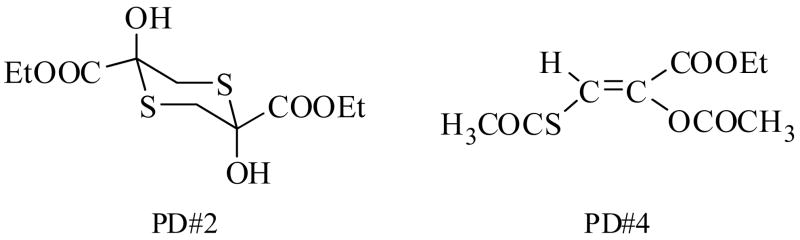Abstract
Historically, antidotal potencies of cyanide antagonists were measured as increases in the experimental LD50 for cyanide elicited by the antidotes. This required the use of high doses of cyanide following pretreatment with the putative antidote. Since IACUC guidelines at our institutions strongly discourage LD50 determinations: we developed a new test paradigm that allowed for maximal survival of cyanide-treated animals with greatly reduced numbers of animals. Symptoms of cyanide toxicity include disruption of neuromuscular coordination, i.e., the righting reflex. Therefore, to establish a dose-response curve, the times required for recovery of this righting reflex with increasing doses of cyanide were measured. A cyanide dose that disrupted this righting reflex for approximately one h with minimal deaths was then selected. Using this paradigm, the current cyanide antidotes, viz., nitrite plus thiosulfate and hydroxocobalamin, as well as some potential cyanide antidotes that we developed, were evaluated pre- and post-cyanide. This allowed, for the first time, the assessment of the post-cyanide effectiveness of the current antidotes against cyanide poisoning in a live animal. In addition, some prototype compounds were found to exhibit antidotal efficacy not only when injected i.p. following cyanide, but also when administered orally 30 min before cyanide. Pre-cyanide oral efficacy suggests that such compounds have the potential of being administered prophylactically before exposure to cyanide. This new test paradigm was found to be a powerful tool for assessing the efficacies of some novel antidotes against cyanide and should be equally applicable for evaluating putative antidotes for other neurotoxins.
Keywords: Cyanide, animal model, recovery, righting reflex, post-cyanide, pre-cyanide, oral efficacy, antidote
1. Introduction
Development of antidotes to central nervous system (CNS) poisons has been difficult, in part, because of the extreme toxicity of neurotoxins over narrow dose ranges. The lack of adequate animal models for testing the efficacies of putative antidotes is a more serious problem. Historically, the most commonly used assessment of an antidote’s effect in vivo against a CNS poison, such as cyanide (Prabhakaran et al., 2006; Way, 1984) has been based on the lethal dose (LD) of the toxin that caused 50% death in a population, i.e., the LD50. Thus, antidotal potencies of antagonists against cyanide were measured as the degree of increase in this cyanide LD50 by antidote co-treatment, defined as the protective index.
The current guidelines of our Institutional Animal Care and Use Committees (IACUC) strongly discourage LD50 determinations and require investigators proposing such experiments to seek alternatives in which death is not an endpoint. Accordingly, it was necessary to devise a new test paradigm that minimized distress, allowed maximal survival of the neurotoxin-treated animals, and enhanced the power of statistical analysis, thereby reducing the number of animals required for each treatment group. Cyanide inhibits the cytochrome c oxidase pathway resulting in neurotoxicity (Isom et al., 1982; Pettersen and Cohen, 1993; Way et al., 1988; Zhang et al., 2007) and a readily observable loss of neuromuscular coordination. The loss of neuromuscular coordination in animals has been used as an indicator of drug neurotoxicity (Quinlan et al., 2002; Werawattanachai et al., 2007). Specifically, the absence (loss) of righting reflex from an inverted wire mesh screen following dosing with the toxin has been used as the primary assessment of such drug toxicity, e.g., with cyanide, soman, chlorpromazine, haloperidol, phenobarbital, and certain sulfur compounds (Baskin et al., 1999; Coughenour et al., 1977; Koplovitz et al., 1989).
We have now expanded this test paradigm by conceptually shifting the emphasis from the observation of dose-related toxicity to the dose-related recovery of neuromuscular coordination following a toxic, but sub-lethal dose of cyanide, as measured by the time required for the animal to “right” itself from an inverted screen, i.e., the recovery time. The validity of recovery as an end point was first examined using the antidotes currently in use, viz., (a) the combination of nitrite plus thiosulfate, and (b) hydroxocobalamin. The latter has recently been approved by the FDA for use as a cyanide antidote in the United States. These were administered both pre- and post-cyanide and their effect on recovery was determined. This was followed by the determination of the efficacy of some novel prototype compounds that we developed as potential cyanide antidotes.
2. Materials and Methods
2.1. Animals
Male ND-4 Swiss-Webster mice weighing 25–34 g were purchased from Harlan Labs, Indianapolis, IN. All animals were group housed in 17×10×8-inch polycarbonate cages with a maximum of eight mice per cage, and placed in a private, temperature-controlled room (21–22°C) within the animal facility barrier, with 12:12 L:D photoperiods (lights on at 0700). These mice, therefore, did not come in contact with female mice or with other experimental animals. The mice were allowed access to Harlan Teklad # 8604 rodent chow, Madison, WI and water ad lib, and the experiments commenced following a week of adaptation to the new environment. Experiments were approved by the University of Minnesota and Department of Veterans Affairs Medical Center IACUC committees, Minneapolis, MN, and conducted in accordance with their guidelines.
2.2. Chemicals
Sodium cyanide, manufactured by Fluka Biochemika, and hydroxocobalamin hydrochloride were purchased from Sigma Aldrich, Inc. St. Louis, MO. Sodium nitrite and sodium thiosulfate were obtained from a Cyanide Antidote Package produced by Taylor Pharmaceuticals, Decatur, IL. Dimethyl sulfoxide was purchased from Burdick and Jackson Laboratories, Inc., Muskegon, MI. The prototype compounds to be evaluated as potential cyanide antidotes, designated as PD#2 and PD#4, were designed and synthesized in our laboratory (Fig. 1). These novel compounds are sulfhydryl-protected derivatives (prodrugs) of 3-mercaptopyruvate (3-MP), which, in a cellular environment, release this natural substrate for the predominantly cytosolic enzyme, 3-mercaptopyruvate: cyanide sulfurtransferase (EC 2.8.1.2), (Nagasawa et al., 2007).
Figure 1.
PD#2 and PD#4 were designed and synthesized in our laboratory. These novel compounds are sulfhydryl-protected derivatives (prodrugs) of 3-mercaptopyruvate (3-MP).
2.3. Mouse Model for Assessing Cyanide Toxicity
The recovery test paradigm is as follows. Mice are treated with a 4.8 mg/kg dose of sodium cyanide intraperitoneally (i.p.), which induces a knockdown state with recovery periods of approximately one h. Before each study, three or more animals are treated with cyanide at this dose to verify that the recovery times are close to one h. A critical aspect of this behavioral test is to correctly assess the optimal time for testing. Premature testing of an animal without full neuromuscular coordination will artificially prolong the recovery period. Signs that the animal is ready to be tested include bodily appearance, both eyes open and bright, head up and moving, steady on feet, un-hunched back and certain movements about the cage, e.g., definite exploration of environment with alert quick ambulation and social interaction. Recognition of these signs, which together indicate the approach of righting reflex recovery, is easily gained by experience. At this point of readiness for testing, the mouse is placed on top of a 10×10 cm fine mesh wire screen. The screen is gently inverted, and the mouse is given one minute to crawl back on top of the screen. The time from cyanide administration to the time the mouse successfully rights itself from the inverted screen is taken as the endpoint and recorded. Animals were checked at 24 and 48 h for lethality.
2.4. Statistical analysis
Data were analyzed by one-factor ANOVA with Scheffe post hoc to compare treatment means. A p-value of <0.05 is considered statistically significant. Data are represented as values ± SE. Correlations were determined using simple or polynomial regressions.
3. Results
3.1. Effect of cyanide dose on recovery time and survival: Dose-Response
Righting reflex recovery time and survival data were collected for each mouse challenged with NaCN. A group of 8 mice were tested with each increasing dose of cyanide to create dose response curves for both righting reflex recovery times and percent survival.
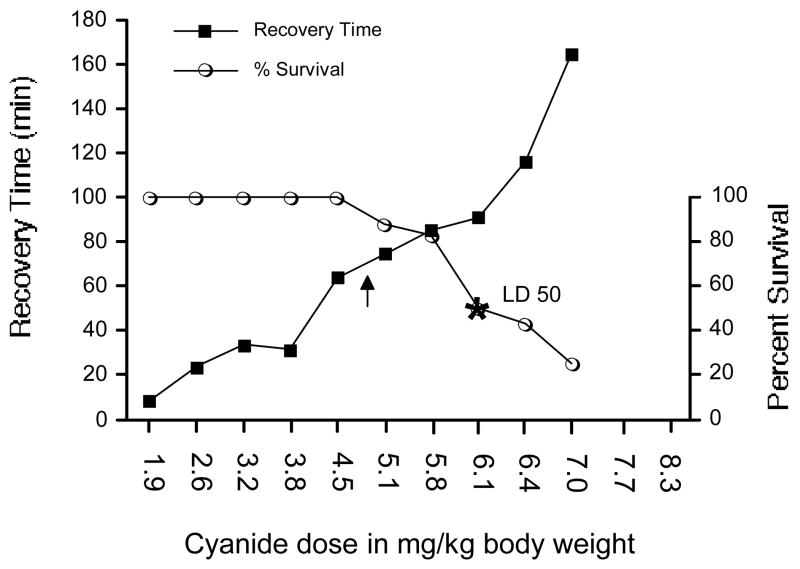
Increasing doses of cyanide increased the recovery time of the righting reflex (Fig. 2). The simple regression analysis of the cyanide dose response slope was Y= −46.638 + 24.688 * X; R2=0.832.
Figure 2.
The effect of cyanide dose on (a) time required for recovery of neuromuscular coordination, and (b) survival. The left y-axis shows the time in min required for the recovery of the righting reflex, while the right y-axis indicates percent survival. The optimum dose for antidote screening was 4.8 mg NaCN/kg body weight, with a resultant 68 min average time required for the recovery of the righting reflex (shown at the arrow). The survival rate at this optimum dose was 94%. The estimated LD50 for NaCN was 6.1 mg/kg (shown at the asterisk). Values reflect means, n= 8/group, except control, i.e., 0 cyanide, n= 20.
Beginning with a NaCN dose of 1.9 mg/kg increasing the dose incrementally had no effect on survival until 5.1 mg/kg, resulting in 85% survival. Subsequently, increasing cyanide doses resulted in decreasing survival to approximately 25% for the maximum 7.0 mg/kg dose. Righting reflex recovery times of survivors over this range increased 2.5-fold from 68 to 170 min. The linear regression of the survival slope for doses ranging from 4.7 to 7.0 mg/kg was Y= 244.943−30.982 * X; R2=0.915. The dose increment of cyanide that reduced survival in a 30 g mouse from essentially 100 to 25% was 77 μg. The estimated LD50 for NaCN was 6.1 mg/kg (Fig. 2).
3.2. Effect of nitrite/thiosulfate in altering the slope of the recovery time and survival with increasing doses of cyanide
Righting reflex recovery time and survival data were collected for each mouse protected with nitrite plus thiosulfate (N/T) administered i.p. 30 min pre-CN at 1.45 and 6.32 mmol/kg, respectively. Groups of 4 mice (except the 34.0 and 39.0 mg/kg doses where n= 8) were tested with each increasing dose of cyanide to create dose response curves for both righting reflex recovery times and percent survival (Fig. 2).
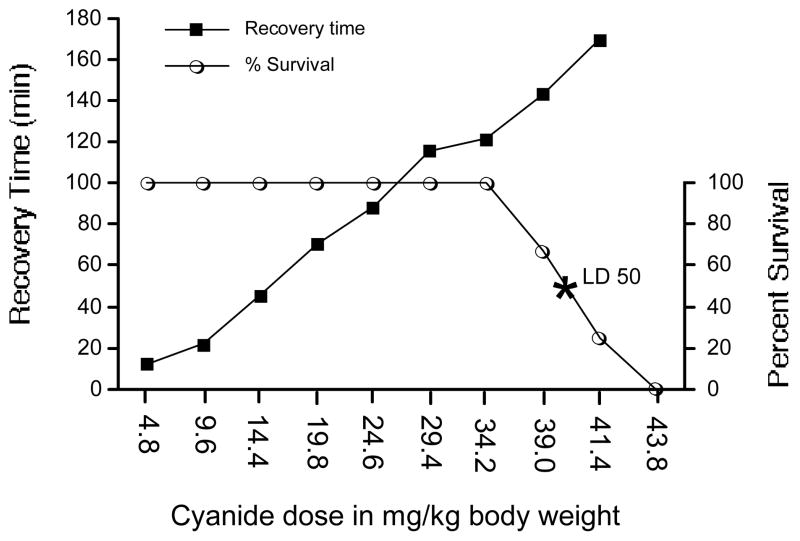
Increasing doses of cyanide increased the recovery times of the righting reflex (Fig. 3). The simple regression analysis of the cyanide dose response slope was Y= −9.4 + 3.957 * X; R2=0.923.
Figure 3.
The effect of cyanide dose on (a) time required for recovery of neuromuscular coordination, and (b) survival when a standard dose of nitrite/thiosulfate was administered as a protective antidote given 30 min pre-cyanide. The left y-axis shows the time in min required for the recovery of the righting reflex, while the right y-axis indicates percent survival. The estimated LD50 for NaCN was 40.0 mg/kg (shown at the asterisk). Values reflect means, n= 4/group, except the 34.2 and 39.0 mg/kg doses, n= 8.
Beginning with a NaCN dose of 4.8 mg/kg, increasing the dose incrementally had no effect on survival until 39.0 mg/kg, resulting in 67% survival. Subsequently, increasing cyanide doses resulted in decreasing survival to 0% for the maximum 43.8 mg/kg dose. Righting reflex recovery times of survivors over this range increased slightly from 143 to 170 min. The linear regression of the survival slope for doses ranging from 4.8 to 43.8 mg/kg was Y= 411.56 −9 * X; R2=0.929. The estimated LD50 for NaCN was 40.0 mg/kg (Fig. 3).
3.3. Absence of effects on righting reflex by antidotes administered alone
All of the antidotes were tested alone for their effect on righting reflex at the highest doses administered i.p. in the cyanide experiments (vide supra). The mice were tested at 0, 1, 5, 30, and 60 min, as well as 24 h after antidote administration. The data collected were animal righting times in sec. The ANOVA repeated measures treatment effect was (F(4,15)=0.956; p=0.460), indicating no significance; hence, these data are not presented.
3.4. Effect of current antidotes administered pre- and post-cyanide
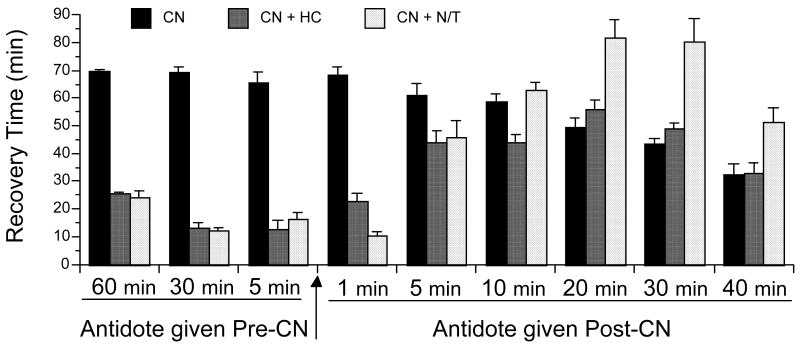
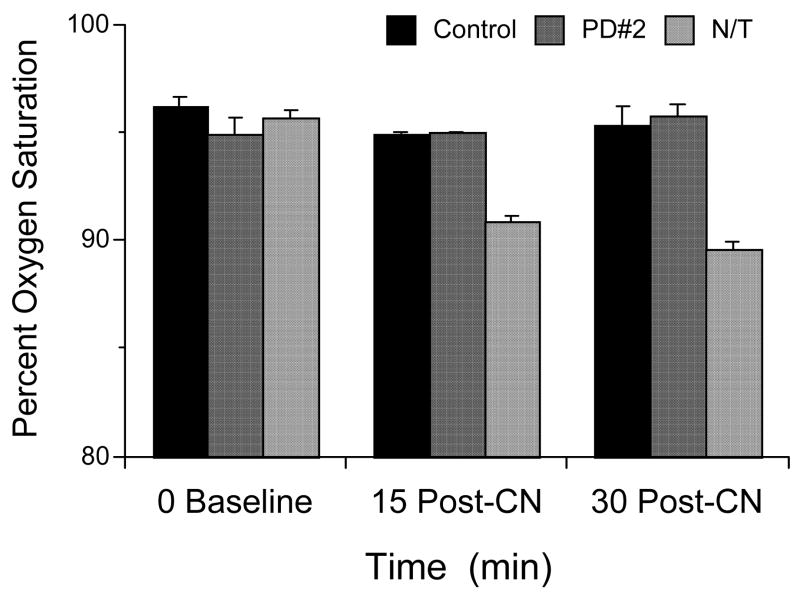
The effect of the current antidotes on the recovery of neuromuscular coordination in cyanide-treated animals was examined when given at 60, 30, and 5 min before cyanide and at 1, 5, 10, 20, 30, and 40 min after cyanide. Pre-CN righting reflex recovery times were measured from the NaCN injection which was given after the antidote or carrier, while post-CN righting reflex recovery times were measured from the carrier or antidote injections, which were given after the NaCN. Therefore, the righting reflex recovery time of the controls decreases with the wait period to saline or antidote injection (Fig. 4). The selected antidote doses are equivalent to human doses and were administered i.p., with hydroxocobalamin (HC) at 0.22 mmol/kg, and nitrite plus thiosulfate (N/T) at 1.45 and 6.32 mmol/kg, respectively. The cyanide was administered i.p. at the optimal 4.8 mg/kg, based on an experimentally determined dose-response curve (vide infra). Cyanide controls were run before each experiment to check for environmental changes that alter animal behavior. The righting reflex recovery times for the current antidotes, when given pre-cyanide or at 1 min post-cyanide, were significantly different from control (p<0.0001; Fig. 4). However, at 5, 10, 20, 30 and 40 min post-cyanide, the antidotal efficacies were compromised drastically; for example, at 10 min post-cyanide the righting times of only HC was significantly different from control (p=0.001). Not only was the efficacy reduced, there was a significant increase in the recovery time with the standard dose of N/T given 20, 30, and 40 min post-cyanide (p<0.0001, p<0.0001, and 0.0030, respectively Fig. 3). All the mice in these groups were cyanotic, with a definite skin discoloration (blue). The percent blood oxygen saturation measured with N/T and PD#2 given 5 min post-CN are presented in Fig. 5. Measurements were taken with a Starr Life Sciences mouse oximeter light cuff and consisted of repeated measures at 0, 15, and 30 min post-CN. A repeated measures ANOVA with Scheffe post hoc showed a significant treatment effect for N/T vs. control (p= 0.0135) (F(2,6)=31.29; p=0.0098), but not for PD#2.
Figure 4.
The effect of current antidotes administered pre- and post-cyanide. Shown is recovery time of the righting reflex in min. Recovery times for post-CN treatments are from the time of antidote administration. The current antidotes, hydroxocobalmin (HC), at 0.22 mmol/kg and nitrite plus thiosulfate (N/T) at 1.45 and 6.32 mmol/kg, respectively, were administered i.p. at the times indicated before and after cyanide (NaCN, 4.8 mg/kg, i.p.). Values reflect means ± SE, n= 8/group, except control, i.e., CN n= 15.
Figure 5.
The percent oxygen saturation of blood as measured using a noninvasive mouse oximeter with repeated measures over multiple time points. The NaCN dose (i.p.) was 4.8 mg/kg administered 5 min before the antidotes. PD#2 was administered at 0.29 mmol/kg and nitrite plus thiosulfate (N/T) at 1.45 and 6.32 mmol/kg, respectively, all i.p. Values reflect means ± SE, n= 2/group with repeated measures.
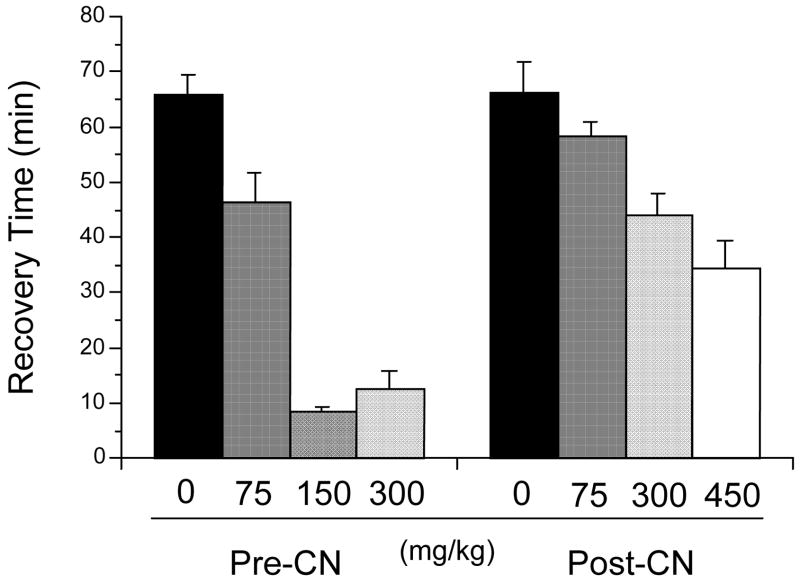
3.5. Effect of hydroxocobalamin dose administered i.p. 5 min pre- and post-cyanide
Given the current interest in hydroxocobalamin, the effect of dose was examined for this agent when administered i.p., both pre- and post-cyanide. The doses selected for administration 5 min pre-cyanide were 0, 75, 150, and 300 mg/kg, or 0, 0.055, 0.11, and 0.22 mmol/kg, respectively. The doses for injection 5 min post-cyanide were similar, except 450 mg/kg (0.33 mmol/kg) was selected as the highest dose. There were significant dose effects for both pre- and post-cyanide, (F(3,37)=47.374, p<0.0001) and (F(3,33)=6.244, p=0.0018), respectively (Fig. 6).
Figure 6.
Dose effect of hydroxocobalamin in mg/kg administered i.p. at 5 min pre- and post-cyanide (4.8 mg/kg). Recovery times of the righting reflex are in min. Values reflect means ± SE, n= 8/group, except control, i.e., CN n= 20.
3. 6. Antidotal properties of the prototype compounds, PD#2 and PD#4, against cyanide
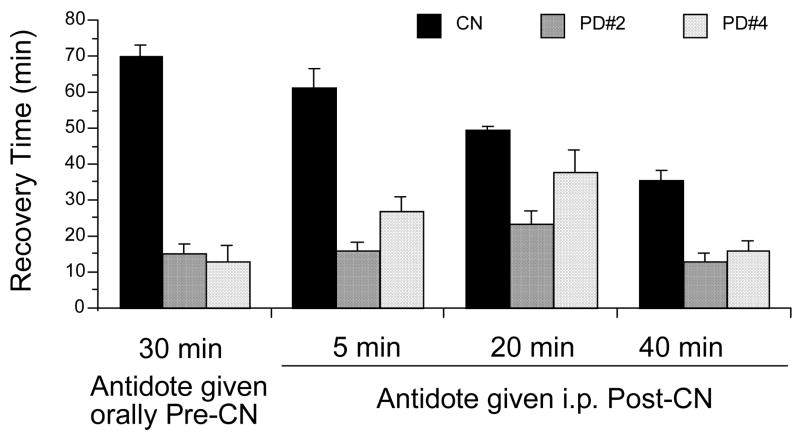
The effect of our prototype cyanide antidotes on the recovery of neuromuscular coordination in cyanide-treated animals was examined when administered 30 min before cyanide and 5, 20, and 40 min after cyanide (Fig. 7). These times were based on antidote efficacy and selected from preliminary studies with the current antidotes (Fig. 4). For pre-cyanide, the prototype compounds were administered orally by gavage at 1.45 mmol/kg. This dose was selected to correspond to the nitrite i.p. dose of 1.45 mmol/kg. For post-cyanide administration, the compounds were given i.p. at 0.29 mmol/kg. This dose is 20% of the oral dose and corresponds to the hydroxocobalamin i.p. dose of 0.22 mmol/kg. Cyanide was administered i.p. at the standard dose of 4.8 mg/kg.
Figure 7.
The antidotal properties of the prototype compounds, PD#2 and PD#4, administered pre- and post-cyanide. The NaCN dose (i.p.) was 4.8 mg/kg. The antidotes were administered i.p. at 0.29 mmol/kg post-CN, and orally pre-CN at 1.45 mmol/kg. Recovery times for post-CN treatments are from the time of antidote administration. Values reflect means ± SE, n= 8/group, except control, i.e., CN n= 15.
These prototype compounds were found to be highly effective in reducing the righting reflex recovery times in all treatment paradigms (Fig. 7), the observed recovery times being significantly different from controls (p<0.0001), except for PD#4 at 20 min post-cyanide (p=0.063).
Discussion
The central nervous system is the primary site of cyanide toxicity (Prabhakaran et al., 2006; Way, 1984). Cyanide-induced histotoxic hypoxia is a method used for cerebral anoxia studies (Amano et al., 1993; Karakida et al., 2007). This hypoxia is effected through cyanide-inhibition of the cytochrome c oxidase pathway primarily in the brain (Isom et al., 1982; Pettersen and Cohen, 1993; Way et al., 1988; Zhang et al., 2007), but also in the skeletal muscles (Zhang et al., 2006). The duration of a 2 mg/kg cyanide-induced coma (Karakida et al., 2007) as measured by the righting reflex assay was consistent with our data. Mice given low doses of cyanide (2–5 mg/kg) display irregular breathing, muscle incoordination, gasping, and convulsions (Isom et al., 1982; Kanthasamy et al., 1994). It is thought that the muscular incoordination and weakness may be explained in part by impaired skeletal muscle mitochondrial function (Haxhiu et al., 1993; Kanthasamy et al., 1994; Zhang et al., 2006). This cyanide-induced transitory muscle incoordination provides an excellent tool for assaying righting reflex recovery.
Our data (Fig. 3) establishes the relationship between the recovery of righting reflex and the LD50 slopes, both slopes shifting in tandem to the right, as demonstrated by the slopes created with cyanide alone versus slopes created with cyanide and the antidote combination of nitrite/thiosulfate. Furthermore, the LD50 values obtained in these studies of 6.1 mg/kg for cyanide alone and 40.0 mg/kg with antidote protection (nitrite/thiosulfate) against cyanide were consistent with those of other investigators (Leung et al., 1986; Schwartz et al., 1979), despite some experimental differences. Mice having a righting reflex recovery time of over 160 min are in the LD75 category. This occurred whether cyanide was administered alone or combined with an antidote, thus demonstrating a definite correlation between the LD50 slope and the righting reflex recovery slope.
These results clearly demonstrate that an animal model focusing on recovery from the effects of a neurological toxin has multiple advantages. The ability to test efficacy of antidotes post-cyanide as well as pre-cyanide is the first important advantage, because a major assumption for the LD50 paradigm was that antidotes showing efficacy given pre-cyanide would also be effective when administered after the toxic insult. Our data demonstrate that although an antidote may be effective when given before cyanide, antidotal efficacy may not prevail when administered after cyanide. Another major advantage of this model is that the recovery is expressed in units of time (min) for multiple animals, versus the single endpoint (death) for the LD50 analysis. Such data can significantly increase the statistical power of the model, allowing comparisons between antidotes with only minor differences in efficacy, and reducing the number of animals required to characterize a putative antidote by 30–60%. In addition, recovery periods may be increased due to antidote-induced toxicity in an animal also undergoing the stress of cyanide poisoning. The third advantage is the narrow range of experimental variability observed for both controls and treated, thus allowing for minimal numbers of animals to achieve statistical significance. These combined features of the model met IACUC guidelines by eliminating death as an endpoint and incorporating a conceptual shift from toxicity to recovery, thereby creating a powerful new paradigm for evaluating the efficacy of putative antidotes against a neurological poison such as cyanide.
The power of this recovery paradigm is demonstrated with our post-cyanide data. When comparing the effect of the current antidotes 5 min post-cyanide to pre-cyanide efficacy, the data showed a drastic reduction in antidotal efficacy (Fig. 4). Indeed, when administered 20 min post-cyanide (and beyond), neither the standard human dose of 300 mg/kg hydroxocobalamin nor the standard nitrite plus thiosulfate doses were protective against 4.8 mg/kg of NaCN. Our data further show that the standard dose of N/T aggravated recovery when given 20, 30, or 40 min post-CN. A significant decrease in percentage of oxygen saturation at 15 and 30 min post-CN was noted following N/T injection as compared to control and PD#2 (Fig. 5). The blue skin coloration of these albino mice treated with the N/T standard antidote at 20–40 min post-CN is further evidence for a decrease in blood percent oxygen saturation. The cyanide is slowly being cleared by various endogenous mechanisms (Buzaleh et al., 1990; Sousa et al., 2003) before the carrier or antidote is administered. Thus, the recovery of righting reflex paradigm allowed the above issues to be addressed both pre- and post-cyanide administration.
In contrast to the prolonged recovery induced by N/T treatment post-CN, PD#2 and PD#4 were highly effective as cyanide antidotes even when administered i.p., 40 min following the dose of cyanide (Fig. 7). This suggests their potential use for the treatment (i.v.) of patients with cyanide poisoning by emergency room personnel. Additionally, PD#2 and PD#4 given orally by gavage 30 min pre-cyanide were equally effective as the current antidotes given i.p. at that same time points. Thus, these compounds hold promise as prophylactic agents against cyanide in impending terrorist or military threat situations.
The ability to test potential antidotes to nerve poisons such as cyanide both pre- and post-toxin allows for the testing of putative antidotes not only singly, but also in combination. This enables the assessment of antidotal cocktails, the next obligatory step in antidote development.
Acknowledgments
We thank Mary Mullett for technical assistance. We thank Ashley Burkhart of Starr Life Sciences Corporation, Oakmont, PA for the excellent mouse oximeter demonstration that provided us with percent oxygen saturation data. This study was supported by a Seed Grant from the Center for Drug Design, Academic Health Center, University of Minnesota, Minneapolis, MN, and by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke (award # 1U01NS058087-01).
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano M, Goto A, Takahashi N, Hasegawa T, Nabeshima T. Effects of BMY-21502 on anoxia in mice. Jpn J Pharmacol. 1993;61:157–163. doi: 10.1254/jjp.61.157. [DOI] [PubMed] [Google Scholar]
- Baskin SI, Porter DW, Rockwood GA, Romano JA, Jr, Patel HC, Kiser RC, Cook CM, Ternay AL., Jr In vitro and in vivo comparison of sulfur donors as antidotes to acute cyanide intoxication. J Appl Toxicol. 1999;19:173–183. doi: 10.1002/(sici)1099-1263(199905/06)19:3<173::aid-jat556>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Buzaleh AM, Vazquez ES, Batlle AM. An experimental assessment of cyanide challenge dose-response, time-course and recovery of indicative toxicity parameters. Gen Pharmacol. 1990;21:839–843. doi: 10.1016/0306-3623(90)90442-o. [DOI] [PubMed] [Google Scholar]
- Coughenour LL, McLean JR, Parker RB. A new device for the rapid measurement of impaired motor function in mice. Pharmacol Biochem Behav. 1977;6:351–353. doi: 10.1016/0091-3057(77)90036-3. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Erokwu B, van Lunteren E, Cherniack NS, Strohl KP. Central and spinal effects of sodium cyanide on respiratory activity. J Appl Physiol. 1993;74:574–579. doi: 10.1152/jappl.1993.74.2.574. [DOI] [PubMed] [Google Scholar]
- Isom GE, Burrows GE, Way JL. Effect of oxygen on the antagonism of cyanide intoxication--cytochrome oxidase, in vivo. Toxicol Appl Pharmacol. 1982;65:250–256. doi: 10.1016/0041-008x(82)90007-2. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Borowitz JL, Pavlakovic G, Isom GE. Dopaminergic neurotoxicity of cyanide: neurochemical, histological, and behavioral characterization. Toxicol Appl Pharmacol. 1994;126:156–163. doi: 10.1006/taap.1994.1102. [DOI] [PubMed] [Google Scholar]
- Karakida F, Ikeya Y, Tsunakawa M, Yamaguchi T, Ikarashi Y, Takeda S, Aburada M. Cerebral protective and cognition-improving effects of sinapic acid in rodents. Biol Pharm Bull. 2007;30:514–519. doi: 10.1248/bpb.30.514. [DOI] [PubMed] [Google Scholar]
- Koplovitz I, Romano JA, Stewart JR. Assessment of motor performance decrement following soman poisoning in mice. Drug Chem Toxicol. 1989;12:221–235. doi: 10.3109/01480548908999155. [DOI] [PubMed] [Google Scholar]
- Leung P, Sylvester DM, Chiou F, Way LL, Way EL, Way JL. Stereospecific effect of naloxone hydrochloride on cyanide intoxication. Toxicol Appl Pharmacol. 1986;83:525–530. doi: 10.1016/0041-008x(86)90235-8. [DOI] [PubMed] [Google Scholar]
- Nagasawa HT, Goon DJW, Crankshaw DL, Vince R, Patterson SE. Novel, Orally Effective Cyanide Antidotes. J Med Chem. 2007 doi: 10.1021/jm7011497. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen JC, Cohen SD. The effects of cyanide on brain mitochondrial cytochrome oxidase and respiratory activities. J Appl Toxicol. 1993;13:9–14. doi: 10.1002/jat.2550130104. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K, Li L, Borowitz JL, Isom GE. Inducible nitric oxide synthase up-regulation and mitochondrial glutathione depletion mediate cyanide-induced necrosis in mesencephalic cells. J Neurosci Res. 2006;84:1003–1011. doi: 10.1002/jnr.20998. [DOI] [PubMed] [Google Scholar]
- Quinlan JJ, Ferguson C, Jester K, Firestone LL, Homanics GE. Mice with glycine receptor subunit mutations are both sensitive and resistant to volatile anesthetics. Anesth Analg. 2002;95:578–582. doi: 10.1097/00000539-200209000-00016. table of contents. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Morgan RL, Way LM, Way JL. Antagonism of cyanide intoxication with sodium pyruvate. Toxicol Appl Pharmacol. 1979;50:437–441. doi: 10.1016/0041-008x(79)90396-x. [DOI] [PubMed] [Google Scholar]
- Sousa AB, Manzano H, Soto-Blanco B, Gorniak SL. Toxicokinetics of cyanide in rats, pigs and goats after oral dosing with potassium cyanide. Arch Toxicol. 2003;77:330–334. doi: 10.1007/s00204-003-0446-y. [DOI] [PubMed] [Google Scholar]
- Way JL. Cyanide Intoxication and its Mechanism of Antagonism. Annu Rev Pharmacol Toxicol. 1984;24:451–481. doi: 10.1146/annurev.pa.24.040184.002315. [DOI] [PubMed] [Google Scholar]
- Way JL, Leung P, Cannon E, Morgan R, Tamulinas C, Leong-Way J, Baxter L, Nagi A, Chui C. The mechanism of cyanide intoxication and its antagonism. Ciba Found Symp. 1988;140:232–243. doi: 10.1002/9780470513712.ch14. [DOI] [PubMed] [Google Scholar]
- Werawattanachai N, Towiwat P, Unchern S, Maher TJ. Neuropharmacological profile of tetrahydrofuran in mice. Life Sci. 2007;80:1656–1663. doi: 10.1016/j.lfs.2007.01.050. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li L, Liu H, Prabhakaran K, Zhang X, Borowitz JL, Isom GE. HIF-1alpha activation by a redox-sensitive pathway mediates cyanide-induced BNIP3 upregulation and mitochondrial-dependent cell death. Free Radic Biol Med. 2007;43:117–127. doi: 10.1016/j.freeradbiomed.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Bruton JD, Katz A, Westerblad H. Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle. J Physiol. 2006;572:551–559. doi: 10.1113/jphysiol.2005.104521. [DOI] [PMC free article] [PubMed] [Google Scholar]