Abstract
Nucleotide oligomerization domain (NOD) 2 functions as a mammalian cytosolic pathogen recognition molecule, and mutant forms have been genetically linked to Crohn's disease (CD). NOD2 associates with the caspase activation and recruitment domain of RIP-like interacting caspase-like apoptosis regulatory protein kinase (RICK)/RIP2 and activates nuclear factor (NF)–κB in epithelial cells and macrophages, whereas NOD2 mutant 3020insC, which is associated with CD, shows an impaired ability to activate NF-κB. To gain insight into the molecular mechanisms of NOD2 function, we performed a functional analysis of deletion and substitution NOD2 mutants. NOD2, but not NOD2 3020insC mutant, associated with cell surface membranes of intestinal epithelial cells. Membrane targeting and subsequent NF-κB activation are mediated by two leucine residues and a tryptophan-containing motif in the COOH-terminal domain of NOD2. The membrane targeting of NOD2 is required for NF-κB activation after the recognition of bacterial muramyl dipeptide in intestinal epithelial cells.
Introduction
Nucleotide oligomerization domain (NOD) 2/caspase activation and recruitment domain (CARD) 15 was the first gene linked to the risk of Crohn's disease (CD; Hugot et al., 2001; Ogura et al., 2001a). NOD2 expression has been found in intestinal epithelial cells (Ogura et al., 2001b; Gutierrez et al., 2002; Hisamatsu et al., 2003), including Paneth cells (Berrebi et al., 2003; Rosenstiel et al., 2003) and monocytes. NOD2 recognizes and reacts to the bacterial component muramyl dipeptide L-Ala, D-Glx (MDP-LD) via its COOH-terminal leucine-rich repeat domain (Girardin et al., 2003; Inohara et al., 2003). In contrast, the 3020insC mutant form of NOD2, which is associated with CD, does not respond to MDP stimulation (Bonen et al., 2003; Chamaillard et al., 2003; Girardin et al., 2003; Inohara et al., 2003). NOD2 activates the nuclear translocation of the transcription nuclear factor (NF)–κB, and RICK (RIP-like interacting caspase-like apoptosis regulatory protein kinase)/RIP2 is essential for this process (Chin et al., 2002; Kobayashi et al., 2002). After the activation of NOD proteins, RICK interacts through homophilic CARD–CARD interaction and leads to the nuclear translocation of NF-κB (Bertin et al., 1999; Inohara et al., 1999; Ogura et al., 2001b; Kobayashi et al., 2002). RICK/RIP2 is involved in the NOD2-dependent activation of NF-κB, and NOD2 activation leads to the ubiquitinylation of the NF-κB essential modulator (NEMO), which is a key component of the NF-κB signaling complex (Abbott et al., 2004). Recent studies do not clearly conclude whether NOD2 is a regulator of TLR2-mediated responses that may be deregulated in the presence of the NOD2 3020insC mutant (Watanabe et al., 2004; Kobayashi et al., 2005; Maeda et al., 2005). Direct interaction between NOD2 and TGF-β–activated kinase 1 and NOD2 and GRIM-19 have also been shown, and each can regulate NOD2-mediated NF-κB activation (Chen et al., 2004; Barnich et al., 2005).
The subcellular localization and trafficking of NOD2/CARD15 during MDP stimulation, which is the minimal bioactive component of the bacterial peptidoglycan, remain unclear. A recent study described regulatory regions and characterized critical residues of NOD2 that are involved in NF-κB activation after MDP stimulation (Tanabe et al., 2004). Several mutations of NOD2 leading to the loss of MDP recognition involve amino acid residues that are predicted to be located in the α-helix/turn regions that form the convex face of the leucine-rich repeat. In this study, we demonstrate that NOD2 can be directed to the intracellular vesicle compartment and cell surface membranes in intestinal epithelial cells. In these cells, the membrane association of NOD2 is necessary for NF-κB activation after MDP stimulation. The ability of NOD2 to function as a bacterial recognition molecule depends on the COOH-terminal protein motif.
Results and discussion
Cellular localization of endogenous NOD2/CARD15 in intestinal epithelial cells
Expression of NOD2/CARD15 was assessed by Western blot analysis in several intestinal epithelial cell lines as well as in COS7 and HEK293 cells. Colo205, SW480, HT29, and LS174 exhibited a strong expression of endogenous NOD2. In contrast, T84 and Caco-2 expressed little or no NOD2 protein. No endogenous expression was observed in COS7 or HEK293 cells (Fig. 1 A). NOD2 that was expressed in HT29 did not contain any of the three common mutations that are associated with CD, and stimulation by MDP-LD induced a 6.2-fold increase in NF-κB activity, indicating functional NOD2 in this cell line (Fig. 1 B).
Figure 1.
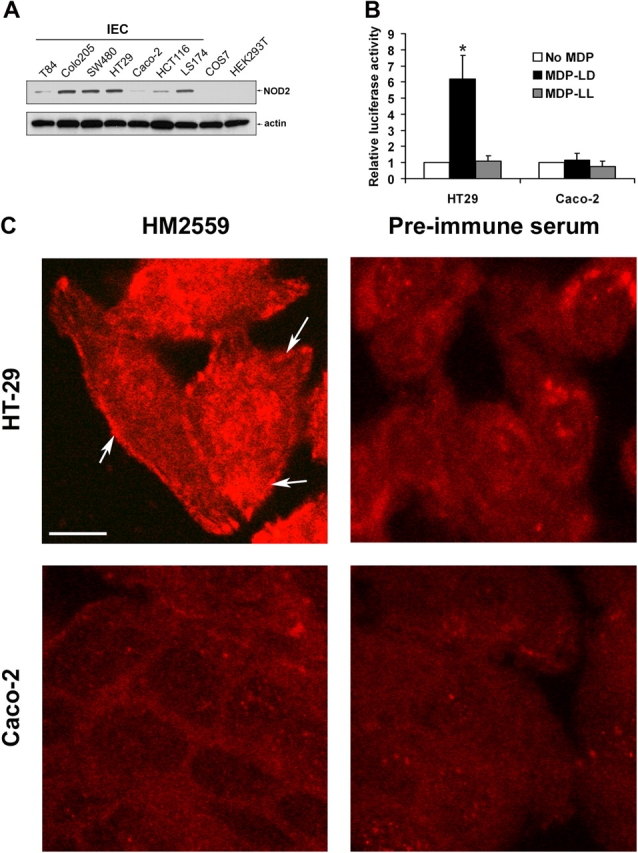
Expression and cellular localization of endogenous NOD2. (A) Western blot analysis using rabbit NOD2 antiserum HM2559 and anti–β-actin. 10 μg of total protein from different intestinal epithelial cells (IEC), COS7, and HEK293 cell lines were loaded onto 4–12% Tris-glycine gel. (B) NF-κB activation in HT29 and Caco-2 cells after stimulation with 1 μg/ml MDP-LD or MDP-LL using NF-κB luciferase reporter assay and normalization with renilla after 18 h of transfection. Error bars represent SEM of at least four separate experiments. *, P < 0.05. (C) Confocal microscopy examination of endogenous NOD2 in HT29 and Caco-2 cells using rabbit NOD2 antiserum HM2559 or preimmune serum and anti–rabbit Texas red as a secondary antibody. Cytosolic and surface membrane localization (arrows) of NOD2 in HT29 cells is shown. Bar, 20 μm.
Immunostaining of HT29 cells with a rabbit NOD2 antiserum revealed cytoplasmic and vesicular staining and membrane association. No membrane staining was observed with preimmune serum. In addition, no staining was observed in Caco-2 cells lacking endogenous NOD2 (Fig. 1 C). In colonic epithelial cells, MDP-LD may be taken up by the hPepT1 transporter (Vavricka et al., 2004). The expression of this transporter is increased in chronically inflamed intestinal mucosa. If MDP-LD is indeed taken up via the hPepT1 transporter, the membrane targeting of NOD2 could play a crucial role in the activation of NF-κB, allowing MDP to interact with NOD2.
The COOH-terminal domain of NOD2 facilitates membrane targeting
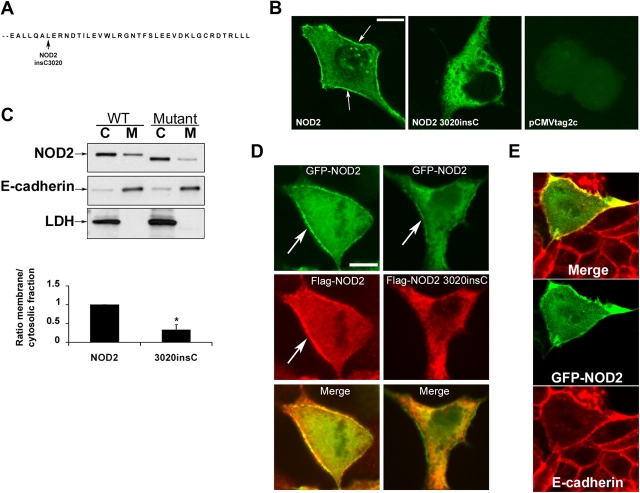
Given the fact that the NOD2 3020insC mutant, which is associated with CD, did not respond to MDP-LD stimulation, the membrane association that was observed for NOD2 led us to examine the subcellular localization of the NOD2 3020insC mutant. Confocal microscopic examination of Caco-2 and COS7 cells that were transfected with Flag or GFP-tagged NOD2 was performed to achieve finer delineation of the spatial distribution of NOD2. Confocal analysis showed that the expressed protein Flag-NOD2 is located in the cytosol and also appears enriched close to the plasma membrane, recapitulating the distribution of endogenous NOD2 (Fig. 2 B). In contrast to wild-type NOD2, Flag-NOD2 3020insC mutant was only present in the cytosol and vesicular compartment. The loss of membrane targeting of the NOD2 3020insC mutant was confirmed by Western blot analysis after separation of cytosolic and membrane fractions. The relative purity of cytosolic and membrane fractions was confirmed by Western blot analysis using antibody against E-cadherin as a membrane marker and antibody anti–lactate dehydrogenase as a cytosolic marker (Fig. 2 C). The ratio of the Flag-tagged NOD2 3020insC protein between membrane and cytosolic fractions was significantly decreased compared with that of the NOD2 wild-type protein, taken as one (Fig. 2 C). GFP-tagged and Flag-tagged NOD2 colocalized in Caco-2 cells (Fig. 2 D), indicating that the nature of the NH2-terminal tag did not affect cellular localization. However, GFP-tagged NOD2 and Flag-tagged NOD2 3020insC mutant did not colocalize near the plasma membrane, confirming the specific membrane association of the NOD2 wild type. This membrane association was confirmed by using anti–E-cadherin antibody, a membrane marker. As shown in Fig. 2 E, the GFP-NOD2 wild type colocalized with E-cadherin in Caco-2 cells.
Figure 2.
Membrane association of expressed NOD2. (A) Amino acid sequences of the COOH-terminal domain of NOD2 and the NOD2 3020insC mutant, which is associated with CD. (B) Confocal microscopy examination of Caco-2 cells that are transfected with Flag-NOD2, Flag-NOD2 3020insC mutant, and empty vector (pCMVtag2C) shows the membrane association of NOD2 (arrows) but not of the NOD2 mutant. NOD2 and 3020insC mutant were detected by using monoclonal anti-Flag antibody followed by fluorescein-conjugated anti–mouse IgG. (C) Western blot analysis using anti-Flag, anti–E-cadherin, or anti–lactate dehydrogenase antibodies. Caco-2 cells were transfected with Flag-NOD2 (WT) or Flag-NOD2 3020insC (mutant). Cytosolic (C) and membrane (M) fractions were separated as described in Materials and methods. Proteins were fractionated through 4–12% Tris-glycine SDS-PAGE and were subjected to Western blot analysis by using anti-Flag antibody to detect NOD2 expression. The ratio in the membrane and cytosolic fractions that were determined after the quantification of NOD2 3020insC mutant was compared with NOD2 wild type. The result is the mean of four separate experiments. Error bar represents SEM. *, P < 0.05. (D) Caco-2 cells were cotransfected with GFP-NOD2 and Flag-NOD2 or with GFP-NOD2 and Flag-NOD2 3020insC mutant and were detected using anti-Flag antibody for Flag-tagged constructs. Only the NOD2 wild type showed plasma membrane association (arrows). (E) Caco-2 cells that were transfected with GFP-NOD2 were stained with anti–E-cadherin antibody, a membrane marker, to confirm the plasma membrane association of the NOD2 wild type. Bars, 20 μm.
The COOH-terminal protein domain directs the membrane targeting of NOD2
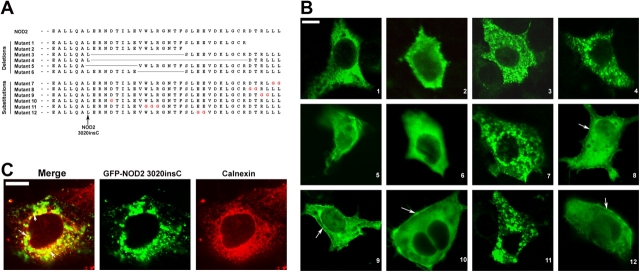
The difference between NOD2 wild type and NOD2 3020insC mutant is the deletion of the final 33 COOH-terminal amino acid residues, indicating that this region can be responsible for membrane targeting. The domain of NOD2 that is responsible for membrane targeting was more specifically identified by serial deletion mutants (Fig. 3 A). Mutants 1 and 2 failed to localize to the cell membranes in Caco-2 cells (Fig. 3 B), indicating that the terminal six amino acid residues of NOD2 are required for membrane targeting. Mutants 3 and 4 were constructed by adding selected COOH-terminal amino acids (last 6 and 17 amino acid residues of NOD2) to the NOD2 3020insC mutant to determine whether these amino acids were sufficient to confer NOD2 membrane association. No membrane targeting in Caco-2 cells that were transfected with these two mutant forms was observed. Instead, these NOD2 mutants were targeted to an intracellular vesicle compartment (Fig. 3 B). Therefore, the last six amino acid residues of NOD2 are necessary but not sufficient to confer membrane targeting. Finally, the deletion of LERNDILE (mutant 5) and VWLRGNTF (mutant 6), which are located in the COOH-terminal domain of NOD2, also resulted in the loss of membrane targeting in transfected Caco-2 cells (Fig. 3 B). The identical distribution of all of these mutant forms of NOD2 was observed when transfected into COS7 cells, including the concomitant loss of membrane targeting (unpublished data) compared with wild-type NOD2, which indicates that the final 33 amino acids play a crucial role for NOD2 membrane recruitment.
Figure 3.
NOD2 membrane association is COOH-terminal dependent. (A) Sequences of Flag-NOD2 COOH-terminal deletion and substitution mutants. (B) Caco-2 cells were transfected with Flag-NOD2 mutant constructs. Mutants 1–12 were detected by confocal microscopy using monoclonal anti-Flag antibody followed by fluorescein-conjugated anti–mouse IgG. Membrane association is still observed for some NOD2 mutants (arrows). (C) COS7 cells were transfected with GFP-NOD2 3020insC and were stained with calnexin (ER marker). Some of the observed vesicles colocalized with calnexin (arrows). Bars, 20 μm.
Several substitution mutations were also introduced in the COOH-terminal domain of NOD2 (Fig. 3 A). Mutant 7—with substitution of amino acids GG for LL at the end of NOD2—and mutant 11—with substitution WLR1017GGG—lost their membrane targeting, whereas the other substitution mutants still showed membrane association (Fig. 3 B). For mutants that failed to colocalize with the plasma membrane, as well as for the NOD2 3020insC mutant, some vesicular staining was observed. By using calnexin, an ER marker, we found that some of these vesicles are within the ER compartment (Fig. 3 C). These results indicate that the two last leucine residues and the WLR motif are required for membrane targeting.
NF-κB activation by MDP-LD is dependent on the membrane targeting of NOD2
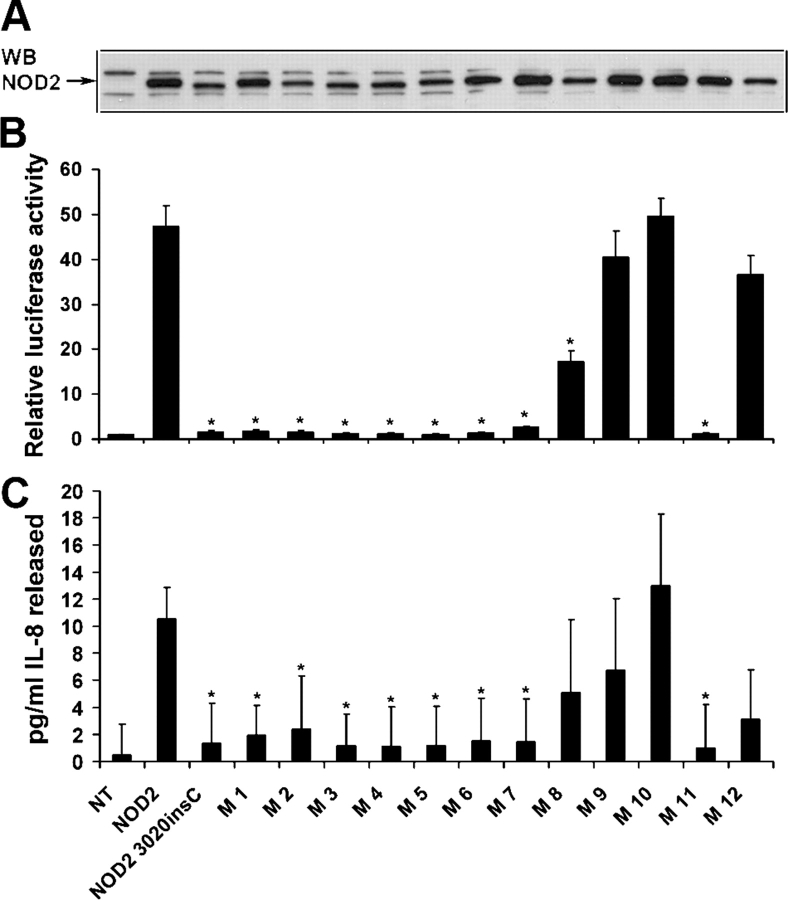
NOD2 is known to activate the nuclear transcription factor NF-κB after MDP-LD stimulation, and the NOD2 3020insC mutant's ability to activate NF-κB is impaired (Girardin et al., 2003). The inability of the NOD2 mutant to respond to MDP-LD and activate NF-κB in CD is paradoxical, considering that NF-κB is responsible for the induction of a large number of inflammatory mediators (O'Neill, 2004). To investigate whether the membrane targeting of NOD2 is required for MDP response, we transfected HEK293 cells with NOD2 wild type and different COOH-terminal mutants without tag. NOD2 expression was confirmed by Western blot analysis using antiserum HM2563 against NOD2 (Fig. 4 A). As shown in Fig. 4 B, NOD2 wild type induced a 47-fold increase in NF-κB activation, whereas mutants 1–7 and 11 induced less than a 3-fold increase in NF-κB. However, mutants 8–10 and 12, which are still membrane associated, activated NF-κB. No NF-κB activation was observed after stimulation with an MDP L-Ala, L-Glx (MDP-LL)–inactive form (unpublished data). The release of IL-8 by HEK293 cells transfected with NOD2 and selected mutants after MDP-LD stimulation was determined by ELISA to confirm the NF-κB activation. The amount of IL-8 found in the supernatant of HEK293 cells that were transfected with NOD2 and stimulated with MDP-LD (10.55 pg/ml) was significantly increased compared with untransfected cells (0.47 pg/ml). The amount of IL-8 released in the supernatant of HEK293 cells that were transfected with the selected NOD2 mutants correlated with NF-κB activation (Fig. 4 C). These results demonstrate that the membrane targeting of NOD2 in intestinal epithelial cells is required for NF-κB activation upon the recognition of MDP. NOD2 binds to RIP2, a cytoplasmic protein, via a CARD–CARD interaction (Kobayashi et al., 2002), suggesting that signaling of NOD2 at the membrane implies either NOD2 redistribution to the cytoplasm after MDP-LD stimulation or recruitment of RIP2 to the membrane.
Figure 4.
Ligand-induced NF-κB activation and IL-8 release are dependent on NOD2 membrane association. (A) Expression of NOD2 and mutants without tag that were transfected into HEK293 cells was determined by Western blot analysis using NOD2 antiserum HM2563. (B) HEK293 cells were transfected with 1 ng NOD2 expression vector or mutants together with 1 μg MDP-LD. NF-κB activity was determined by using an NF-κB luciferase reporter assay and was normalized with renilla after 18 h of transfection. Fold increase of NF-κB activation was determined by comparing untransfected and nonstimulated HEK293 with MDP. (C) IL-8 released in the supernatant of HEK293 cells that were transfected with mutants (M) of NOD2 and stimulated 18 h with 1 μg MDP-LD was measured by ELISA. Error bars represent SEM of at least four separate experiments. NT, nontransfected. *, P < 0.05.
Role of WLR1017, R702, and G908 in MDP-LD recognition
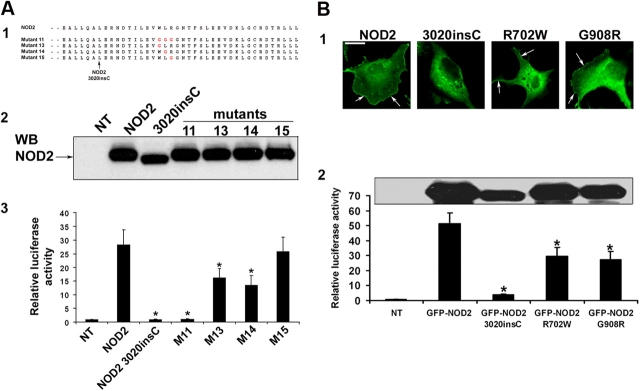
To determine which amino acid residues in the WLR motif are required for MDP recognition, we constructed three additional mutants (Fig. 5 A). NF-κB activation showed that both W1017G (mutant 13) and L1018G (mutant 14) play vital roles in MDP-LD recognition because NF-κB activation is significantly decreased compared with wild-type NOD2. However, R1019G substitution (mutant 15) had no effect on NOD2 MDP-LD recognition. These results were confirmed by determining the amounts of IL-8 released in the supernatant of HEK293 cells that were transfected with these mutants (unpublished data). Substitutions W1017G and L1018G led only to a twofold decrease of NF-κB, whereas WLR1017GGG substitutions had impaired NF-κB activation, indicating that both W and L amino acid residues are important but not sufficient to completely abrogate NOD2 function. However, the two other NOD2 mutants that are associated with CD—NOD2 R702W and NOD2 G908R—still showed membrane association in transfected COS7 cells, whereas these two mutants showed decreased levels of NF-κB activation after MDP-LD stimulation. This suggested that these two amino acids are required for MDP-LD recognition but did not play any role in the membrane targeting of NOD2 (Fig. 5 B).
Figure 5.
Ligand-induced NF-κB activation are dependent on a three–amino acid motif that is required for membrane association. (A, 1) Amino acid sequences of Flag-NOD2 COOH-terminal substitution mutants. (2) Expression of these mutants was determined by Western blot analysis using NOD2 antiserum HM2563. (3) Fold increase of NF-κB activation was determined as described in Fig. 4. (B, 1) GFP-NOD2 wild type and the three main NOD2 mutations that are associated with CD (GFP-NOD2 3020insC, GFP-NOD2 R702W, and GFP-NOD2 G908R) were transfected in COS7 cells. Only NOD2 3020insC failed to colocalize with the plasma membrane, whereas the two other NOD2 mutant forms still showed membrane association (arrows). Bar, 20 μm. (2) Expression of NOD2 mutants and NF-κB activation were determined and compared with untransfected and nonstimulated HEK293 with MDP-LD, as described in Fig. 4. Error bars represent SEM of at least four separate experiments. *, P < 0.05.
In summary, the subcellular localization of NOD2 is important for ligand binding because cell membrane localization in intestinal epithelial cells was required for NOD2 to recognize MDP-LD after its transport across the cell membrane. However, in contrast to Toll-like receptors that recognize bacterial components outside the cell, NOD2 does not contain a transmembrane domain and does not act as a membrane receptor. Furthermore, the observed membrane association of NOD2 could be an interaction with the inner side of the plasma membrane but not with cell surface expression. This study suggested that NOD2 membrane association is necessary for NOD2 function as an innate immune receptor, which is impaired for the most common NOD2 mutant associated with CD.
Materials and methods
Cell culture and transfection
Caco-2, T84, HT29, HCT116, Colo205, SW480, LS174, COS7, and HEK293 cells were obtained from the American Type Culture Collection and were cultured as described previously (Hisamatsu et al., 2003). For NF-κB assay or protein expression, cells were transfected using LipofectAMINE 2000 (Invitrogen), and for immunostaining experiments, cells were transfected using TransIT Transfection Reagent Kit (Mirus Bio Corp.) according to the manufacturer's protocols.
Construction of expression plasmids
Flag-tagged (pCMVtag2c-NOD2) and GFP-tagged (pEGFPC1-NOD2) NOD2 mammalian expression vectors were described previously (Hisamatsu et al., 2003). NOD2 mammalian expression vector without tag was provided by M. Chamaillard (Institut National de la Santé et de la Recherche Medicale, Lille, France). All NOD2 mutants were generated by PCR using the QuickChange Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer's protocols and were sequenced. Expression was confirmed by Western blot analysis using mouse anti-Flag M2 mAb (Sigma-Aldrich) or rabbit antiserum HM2559 or HM2563 against NOD2 (Hisamatsu et al., 2003).
Confocal microscopy
HT29, Caco-2, or COS7 cells were seeded on sterile permanox coverslips and were transfected after 24 h with various NOD2 constructs. After 48 h, cells were washed twice with ice-cold PBS, fixed 10 min with cold methanol at −20°C, washed three times with ice-cold PBS, and blocked 30 min with 5% donkey serum in PBS. Cells were stained using rabbit NOD2 antiserum HM2559 and Texas red–conjugated anti–rabbit IgG (Vector Laboratories) secondary antibodies for endogenous NOD2 or using mouse anti-Flag M2 mAb (Sigma-Aldrich) and FITC-conjugated anti–mouse IgG secondary antibodies. The coverslips were mounted in Vectashield (Vector Laboratories) and examined at RT with a confocal microscope (model Radiance 2000; Bio-Rad Laboratories) using multitracking (line switching) for two-color imaging (40×). Image acquisition was performed with LaserSharpScanning software (Bio-Rad Laboratories). For vesicular staining, anticalnexin rabbit pAb (StressGen Biotechnologies) was used in COS7 cells followed by Texas red–conjugated anti–rabbit IgG secondary antibodies.
NF-κB activation assay and detection of cytokine IL-8 in the supernatant of transfected cells
HEK293 cells were transfected overnight with 1 ng NOD2, 10 ng pIV luciferase reporter plasmid, and 1 ng of renilla plasmid together with 1 μg MDP-LD or MDP-LL (Sigma-Aldrich). After 18 h, luciferase activity was measured by using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions and was normalized to renilla activity.
The amount of human cytokine IL-8 released in the supernatant of HEK293 cells that were transfected with 1 ng NOD2 or different NOD2 mutants was determined by ELISA (BD Biosciences). The optical density was determined at a wavelength of 450 nm, and cytokine concentrations in picograms per milliliter were assessed according to the manufacturer's instructions.
Protein extraction and immunoblotting experiments
Cells were seeded on 6-well plates and were transfected 24 h later with 1 μg DNA. Medium was removed, and 300 μl of lysis buffer (1% Triton X-100, 0.1 M NaCl, 10 mM Hepes, pH 5.6, 2 mM EDTA, 4 mM Na3VO4, and 40 mM NaF) supplemented with protease inhibitor cocktail (Complete Mini; Roche Diagnostics) was added. Lysates were harvested and passed through a 21-gauge needle 10 times. The cytosolic fraction was obtained by centrifugation at 10,000 g for 30 min at 4°C. The pellet was resuspended in 200 μl of lysis buffer containing 1% SDS and was sonicated twice for 20 s. After 5 min of centrifugation at 10,000 g, the resulting supernatant was collected. The protein concentration was determined by using the DC Protein Assay Kit (Bio-Rad Laboratories).
10 μg of proteins from all intestinal epithelial cells were separated on 4–12% Tris-glycine gels (Invitrogen), blotted onto polyvinylidene difluoride membranes, and stained for NOD2 using rabbit NOD2 antiserum HM2559. Proteins from Caco-2 cells that were transfected with Flag-tagged NOD2 and all mutants were separated on 4–12% Tris-glycine gel (Invitrogen), blotted onto polyvinylidene difluoride membranes, and stained using anti-Flag M2 mAb (Sigma-Aldrich), anti–E-cadherin mAb (BD Biosciences), anti–lactate dehydrogenase (Abcam), or anti–β-actin (C-11; Santa Cruz Biotechnology, Inc.).
Statistical analysis
For the analysis of significant differences in NF-κB activation or IL-8 release, a t test was used. All experiments were repeated at least four times. P ≤ 0.05 was considered to be statistically significant.
Acknowledgments
We are grateful to Dr. Mathias Chamaillard for NOD2 construct and to Dr. Ian Rosenberg, Jiri Kalabis, Jesus Yamamoto-Furusho, Emiko Mizoguchi, and Kathryn Devaney for helpful discussions and critical reading of the manuscript.
This work was supported by grants DK54429, DK33506 (to H.-C. Reinecker); and DK069344, DK043351, and DK07191 from the National Institutes of Health (to D.K. Podolsky).
Abbreviations used in this paper: CARD, caspase activation and recruitment domain; CD, Crohn's disease; MDP, muramyl dipeptide; NOD2, nucleotide oligomerization domain 2; NF, nuclear factor; RICK, RIP-like interacting caspase-like apoptosis regulatory protein kinase.
References
- Abbott, D.W., A. Wilkins, J.M. Asara, and L.C. Cantley. 2004. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr. Biol. 14:2217–2227. [DOI] [PubMed] [Google Scholar]
- Barnich, N., T. Hisamatsu, J.E. Aguirre, R. Xavier, H.C. Reinecker, and D.K. Podolsky. 2005. GRIM-19 interacts with NOD2 and serves as down-stream effector of anti-bacterial function in intestinal epithelial cells. J. Biol. Chem. 19:19021–19026. [DOI] [PubMed] [Google Scholar]
- Berrebi, D., R. Maudinas, J.P. Hugot, M. Chamaillard, F. Chareyre, P. De Lagausie, C. Yang, P. Desreumaux, M. Giovannini, J.P. Cezard, et al. 2003. Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn's disease colon. Gut. 52:840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin, J., W.J. Nir, C.M. Fischer, O.V. Tayber, P.R. Errada, J.R. Grant, J.J. Keilty, M.L. Gosselin, K.E. Robison, G.H. Wong, et al. 1999. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. J. Biol. Chem. 274:12955–12958. [DOI] [PubMed] [Google Scholar]
- Bonen, D.K., Y. Ogura, D.L. Nicolae, N. Inohara, L. Saab, T. Tanabe, F.F. Chen, S.J. Foster, R.H. Duerr, S.R. Brant, et al. 2003. Crohn's disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. 124:140–146. [DOI] [PubMed] [Google Scholar]
- Chamaillard, M., D. Philpott, S.E. Girardin, H. Zouali, S. Lesage, F. Chareyre, T.H. Bui, M. Giovannini, U. Zaehringer, V. Penard-Lacronique, et al. 2003. Gene-environment interaction modulated by allelic heterogeneity in inflammatory diseases. Proc. Natl. Acad. Sci. USA. 100:3455–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.M., Y. Gong, M. Zhang, and J.J. Chen. 2004. Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J. Biol. Chem. 279:25876–25882. [DOI] [PubMed] [Google Scholar]
- Chin, A.I., P.W. Dempsey, K. Bruhn, J.F. Miller, Y. Xu, and G. Cheng. 2002. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 416:190–194. [DOI] [PubMed] [Google Scholar]
- Girardin, S.E., I.G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D.J. Philpott, and P.J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869–8872. [DOI] [PubMed] [Google Scholar]
- Gutierrez, O., C. Pipaon, N. Inohara, A. Fontalba, Y. Ogura, F. Prosper, G. Nunez, and J.L. Fernandez-Luna. 2002. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J. Biol. Chem. 277:41701–41705. [DOI] [PubMed] [Google Scholar]
- Hisamatsu, T., M. Suzuki, H.C. Reinecker, W.J. Nadeau, B.A. McCormick, and D.K. Podolsky. 2003. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 124:993–1000. [DOI] [PubMed] [Google Scholar]
- Hugot, J.P., M. Chamaillard, H. Zouali, S. Lesage, J.P. Cezard, J. Belaiche, S. Almer, C. Tysk, C.A. O'Morain, M. Gassull, et al. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 411:599–603. [DOI] [PubMed] [Google Scholar]
- Inohara, N., T. Koseki, L. del Peso, Y. Hu, C. Yee, S. Chen, R. Carrio, J. Merino, D. Liu, J. Ni, and G. Nunez. 1999. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J. Biol. Chem. 274:14560–14567. [DOI] [PubMed] [Google Scholar]
- Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, et al. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509–5512. [DOI] [PubMed] [Google Scholar]
- Kobayashi, K., N. Inohara, L.D. Hernandez, J.E. Galan, G. Nunez, C.A. Janeway, R. Medzhitov, and R.A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 416:194–199. [DOI] [PubMed] [Google Scholar]
- Kobayashi, K.S., M. Chamaillard, Y. Ogura, O. Henegariu, N. Inohara, G. Nunez, and R.A. Flavell. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 307:731–734. [DOI] [PubMed] [Google Scholar]
- Maeda, S., L.C. Hsu, H. Liu, L.A. Bankston, M. Iimura, M.F. Kagnoff, L. Eckmann, and M. Karin. 2005. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1beta processing. Science. 307:734–738. [DOI] [PubMed] [Google Scholar]
- Ogura, Y., D.K. Bonen, N. Inohara, D.L. Nicolae, F.F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R.H. Duerr, et al. 2001. a. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 411:603–606. [DOI] [PubMed] [Google Scholar]
- Ogura, Y., N. Inohara, A. Benito, F.F. Chen, S. Yamaoka, and G. Nunez. 2001. b. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276:4812–4818. [DOI] [PubMed] [Google Scholar]
- O'Neill, L.A. 2004. How NOD-ing off leads to Crohn disease. Nat. Immunol. 5:776–778. [DOI] [PubMed] [Google Scholar]
- Rosenstiel, P., M. Fantini, K. Brautigam, T. Kuhbacher, G.H. Waetzig, D. Seegert, and S. Schreiber. 2003. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 124:1001–1009. [DOI] [PubMed] [Google Scholar]
- Tanabe, T., M. Chamaillard, Y. Ogura, L. Zhu, S. Qiu, J. Masumoto, P. Ghosh, A. Moran, M.M. Predergast, G. Tromp, et al. 2004. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 23:1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavricka, S.R., M.W. Musch, J.E. Chang, Y. Nakagawa, K. Phanvijhitsiri, T.S. Waypa, D. Merlin, O. Schneewind, and E.B. Chang. 2004. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 127:1401–1409. [DOI] [PubMed] [Google Scholar]
- Watanabe, T., A. Kitani, P.J. Murray, and W. Strober. 2004. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 5:800–808. [DOI] [PubMed] [Google Scholar]