Abstract
Alzheimer's disease (AD) is the most common form of dementia among older people. It is characterized by the extracellular accumulation of β-amyloid (Aβ) deposits called senile or neuritic plaques. Aβ is generated by the proteolytic cleavage of Aβ precursor protein (APP) by β and γ-secretases localized in the secretory and endocytic compartments. In this issue, Yu et al. (on p. 87) report a novel mechanism for the generation of Aβ peptides, which takes place in autophagic vacuoles (AVs) that accumulate in AD brains.
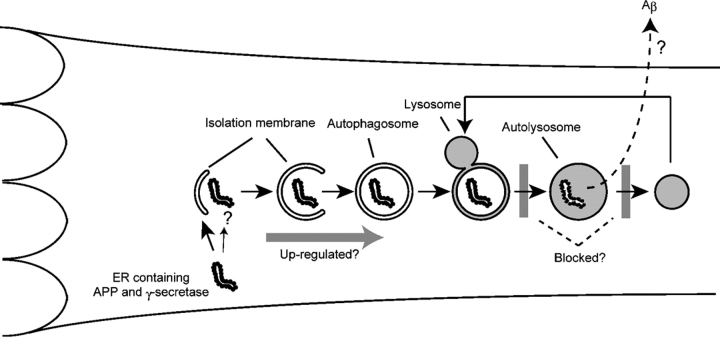
Autophagy is a generic term for pathways that transport cytosolic contents to lysosomes. Three types of autophagy have been proposed: macroautophagy, microautophagy, and chaperone-mediated autophagy (Cuervo, 2004; Levine and Klionsky, 2004). Among these, macroautophagy is believed to be responsible for the majority of intracellular protein degradation. When macroautophagy (hereafter simply referred to as autophagy) is induced by nutrient starvation and other stresses or chemical agents, cytoplasmic constituents, including organelles, are enclosed by membrane cisternae known as isolation membranes (Fig. 1). Closure of these membranes results in the formation of double membrane structures called autophagosomes. The autophagosomes eventually fuse with lysosomes to become autolysosomes. Lysosomal hydrolases degrade the cytoplasm-derived contents of the autophagosome together with its inner membrane. The primary role of autophagy is adaptation to nutrient starvation; autophagy is extensively induced after food withdrawal (Mizushima et al., 2004) and during the early neonatal period (Kuma et al., 2004). Besides this fundamental role, autophagy plays pleiotropic roles in intracellular clearance, degradation of invading bacteria, antigen presentation, and possibly cell death (Debnath et al., 2005). In addition, the relationship of autophagy to various diseases is now attracting attention.
Figure 1.
Aβ production in AVs. The membrane dynamics of autophagy and its proposed role in Aβ generation in the AD brain are depicted. A portion of cytoplasm is enclosed by the isolation membrane to form an autophagosome. The outer membrane of the autophagosome then fuses with a lysosome to degrade the inside materials. Organelles can be degraded by this pathway. After digestion is completed, autolysosomes are thought to become dense lysosomes. The origin of the isolation membrane is not known, but vesicles might be delivered from the ER. Thus, APP and the γ-secretase complex could be incorporated into the autophagosome membrane. Alternatively, ER and the Golgi apparatus containing APP and the γ-secretase complex may be engulfed by autophagosomes. In AD, some steps of autophagosome/autolysosome maturation are blocked, which may promote Aβ production.
The accumulation of AVs or related structures has been reported in several human diseases. Intriguingly, the list includes typical neurodegenerative diseases, including AD (Okamoto et al., 1991; Cataldo et al., 1996), polyglutamine (CAG repeat) diseases (Petersen et al., 2001; Ravikumar et al., 2002), and Parkinson's disease (Anglade et al., 1997). It should be noted that autophagic activity is maintained at low levels in the brain even with nutrient starvation, which is a condition that induces autophagy in most other organs (Mizushima et al., 2004). Thus, neural cells have the ability to induce autophagy in response to factors other than nutrient limitation.
It has been suggested that autophagy may be upregulated to eliminate abnormal intracellular proteins that would otherwise accumulate within cells as aggregates or inclusions (Ravikumar et al., 2004). In addition, it was recently shown that protein aggregates accumulate extensively in the hepatocytes of liver-specific Atg7 knockout mice without the expression of mutant proteins, suggesting that autophagy indeed plays a critical role in intracellular clearance (Komatsu et al., 2005).
In contrast, there is an opposing idea that continuous autophagy might promote cell death; however, this is still a controversial issue (Debnath et al., 2005). It has been shown that autophagy is induced in certain types of cell death (mainly nonapoptotic), but only a limited number of studies have demonstrated that autophagy is required for cell death (Shimizu et al., 2004; Yu et al., 2004). Most cell death that is associated with autophagy may not be caused by the activation of autophagy (Degterev et al., 2005).
In this issue, Yu et al. (2005) report a novel role of autophagy in the pathogenesis of AD. They have shown that AVs accumulate in the brains of AD patients and presenilin-1 (PS1)/APP mice, which is an AD model in which both human mutant PS1 and mutant APP are expressed. A large number of AVs were observed in dystrophic neurites before the appearance of extracellular Aβ deposition, indicating that AV accumulation is a very early event. In contrast to the larger number of AVs, the increase in dense bodies (dense lysosomes) was much smaller. Accordingly, they have concluded that there is an impairment of autolysosome maturation into lysosomes in the AD brain (Fig. 1).
Furthermore, Yu et al. (2005) found not only that AVs accumulate in AD neurons but also that Aβ is produced within the AVs. First, they detected Aβ peptides (both Aβ40 and Aβ42) and γ-secretase complex components such as PS1 and nicastrin on the membranes of and inside AVs in the brains of AD patients, AD model mice, and several cell lines expressing APP. By cell fractionation analysis, they confirmed that these proteins were highly enriched in AVs. Second, they observed a clear correlation between autophagic activity and Aβ secretion into the medium. When autophagy was upregulated in cell lines by amino acid starvation or mammalian target of rapamycin inhibition, Aβ generation was accelerated. In contrast, Aβ generation was suppressed when autophagy was inhibited by 3-methyladenine or an excess of amino acids. Finally, they detected PS1-dependent γ-secretase activity in isolated AV fractions. Under normal growth conditions, the highest γ-secretase activity was detected in the ER/Golgi/endosome fraction. However, the highest γ-secretase activity was detected in AVs when autophagy was induced by starvation. Altogether, Yu et al. (2005) propose that AVs are a novel site of Aβ peptide production.
The idea that persistent and/or incomplete autophagic degradation participates in the excess production of Aβ peptides is very attractive. The findings of Yu et al. (2005) raise several issues that merit further investigation. Can Aβ peptides that are produced in AVs be efficiently secreted into the extracellular space and contribute to plaque formation? It is currently thought that the lysosome is not a terminal organelle and that lysosomal contents can be secreted through exocytosis or exosome-mediated secretion. How are APP/Aβ and the γ-secretase complex incorporated into AVs? Because PS1 is detected on the limiting membrane of AVs (Yu et al., 2005), PS1 might be recruited on the source membrane of autophagosomes. In addition, as ER fragments are frequently sequestered into autophagosomes (Hamasaki et al., 2005), APP and the γ-secretase complex might be engulfed together with ER fragments as substrates (Fig. 1). Golgi apparatus containing APP and secretases also could be sequestered by autophagosomes. Alternatively, these proteins may be delivered to endosomes and lysosomes via the conventional secretory pathway and may then join the autophagic pathway upon AV–lysosome fusion. Is Aβ in AVs toxic for cells? It has been reported that the number of extracellular plaques does not correlate with AD symptoms, whereas intracellular Aβ is toxic to neurons (Billings et al., 2005). The final, and probably most important, question is to determine what primarily causes the AV maturation defect in AD. This question may well be related to the question of why APP is specifically processed into Aβ instead of being completely degraded to amino acids by lysosomal proteases.
In general, little is known about the involvement of autophagy in neurodegenerative diseases. However, the new data reported by Yu et al. (2005) provides new insights into the role of AVs and the mechanism of Aβ generation in AD.
Abbreviations used in this paper: Aβ, β-amyloid; AD, Alzheimer's disease; APP, Aβ precursor protein; AV, autophagic vacuole; PS1, presenilin-1.
References
- Anglade, P., S. Vyas, F. Javoy-Agid, M.T. Herrero, P.P. Michel, J. Marquez, A. Mouatt-Prigent, M. Ruberg, E.C. Hirsch, and Y. Agid. 1997. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol. Histopathol. 12:25–31. [PubMed] [Google Scholar]
- Billings, L.M., S. Oddo, K.N. Green, J.L. McGaugh, and F.M. Laferla. 2005. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 45:675–688. [DOI] [PubMed] [Google Scholar]
- Cataldo, A.M., D.J. Hamilton, J.L. Barnett, P.A. Paskevich, and R.A. Nixon. 1996. Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer's disease. J. Neurosci. 16:186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo, A.M. 2004. Autophagy: in sickness and in health. Trends Cell Biol. 14:70–77. [DOI] [PubMed] [Google Scholar]
- Debnath, J., E.H. Baehrecke, and G. Kroemer. 2005. Does autophagy contribute to cell death? Autophagy. 1:66–74. [DOI] [PubMed] [Google Scholar]
- Degterev, A., Z. Huang, M. Boyce, Y. Li, P. Jagtap, N. Mizushima, G.D. Cuny, T.J. Mitchison, M.A. Moskowitz, and J. Yuan. 2005. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1:112–119. [DOI] [PubMed] [Google Scholar]
- Hamasaki, M., T. Noda, M. Baba, and Y. Ohsumi. 2005. Starvation triggers the delivery of the endoplasmic reticulum to the vacuole via autophagy in yeast. Traffic. 6:56–65. [DOI] [PubMed] [Google Scholar]
- Komatsu, M., S. Waguri, T. Ueno, J. Iwata, S. Murata, I. Tanida, J. Ezaki, N. Mizushima, Y. Ohsumi, Y. Uchiyama, et al. 2005. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 169:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma, A., M. Hatano, M. Matsui, A. Yamamoto, H. Nakaya, T. Yoshimori, Y. Ohsumi, T. Tokuhisa, and N. Mizushima. 2004. The role of autophagy during the early neonatal starvation period. Nature. 432:1032–1036. [DOI] [PubMed] [Google Scholar]
- Levine, B., and D.J. Klionsky. 2004. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 6:463–477. [DOI] [PubMed] [Google Scholar]
- Mizushima, N., A. Yamamoto, M. Matsui, T. Yoshimori, and Y. Ohsumi. 2004. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell. 15:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, K., S. Hirai, T. Iizuka, T. Yanagisawa, and M. Watanabe. 1991. Reexamination of granulovacuolar degeneration. Acta Neuropathol. (Berl.). 82:340–345. [DOI] [PubMed] [Google Scholar]
- Petersen, A., K.E. Larsen, G.G. Behr, N. Romero, S. Przedborski, P. Brundin, and D. Sulzer. 2001. Expanded CAG repeats in exon 1 of the Huntington's disease gene stimulate dopamine-mediated striatal neuron autophagy and degeneration. Hum. Mol. Genet. 10:1243–1254. [DOI] [PubMed] [Google Scholar]
- Ravikumar, B., R. Duden, and D.C. Rubinsztein. 2002. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 11:1107–1117. [DOI] [PubMed] [Google Scholar]
- Ravikumar, B., C. Vacher, Z. Berger, J.E. Davies, S. Luo, L.G. Oroz, F. Scaravilli, D.F. Easton, R. Duden, C.J. O'Kane, and D.C. Rubinsztein. 2004. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 36:585–595. [DOI] [PubMed] [Google Scholar]
- Shimizu, S., T. Kanaseki, N. Mizushima, T. Mizuta, S. Arakawa-Kobayashi, C.B. Thompson, and Y. Tsujimoto. 2004. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 6:1221–1228. [DOI] [PubMed] [Google Scholar]
- Yu, L., A. Alva, H. Su, P. Dutt, E. Freundt, S. Welsh, E.H. Baehrecke, and M.J. Lenardo. 2004. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 304:1500–1502. [DOI] [PubMed] [Google Scholar]
- Yu, W.H., A.M. Cuervo, A. Kumar, C.M. Peterhoff, S.D. Schmidt, J.H. Lee, P.S. Mohan, M. Mercken, M.R. Farmery, L.O. Tjernberg, et al. 2005. Macroautophagy—a novel β-amyloid peptide-generating pathway activated in Alzheimer's disease. J. Cell Biol. 171:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]