Abstract
Organs and tissues adapt to acute or chronic mechanical stress by remodeling their actin cytoskeletons. Cells that are stimulated by cyclic stretch or shear stress in vitro undergo bimodal cytoskeletal responses that include rapid reinforcement and gradual reorientation of actin stress fibers; however, the mechanism by which cells respond to mechanical cues has been obscure. We report that the application of either unidirectional cyclic stretch or shear stress to cells results in robust mobilization of zyxin from focal adhesions to actin filaments, whereas many other focal adhesion proteins and zyxin family members remain at focal adhesions. Mechanical stress also induces the rapid zyxin-dependent mobilization of vasodilator-stimulated phosphoprotein from focal adhesions to actin filaments. Thickening of actin stress fibers reflects a cellular adaptation to mechanical stress; this cytoskeletal reinforcement coincides with zyxin mobilization and is abrogated in zyxin-null cells. Our findings identify zyxin as a mechanosensitive protein and provide mechanistic insight into how cells respond to mechanical cues.
Introduction
The ability to respond to mechanical cues, such as stretch and shear, is a fundamental cellular attribute that is conserved from invertebrates to vertebrates (Gillespie and Walker, 2001). In higher animals, organs and tissues adapt their morphologies and functions in response to acute or chronic mechanical stress (Frangos, 1993). For instance, pressure overload causes cardiovascular hypertrophy, and the disuse of muscles results in atrophy. Mechanical stress induces adaptive remodeling of the actin cytoskeleton to yield changes in cell shape, orientation, and phenotype (Helmke and Davies, 2002; Ingber, 2003; Albinsson et al., 2004). Although the physiology of mechanically induced tissue remodeling is well documented, the molecular mechanisms by which cells sense mechanical stress and convert the physical information into biological signals have remained elusive.
Two general models have been proposed to explain how cells sense physical forces. First, stretch-activated ion channels have been postulated to facilitate ion fluxes that activate signaling cascades in response to mechanical cues (Martinac, 2004). Alternatively, constituents of cell adhesion sites and the actin cytoskeleton might be altered in response to physical forces and activate signaling cascades (Bershadsky et al., 2003; Ingber, 2003). Based on this perspective, membrane–substratum interaction sites called focal adhesions, which serve to bridge integrins to the actin cytoskeleton, have been extensively studied in an effort to understand mechanotransduction (Naruse et al., 1998; Chen et al., 1999; Helmke and Davies, 2002; Bershadsky et al., 2003; Katsumi et al., 2004).
Recent work has highlighted the significance of channel-independent elements of the mechanosensing machinery that appear to be localized at focal adhesions (Bershadsky et al., 2003). Exposure of detergent-extracted cells to cyclic strain activates a signaling cascade involving the focal adhesion constituent p130Cas (Crk-associated substrate) under conditions in which changes in ion permeability were eliminated (Tamada et al., 2004). This study suggests that mechanical stress could alter the molecular composition of focal adhesions or the conformation of focal adhesion constituents to affect a cellular response.
Zyxin is a LIM protein with several features that suggest a role in the cellular response to mechanical cues. First, zyxin is localized prominently at focal adhesions, the site where the mechanosensor is thought to reside (Beckerle, 1997; Bershadsky et al., 2003). Second, zyxin interacts directly with the stretch-sensitive protein p130Cas (Yi et al., 2002). Finally, zyxin's association with stress fiber termini and its ability to promote actin filament assembly (Fradelizi et al., 2001) are consistent with a role in the cytoskeletal remodeling that occurs upon the application of mechanical load.
We report that the unidirectional cyclic stretch of cells results in a rapid and robust mobilization of zyxin from focal adhesions to actin filaments, whereas other focal adhesion proteins and zyxin family members remain concentrated at the substratum attachment sites. Cyclic stretch also induces a rapid comobilization of vasodilator-stimulated phosphoprotein (VASP) from focal adhesions to actin filaments, and this depends on the presence of zyxin. Coincident with the redistribution of zyxin, there was a generalized thickening of actin filaments (i.e., stress fiber reinforcement), and this response was significantly attenuated in zyxin-null fibroblasts. Our studies establish zyxin as a mechanosensitive molecule and demonstrate that it plays a key role in actin remodeling and reinforcement in response to mechanical cues.
Results and discussion
Cyclic stretch induces bimodal actin cytoskeletal remodeling: reinforcement and reorientation
To investigate mechanically stimulated molecular changes within individual cells, we subjected fibroblasts adhering to ECM on elastic silicone membranes to cyclic stretch. Phalloidin staining of actin filaments after unidirectional cyclic stretch revealed two significant responses: rapid thickening (reinforcement) of actin filaments and gradual reorientation of actin stress fibers that were perpendicular to the stretch axis, which resulted in cell alignment (Fig. 1, A and B). In this study, we have applied novel methods to quantitate the actin cytoskeletal remodeling that occurs in response to stretch. The actin thickening was quantitated based on the “erosion” rank filter to generate a stress fiber thickness index (SFTI) that reflects the thickness of actin filaments in regions, not on certain lines or points. The SFTI analysis demonstrated that 15 min of cyclic stretch induced significant thickening of actin filaments (Fig. 1 A). The gradual actin reorientation was also quantitated by using alignment index analysis (Yoshigi et al., 2003). We observed that cells dramatically reoriented their actin stress fibers from a random to a perpendicular alignment relative to the stretch axis (Fig. 1 B). As demonstrated below (see Fig. 5), our ability to separate and quantitate the two critical responses to stretch—cytoskeletal reinforcement and reorientation—allowed us to evaluate whether the two processes were parallel or sequential and whether they had distinct molecular requirements (Fig. 1 C).
Figure 1.
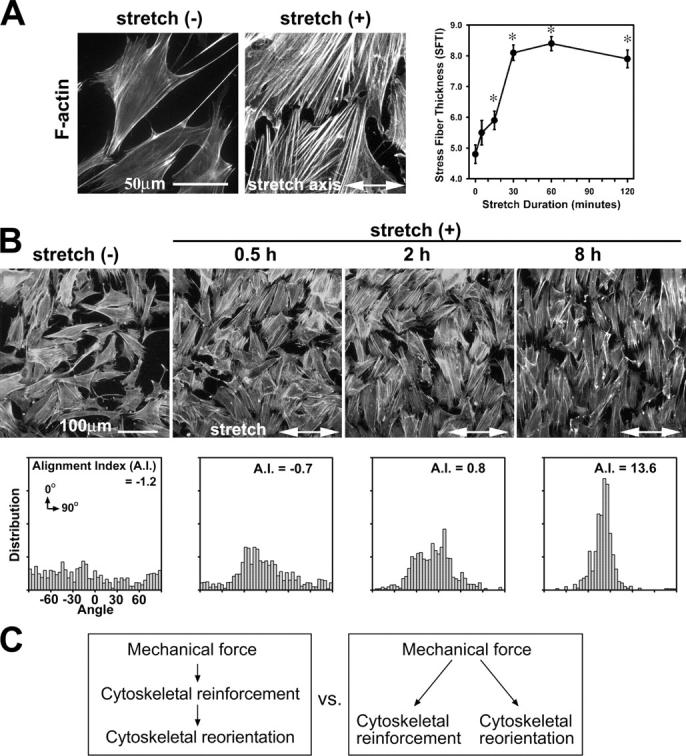
Uniaxial cyclic stretch induces bimodal actin cytoskeletal remodeling: actin stress fiber reinforcement and reorientation. (A) Phalloidin staining of mouse fibroblasts on unstretched membranes (−) or membranes exposed to 1 h of cyclic stretch (+; 15% at 0.5 Hz). The stress fiber thickness index (SFTI) analysis showed actin thickening in a stretch duration–dependent manner (*, P < 0.05). Error bars represent SEM. (B) Labeling of F-actin in fibroblasts exposed to unidirectional cyclic stretch. Quantitative analysis illustrated progressive alignment of the actin cytoskeleton, which was perpendicular to the stretch axis, as a function of stretch durations. (C) Mechanical force induces both cytoskeletal reinforcement and reorientation. These two responses may result from sequential or parallel pathways that are mechanistically related or distinct.
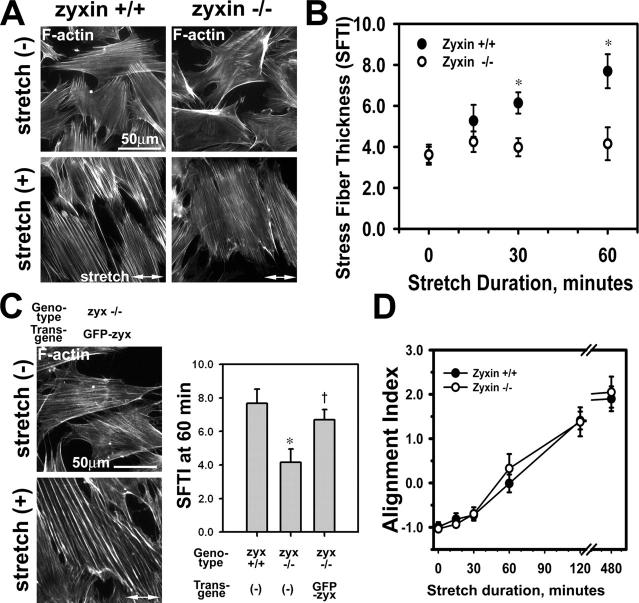
Figure 5.
Zyxin is required for actin stress fiber reinforcement in response to mechanical stimulation. (A) F-actin (phalloidin) in zyxin +/+ and −/− fibroblasts before (−) or after (+) cyclic stretch. Note the thinner stress fibers in stretched zyxin −/− cells. (B) SFTI was measured over a 1-h time course of stretch stimulation. Zyxin-null (−/−) fibroblasts showed an impaired actin thickening response (*, P < 0.05). (C) Phalloidin staining and SFTI analysis indicated that the expression of GFP-zyxin in zyxin −/− cells rescued the stress fiber reinforcement response (*, P < 0.05 vs. +/+; †, P < 0.05 vs. −/−). (D) Comparison of the alignment index of zyxin +/+ and −/− cells over an 8-h time course of stretch stimulation. Error bars represent SEM.
Zyxin mobilizes from focal adhesions to actin filaments in response to mechanical stress
To explore whether the molecular composition of the focal adhesion is modulated in response to physical force, we characterized the subcellular localization of a number of focal adhesion constituents in response to cyclic stretch. After stretch for 15 min–1 h, vinculin-containing focal adhesions increased in number and size (Fig. 2 A), which is consistent with previous reports that mechanical force induces the remodeling of integrin-based adhesions (Davies et al., 1994; Mack et al., 2004). Like vinculin, many other focal adhesion constituents, including paxillin, talin, and FAK, remained concentrated in focal adhesions of both unstretched and stretched cells (unpublished data). In striking contrast to vinculin, the focal adhesion protein zyxin was rapidly mobilized from focal adhesions to the remodeling actin filaments in response to cyclic stretch (Fig. 2 B). We obtained similar results with multiple, independently derived antizyxin antibodies (not depicted). Double labeling of vinculin and zyxin clearly revealed that vinculin-rich focal adhesions remained intact even as zyxin displayed a dramatic redistribution to actin filaments (Fig. 2 C). In a recent study in which vascular smooth muscle cells were stretched equibiaxially, zyxin moved from focal adhesions to cell nuclei, but no accumulation of zyxin on actin filaments was reported (Cattaruzza et al., 2004). The variance may be a result of differences in cell type or of the protocols used for mechanical stimulation.
Figure 2.
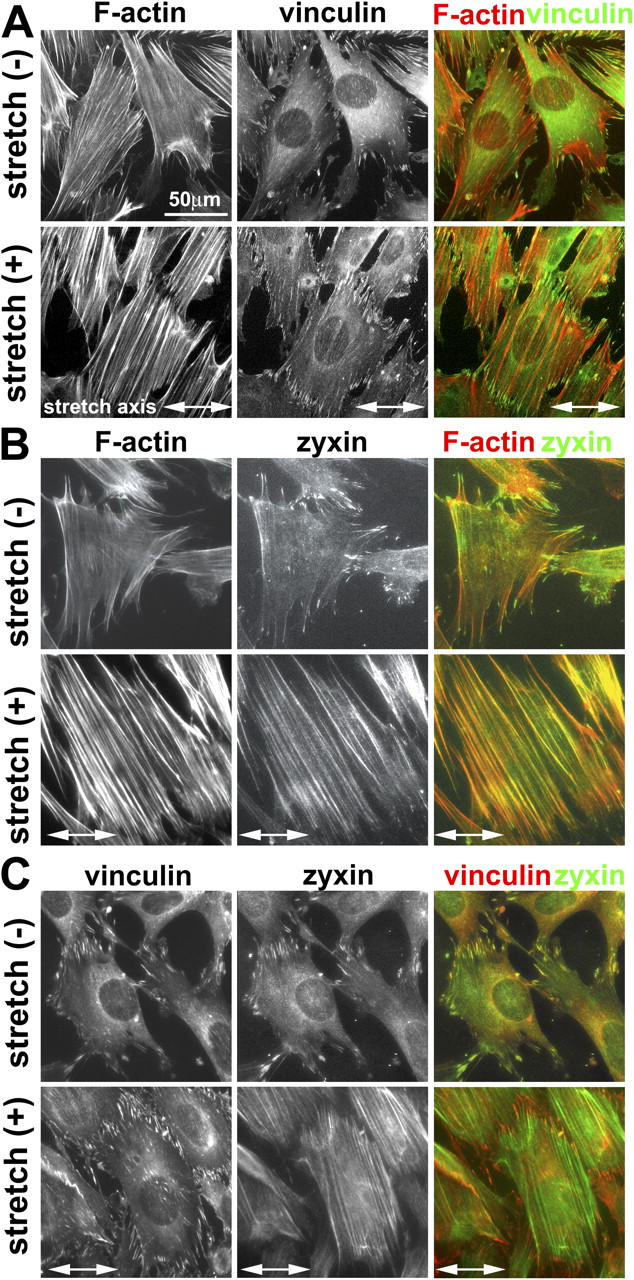
Cyclic stretch induces zyxin mobilization from focal adhesions to the actin cytoskeleton. (A) Fibroblasts on unstretched membranes (−) or subjected to uniaxial cyclic stretch (+; 1 h at 15% and 0.5 Hz) aligned and reinforced their actin filaments (phalloidin staining), whereas the focal adhesion protein vinculin remained at adhesion sites. (B) In contrast to vinculin, unidirectional cyclic stretch resulted in the mobilization of zyxin from focal adhesions to actin filaments. (C) Double labeling of vinculin and zyxin revealed their colocalization at focal adhesions in unstretched cells. Detection of vinculin in focal adhesions of stretched cells in which zyxin has been mobilized to actin filaments clearly illustrates that focal adhesions persist after stretch and highlight the reduced zyxin levels at those sites.
To validate that the altered distribution of zyxin in response to stretch accurately reflects a physiological change and does not result from altered antibody accessibility in the stretched cells, we examined the impact of cyclic stretch on the distribution of GFP-zyxin that was expressed in fibroblasts derived from zyxin −/− mice (Fig. 3 A; Hoffman et al., 2003). The phenotype of zyxin −/− fibroblasts will be described in detail elsewhere (unpublished data). Direct visualization of GFP-zyxin revealed strong zyxin accumulation in focal adhesions before stretch and a mobilization of zyxin to stress fibers with a corresponding reduction in the focal adhesion resident zyxin after stretch (Fig. 3 B). Collectively, these data identify zyxin as a mechanosensitive protein at cell–substratum attachment sites. Given this evidence that the subcellular distribution of zyxin is dynamic and sensitive to physical cues, the validity of using GFP-zyxin as a focal adhesion marker should be reevaluated.
Figure 3.
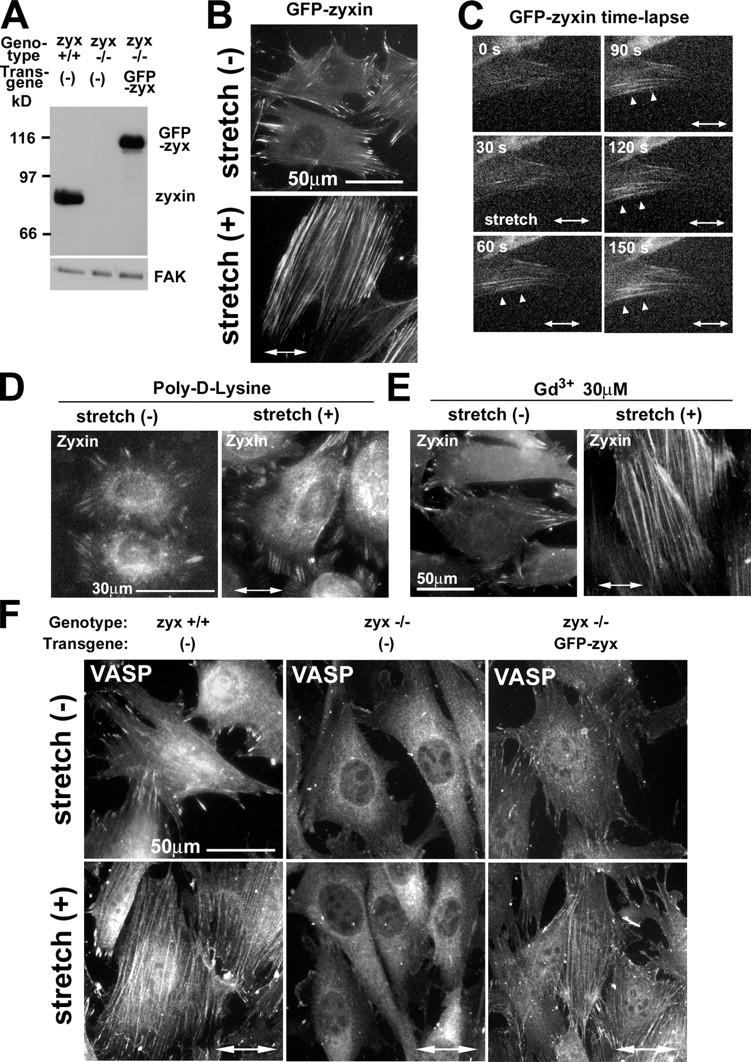
Stretch-induced kinetics of GFP-zyxin, effects of inhibitors, and VASP localization. (A) Immunoblot analysis with antizyxin antibody in wild-type, zyxin −/−, and zyxin −/− cells expressing full-length GFP-tagged zyxin (GFP-zyx). GFP-zyxin was expressed at a level that was comparable to wild-type cells. FAK immunoblot illustrates equal protein loading. (B) Direct visualization of GFP-zyxin in unstretched and stretched cells. (C) Kinetics of zyxin mobilization to stress fibers in response to stretch. By using a live cell stretching system, GFP-zyxin signals were recorded during 150 s of cyclic stretching. Arrowheads indicate GFP-zyxin accumulation along actin filaments. (D) Cells were allowed to spread on poly-d-lysine–coated silicone for 3 h, stretched for 60 min, and immunostained to detect zyxin. (E) Cells were stretched in the presence of Gd3+, and zyxin was localized by immunostaining. (F) Wild-type (+/+), zyxin-null (−/−), and zyxin-null cells expressing GFP-zyxin were stimulated by cyclic stretch (+; 1 h at 15% and 0.5 Hz) and were immunostained to detect VASP.
To examine the kinetics of zyxin's mobilization in response to stretch, we studied GFP-zyxin–expressing fibroblasts by using a stretch device that was custom designed for live cell imaging. The response to stretch was extremely rapid, as merely 60–120 s of cyclic stretch (15% at 0.5 Hz) resulted in a significant accumulation of GFP-zyxin along actin filaments (Fig. 3 C, arrowheads). The earliest stretch-induced mobilization of zyxin was detected in cells that were oriented with their primary cell axis parallel to the stretch axis, likely because the largest strain that was generated along actin filaments was parallel to the stretch axis (McKnight and Frangos, 2003).
To explore whether the stretch-induced mobilization of zyxin depends on integrin functions, cells were plated on the nonspecific attachment factor poly-d-lysine. Under this condition, zyxin did not relocalize in response to stretch (Fig. 3 D), demonstrating that integrin-mediated adhesion is required for the stretch-induced zyxin response. We also tested whether the stretch-induced mobilization of zyxin was dependent on stretch-activated ion channels by using Gd3+, which is a well-characterized inhibitor of stretch-activated ion channels (Martinac, 2004). Gd3+ treatment did not affect stretch-induced zyxin mobilization from focal adhesions to stress fibers (Fig. 3 E), suggesting that stretch-activated ion channels are not required for this response.
Interestingly, two proteins that are closely related to zyxin, the lipoma preferred partner (Petit et al., 2000) and thyroid receptor–interacting protein 6 (Yi et al., 2002), failed to mobilize in response to stretch but remained concentrated at focal adhesions (unpublished data), whereas another cytoskeletal LIM protein, Hic-5, responded to mechanical cues in a fashion that was similar to zyxin (Kim-Kaneyama et al., 2005). In future studies, it will be important to assess whether zyxin and Hic-5 display common features that contribute to their shared capacities for mechanosensitivity.
Zyxin is required for the ability of VASP to accumulate on stress fibers after mechanical stimulation
We showed previously that zyxin is primarily responsible for the localization of Ena/VASP proteins at focal adhesions (Drees et al., 1999). Zyxin displays four proline-rich ActA repeats that bind to the ena/VASP family members (Krause et al., 2003). Ena/VASP proteins promote actin polymerization on the surface of Listeria monocytogenes and within mammalian cells (Beckerle, 1998). Because VASP is coexpressed with zyxin in mechanosensitive tissues like vascular smooth muscle, we postulated that zyxin might recruit VASP to the actin cytoskeleton in response to stretch and might facilitate stress fiber reinforcement.
We found that zyxin and VASP were coordinately mobilized from focal adhesions to actin filaments in response to cyclic stretch (Fig. 3 F). Another zyxin-binding protein, α-actinin, was present on stress fibers both before and after stretch (not depicted). To evaluate whether the redistribution of VASP to stress fibers after stretch was dependent on the presence of zyxin, we tested whether VASP in zyxin −/− cells would relocalize to actin filaments after stretch. We found that VASP in zyxin −/− cells failed to mobilize to actin filaments (Fig. 3 F). To confirm that zyxin is responsible for targeting VASP to actin filaments during the cellular response to stretch, we reintroduced full-length GFP-tagged zyxin into zyxin −/− fibroblasts. Reexpression of zyxin restored the ability of VASP to accumulate along actin filaments in response to stretch (Fig. 3 F). Thus, zyxin plays an essential role in the recruitment of VASP to actin filaments during the adaptive response to mechanical stress.
Generality of mechanically induced zyxin mobilization from focal adhesions
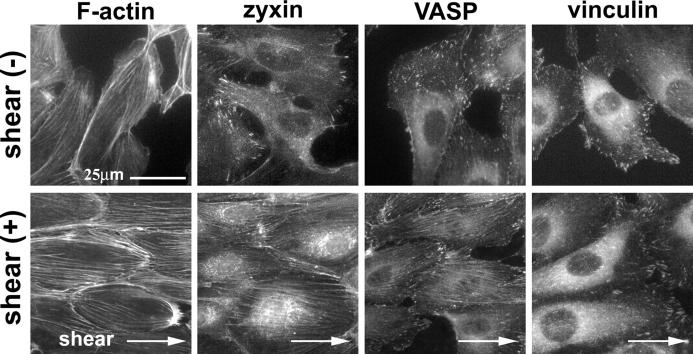
Cyclic stretch experiments have been used to mimic the dynamic environment of the vascular wall, which is expanded by pulsatile pressure (Kakisis et al., 2004). Endothelial cells lining the vascular wall also experience shear stress as a major determinant of endothelial pathophysiology (Cunningham and Gotlieb, 2005). To evaluate whether zyxin mobilization is a general response to mechanical stress or is limited to cyclic stretching, we tested the effect of physiological shear stress (15 dyne/cm2) on zyxin localization. Human umbilical vein endothelial cells that were subjected to laminar shear stress reoriented their actin filaments to align parallel to the fluid flow, as seen by phalloidin staining (Fig. 4). Concomitant with actin realignment, shear stress induced the mobilization of zyxin and VASP onto actin filaments, whereas the focal adhesion protein vinculin was not recruited to stress fibers. Similar results were obtained in human aortic smooth muscle cells upon the application of shear stress (unpublished data). Although the shear-induced accumulation of zyxin and VASP on stress fibers of endothelial cells was apparent, we commonly observed residual zyxin and VASP in focal adhesions as well. The incomplete mobilization of zyxin and VASP may reflect cell type differences or the fact that fluid shear stress represents a milder mechanical stress than cyclic stretch. Together, these data indicate that zyxin and VASP mobilization to stress fibers is a general response to mechanical stress that is conserved in multiple cell types.
Figure 4.
Fluid shear stress leads to actin reinforcement, realignment, and mobilization of zyxin and VASP to actin stress fibers. Vascular endothelial cells that were subjected to fluid shear stress (2 h) aligned their actin filaments parallel to the flow direction (arrow). Zyxin and VASP mobilized to actin filaments, whereas vinculin remained at focal adhesions.
Lack of zyxin results in an impaired cellular response to mechanical stress
The striking mobilization of zyxin and its binding partner VASP in response to stretch led us to postulate that zyxin is critical for reinforcement of the actin cytoskeleton in response to physical stress. To test this hypothesis, we evaluated the stretch response of zyxin null (−/−) fibroblasts. Actin filament arrays in wild-type (+/+) and zyxin-null (−/−) fibroblasts before and after 15, 30, and 60 min of cyclic stretch were compared. Actin filaments in zyxin −/− fibroblasts were significantly thinner as compared with actin filaments in zyxin +/+ cells; representative images at 60 min of stretch are shown in Fig. 5 A. The SFTI analysis comparing zyxin +/+ with −/− cells confirmed that cyclic stretch resulted in robust thickening of stress fibers in zyxin +/+ cells in a duration-dependent manner, and this response was significantly attenuated in zyxin −/− cells (Fig. 5 B). Reintroduction of GFP-zyxin into zyxin −/− cells rescued their ability to reinforce the actin cytoskeleton in response to stretch (Fig. 5 C). These data demonstrate that zyxin is essential for the mechanically induced reinforcement of the actin cytoskeleton. It is intriguing to consider the possibility that the redistribution of zyxin to stress fibers in response to mechanical cues is driven by actin polymerization that depends on zyxin and partners such as VASP.
As described in Fig. 1, cells both reinforce and reorient their actin cytoskeletons in response to uniaxial stretch. Using the alignment index analysis (Fig. 1 B), we tested whether the lack of zyxin disturbed cytoskeletal reorientation in response to stretch. In this case, no significant difference in the alignment response was detected when comparing zyxin +/+ with −/− cells (Fig. 5 D). These results illustrate that zyxin is not essential for cells to reorient the cytoskeleton in response to directional mechanical cues; thus, at least some components of the mechanotransduction remain intact in zyxin −/− cells. A comparison of the responses of wild-type and zyxin −/− cells to mechanical stress provides the first experimental evidence that mechanically induced cytoskeletal reinforcement and reorientation responses are independent, mechanistically distinct events (Fig. 1 C).
Physiological implications
Our data highlight the importance of zyxin for the cellular response to mechanical forces, such as cyclic stretch and shear stress. Zyxin is expressed at high levels in mechanosensitive tissues such as lung, bladder, and the vasculature (unpublished data), indicating that zyxin plays a key role in the response to mechanical stress. Although zyxin-null mice are viable and fertile (Hoffman et al., 2003), the compromised response of zyxin −/− cells to mechanical cues suggests that the null mice may exhibit pathophysiological defects if subjected to mechanical challenges.
In this study, we have shown that zyxin is rapidly mobilized from focal adhesions to actin stress fibers in response to mechanical cues in a manner that is dependent on integrin function and is independent of stretch-activated ion channels. Moreover, zyxin is essential for the ability of cells to reinforce actin cytoskeleton in response to mechanical cues. The zyxin-dependent recruitment of the actin assembly–promoting factor VASP to actin filaments provides a potential mechanism for the mechanically induced reinforcement of stress fibers. Identification of the molecular triggers that result in the mobilization of zyxin and its stimulation of stress fiber remodeling will provide important insights into how cells respond to physical forces.
Materials and methods
Materials
Silicone membranes (durometer, 40; thickness, 0.5 mm) were obtained from AAA-Acme Rubber. Rat tail collagen (type I), poly-d-lysine, and bovine plasma fibronectin were purchased from BD Biosciences and Sigma-Aldrich, respectively. AlexaFluor phalloidin and DAPI were obtained from Invitrogen. Antizyxin (B71 and B38) and antithyroid receptor–interacting protein 6 (B65) antibodies were described previously (Hoffman et al., 2003). Other scientists provided antilipoma preferred partner MP2 (M. Petit, University of Leuven, Leuven, Belgium) and anti-VASP 2010 (F. Gertler, Massachusetts Institute of Technology, Cambridge, MA) antibodies. Antivinculin (Sigma-Aldrich), paxillin (Chemicon), FAK, VASP (BD Biosciences), and AlexaFluor secondary antibodies (Invitrogen) were used as recommended by the manufacturers.
Cell culture and transfection
Mouse fibroblasts were isolated as described previously (Bockholt and Burridge, 1995). Both early passage (<p5) mouse embryo fibroblasts and late passage (>p20) spontaneously immortalized fibroblasts were used with similar results. Primary human umbilical vein endothelial cells were used between passage p2 and p4 as recommended by the manufacturer (Cambrex).
Rectangular (3 × 4.5 cm) silicone membranes were coated by a mixture of 25 μg/ml type I collagen and 2 μg/ml fibronectin solution for 6 h. Cells were plated at a density of 10,000 cells/cm2, and experiments were performed in growth medium 24 h after plating. Poly-d-lysine experiments were performed in reduced serum (0.4%) medium. For complementation studies, an expression construct encoding full-length zyxin tagged with NH2-terminal eGFP and COOH-terminal myc was introduced into zyxin-null fibroblasts by using the retroviral vector LINXz (provided by F.H. Gage, Salk Institute, San Diego, CA; Hoshimaru et al., 1996) and retrovirus-producing Phoenix cells (provided by G.P. Nolan, Stanford University, Stanford, CA).
In vitro mechanical stimulation systems
A custom-made unidirectional stretch device was developed (provided by P. Murdock, University of Utah, Salt Lake City, UT; Yoshigi et al., 2003). The rectangular silicone membrane was clamped at both ends and pulled by a step motor. Strain patterns on the silicone membrane were analyzed by the finite element method to exclude areas where shear/longitudinal strain was > ±5%. Parameters of physiological linear strain were set to 15% and 0.5 Hz, as used by others previously (Cattaruzza et al., 2004; Kim-Kaneyama et al., 2005). Similar results were obtained by using 10% stretch. A shear stress device based on cone and plate viscometer was fabricated as described previously (Frangos, 1993). Laminar fluid shear was applied at 15 dyne/cm2.
Protein detection
Cell staining was based on standard protocols. In brief, cells were washed in warmed Hepes-buffered saline solution, fixed in 3.7% PFA for 10 min, and permeabilized in 0.5% Triton X-100 for 10 min. Cells were incubated in primary antibody overnight at 4°C followed by secondary antibody for 1 h at RT. Phalloidin staining was performed as recommended by the manufacturer (Invitrogen). Cell images were captured with a fluorescence microscope (Axioplan-2; Carl Zeiss MicroImaging, Inc.), a 40× plan NA 1.3 Neofluar objective, and a CCD camera (CoolSNAP; Roper Scientific) equipped with QED software (QED Imaging). For immunoblotting, proteins were resolved by 10% SDS-PAGE and were transferred to nitrocellulose membranes for ECL detection (GE Healthcare) using standard protocols.
Quantitative image processing
The alignment index of the actin cytoskeleton was equivalent to the kurtosis of angle distributions as described previously (Yoshigi et al., 2003). An analysis of actin filament thickness was based on erosion rank filtering as described previously (Russ, 2002). In brief, the sequential application of an erosion filter yielded brightness decay that was linearly related to the thickness of actin filaments. The decay constant was termed SFTI and reflects regional thickness of actin filaments. Phalloidin-stained actin filaments in 20 40× images were randomly selected, and 100 regions of interests (round shape; Φ = 20 μm; all inside cells) were analyzed and averaged. The source code was implemented by using LabVIEW (National Instruments).
Statistical analysis
Data were expressed as means ± SEM. Analysis of variance was performed followed by Dunnet's tests. P < 0.05 was defined as significant.
Acknowledgments
We thank M. Petit and F. Gertler for antibodies, F. Gage for the retroviral vector, G. Nolan for virus-producing cells, K. Andrews for the construction of GFP-zyxin, P. Murdock for the live cell stretch device, and K. Yasuda for experiments using endothelial cells.
This work was supported by the National Institutes of Health (grant GM50877 to M.C. Beckerle and grant K12HDO1410 Scholar to M. Yoshigi), the Children's Health Research Center of the University of Utah, and the Huntsman Cancer Foundation.
M. Yoshigi and L.M. Hoffman contributed equally to this work.
Abbreviations used in this paper: SFTI, stress fiber thickness index; VASP, vasodilator-stimulated phosphoprotein.
References
- Albinsson, S., I. Nordstrom, and P. Hellstrand. 2004. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J. Biol. Chem. 279:34849–34855. [DOI] [PubMed] [Google Scholar]
- Beckerle, M.C. 1997. Zyxin: zinc fingers at sites of cell adhesion. Bioessays. 19:949–957. [DOI] [PubMed] [Google Scholar]
- Beckerle, M.C. 1998. Spatial control of actin filament assembly: lessons from Listeria. Cell. 95:741–748. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A.D., N.Q. Balaban, and B. Geiger. 2003. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19:677–695. [DOI] [PubMed] [Google Scholar]
- Bockholt, S.M., and K. Burridge. 1995. An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src-, fyn-, and yes- mice. Cell Adhes. Commun. 3:91–100. [DOI] [PubMed] [Google Scholar]
- Cattaruzza, M., C. Lattrich, and M. Hecker. 2004. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension. 43:726–730. [DOI] [PubMed] [Google Scholar]
- Chen, K.D., Y.S. Li, M. Kim, S. Li, S. Yuan, S. Chien, and J.Y. Shyy. 1999. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J. Biol. Chem. 274:18393–18400. [DOI] [PubMed] [Google Scholar]
- Cunningham, K.S., and A.I. Gotlieb. 2005. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Invest. 85:9–23. [DOI] [PubMed] [Google Scholar]
- Davies, P.F., A. Robotewskyj, and M.L. Griem. 1994. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J. Clin. Invest. 93:2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees, B.E., K.M. Andrews, and M.C. Beckerle. 1999. Molecular dissection of zyxin function reveals its involvement in cell motility. J. Cell Biol. 147:1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradelizi, J., V. Noireaux, J. Plastino, B. Menichi, D. Louvard, C. Sykes, R.M. Golsteyn, and E. Friederich. 2001. ActA and human zyxin harbour Arp2/3-independent actin-polymerization activity. Nat. Cell Biol. 3:699–707. [DOI] [PubMed] [Google Scholar]
- Frangos, J.A, editor. 1993. Physical Forces and the Mammalian Cell. Academic Press, San Diego, CA. 400 pp.
- Gillespie, P.G., and R.G. Walker. 2001. Molecular basis of mechanosensory transduction. Nature. 413:194–202. [DOI] [PubMed] [Google Scholar]
- Helmke, B.P., and P.F. Davies. 2002. The cytoskeleton under external fluid mechanical forces: hemodynamic forces acting on the endothelium. Ann. Biomed. Eng. 30:284–296. [DOI] [PubMed] [Google Scholar]
- Hoffman, L.M., D.A. Nix, B. Benson, R. Boot-Hanford, E. Gustafsson, C. Jamora, A.S. Menzies, K.L. Goh, C.C. Jensen, F.B. Gertler, et al. 2003. Targeted disruption of the murine zyxin gene. Mol. Cell. Biol. 23:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshimaru, M., J. Ray, D.W. Sah, and F.H. Gage. 1996. Differentiation of the immortalized adult neuronal progenitor cell line HC2S2 into neurons by regulatable suppression of the v-myc oncogene. Proc. Natl. Acad. Sci. USA. 93:1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber, D.E. 2003. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 116:1397–1408. [DOI] [PubMed] [Google Scholar]
- Kakisis, J.D., C.D. Liapis, and B.E. Sumpio. 2004. Effects of cyclic strain on vascular cells. Endothelium. 11:17–28. [DOI] [PubMed] [Google Scholar]
- Katsumi, A., A.W. Orr, E. Tzima, and M.A. Schwartz. 2004. Integrins in mechanotransduction. J. Biol. Chem. 279:12001–12004. [DOI] [PubMed] [Google Scholar]
- Kim-Kaneyama, J.R., W. Suzuki, K. Ichikawa, T. Ohki, Y. Kohno, M. Sata, K. Nose, and M. Shibanuma. 2005. Uni-axial stretching regulates intracellular localization of Hic-5 expressed in smooth-muscle cells in vivo. J. Cell Sci. 118:937–949. [DOI] [PubMed] [Google Scholar]
- Krause, M., E.W. Dent, J.E. Bear, J.J. Loureiro, and F.B. Gertler. 2003. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 19:541–564. [DOI] [PubMed] [Google Scholar]
- Mack, P.J., M.R. Kaazempur-Mofrad, H. Karcher, R.T. Lee, and R.D. Kamm. 2004. Force-induced focal adhesion translocation: effects of force amplitude and frequency. Am. J. Physiol. Cell Physiol. 287:C954–C962. [DOI] [PubMed] [Google Scholar]
- Martinac, B. 2004. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 117:2449–2460. [DOI] [PubMed] [Google Scholar]
- McKnight, N.L., and J.A. Frangos. 2003. Strain rate mechanotransduction in aligned human vascular smooth muscle cells. Ann. Biomed. Eng. 31:239–249. [DOI] [PubMed] [Google Scholar]
- Naruse, K., T. Yamada, X.R. Sai, M. Hamaguchi, and M. Sokabe. 1998. Pp125FAK is required for stretch dependent morphological response of endothelial cells. Oncogene. 17:455–463. [DOI] [PubMed] [Google Scholar]
- Petit, M.M., J. Fradelizi, R.M. Golsteyn, T.A. Ayoubi, B. Menichi, D. Louvard, W.J. Van de Ven, and E. Friederich. 2000. LPP, an actin cytoskeleton protein related to zyxin, harbors a nuclear export signal and transcriptional activation capacity. Mol. Biol. Cell. 11:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ, J.C. 2002. The Image Processing Handbook. Fourth edition. CRC Press, Boca Raton, FL. 732 pp.
- Tamada, M., M.P. Sheetz, and Y. Sawada. 2004. Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell. 7:709–718. [DOI] [PubMed] [Google Scholar]
- Yi, J., S. Kloeker, C.C. Jensen, S. Bockholt, H. Honda, H. Hirai, and M.C. Beckerle. 2002. Members of the Zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J. Biol. Chem. 277:9580–9589. [DOI] [PubMed] [Google Scholar]
- Yoshigi, M., E.B. Clark, and H.J. Yost. 2003. Quantification of stretch-induced cytoskeletal remodeling in vascular endothelial cells by image processing. Cytometry A. 55:109–118. [DOI] [PubMed] [Google Scholar]