the term pre-GFPΔTPP. To assess the specificity of Tat-mediated translocation, we generated two mutants in which the essential RR motif in the signal peptide (Chaddock et al., 1995) was converted to twin-lysine (KK; pre–KK-GFP and pre–KK-GFPΔTPP). This mutation completely blocks translocation by the Tat pathway. Finally, we prepared a second set of constructs in which all the aforementioned mutations were introduced in the background of an authentic Tat substrate, pea pre-23K (Fig. 1). Two Met residues were incorporated at the extreme COOH terminus of the 23K protein (see Materials and methods) to facilitate labeling with [35S]-Met because wild-type pea 23K does not contain Met residues (indicated by “MM” in the pre-23K structure in Fig. 1). The extra Met residues do not affect the targeting characteristics of pre-23K in chloroplast import assays (unpublished data).
Figure 1.
GFP and 23K constructs used in this study. In these constructs, 23K and GFP are preceded by bipartite presequences containing a chloroplast-import (“transit”) signal followed by an RR signal peptide. The figure shows the NH2-terminal sections of the signal peptides with the RR motifs in bold (mutated to KK in some mutants), together with the COOH-terminal regions of the signal peptides ending with the AXA motif specifying cleavage by TPP (TPP site). In the GFPΔTPP and 23KΔTPP mutants, the terminal Ala is deleted to block cleavage by TPP. The region underlined in the GFP constructs is the linker peptide; two Mets were introduced at the extreme COOH terminus of the 23K protein (bold) to aid in labeling the protein.
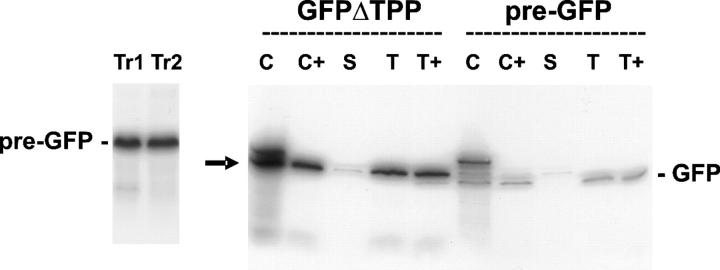
Before expression in vivo, we tested whether the GFP constructs behave as typical Tat substrates in standard in vitro chloroplast protein import assays. Pre-GFP and pre-GFPΔTPP were synthesized in vitro and imported into chloroplasts, and the organelles were fractionated to assess the locations of the polypeptides. Fig. 2 shows that both the pre-GFP and pre-GFPΔTPP translation products (lanes Tr1 and 2) are imported into the chloroplasts and processed to smaller forms that are resistant to proteolysis of the organelles (lanes C and C+). Pre-GFP is processed primarily to the mature form, which is found in the thylakoid fraction (Fig. 2, lane T), where it is resistant to protease treatment (T+), confirming a lumenal location. When the mutant lacking the consensus site for TPP (pre-GFPΔTPP) is imported, the precursor is targeted with equal efficiency to the thylakoid lumen, where it accumulates exclusively as the intermediate GFP (iGFP). This result shows that the mutation completely blocks processing to the mature size, as expected based on a previous study (Shackleton and Robinson, 1991), but does not affect translocation. It is known that Tat-mediated translocation into the thylakoid lumen is completely blocked when the ΔpH across the thylakoid membrane is dissipated in vitro (Mould and Robinson, 1991; Cline et al., 1992), and the same applies to these GFP constructs (unpublished data). Pre-GFP and pre-GFPΔTPP thus behave as absolutely typical Tat substrates in these in vitro import assays. As with every Tat substrate analyzed in vitro to date, translocation is unidirectional, and the mature 23K and GFP are found exclusively in the thylakoid lumen.
Figure 2.
Import of pre-GFP and pre-GFPΔTPP into intact chloroplasts. Pre-GFP and pre-GFPΔTPP were synthesized in vitro in the presence of [35S]-Met, and the translation products (lanes Tr1 and 2) were incubated with intact pea chloroplasts. After incubation, we analyzed samples of the chloroplast (C), the chloroplasts after treatment with thermolysin (C+), and the stromal (S) and thylakoid (T) fractions after lysis of the organelles. Lanes T+, trypsin-treated thylakoids; GFP, mature GFP; arrow, iGFP; pre-GFP, full precursor form.
The GFP constructs are correctly targeted to chloroplasts when expressed in protoplasts and are processed to the expected sizes
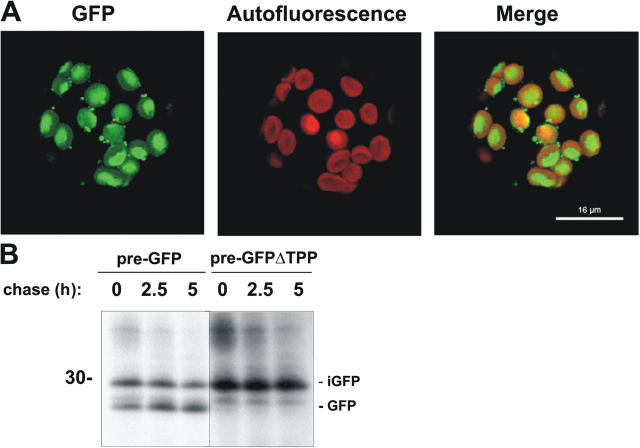
To study their targeting characteristics in vivo, we transiently expressed the GFP chimeras in tobacco protoplasts. 18 h after transfection, cells expressing pre-GFP and pre-GFPΔTPP constructs were analyzed by fluorescence confocal microscopy. A typical transfected protoplast expressing pre-GFP is shown in Fig. 3 A, with the GFP localized almost exclusively within the chloroplasts. The natural autofluorescence of the chlorophyll is a good visual marker for the thylakoids within the chloroplasts (Fig. 3 A, right, red channel). Perhaps surprisingly, the merged images show that GFP is present in the regions of low red fluorescence that correspond to the stroma. GFP is also visible in smaller punctate structures outside the chloroplasts; these are “stromules,” which protrude from, and form links with, other chloroplasts, as observed in a previous study (Kwok and Hanson, 2004).
Figure 3.
Pre-GFP and pre-GFPΔTPP are correctly targeted to chloroplasts in transfected tobacco protoplasts. Tobacco protoplasts were transfected with constructs expressing pre-GFP and pre-GFPΔTPP. (A) Confocal microscopy data after expression of pre-GFP for 24 h; the individual images were obtained using the red channel (shows pigment autofluorescence) or green channel for GFP fluorescence. The merged images are shown on the right. Bar, 16 μm. (B) Protoplasts expressing pre-GFP or pre-GFPΔTPP were pulse labeled with 35S-Met and 35S-Cys for 1 h and then chased with a mixture of cold Met and cold Cys for the times indicated above the lanes. The protoplasts were then lysed and subjected to immunoprecipitation using antibodies to GFP. The mobility of mature GFP is indicated (GFP) together with iGFP. Mobility of a 30-kD marker protein is indicated on the left of the autoradiograph.
The targeting of the GFP chimeras was also analyzed using pulse-chase techniques, and the data are shown in Fig. 3 B. Protoplasts expressing pre-GFP and pre-GFPΔTPP were pulse labeled for 1 h using a mixture of 35S-Met and 35S-Cys and subjected to chase periods of 0, 2.5, or 5 h. Protoplast homogenates were then immunoprecipitated with anti-GFP antiserum, and the selected polypeptides were analyzed by SDS-PAGE and fluorography. When pre-GFP is expressed, two immunoreactive bands are apparent (Fig. 3 B), which comigrate with the intermediate and mature forms observed during import reactions (unpublished data). Fig. 3 B shows that the intermediate form undergoes time-dependent processing and the mature form accumulates. In contrast, expression of pre-GFPΔTPP generates only one immunoreactive band that comigrates with the intermediate form observed after import of pre-GFP, and this polypeptide is stable over the chase period. These data resemble those obtained during import experiments in the sense that the same intermediate- and mature-size bands are evident, although the confocal data provide evidence that significant amounts of GFP are localized in the stroma.
Pre-GFP and pre-23K are processed in the lumen but accumulate primarily in the stroma
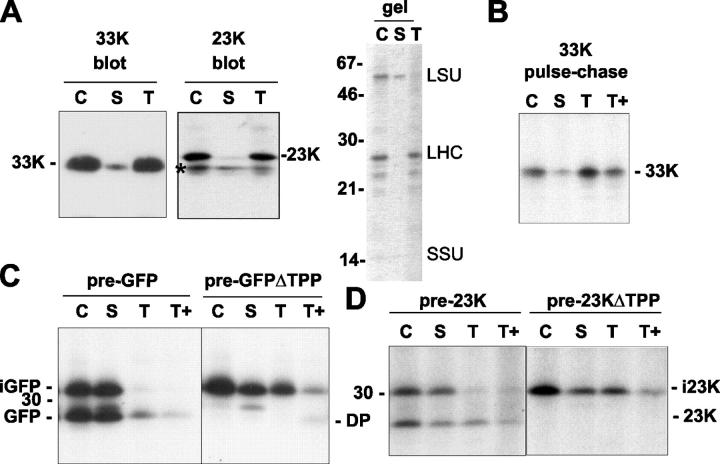
To determine the locations of the GFP forms, intact chloroplasts were isolated from transfected protoplasts and fractionated into stroma and thylakoids, after which the GFP polypeptides were again detected by immunoprecipitation. Control experiments for the effectiveness of the fractionation procedure and intactness of the thylakoid fraction are shown in Fig. 4 A. First, we immunoblotted samples of the intact chloroplasts and the stromal and thylakoid fractions obtained after lysis of the chloroplasts (Fig. 4 A, left, lanes C, S, and T) using antibodies to 23K and the lumenal 33-kD photosystem II subunit (33K, a Sec substrate). These data show that the majority of 33K and 23K are found in the thylakoid fraction, confirming that minimal breakage of thylakoids occurs during fractionation. The efficiency of the fractionation protocol is confirmed by the stained gel in this panel, which shows that the abundant large and small subunits of the stromal enzyme Rubisco are present in the stromal fraction as expected, whereas the abundant 26-kD polypeptide of the light-harvesting complex is only found in the thylakoid fraction.
Figure 4.
Both the intermediate and mature forms of GFP and 23K accumulate in the stroma. (A) Controls for thylakoid intactness and fractionation efficiency. Intact chloroplasts were isolated from tobacco protoplasts expressing pre-GFP and fractionated into stromal and thylakoid samples. The panel shows immunoblots of chloroplast (C), stromal (S), and thylakoid (T) samples using antibodies to the lumenal 33-kD photosystem II protein (33K) and to 23K. Asterisk denotes a polypeptide nonspecifically recognized by 23K antibodies in all fractions. Also shown is a Coomassie-stained gel of the same fractions, with the mobilities of molecular mass markers (kD) indicated on the left. The Rubisco large and small subunit bands (LSU and SSU) are indicated on the right together with the major component of the thylakoid light-harvesting complex (LHC). (B) A pulse-chase analysis of the transport of endogenous 33K in protoplasts that were mock transfected with empty vector. The protoplasts were pulsed with 35S-Met and 35S-Cys for 1 h and chased for 2 h. The chloroplasts were then isolated and processed to yield stromal, thylakoid, and protease-treated thylakoid (T+) samples, as detailed in Materials and methods, and subjected to immunoprecipitation using antibodies to wheat 33K protein. (C) Pre-GFP and pre-GFPΔTPP were expressed in protoplasts for 18 h as detailed in Fig. 3, after which the protoplasts were pulsed with 35S-Met and 35S-Cys for 1 h and chased for 2 h. The chloroplasts were isolated and fractionated as in B. GFP, mobility of mature GFP marker. (D) Protoplasts expressing pre-23K and pre-23KΔTPP for 18 h were then pulse labeled for 3 h, after which the chloroplasts were isolated and subsequently fractionated exactly as for GFP constructs in C. Samples were then immunoprecipitated using antibodies to 23K. i23K, intermediate form of 23K.
We also performed a pulse-chase assay to study the targeting of 33K to assess the functioning of the Sec pathway (for comparative purposes) and the integrity of the thylakoid system as a whole (Fig. 4 B). Protoplasts were mock transfected using empty vector, and 18 h later they were pulsed for 1 h with a mixture of 35S-Met and 35S-Cys and then chased for 2 h. Chloroplasts, stroma, thylakoids, and protease-treated thylakoids were isolated and subjected to immunoprecipitation with antibodies to 33K. The data show the presence of mature 33K within the chloroplasts, the majority of which is present in thylakoids, where it is protease resistant, confirming a lumenal location (Fig. 4 B, lane T+). The thylakoids are thus import competent for Sec substrates, and the fractionation procedure is effective because the level of stromal mature 33K is low. The absence of Met or Cys in the three highly expressed Tat substrates (23K, 16K, and photosystem I subunit N) precludes any analysis of endogenous Tat substrates in this manner (and labeling with 3H-labeled leucine was not successful).
Fig. 4 C shows the distribution of imported pre-GFP forms after expression in transfected protoplasts as in Fig. 3. Both the intermediate and mature forms of GFP are again apparent, and the surprising finding is that both are predominantly found in the stromal fraction. A very small proportion of mature GFP is associated with thylakoids, and some of this protein is resistant to protease (Fig. 4 C, lane T+), which is indicative of a lumenal location. These data suggest that GFP is initially targeted to the lumen and processed to the mature size by TPP but is then unexpectedly returned to the stroma.
When the fractionation was performed on pre-GFPΔTPP–expressing protoplasts, much more of the imported protein (all in intermediate form) is found associated with the thylakoids, and the protein is almost equally distributed between stroma and thylakoids. It is also notable that some of the thylakoid-associated protein is digested to a degradation product (DP) that is marginally smaller than mature GFP.
Similar tests were performed on pre-23K and the TPP cleavage site mutant of this precursor (pre-23KΔTPP), as shown in Fig. 4 D. The data for pre-23K show that at the end of the pulse period, the levels of intermediate and mature form in the chloroplasts are approximately equal in the isolated chloroplast fraction (Fig. 4 D, lane C). Almost all of the intermediate form is in the stroma, as expected, but so too is a large proportion of the mature protein. Just over half of the mature protein is found in the thylakoids, where it is largely resistant to proteolysis (Fig. 4 D, lane T+). These data generally resemble those obtained for pre-GFP, except that a much larger proportion of imported protein is found in the thylakoid fraction as the mature size. Again, the major surprise is the presence of mature protein in the stroma. The data obtained with pre-23KΔTPP generally resemble those obtained with pre-GFPΔTPP: all of the imported protein is intermediate and present in the stromal and thylakoid fractions to essentially equal extents. Proteolysis of thylakoids does not generate a protected shifted band (Fig. 4 D, lane T+), but the mature protein only contains Mets at the extreme COOH terminus, and these may be lost during proteolysis. The single Cys residue may not be sufficiently labeled to allow detection.
The repartitioning of processed GFP and 23K to the stroma depends on an intact RR motif, indicating partial translocation by the Tat machinery
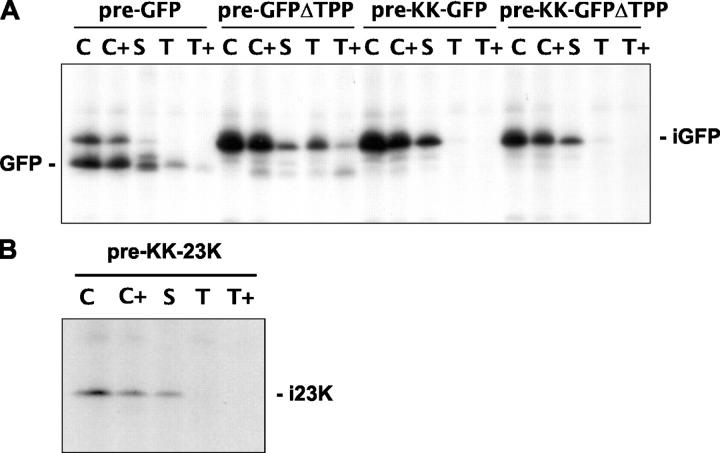
The GFP observed in Fig. 4 is mature, which strongly suggests that it is generated by TPP, but we cannot exclude the possibility that this species is in fact generated by general stromal proteases before engaging the Tat machinery. This is unlikely because pre-GFPΔTPP is not cleaved to the mature size and this construct contains only two mutations (deletion of the terminal Ala and the NH2-terminal residue of the mature protein). Nevertheless, we substituted the RR motifs in both pre-GFP and pre-GFPΔTPP to completely block any interaction with, or translocation by, the Tat system (Fig. 1). These RR motifs are far removed from the site of cleavage and should not be involved in recognition by a hypothetical nonspecific protease that cleaves at the presequence-mature protein junction. Fig. 5 shows chloroplast fractionations from protoplasts expressing these KK mutants, denoted pre–KK-GFP and pre–KK-GFPΔTPP. Pre-GFP and pre-GFPΔTPP were analyzed simultaneously to provide markers for the intermediate and mature sizes; these behave as in Fig. 4, with the majority of imported pre-GFP found as mature GFP in the stroma and the pre-GFPΔTPP found as a mixture of the stromal- and thylakoid-bound intermediate forms. Completely different results are obtained with the KK mutants: both proteins are found exclusively as intermediate proteins in the stroma. We also analyzed a pre-23K mutant in which the RR motif was substituted by KK, and the data are shown in Fig. 5 B. As with the GFP mutant depicted in Fig. 5 A, the presence of the KK motif leads to a complete block in maturation and the intermediate protein accumulates in the stroma.
Figure 5.
Complete maturation of pre-GFP and pre-23K is totally dependent on an intact RR motif. Pre-GFP, pre-GFPΔTPP, pre–KK-GFP, pre–KK-GFPΔTPP, and pre–KK-23K were expressed in protoplasts, followed by pulse and chase treatments as described in Fig. 4. The chloroplasts were isolated and samples prepared of chloroplasts (C), protease-treated chloroplasts (C+), stroma (S), thylakoids (T), and thermolysin-treated thylakoids (T+) as for chloroplast import experiments in Fig. 2. Samples were immunoprecipitated using antibodies to GFP (A) or 23K (B).
These data indicate two important points. First, the mature GFP generated during expression of pre-GFP (Fig. 4) is indeed generated by TPP and not by nonspecific proteases in the stroma. The protein must therefore be returned to the stroma after partial translocation across the membrane by the Tat system and processing by TPP. Second, expression of pre-GFPΔTPP leads to substantial association of the intermediate with the thylakoids, to the extent that about half of the protein is bound, whereas Fig. 5 shows that the noncleavable KK mutant (pre–KK-GFPΔTPP) does not associate with the thylakoid membrane at all. This indicates an involvement of the Tat system in the strong association of the pre-GFPΔTPP intermediate with the membrane.
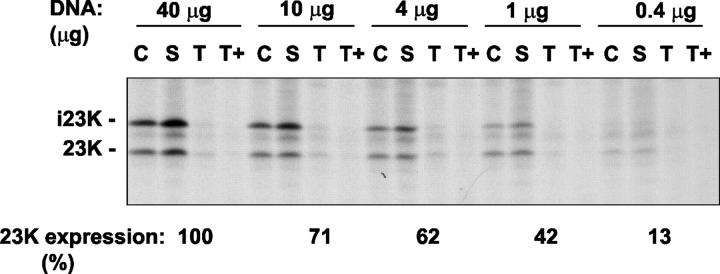
The extensive repartitioning of mature GFP and 23K to the stroma is unexpected, and an important question is whether this process occurs at normal expression levels. The promoter used is known to drive high-level expression, and the translocation reversal may stem from overloading of the system (but see Discussion). We cannot analyze the targeting of the endogenous Tat proteins by similar pulse-chase methods, but we have tested whether the extent of translocation reversal is linked to expression level by systematically reducing the amount of DNA used in the transfection process (Fig. 6). Previous experiments involved transfection of 106 protoplasts with 40 μg DNA, and Fig. 6 (left) shows an identical experiment in which the i23K and 23K polypeptides were immunoprecipitated. The i23K and 23K bands were quantitated by phosphorimager analysis, and the combined 23K expression level is indicated as 100%. Reducing the quantity of DNA used for transfection results in a gradual reduction in 23K expression levels (indicated under the autoradiograph), and with the smallest amount used (0.4 μg), expression is reduced to 13% of the control value. Nevertheless, mature 23K is still found primarily in the stroma, showing that translocation reversal is not significantly affected. Translocation reversal is thus not a simple response to the levels of Tat substrate present.
Figure 6.
Translocation reversal is not strictly linked to expression levels. Protoplasts were transfected with DNA encoding pre-23K under standard conditions (40 μg DNA per incubation) or with smaller amounts of DNA as indicated. Cells were labeled for 3 h with 35S-Met and 35S-Cys and then fractionated as in Fig. 5. Samples were subjected to immunoprecipitation with antibodies to 23K and the i23K, and 23K bands were quantitated using a phosphorimager; the combined radiolabeled contents (arbitrary units) are shown under the autoradiogram, with 100% representing the expression level obtained with 40 μg DNA.
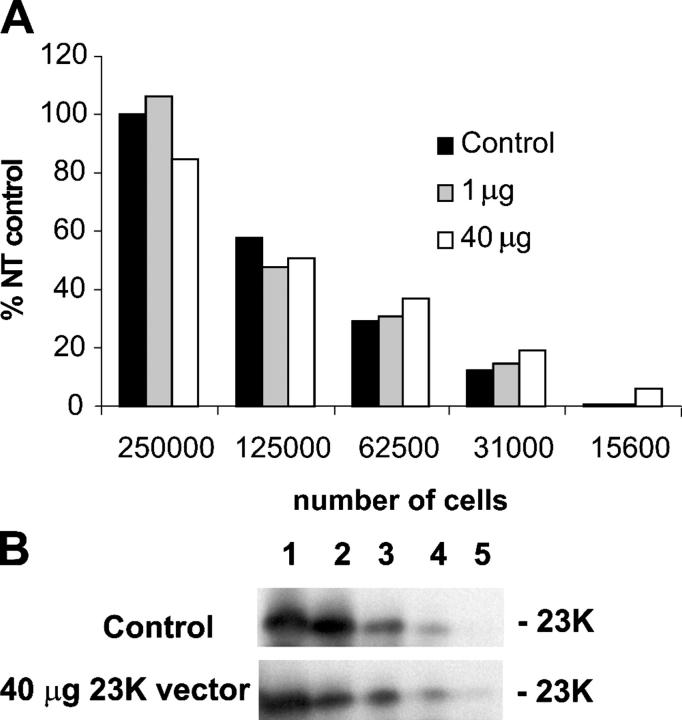
A quantitative assessment of the overexpression levels is shown in Fig. 7. Here, we transfected the standard protoplasts (106) with 40 μg of empty vector (control) with 1 μg of vector encoding pre-23K + 39 μg of empty vector, or with 40 μg of pre-23K vector as used in Fig. 6. An identical number of protoplasts was simultaneously transfected with the same vector expressing pre-GFP, and after expression for 24 h, confocal microscopy revealed that 3.4 or 11% of cells were transfected with 1 or 40 μg of vector, respectively (unpublished data). We analyzed varying numbers of the protoplasts expressing pre-23K by immunoblotting with 23K antibodies, and the calculated signal intensities are shown plotted against number of protoplasts in Fig. 7, together with the immunoblot of samples from protoplasts transfected with empty vector or 40 μg of pre-23K vector. The steady decrease in signal intensity with dilution shows that the signals are within the linear range, and the plotted data show that none of the pre-23K overexpressing samples yields a signal that is significantly higher than the equivalent control sample. Even allowing for the facts that only 11% of the cells were transfected and that the stromal 23K is slightly less stable than the thylakoid-localized protein, this finding clearly indicates that the protein is not grossly overexpressed relative to the wild-type endogenous protein. An increased signal intensity would be clearly evident if the pre-23K were overexpressed by 10-fold or greater.
Figure 7.
23K is not highly overexpressed in transfected protoplasts. (A) Tobacco protoplasts (106) were transfected with 40 μg of empty vector (Control) or 1 μg/40 μg of vector encoding pre-23K exactly as in Fig 6. After incubation for 24 h, the indicated number of cells from each incubation were analyzed by immunoblotting using antisera to spinach 23K, after which the 23K band signal intensities were calculated using a densitometer. All values are shown plotted relative to the 23K signal obtained with 250,000 cells from the sample transfected with empty vector. (B) Immunoblot of samples from control (empty vector) transfections and protoplasts transfected with 40 μg of pre-23K vector. Lanes 1–5 represent sample loadings of 250,000 cells down to 15,600 cells as in A.
On the basis of these findings it is clear that the reversal process may occur to some extent under normal growth conditions. The immunoblots in Fig. 4 indicate that the majority of 23K is indeed found in the thylakoid fraction at steady state, but the low steady-state levels of mature 23K in the stroma may reflect rapid degradation by stromal proteases. We addressed this question by analyzing the stability of stromal 23K in transfected protoplasts using longer chase periods (unpublished data) and have determined that the stromal protein has a half-life of ∼3 h, whereas unassembled lumenal 23K has a half-life of ∼8 h (Hashimoto et al., 1996).
Thylakoid-associated GFPΔTPP intermediate is delayed in repartitioning and possibly trapped in the translocation machinery
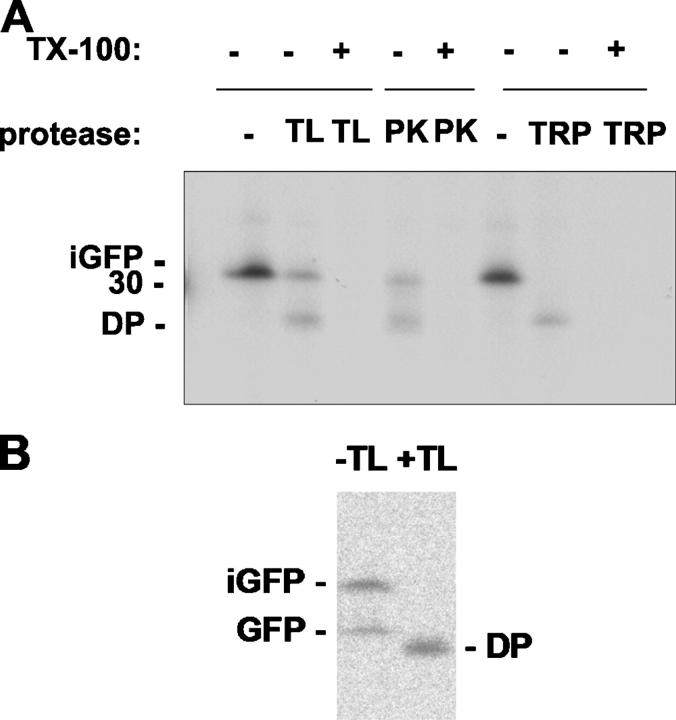
The data shown in Figs. 4 and 5 point to interesting differences in the fate of pre-GFP and the pre-GFPΔTPP mutant. The latter associates to a significant extent with the thylakoid membrane, and this is dependent on a functional RR signal peptide (the corresponding KK mutant does not associate at all). This membrane association was investigated further by a more detailed analysis of the protease sensitivity of the bound intermediate as shown in Fig. 8. Thylakoids were isolated after the pulse period and incubated in buffer (Fig. 8, lanes denoted by minus sign) or with thermolysin, proteinase K, or trypsin. Parallel protease incubations were performed in the presence of the detergent Triton X-100 to allow full access of the protease to the lumenal contents. The data show that a significant proportion of the GFPΔTPP is resistant to digestion by thermolysin or proteinase K, with a further population of molecules digested to a DP that is marginally smaller than mature GFP. The presence of Triton X-100 results in complete digestion of the protein, and our conclusion is that the GFPΔTPP is associated with the membrane in a location that protects the protein, either wholly or substantially, from digestion. Trypsin, on the other hand, digests all of the GFPΔTPP to the DP form and/or smaller products that are not detectable.
Figure 8.
Thylakoid-associated GFPΔTPP intermediate is partially protected from proteolysis and buried within the membrane. (A) Tobacco protoplasts transfected with a plasmid encoding pre-GFPΔTPP were pulsed for 3 h with 35S-Met and 35S-Cys. Chloroplasts were isolated and fractionated, and purified thylakoids were split into eight identical aliquots and incubated either in the absence or in the presence of different proteases and detergent (Triton X-100), as indicated. TL, thermolysin; PK, proteinase K; TRP, trypsin. (B) Protoplasts expressing pre-GFP were pulsed with 35S-Met and 35S-Cys as in A, after which the stromal fraction was prepared and analyzed directly after immunoprecipitation with GFP antibodies (−TL) or after incubation with thermolysin as in A (+TL). Proteins were immunoprecipitated using anti-GFP antisera and analyzed by SDS-PAGE and fluorography.
As a further control for the protease sensitivity for the intermediate GFPΔTPP and mature GFP proteins, we isolated the stromal fraction from cells expressing pre-GFP and incubated this fraction with the same concentration of thermolysin (Fig. 8 B). The results show that all of the iGFP and GFP is degraded, with a major DP formed. The intermediate form is thus inherently susceptible to digestion, confirming that a significant pool of the intermediate GFPΔTPP in Fig. 8 A is in a protected location within the thylakoid membrane. Because this membrane association is the result of Tat-dependent activity, the data strongly suggest that the GFPΔTPP is associated with the Tat machinery. A further pool is apparently exposed to the stromal face of the membrane and digested to a DP that remains associated with the thylakoid when these membranes are pelleted; these molecules may also be partially protected by association with the translocation machinery. It is notable that the presence of Triton X-100 leads to complete digestion rather than the generation of a DP; this presumably reflects a nonspecific effect of Triton X-100 binding. It is also notable that trypsin is able to digest all GFP forms to the stable GFP “core” fragment, and this may reflect an ability of trypsin to digest protein regions on the trans face of chloroplastic membranes; it has been shown that trypsin is able to digest even inner envelope membrane proteins when incubated with intact chloroplasts (Cline et al., 1984).
Discussion
The Tat system has been extensively studied using in vitro assays, and important points have emerged regarding the overall operating mechanism. These assays often faithfully reproduce the bona fide operation of protein translocation systems, but in the case of the Tat system there are already hints that they may not fully reflect the in vivo situation. In vitro assays have invariably shown a complete reliance on the thylakoidal ΔpH (Mould and Robinson, 1991; Cline et al., 1992), but Finazzi et al. (2003) recently reported that Tat substrates in C. reinhardtii show no ΔpH dependence in vivo. We judged it important to study the Tat system in higher plants, and the transfected protoplast system is ideal because kinetic studies can be performed on approximately the same time scales as those used in standard import assays. Moreover, transfected protoplasts have been widely used to study the targeting of proteins to chloroplasts, the endomembrane system, and other organelles (Denecke and Vitale, 1995; Lee et al., 2002). In the vast majority of studies, passenger proteins have been targeted with complete fidelity.
The GFP constructs used in this study have been tested in chloroplast and thylakoid import assays in vitro, and they behave as typical Tat substrates in that translocation across the thylakoid membrane is unidirectional and ΔpH dependent. However, the in vivo studies report a very different picture, and we have invariably found that the majority of imported pre-GFP is present as mature protein in the stroma. Three lines of evidence demonstrate that this results from partial translocation by the Tat system, processing to the mature size on the trans side of the membrane, and then return of the substrate to the stroma. First, removal of the terminal Ala in the signal peptide leads to a complete block in maturation, which is consistent with the known substrate specificity of the lumen-facing TPP. Second, mutagenesis of the RR motif likewise blocks maturation, indicating that the Tat system is responsible for translocating the protein to the point where TPP can access the cleavage site. The only other scenario is that a stromal protease nonspecifically recognizes all of the substrate before it is able to access the Tat system and that the protease absolutely requires the presence of both the −1 Ala and the RR motif (20/21 residues distant) to effect cleavage. This is ruled out by a third observation: the pre-GFPΔTPP and pre-23KΔTPP mutants associate very strongly with the thylakoid membrane, whereas the KK mutants do not associate at all. As discussed later in this section, this is firm evidence for a prolonged interaction of the noncleavable substrates with the Tat machinery, which is prevented by the KK mutations.
The active site of TPP is known to be on the trans side of the thylakoid membrane (Kirwin et al., 1988), as with other members of the signal peptidase family (for review see Dalbey and Von Heijne, 1992), and this shows that the NH2 terminus of the mature protein must have been translocated across the entire bilayer to become accessible to this peptidase. If GFP is transported in a folded state as expected, this would place the entire mature protein within the confines of the membrane bilayer at this point. This shows that the translocation process must be aborted at a very late stage in translocation, providing the first evidence that translocation within the Tat translocon is reversible. There are no indications that the protein is returned to the stroma after complete translocation into the lumen, although this possibility cannot be excluded at present. Importantly, the same process occurs to a significant extent with pre-23K, an authentic Tat substrate, demonstrating that this process is not restricted to heterologous proteins.
It has not been possible to probe the targeting of endogenous Tat substrates in vivo because of the absence of Met residues in 23K or the only other abundant Tat substrates, 16K and PsaN. None of these proteins are sufficiently labeled with [3H] amino acids for this form of analysis (unpublished data). We therefore have no information on the extent to which this translocation reversal process occurs under typical growth conditions in wild-type plants. It seems unlikely that the translocation process is normally so inefficient; it is more probable that translocation reversal is triggered by the overproduction of Tat substrates in the transfected protoplasts. The promoter used is particularly strong and able to drive high rates of expression. However, this does not fully explain our observations because it is possible to completely saturate the Tat system in vitro, using either chloroplast or thylakoid import assays in which recombinant precursor protein is present as a competitor for a radiolabeled precursor. Several studies have been published using this approach (Cline et al., 1993; Bogsch et al., 1997), and it has been clearly shown that the unlabeled recombinant protein competes with the radiolabeled precursor protein for translocation. In every case reported, the result is an accumulation of the intermediate protein in the stroma. Most important, no mature Tat substrate is detected in the stroma at any stage. In other words, translocation reversal on any scale has never been detected in vitro, even when the Tat system is massively overloaded. A study by Leheny et al. (1998) did observe partial translocation of the 16K in response to azide, which inhibits SecA but not the Tat pathway, but this was almost certainly attributable to nonproductive interaction with the Sec pathway under the conditions used.
It is also relevant that the reversal process occurs even when the expression level is markedly reduced to the point where the substrate is only just detectable. This again argues against a simple effect of substrate concentration and suggests that the overexpression of substrates under these conditions may lead to more complex effects on the translocation pathway. A possible example is the triggering of some form of quality control system that could be exacerbated by the protoplast stress. However, we have no direct evidence for such effects. Given that the reversal process is evident with even the lowest detectable levels of pre-23K, we believe that this process must occur during the normal operation of the Tat system in vivo, although possibly under specific conditions or to a lower extent.
Why is translocation reversal so extensive in the in vivo situation described in this study, yet undetectable during import assays? Apart from possible stress effects, there are other potential explanations, including the absence of critical ions in isolated chloroplasts and the differing pH or redox states in the in vitro and in vivo states, as well as others. It is possible that the missing factors are the same as those that cause translocation in vivo to be ΔpH independent in C. reinhardtii (Finazzi et al., 2003). Whatever the explanation for the observed translocation reversal, this process can clearly occur on a large scale in vivo, and this has major implications for the translocation mechanism. Cross-linking studies have shed light on some of the early events in the translocation process, but little is known about the actual translocation process. Several points have emerged from this study. The first, obvious point is that translocation is, in principle, reversible to a significant extent, and it will be of particular interest to determine the means by which translocation is halted at such a late stage and then reversed. Our data represent strong evidence that the formation of the translocation complex does not automatically lead to unidirectional translocation.
The second point is that our data obtained with the TPP cleavage site mutants have interesting implications with respect to the translocation process. Although the pre-GFP construct is processed and rapidly returned to the stroma, it is notable that a large proportion of the pre-GFPΔTPP mutant is found to be associated with the thylakoid membrane. This association is completely dependent on, and therefore presumably mediated by, the Tat system because KK mutants show no propensity for binding to the thylakoid membrane. Protease protection studies show that a significant proportion of the thylakoid-associated, intermediate form of the pre-GFPΔTPP is cleaved to a product that is slightly smaller than mature GFP, which strongly suggests that the protein is largely buried within the membrane. Because this protein must interact with the Tat system at some stage, there is a strong possibility that the protein is actually buried within the Tat complex to the extent that only part of the molecule is accessible to protease. We believe that this substrate is probably undergoing translocation reversal, as with pre-GFP, but the presence of the uncleavable signal peptide somehow slows down the rate of reversal by virtue of its interaction with either the membrane bilayer or the Tat machinery. If this is the case, the data represent evidence of a transmembrane translocation intermediate where the (mutated) signal peptidase site has reached the lumen while part of the protein is accessible to proteolysis from the cis side.
Materials and methods
Plasmid construction
Constructs used in this work are shown in Fig. 1. All 23K sequences derive from the pea pre-23K cDNA (available from GenBank/EMBL/DDBJ under accession no. X15552; Wales et al., 1989) and were cloned in the cauliflower mosaic virus 35S promoter–driven expression vector pDHA (Tabe et al., 1995). Full-length pre-23K was amplified by PCR using the oligonucleotides 5′-TCTAGATTACATCATGCAGTGTCCTCCAC-3′ (forward) and 5′-CTCGAGCTCTAGACATGGCATCTACAC-3′ (reverse) and subcloned as an XbaI fragment into XbaI-cut pDHA, and two extra Met residues were incorporated at the COOH terminus just before the stop codon. The Ala 73 deletion (pre-23KΔTPP) was introduced into the presequence of pre-23K using the oligonucleotides 5′-GGTTTCACCTGCAGATTATGGAGAAGCTGC-3′ and 5′-GCAGCTTCTCCATAATCTGCAGGTGAAACC-3′ and the Quick-Change mutagenesis system (Stratagene), following the manufacturer's instructions.
The arginine 51–52 to lysine mutations (pre–KK-GFP and pre–KK-23K) were introduced by mutagenesis into the presequence of the pre-23K and pre-GFP using the following oligonucleotides: 5′-GCTGTTGTGTCTAAGAAGTTAGCACTTTCTG-3′ and 5′-CAGAAAGTGCTAACTTCTTAGACACAACAGC-3′.
Pre-GFP was constructed starting from the wild-type pre-23K (Wales et al.,1989), the sequence coding for the mature protein, and amino acids 74–259 were removed and substituted with a sequence coding for a 13-residue linker (PEKKLGPLQGSGI) by PCR amplification using primers 5′-TCTAGATTACATCATGCAGTGTCCTCCAC-3′ (forward) and 5′-GATGCCGGATCCTTGAAGGGGACCCTTTTTTTTCTCGGGAACATTGAAAGAACTTGC-3′. The amplified fragment was used as a template to generate the fusion between the 23K signal peptide-coding region and GFP. This fusion was then obtained by PCR using the complementary oligonucleotides 5′-ggatccggcatcAGTAAAGGAG-3′ and 5′-CTCCTTTACTgatgccggatcc-3′, in which the bases shown in uppercase letters anneal to GFP and the bases shown in lowercase letters anneal to the 23K presequence linker. The GFP used for this cloning is a cytosolic version of GFP, named sGFP (provided by L. Frigerio, University of Warwick, Coventry, UK), which has been described previously (Foresti et al., 2003).
Transient transformation of leaf protoplasts
Protoplasts were prepared from 4- to 7-cm-long axenic leaves of Nicotiana tabacum cv Petit Havana SR1. Protoplasts were subjected to polyethylene glycol-mediated transfection as described previously (Pedrazzini et al., 1997) and were incubated overnight at 25°C in the dark before pulse labeling.
In vivo labeling of protoplasts and analysis of expressed polypeptides
Pulse-chase labeling of protoplasts using Pro-Mix (a mixture of 35S-Met and 35S-Cys; GE Healthcare) was performed as described by Frigerio et al. (1998). At desired time points, three volumes of W5 medium (Frigerio et al., 1998) were added, and protoplasts were pelleted at 100 g for 5 min. Cells were frozen in liquid nitrogen and stored at −80°C. Homogenization of protoplasts and incubation media was performed by adding two volumes of ice-cold homogenization buffer (150 mM Tris-Cl, 150 mM NaCl, 1.5 mM EDTA, and 1.5% [wt/vol] Triton X-100, pH 7.5) with a complete protease inhibitor cocktail (Boehringer). Immunoprecipitation of expressed polypeptides was performed as in Frigerio et al. (1998), using rabbit polyclonal antisera raised against pea 23K and GFP (Invitrogen). Immunoselected proteins were analyzed by SDS-PAGE and fluorography. For Western blotting, chloroplast samples were resolved by SDS-PAGE and blotted onto a polyvinylidene difluoride membrane (GE Healthcare), probed with rabbit polyclonal antisera raised against wheat 23K or pea 33K, and revealed by ECL (GE Healthcare).
Confocal microscopy
Tobacco protoplasts were transfected with GFP constructs as described in the previous section. Cells were incubated in the dark overnight at 25°C before observation. Images were collected at RT with a confocal system (Digital Module R, TCS SP2; Leica), with the 63× objective: HC×PL APO 63×/1.40–0.60 oil NA 1.4. The acquired images were subjected to three-dimensional reconstruction using LCS software (Leica).
Chloroplast isolation and fractionation
Protoplast pellets (from 3,000,000 cells) obtained during pulse-chase were resuspended in 150 μl HS buffer (50 mM Hepes-KOH and 330 mM sorbitol, pH 8.0) and homogenized by repeated passage through a 23-gauge needle. The lysate was diluted to 4 ml with HS buffer and loaded on top of a 35% (vol/vol) percoll pad in 5× HS and centrifuged at 1,400 g for 9 min at 4°C. Pellets (chloroplasts) were washed once in HS buffer, pelleted at 3,300 g for 2 min, and resuspended in 120 μl HS. A 30-μl aliquot was saved and used for immunoprecipitation, and the rest was either incubated for 1 h on ice with 2 μg/μl of thermolysin (Sigma-Aldrich) at the final concentration of 0.2 μg/μl or lysed in HM buffer (10 mM Hepes-KOH, pH 8.0, and 5 mM MgCl) supplemented with 10 μM EDTA, for 10 min on ice, after which the lysate was centrifuged for 10 min at 20,000 g. Supernatants (stroma) were saved and used for immunoprecipitation, whereas pellets (thylakoids) were washed in HM and then resuspended in 60 μl HM buffer containing 3 μM CaCl2. 30 μl were used for immunoprecipitation, and the rest was incubated for 20 min on ice with 0.2 μg/μl of thermolysin. At the end of the protease treatment, 10 mM EDTA was added, and the last aliquot (30 μl) was kept for immunoprecipitation.
Chloroplast isolation and import assays
Chloroplasts were isolated from 8- to 9-d-old pea seedlings (Pisum sativum, var. Feltham First) by Percoll pad centrifugation as described by Mould and Robinson (1991). Pea pre-23K was synthesized by in vitro transcription of cDNA clones (Wales et al., 1989) followed by translation in a wheat germ system in the presence of 35S-Met. Assays for chloroplast protein import were conducted as described by Mould and Robinson (1991). After import incubations, chloroplasts were fractionated as described in the previous section and the different fractions analyzed by SDS-PAGE and fluorography.
Protease protection assay
Protoplast pellets (3 × 106 cells) were homogenized (as described in section In vivo labeling…), and thylakoids were isolated from lyzed chloroplasts (also as described in section In vivo labeling…), only without EDTA. Pelleted thylakoids were resuspended in HM buffer, divided into eight aliquots, and incubated for 30 min at 4°C in HM (as control), or with 60 μg/ml proteinase K (Calbiochem), 200 μg/ml thermolysin (Sigma-Aldrich), or 200 μg/ml trypsin (Sigma-Aldrich), in the presence or in the absence of 1% Triton X-100. Each protease was inhibited before immunoprecipitation as follows: thermolysin, by addition of 10 mM EDTA; proteinase K, by addition of 1 mM PMSF; and trypsin, by addition of 60 μg/ml trypsin inhibitor.
Acknowledgments
We gratefully acknowledge the help of Lorenzo Frigerio and Anja Nenninger in the confocal microscopy. We thank Lorenzo Frigerio for the gift of the sGFP construct.
This work was supported by Biotechnology and Biological Sciences Research Council grants C15310 and C18313 to C. Robinson.
Abbreviations used in this paper: DP, degradation product; iGFP, intermediate GFP; Tat, twin-arginine translocase; TPP, thylakoidal processing peptidase.
References
- Alami, M., I. Luke, S. Deitermann, G. Eisner, H.G. Koch, J. Brunner, and M. Müller. 2003. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell. 12:937–946. [DOI] [PubMed] [Google Scholar]
- Berks, B. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22:393–404. [DOI] [PubMed] [Google Scholar]
- Bogsch, E., S. Brink, and C. Robinson. 1997. Pathway specificity for a ΔpH-dependent precursor thylakoid lumen protein is governed by a “Sec-avoidance” motif in the transfer peptide and a “Sec-incompatible” mature protein. EMBO J. 16:3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogsch, E.G., F. Sargent, N.R. Stanley, B.C. Berks, C. Robinson, and T. Palmer. 1998. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J. Biol. Chem. 273:18003–18006. [DOI] [PubMed] [Google Scholar]
- Bolhuis, A., J.E. Mathers, J.D. Thomas, C.M.L. Barrett, and C. Robinson. 2001. TatB and TatC form a structural and functional unit of the twin-arginine translocase of Escherichia coli. J. Biol. Chem. 276:20213–20219. [DOI] [PubMed] [Google Scholar]
- Chaddock, A.M., A. Mant, I. Karnauchov, S. Brink, R.G. Herrmann, R.G. Klösgen, and C. Robinson. 1995. A new type of signal peptide: central role of a twin-arginine motif in transfer signals for the ΔpH-dependent thylakoidal protein translocase. EMBO J. 14:2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.A., and S.M. Theg. 1997. A folded protein can be transported across the chloroplast envelope and thylakoid membranes. Mol. Biol. Cell. 8:923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, K., and H. Mori. 2001. Thylakoid ΔpH-dependent precursor proteins bind to a cpTatC–Hcf106 complex before Tha4-dependent transport. J. Cell Biol. 154:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, K., M. Werner-Washurne, J. Andrew, and K. Keegstra. 1984. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 75:675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, K., W.F. Ettinger, and S.M. Theg. 1992. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J. Biol. Chem. 267:2688–2696. [PubMed] [Google Scholar]
- Cline, K., R. Henry, C. Li, and J. Yuan. 1993. Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J. 12:4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey, R.E., and G. Von Heijne. 1992. Signal peptidases in prokaryotes and eukaryotes—a new protease family. Trends Biochem. Sci. 17:474–478. [DOI] [PubMed] [Google Scholar]
- Denecke, J., and A. Vitale. 1995. The use of protoplasts to study protein synthesis and transport by the plant endomembrane system. Methods Cell Biol. 50:335–348. [DOI] [PubMed] [Google Scholar]
- Finazzi, G., C. Chasen, F.-A. Wollman, and C. de Vitry. 2003. Thylakoid targeting of Tat passenger proteins shows no delta pH dependence in vivo. EMBO J. 22:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., A. Vitale, J.M. Lord, A. Ceriotti, and L.M. Roberts. 1998. Free ricin A chain, proricin, and native toxin have different cellular fates when expressed in tobacco protoplasts. J. Biol. Chem. 273:14194–14199. [DOI] [PubMed] [Google Scholar]
- Foresti, O., L. Frigerio, H. Holkeri, M. de Virgilio, S. Vavassori, and A. Vitale. 2003. A phaseolin domain involved directly in trimer assembly is a determinant for binding by the chaperone BiP. Plant Cell. 15:2464–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, A., Y. Yamamoto, and S.M. Theg. 1996. Unassembled subunits of the photosynthetic oxygen-evolving complex present in the thylakoid lumen are long-lived and assembly-competent. FEBS Lett. 391:29–34. [DOI] [PubMed] [Google Scholar]
- Hynds, P.J., D. Robinson, and C. Robinson. 1998. The Sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J. Biol. Chem. 273:34868–34874. [DOI] [PubMed] [Google Scholar]
- Kirwin, P.M., P.D. Elderfield, R.S. Williams, and C. Robinson. 1988. Transport of proteins into chloroplasts. Organization, orientation, and lateral distribution of the plastocyanin processing peptidase in the thylakoid network. J. Biol. Chem. 263:18128–18132. [PubMed] [Google Scholar]
- Kwok, E.Y., and M.R. Hanson. 2004. GFP-labelled Rubisco and aspartate aminotransferase are present in plastid stromules and traffic between plastids. J. Exp. Bot. 55:595–604. [DOI] [PubMed] [Google Scholar]
- Lee, K.H., D.H. Kim, S.W. Lee, Z.H. Kim, and I. Hwang. 2002. In vivo import experiments in protoplasts reveal the importance of the overall context but not specific amino acid residues of the transit peptide during import into chloroplasts. Mol. Cells. 14:388–397. [PubMed] [Google Scholar]
- Leheny, E.A., S.A. Teter, and S.M. Theg. 1998. Identification of a role for an azide-sensitive factor in the thylakoid transport of the 17-kilodalton subunit of the photosynthetic oxygen-evolving complex. Plant Physiol. 116:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, H., and K. Cline. 2002. A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid ΔpH/Tat translocase. J. Cell Biol. 157:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi, R., N. Nagata, T. Ito, S. Takahashi, T. Hobo, S. Yoshida, and K. Shinozaki. 2001. An essential role of a TatC homologue of a Delta pH-dependent protein transporter in thylakoid membrane formation during chloroplast development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 98:10499–10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould, R.M., and C. Robinson. 1991. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J. Biol. Chem. 266:12189–12193. [PubMed] [Google Scholar]
- Oates, J., C.M.L. Barrett, J.P. Barnett, K.G. Byrne, A. Bolhuis, and C. Robinson. 2005. The Escherichia coli twin-arginine translocation apparatus incorporates a distinct form of TatABC complex, spectrum of modular TatA complexes and minor TatAB complex. J. Mol. Biol. 346:295–305. [DOI] [PubMed] [Google Scholar]
- Pedrazzini, E., G. Giovinazzo, A. Bielli, M. de Virgilio, L. Frigerio, M. Pesca, F. Faoro, R. Bollini, A. Ceriotti, and A. Vitale. 1997. Protein quality control along the route to the plant vacuole. Plant Cell. 9:1869–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli, I., E. de Leeuw, R. Wallis, E. van den Brink-van der Laan, B. de Kruijff, B.A. Wallace, T. Palmer, and B.C. Berks. 2002. Characterization and membrane assembly of the TatA component of the Escherichia coli twin-arginine protein transport system. Biochemistry. 41:13690–13697. [DOI] [PubMed] [Google Scholar]
- Robinson, C., and A. Bolhuis. 2004. Tat-dependent protein targeting in prokaryotes and chloroplasts. Biochim. Biophys. Acta. 1694:135–147. [DOI] [PubMed] [Google Scholar]
- Santini, C.L., B. Ize, A. Chanal, M. Müller, G. Giordano, and L.F. Wu. 1998. A novel Sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent, F., E.G. Bogsch, N.R. Stanley, M. Wexler, C. Robinson, B.C. Berks, and T. Palmer. 1998. Overlapping functions of components of a bacterial Sec-independent export pathway. EMBO J. 17:3640–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles, A.M., A. Yonetani, A. Baron, D.R. Bush, K. Cline, and R. Martienssen. 1997. Sec-independent protein translocation by the maize Hcf106 protein. Science. 278:1467–1470. [DOI] [PubMed] [Google Scholar]
- Shackleton, J.B., and C. Robinson. 1991. Transport of proteins into chloroplasts. The thylakoidal processing peptidase is a signal-type peptidase with stringent substrate requirements at the -3 and -1 positions. J. Biol. Chem. 266:12152–12156. [PubMed] [Google Scholar]
- Siemering, K.R., R. Golbik, R. Sever, and J. Haseloff. 1996. Mutations that suppress the thermosensitivity of green fluorescent protein. Curr. Biol. 6:1653–1663. [DOI] [PubMed] [Google Scholar]
- Stanley, N.R., T. Palmer, and B.C. Berks. 2000. The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli. J. Biol. Chem. 275:11591–11596. [DOI] [PubMed] [Google Scholar]
- Tabe, L.M., T. Wardley-Richardson, A. Ceriotti, A. Aryan, W. McNabb, A. Moore, and T.J.V. Higgins. 1995. A biotechnological approach to improving the nutritive value of alfalfa. J. Anim. Sci. 73:2752–2759. [DOI] [PubMed] [Google Scholar]
- Voelker, R., and A. Barkan. 1995. Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J. 14:3905–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wales, R., B.J. Newman, S.A. Rose, D. Pappin, and J.C. Gray. 1989. Characterization of cDNA clones encoding the extrinsic 23 kDa polypeptide of the oxygen-evolving complex of photosystem II in pea. Plant Mol. Biol. 13:573–582. [DOI] [PubMed] [Google Scholar]
- Walker, M.B., L.M. Roy, E. Coleman, R. Voelker, and A. Barkan. 1999. The maize tha4 gene functions in sec-independent protein transport in chloroplasts and is related to hcf106, tatA, and tatB. J. Cell. Biol. 147: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, J.H., P.T. Bilous, G.M. Shaw, S.P. Lubitz, L. Frost, G.H. Thomas, J.A. Cole and R.J. Turner. 1998. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell. 93:93–101. [DOI] [PubMed] [Google Scholar]