Abstract
The aggregation of high affinity IgE receptors (Fcɛ receptor I [FcɛRI]) on mast cells is potent stimulus for the release of inflammatory and allergic mediators from cytoplasmic granules. However, the molecular mechanism of degranulation has not yet been established. It is still unclear how FcɛRI-mediated signal transduction ultimately regulates the reorganization of the cytoskeleton and how these events lead to degranulation. Here, we show that FcɛRI stimulation triggers the formation of microtubules in a manner independent of calcium. Drugs affecting microtubule dynamics effectively suppressed the FcɛRI-mediated translocation of granules to the plasma membrane and degranulation. Furthermore, the translocation of granules to the plasma membrane occurred in a calcium-independent manner, but the release of mediators and granule–plasma membrane fusion were completely dependent on calcium. Thus, the degranulation process can be dissected into two events: the calcium-independent microtubule-dependent translocation of granules to the plasma membrane and calcium-dependent membrane fusion and exocytosis. Finally, we show that the Fyn/Gab2/RhoA (but not Lyn/SLP-76) signaling pathway plays a critical role in the calcium-independent microtubule-dependent pathway.
Introduction
Mast cells and basophils are granulated cells that play a pivotal role in allergy and inflammation. Their granules contain inflammatory mediators such as histamine, proteases, lipid mediators, and cytokines. The activation of mast cells induces exocytosis and fusion of cytoplasmic granules with the plasma membrane, followed by the release of inflammatory mediators within minutes of stimulation. One potent stimulus is the aggregation of high affinity receptors (Fcɛ receptor I [FcɛRI]) by the Ag–IgE complexes.
FcɛRI stimulation initiates a signaling cascade that includes activation of tyrosine kinases, such as Syk, Lyn, Fyn, and BTK, and phosphorylation of numerous adaptor proteins (Eiseman and Bolen, 1992; Hata et al., 1998; Nadler et al., 2000; Rivera, 2002; Siraganian et al., 2002). These adapters include the linker for the activation of T cells (LAT), SH2 domain–containing leukocyte protein of 76 kD (SLP-76), Grb2-associated binder 2 (Gab2), MIST/Clnk, 3BP2, and adhesion- and degranulation-promoting adaptor protein (ADAP) (Hamawy et al., 1997; Cao et al., 1999; Goitsuka et al., 2000; Geng et al., 2001; Gu et al., 2001; Kimura et al., 2001; Sada et al., 2002; Xie et al., 2002).
Gab2 is a member of the Gab family of adaptor proteins and displays sequence similarity with Drosophila DOS, a substrate for Corkscrew, a homologue of SHP2, a mammalian protein tyrosine phosphatase (Hibi and Hirano, 2000; Liu and Rohrschneider, 2002; Gu and Neel, 2003; Nishida and Hirano, 2003). The Gab family adaptor proteins are involved in signal transduction through a variety of cytokine and growth factor receptors including c-kit (Nishida et al., 1999, 2002). These FcɛRI-associated adaptor proteins regulate the generation of downstream second messengers, such as phosphatidylinositol-3,4,5-trisphosphate and the induction of calcium influx. The deletion of adaptor proteins LAT, SLP-76, or Gab2 results in decreased FcɛRI-mediated mast cell activation (Pivniouk et al., 1999; Saitoh et al., 2000, 2003; Gu et al., 2001; Gonzalez-Espinosa et al., 2003; Kettner et al., 2003). Collectively, these reports indicate that adaptor proteins play important roles in mast cell degranulation.
Recent studies suggest that FcɛRI-mediated signaling occurs through two distinct adaptor complexes (Nadler and Kinet, 2002; Parravicini et al., 2002; Blank and Rivera, 2004). LAT forms a molecular complex that includes the adapters SLP-76, Grb2, phospholipase Cγ1, and the guanine nucleotide exchange factor, Vav1. Assembly of the LAT complex requires calcium signaling through PLCγ1 and PLCγ2. In addition, the calcium response is partially impaired in Lyn-deficient mast cells (Nishizumi and Yamamoto, 1997). On the other hand, the adaptor molecule Gab2 has been shown to be critical for mast cell signaling due to its ability to recruit PI-3 kinase (Gu et al., 2001). Fyn is required for the FcɛRI-induced phosphorylation of Gab2 and mast cell degranulation, but not for calcium signaling (Parravicini et al., 2002). These reports suggest the presence of a calcium-independent pathway in mast cell degranulation, in addition to the well-known calcium-dependent pathway.
The FcɛRI-mediated signal pathway controls cytoskeletal rearrangements that influence and accompany mast cell degranulation. Two observations underscore the importance of the microtubules in mast cell degranulation. First, tubulin polymerization-inhibiting agents can suppress FcɛRI-induced degranulation (Nielsen and Johansen, 1986; Tasaka et al., 1991; Martin-Verdeaux et al., 2003). Second, FcɛRI-mediated movement of granules is dependent on microtubules in the mast cell line, RBL (Smith et al., 2003). These reports suggested that microtubules play an important role for mast cell degranulation. However, the precise roles and molecular mechanisms of cytoskeletal rearrangements in the degranulation process were not well known.
In this study, we demonstrated that the formation of microtubules is important for FcɛRI-induced granule translocation to the plasma membrane. Furthermore, we showed that this process is dependent on the Fyn/Gab2/RhoA signaling pathway, but independent of calcium, Lyn, and SLP-76.
Results
FcɛRI-induced microtubule formation is independent of calcium
Microtubule and actin filament networks function cooperatively in the process of vesicle and organelle transport (Goode et al., 2000). We examined whether FcɛRI stimulation generates changes in cytoskeletal proteins such as tubulin or actin in bone marrow–derived mast cells (BMMCs). After FcɛRI stimulation, we observed an enhancement of the intensity of tubulin staining (Fig. 1, A and B). We also observed the microtubule structures between the cortical layer and cytoplasmic region of the cells (Fig. 1 C). At the same time, we noted disassembly of the cortical F-actin fluorescent ring (Fig. 1, D–I). FcɛRI stimulation increased the number of cells displaying fragmentation of the cortical F-actin rings (from 4.5 ± 4.4% in unstimulated cells to 55.2 ± 1.7% in stimulated cells, n = 300 cells; Fig. 2 H). Microtubule and F-actin did not appear to colocalize after FcɛRI stimulation (Fig. 1, J and K). Microtubule was localized to the area into which the F-actin ring had collapsed (Fig. 1 K).
Figure 1.
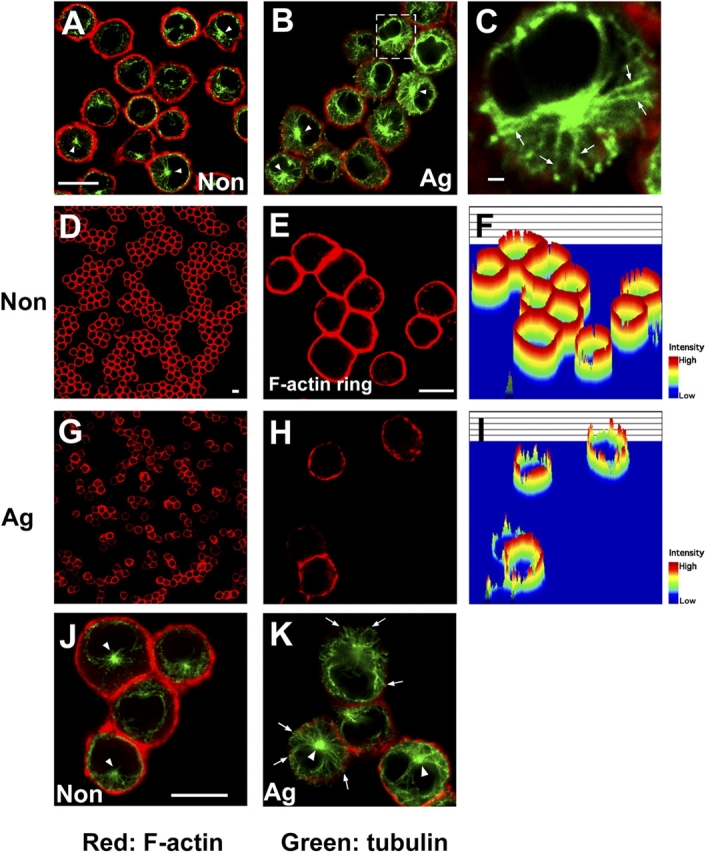
FcɛRI stimulation induces cytoskeletal rearrangement. (A–C) FcɛRI stimulation induces microtubule formation. IgE-sensitized BMMCs were stimulated with either vehicle (A) or DNP-HSA (B) for 5 min and fixed, then double stained with phalloidin-rhodamine (red fluorescence) and antibody to α-tubulin (green fluorescence). Representative images by confocal microscopy are shown. C is a magnified version of the region delineated by the dotted line in B. Arrows indicate the structure of microtubules in BMMCs. Arrowheads indicate the position of MTOC. Bar, 10 μm. (D–I) FcɛRI stimulation induces F-actin ring disassembly. IgE-sensitized BMMCs were stimulated with either vehicle (D–F) or DNP-HSA (G–I) for 5 min. Cells were stained with phalloidin-rhodamine (red fluorescence). E and H show magnified images for D and G, respectively. F and I show intensity of F-actin by pseudo-3D analysis. Bar, 10 μm. (J and K) Microtubule and F-actin do not colocalize after stimulation. IgE-sensitized BMMCs were stimulated with either vehicle (J) and DNP-HSA (K). Cells were stained with phalloidin-rhodamine (red fluorescence) and antibody to α-tubulin (green fluorescence). Arrowheads indicate the position of MTOC. Arrows indicate microtubules at the cortical layer. Bar, 10 μm.
Figure 2.
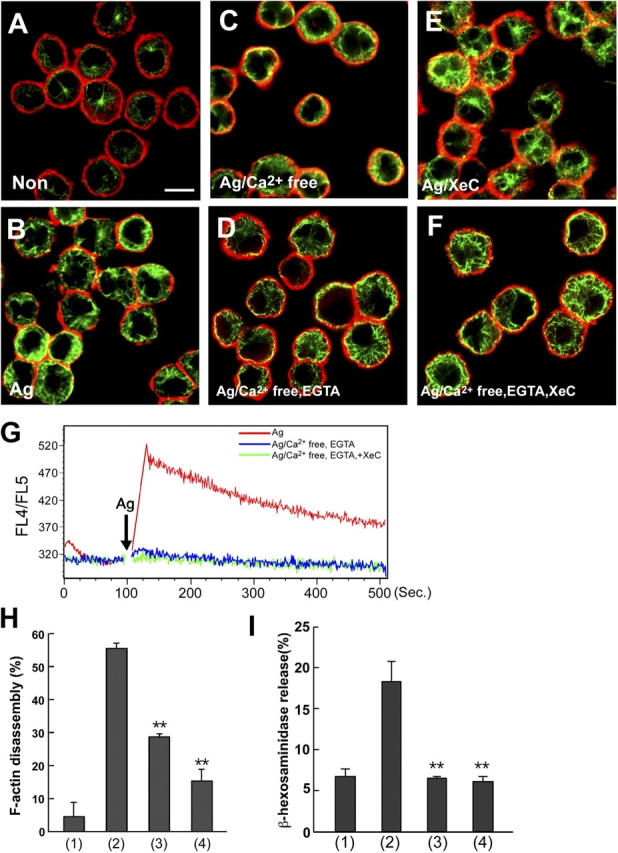
Calcium is dispensable for microtubule formation, but essential for F-actin disassembly. (A–F) Microtubule formation is calcium independent. IgE-sensitized BMMCs were stimulated with either vehicle (A), DNP-HSA in normal medium (B), DNP-HSA in calcium-free medium (C), DNP-HSA in calcium-free medium containing EGTA (D), DNP-HSA in normal medium containing xestospongin C (XeC, 10 μM) (E), or DNP-HSA in calcium-free medium containing EGTA and XeC (F). 5 min after stimulation, cells were fixed and processed for double staining with phalloidin-rhodamine (red fluorescence) and antibody to α-tubulin (green fluorescence). Representative images by confocal microscopy are shown. Bar, 10 μm. (G) Measurement of intracellular calcium IgE-sensitized BMMCs were stimulated with either DNP-HSA (red line), DNP-HSA in calcium-free medium containing EGTA (blue line), or DNP-HSA in calcium-free medium containing EGTA and XeC (green line) for indicated periods. Intracellular calcium was measured as described in Materials and methods. (H) F-actin disassembly is dependent on calcium. IgE-sensitized BMMCs were incubated with either vehicle (1), DNP-HSA in normal medium (2), DNP-HSA in calcium-free medium (3), or DNP-HSA in calcium-free medium containing EGTA (4) for 5 min. The F-actin ring was detected by phalloidin-rhodamine. The number of cells showing F-actin disassembly was estimated using a quantitative intensity analysis of pseudo-3D image. The values indicate means ± SD of three separate experiments. Statistical analysis was performed using the t test. Double asterisk indicates P < 0.01 vs. antigen (Ag)-stimulated BMMCs in normal medium. (I) Intracellular calcium is essential for mast cell degranulation. IgE-sensitized BMMCs were stimulated with either vehicle (1), DNP-HSA in normal medium (2), DNP-HSA in calcium-free medium (3), or DNP-HSA in calcium-free medium containing EGTA (4) for 30 min. β-Hexosaminidase release was measured for indication of mast cell degranulation. The values indicate means ± SD of three separate experiments. Statistical analysis was performed using the t test. Double asterisk indicates P < 0.01 vs. Ag-stimulated BMMCs in normal medium.
Because intracellular calcium is important for mast cell degranulation, we examined whether intracellular calcium was required for the formation of microtubules and F-actin ring disassembly. As shown in Fig. 2 (H and I), intracellular calcium was essential for the FcɛRI-mediated F-actin ring disassembly and degranulation. However, the FcɛRI-mediated microtubule formation was observed in BMMCs in calcium-free medium or medium containing EGTA (Fig. 2, A–D). Furthermore, we showed that EGTA/xestospongin C (IP3 receptor inhibitor) treatment, which completely inhibited the increase of intracellular calcium in BMMCs (Fig. 2 G), did not inhibit FcɛRI-induced microtubule formation (Fig. 2, E and F). These results indicated that the FcɛRI-mediated microtubule formation was calcium independent, but the F-actin disassembly was dependent on calcium.
Microtubule dynamics are involved in mast cell degranulation
We next demonstrated the need for both microtubule formation and F-actin ring disassembly in FcɛRI-mediated degranulation using various cytoskeletal inhibitors. First, we tested drugs affecting F-actin, latrunculin B (which disrupts F-actin), and jasplakinolide (which stabilizes F-actin). Latrunculin B treatment induced the disassembly of the F-actin rings (Fig. 3, B and G) and significantly enhanced β-hexosaminidase release (Fig. 3 K). In contrast, jasplakinolide suppressed F-actin ring disassembly (Fig. 3, C and H) and slightly reduced β-hexosaminidase release (Fig. 3 L). These results are consistent with the idea that the disassembly of the F-actin ring is required, at least in part, for mast cell degranulation in a calcium-dependent manner.
Figure 3.
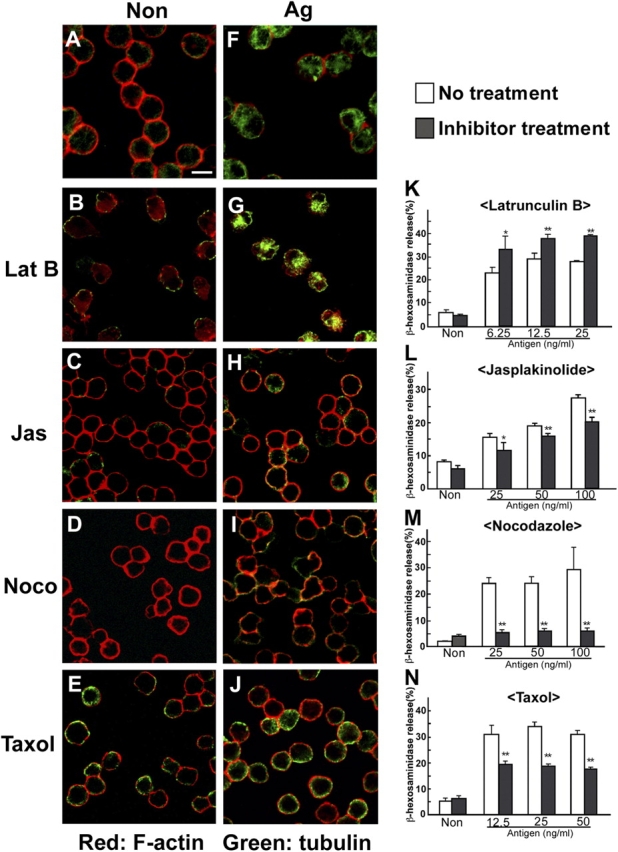
Microtubule dynamics are required for mast cell degranulation. (A–J) The effect of cytoskeletal inhibitors on microtubule formation and F-actin disassembly. IgE-sensitized BMMCs were pretreated with either vehicle (A and F), latrunculin B (B and G), jasplakinolide (C and H), nocodazole (D and I), or Taxol (E and J) as described in Materials and methods. Then, cells were stimulated with either vehicle (A–E) or DNP-HSA (F–J) in the presence of each inhibitor. Cells were visualized by confocal microscopy. Bar, 10 μm. (K–N) The effect of cytoskeletal inhibitors on mast cell degranulation. IgE-sensitized BMMCs were pretreated with either latrunculin B (K), jasplakinolide (L), nocodazole (M), or Taxol (N) as described in Materials and methods. Then, cells were stimulated with various concentrations of DNP-HAS as indicated for 30 min in the presence of each inhibitor. β-Hexosaminidase release was measured for indication of mast cell degranulation. The values indicate means ± SD of three separate experiments. Statistical analysis was performed using the t test. Single asterisk indicates P < 0.05 vs. without inhibitor. Double asterisk indicates P < 0.01 vs. without inhibitor.
We next examined whether drugs affecting microtubules, such as nocodazole (which disrupts microtubules) and Taxol (which stabilizes microtubules), inhibited the FcɛRI-mediated microtubule formation and degranulation. As shown in Fig. 3 (D, I, and M), nocodazole inhibited not only microtubule formation but also degranulation in BMMCs, indicating an important role for microtubule formation in mast cell degranulation. Furthermore, Taxol stabilized microtubule formation and inhibited mast cell degranulation (Fig. 3, E, J, and N). These results indicated that the dynamics of microtubules play a critical role in mast cell degranulation.
FcɛRI-mediated granule translocation depends on microtubule dynamics, but not calcium
To visualize the granules, we introduced a CD63-GFP fusion protein into BMMCs. Before FcɛRI stimulation, we observed that CD63-containing granule structures were retained in cytoplasm (Fig. 4 A, left) as reported by Nishikata et al. (1992). After FcɛRI stimulation, the CD63-containing granules were translocated to the plasma membrane (Fig. 4 A, middle; Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200501111/DC1). Consistent with this, FACS analysis showed the increase of CD63 cell surface expression, indicating that granule–plasma membrane fusion occurred (Fig. 4 B, middle).
Figure 4.
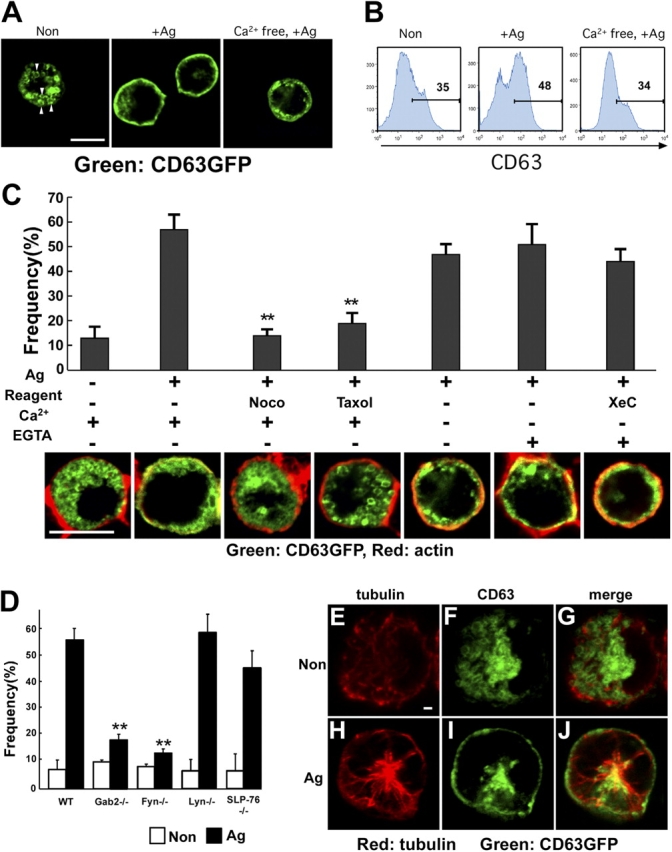
Granule translocation does not require calcium, but does microtubule formation, Fyn, and Gab2. (A) FcɛRI stimulation induces the translocation of granules to the plasma membrane. BMMCs expressing CD63-GFP were sensitized for 6 h with IgE and stimulated with either vehicle (left), DNP-HSA in normal medium (middle), or DNP-HSA in calcium-free medium (right) for 10 min. Cells were fixed with 4% PFA for 30 min, and then attached to glass slides by using cytospin. CD63-GFP was visualized by confocal microscopy. Representative images are shown. Bar, 10 μm. Arrowheads show the structure of granules in the BMMC. (B) The fusion of CD63-containing granules to the plasma membrane is calcium dependent. IgE-sensitized BMMCs were stimulated with either vehicle (left), DNP-HSA in normal medium (middle), or DNP-HSA in calcium-free medium (right) for 10 min. Cell surface expression of CD63 was detected by FACS using anti-CD63. The number in the figures indicates the percentage of CD63-positive cells. (C) Calcium is not required for FcɛRI-induced granule translocation. BMMCs expressing CD63-GFP were sensitized for 6 h with IgE and stimulated with DNP-HSA (Ag) in various conditions as indicated for 10 min. Cells were fixed with 4% PFA for 30 min, and attached to glass slides by using cytospin. Cells were stained with phalloidin-rhodamine (red fluorescence) to detect F-actin. Both F-actin and CD63-GFP were visualized by confocal microscopy. We calculated the frequency of cells showing granule translocation to the plasma membrane according to the following criteria. The first criterion was the increase of yellow-color fluorescence around the plasma membrane, which was a result of the merge of phalloidin-rhodamine and CD63-GFP signal. The second was the obvious decrease of the cytoplasmic area containing CD63-GFP as compared with that of nonstimulated cells. The cells that satisfied both criteria were considered to be positive for granule translocation. Representative images obtained in various conditions were shown in the bottom panel. We counted at least 90 independent GFP-positive cells for each experiment. Statistical analysis was performed using the t test. Double asterisk indicates P < 0.01 vs. antigen-induced BMMCs in normal condition. Bar, 10 μm. (D) Fyn and Gab2 are required for FcɛRI-induced granule translocation. Either wild-type, Gab2-, Fyn-, Lyn-, or SLP-76–deficient BMMCs introduced with CD63-GFP were sensitized for 6 h with IgE and stimulated with DNP-HSA for 10 min. The frequency of cells showing granule translocation to the plasma membrane was determined as described in the figure legend for panel C. The values are shown as means ± SD of three separate experiments. Statistical analysis was performed using the t test. Double asterisk indicates P < 0.01 vs. wild-type mast cells. (E–J) Partial colocalization of CD63-containing granule with microtubules. IgE-sensitized BMMCs expressing CD63-GFP were stimulated with either vehicle (E–G) or DNA-HSA (H–J) for 5 min. Cells were stained with antibody to α-tubulin (red fluorescence). Bar, 1 μm.
Next, we investigated the role of microtubules in the translocation of granules to the plasma membrane. As shown in Fig. 4 C, treatment with nocodazole and Taxol decreased the frequency of FcɛRI-induced granule translocation, revealing a role for microtubules in this process. Consistent with this idea, tubulin- and CD63-containing granules were partially colocalized after stimulation (Fig. 4, E–J). Because microtubule formation was independent of calcium as described in Fig. 2, we asked whether the translocation of granules to the plasma membrane is also calcium independent. As shown in Fig. 4 A (right), Fig. 4 C, and Fig. S1 B, the translocation of granules to the plasma membrane still occurred in either calcium-free medium or medium containing EGTA, conditions under which degranulation was completely abolished (Fig. 2 I). Furthermore, we showed that EGTA/xestospongin C treatment, which completely inhibited the increase of intracellular calcium in BMMCs (Fig. 2 G), did not inhibit the translocation of granules to the plasma membrane (Fig. 4 C). In the absence of calcium, we could not observe FcɛRI-induced increase of surface expression of CD63 by FACS analysis (Fig. 4 B, right), indicating that CD63-containing granules were translocated to the plasma membrane without granule–plasma membrane fusion. Together, these data clearly showed that the translocation of granules is microtubule-dependent and calcium-independent, but the fusion of granules to the plasma membrane is calcium dependent.
The Fyn/Gab2/RhoA- but not Lyn/SLP-76–mediated pathway is required for the FcɛRI-mediated formation of microtubules and translocation of granules to the plasma membrane
Fyn-deficient BMMCs are defective in degranulation, but they have an intact calcium influx. The degranulation process is known to be associated with phosphorylation of Gab2 in a Fyn-dependent manner (Parravicini et al., 2002). Therefore, we examined the possibility that Fyn/Gab2 signaling is involved in the translocation of granules to the plasma membrane.
Gab2- or Fyn-deficient BMMCs showed a decreased β-hexosaminidase release (Fig. 5 A). We further confirmed that the FcɛRI-induced phosphorylation of Gab2 was decreased in Fyn-deficient BMMCs (Fig. 5 B). More importantly, the FcɛRI-induced formation of polymeric tubulin was decreased in Fyn- and Gab2-deficient BMMCs, as shown in Fig. 5 C. Consistent with this, the microtubule formation was impaired in Fyn- or Gab2-deficient BMMCs, but was intact in Lyn- and SLP-76–deficient BMMCs (Fig. 5, D–M).
Figure 5.
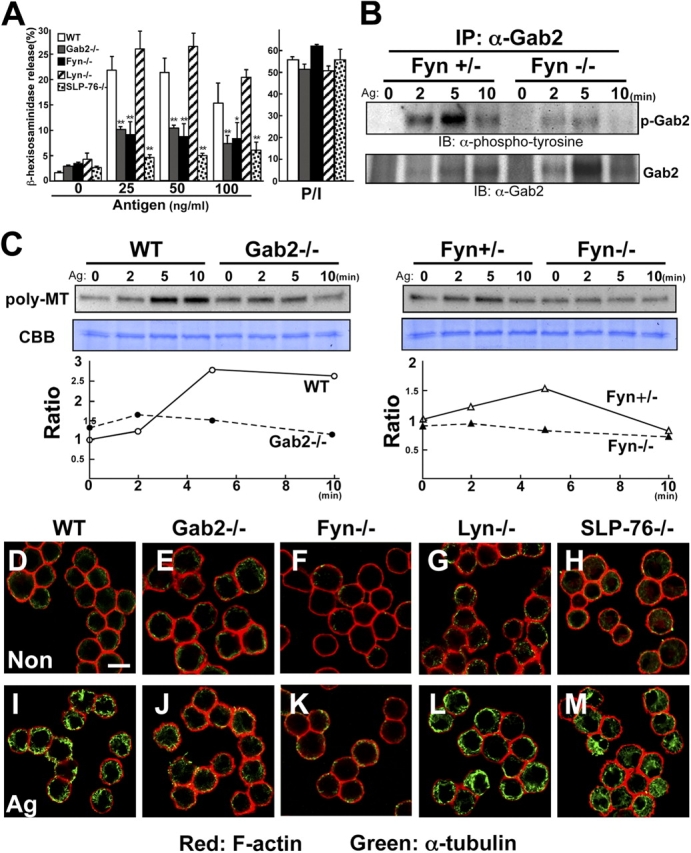
The Fyn/Gab2-mediated pathway controls microtubule formation. (A) Fyn and Gab2 are required for mast cell degranulation. Either wild-type, Gab2-, Fyn-, Lyn-, or SLP-76–deficient BMMCs were sensitized with IgE and then stimulated with various concentrations of DNP-HSA (Antigen) as indicated for 30 min. β-Hexosaminidase release was measured for indication of mast cell degranulation. The values indicate means ± SD of three separate experiments. Statistical analysis was performed using the t test. Single asterisk indicates P < 0.05 vs. wild-type BMMCs. Double asterisk indicates P < 0.01 vs. antigen-induced wild-type BMMCs. (B) Fyn-dependent Gab2 phosphorylation. Either wild-type or Fyn-deficient BMMCs were sensitized with IgE and then stimulated with 100 ng/ml DNP-HSA. Immunoprecipitates of anti-Gab2 antibody were probed with anti-phosphotyrosine (top) or anti-Gab2 antibody (bottom). (C) FcɛRI stimulation increases polymerization of tubulin. Either wild-type, Gab2-, or Fyn-deficient BMMCs were sensitized with IgE and stimulated with 100 ng/ml DNP-HSA for the times indicated. Stimulated cells were lysed in 0.1% Triton X-100. Triton X-100–insoluble fraction was subjected to Western blot analysis using anti-α-tubulin antibody. Lysate from each of the same samples were stained with CBB for loading control. One representative of three experiments is shown for each panel. Ratio is indicated as relative intensity (versus intensity in unstimulated cells). Poly-MT, polymeric tubulin. (D–M) Fyn and Gab2 are required for FcɛRI-induced microtubule formation. Either wild-type, Gab2-, Fyn-, Lyn-, or SLP-76–deficient BMMCs were sensitized with IgE and then stimulated with vehicle (D–H) or DNP-HSA (I–M) for 5 min. Stimulated cells were processed for double staining with phalloidin-rhodamine (red fluorescence) and antibody to α-tubulin (green fluorescence). Representative images are shown. Bar, 10 μm.
Cytoskeletal organization is regulated by small G proteins such as Rho, Rac, and Cdc42 (Etienne-Manneville and Hall, 2002). We examined RhoA and Rac activation in wild-type and Gab2-deficient BMMCs, by measuring the GTP-bound (active) RhoA and Rac after FcɛRI stimulation. The amount of GST-Rhotekin–bound RhoA was significantly decreased in Gab2-deficient BMMCs compared with wild-type cells at 5 min after stimulation (Fig. 6 A). We did not, however, observe any difference in the time course of Rac activation between Gab2-deficient and wild-type BMMCs (Fig. 6 A).
Figure 6.
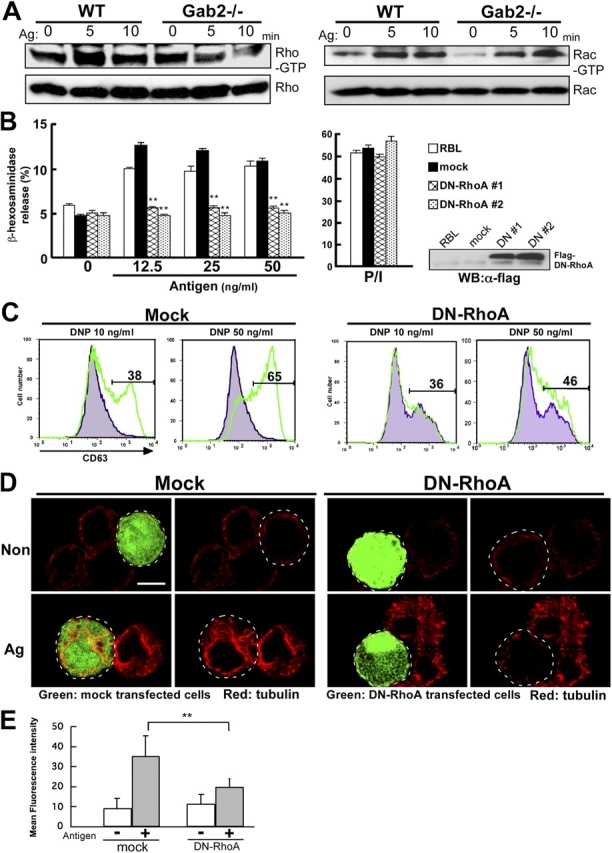
RhoA is required for FcɛRI-induced microtubule formation and degranulation. (A) FcɛRI stimulation induces RhoA and Rac activation. RhoA and Rac activity were determined by Rhotekin RBD and PAK CRIB pull-down assay, respectively. Either wild-type or Gab2-deficient BMMCs were sensitized with IgE and then stimulated with DNP-HSA (Ag) for the times indicated. Cell lysates were incubated with GST-Rhotekin RBD (left) or GST-PAK CRIB (right) fusion protein. RhoA-GTP and Rac-GTP forms were detected by anti-RhoA and anti-Rac antibodies, respectively. One representative of three experiments is shown for each panel. (B) RhoA is involved in mast cell degranulation. RBL-2H3 cells were stably transfected with Flag-RhoA N19 (DN-RhoA) and pcDNA3 (mock). DN-RhoA or mock-introduced cells were sensitized with 0.5 μg/ml IgE for 12 h, and stimulated with various concentrations of DNP-HSA (Antigen) as indicated for 30 min. β-Hexosaminidase release was measured for indication of mast cell degranulation. The values indicate means ± SD of three separate experiments. Statistical analysis was performed using the t test. Double asterisk indicates P < 0.01 vs. mock-introduced RBL-2H3 cells. Expression of Flag-tagged DN-RhoA in RBL-2H3 stable transfectants were visualized immunoblotting with anti-Flag antibodies (right). (C) RhoA is required for FcɛRI-induced increase of cell surface expression of CD63. BMMCs transfected with retroviral vector encoding IRES-GFP (Mock) or IRES-GFP Flag-RhoA N19 (DN-RhoA) were sensitized for 6 h with IgE. Cells were stimulated with either vehicle (gray histogram) or DNP-HSA (green line) for 10 min. Antigen-induced surface expression of CD63 was detected with anti-CD63 antibody, followed by FACS analysis. The number in the figures indicates the percentage of CD63-positive cells. (D) RhoA controls FcɛRI-mediated microtubule formation. BMMCs transfected with retroviral vector encoding IRES-GFP (Mock) or IRES-GFP Flag-RhoA N19 (DN-RhoA) were sensitized with IgE for 6 h. Cells were stimulated with DNP-HSA for 5 min. Cells were stained with antibody to α-tubulin (red fluorescence). The region delineated by the dotted line indicates mock-transfected cells (left) and RhoA N19–transfected cells (right), respectively. Representative images are shown. Bar, 10 μm. (E) Quantification of effect of DN-RhoA on microtubule formation BMMCs transfected with retroviral vector encoding IRES-GFP (mock) or IRES-GFP Flag-RhoA N19 (DN-RhoA) were sensitized with IgE for 6 h. Cells were stimulated with DNP-HSA for 5 min. Cells were stained with antibody to α-tubulin (red fluorescence). Mock or DN-RhoA transfected BMMCs were selected and mean fluorescence intensity of them was measured by Leica confocal software version 2.5. Statistical analysis was performed using the t test. Double asterisk indicates P < 0.01 vs. mock-infected BMMCs.
We next examined the need for RhoA activation in mast cell degranulation. We established RBL cells and BMMCs that stably expressed a dominant-negative form of RhoA (DN-RhoA), and found that they showed a decrease in FcɛRI-mediated degranulation and surface expression of CD63, compared with mock-transfected mast cells (Fig. 6, B and C). We also showed that DN-RhoA inhibited FcɛRI-induced microtubule formation, whereas F-actin disassembly was not affected (Fig. 6, D and E; unpublished data). Collectively, these data indicated that RhoA protein is required for FcɛRI-induced microtubule formation and degranulation.
Finally, we showed that there was a decrease in granule translocation in Fyn- and Gab2-deficient BMMCs, whereas granule translocation still occurred in Lyn- and SLP-76–deficient BMMCs (Fig. 4 D). Together, all these data indicate that the Fyn/Gab2/RhoA signaling pathway is required for the microtubule-dependent FcɛRI-induced translocation of granules to the plasma membrane.
Discussion
The calcium-independent microtubule-dependent pathway in FcɛRI-mediated mast cell degranulation
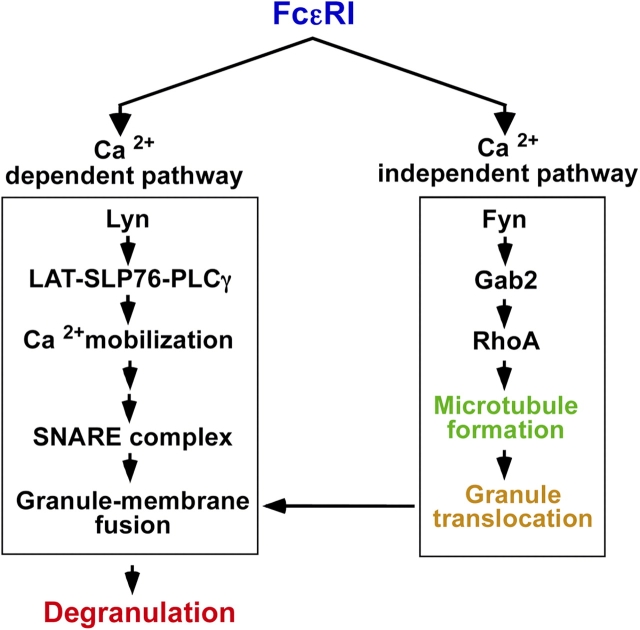
FcɛRI-mediated degranulation has been thought to be dependent on calcium influx. Studies of exocytosis in mast cells have demonstrated that members of the SNARE family, including SNAP-23, synaptotagmin, syntaxin (t-SNARE), and molecules of the VAMPs family (v-SNARE) regulate granule-to-plasma or granule-to-granule membrane fusion in response to elevated cytosolic calcium concentrations (Castle et al., 2002). In addition to the calcium-dependent pathway, we here showed that the calcium-independent and microtubule-dependent pathway play a critical role in mast cell degranulation. Furthermore, this pathway is regulated by Fyn/Gab2/RhoA signaling and is required for granule translocation to the plasma membrane. These conclusions were drawn from the following results: (1) FcɛRI stimulation induced the formation of microtubules; (2) agents affecting microtubule dynamics inhibited FcɛRI stimulation–induced degranulation and the translocation of granules to the plasma membrane; (3) neither the microtubule formation nor the translocation of granules to the plasma membrane was dependent on calcium; (4) Fyn/Gab2/RhoA signaling was not only involved in microtubule formation but also was required for degranulation and the translocation of granules to the plasma membrane; and (5) Lyn and SLP-76 were neither involved in microtubule formation nor the translocation of granules to the plasma membrane.
The presence of a calcium-independent pathway was suggested previously by a study showing that Fyn-deficient BMMCs have an impaired FcɛRI-induced mast cell degranulation, but an intact calcium influx (Parravicini et al., 2002). Taking this and our findings together, we propose that the mast cell degranulation process can be dissected into two steps: the calcium-independent and microtubule-dependent process and the calcium-dependent one. The former step is involved in the translocation of granules to the plasma membrane, the latter involved in the fusion of granule with the plasma membrane and exocytosis (Fig. 7).
Figure 7.
Calcium-independent microtubule-dependent pathway is required for FcɛRI-mediated degranulation in mast cells. The FcɛRI-mediated signaling pathway can be dissected into calcium-dependent and calcium-independent pathways. The calcium-independent pathway is critical for microtubule-dependent translocation of granules to the plasma membrane, and this pathway is regulated by Fyn/Gab2/RhoA signaling.
Role of cytoskeletal rearrangements in mast cell degranulation
Several studies have suggested the involvement of cytoskeletal rearrangements in mast cell degranulation. Actin polymerization–inhibiting agents increase both the rate and extent of FcɛRI-induced degranulation (Frigeri and Apgar, 1999; Oka et al., 2002), and biochemical studies indicated that FcɛRI stimulation causes a rapid increase in the level of F-actin in RBL cells (Pfeiffer et al., 1985; Frigeri and Apgar, 1999). Furthermore, activation-induced rearrangement of microtubules is observed in rat peritoneal mast cells while tubulin polymerization-inhibiting agents inhibit degranulation (Nielsen and Johansen, 1986; Tasaka et al., 1991; Martin-Verdeaux et al., 2003). However, the precise roles of the cytoskeletal rearrangements in the mast cell degranulation process have not been established.
We found that FcɛRI stimulation triggers F-actin ring disassembly in a calcium-dependent manner in mast cells, and that disruption of the F-actin cytoskeleton by latrunculin B enhances the FcɛRI-mediated mast cell degranulation. Others have described a similar effect in RBL cells and neuroblastoma cell lines (Frigeri and Apgar, 1999; Holowka et al., 2000; Ohnishi et al., 2001). Together, these data suggest that F-actin disassembly is involved in a mast cell exocytotic step. One explanation is that the removal of cortical F-actin permits granules greater access to the plasma membrane. Alternatively, as shown in chromaffin cells, cortical F-actin may act as a barrier between the reserve and the release-ready secretory vesicle pools (Cheek and Burgoyne, 1986; Vitale et al., 1995). F-actin disassembly may act to disrupt this actin barrier. At present, we do not have conclusive data indicating the precise role of F-actin disassembly in mast cell degranulation.
We demonstrated that FcɛRI stimulation triggers the formation of microtubules in a calcium-independent manner in mast cells. We observed a rapid increase in the polymeric form of tubulin soon after FcɛRI stimulation and an enhanced rate of polymer formation over the next 15 min. FcɛRI-induced microtubule formation returned to the basal level at 20 min after stimulation. We found that nocodazole, which induces the depolymerization of the microtubule, significantly inhibited the FcɛRI-mediated mast cell degranulation. We obtained similar results with Taxol, a stabilizer of microtubules. These data indicated that a dynamic microtubule network is required for mast cell degranulation.
We observed that granules containing CD63-GFP translocated to the plasma membrane within 10 min after FcɛRI stimulation. The translocation of CD63-containing granule to the plasma membrane could be induced as a result of granule–granule fusion. However, this is unlikely because (1) the translocation of granule to the plasma membrane is calcium independent; (2) it is considered that the membrane fusion is calcium dependent in general (Lin and Scheller, 2000); and (3) it is reported that granule–granule fusion is calcium dependent, like the fusion of granule with the plasma membrane (Guo et al., 1998). Actually, we observed the translocation of CD63-containing granules to the plasma membrane in the absence of calcium, under the condition that we did not observe FcɛRI-induced increase of cell surface expression of CD63 by FACS analysis. Furthermore, the observed translocation of granules to the plasma membrane was totally dependent on a dynamic microtubule network. These results support the idea that FcɛRI stimulation induces the translocation of granules to the plasma membrane in a manner dependent on microtubule and independent of calcium, although the involvement of granule–granule fusion in a part of the translocation process is not excluded. Anyway, this is consistent with recent studies showing that granules are translocated to the plasma membrane in RBL cells after stimulation (Amano et al., 2001; Smith et al., 2003).
The Fyn/Gab2/RhoA-mediated pathway controls the FcɛRI-induced polymerization of tubulin and translocation of granules to the plasma membrane
Mast cell degranulation depends on the FcɛRI-induced formation of a complex consisting of several adaptor molecules and catalytic enzymes, including src family kinases. However, little is known about the molecular mechanism that relays the early FcɛRI-activated signals to cytoskeletal changes that occur subsequently. Our study provides the first direct evidence for a critical role of adaptor proteins such as Gab2 in FcɛRI-dependent microtubule organization. Using Gab2- and Fyn-deficient BMMCs, we showed that the Fyn/Gab2-mediated pathway controlled microtubule formation. We then found that FcɛRI stimulation induced the activation of RhoA and Rac, and that the activation of RhoA was decreased in Gab2-deficient BMMCs. We also found that dominant-negative RhoA, when introduced into mast cells, inhibited FcɛRI-induced microtubule formation and degranulation. Consistent with our findings, another group reported that Rho regulates mast cell secretion (Price et al., 1995; Mariot et al., 1996). These data indicated that the Rho GTPase activity is critical for microtubule organization and degranulation in mast cells. There are two potential effects of Rho on microtubule. One is that Rho activation leads to microtubule stabilization. The other is that Rho activation inactivates a microtubule-destabilizing protein. Some reports have showed that Rho promotes the stabilization of microtubule through its target mDia (Ishizaki et al., 2001; Palazzo et al., 2001). Further biochemical analyses will clarify the mechanisms by which RhoA controls microtubule organization.
In summary, our results in this paper showed that the FcɛRI-mediated degranulation process can be dissected into two steps: the calcium-independent microtubule-dependent translocation of granules to the membrane, and the calcium-dependent degranulation accompanied with granule–plasma membrane fusion. We further showed that the Fyn/Gab2/RhoA signaling pathway, but not the Lyn/SLP-76-mediated one, plays a critical role in the calcium-independent microtubule-dependent pathway (Fig. 7). These novel results inspire new appreciation for the role of the microtubule cytoskeleton in supporting secretory cell functions such as FcɛRI-mediated degranulation in mast cells, and most likely in cells of other lineages cells as well.
Materials and methods
Mice
The generation of Gab2-deficient mice from a C57BL6 and 129Sv mixed background was reported by Nishida et al. (2002). Fyn- and Lyn-deficient mice were derived from a C57BL/6 background (Yagi et al., 1993; Nishizumi et al., 1995). SLP-76–deficient mice were of a C57BL6 and 129Sv mixed background. (Pivniouk et al., 1999).
Antibodies and reagents
Anti-Gab2 antibodies were generated as described previously (Nishida et al., 1999). Anti-Flag (M2) and anti-phosphotyrosine (4G10) antibodies were purchased from Sigma-Aldrich and Upstate Biotechnology, respectively. Latrunculin B, nocodazole, paclitaxel (Taxol), and xestospongin C were purchased from Sigma-Aldrich. Jasplakinolide was purchased from Molecular Probes, Inc.
Primary mast cell cultures and cell lines
BMMCs were selectively grown in RPMI 1640 medium supplemented with IL-3 (supernatant of a mIL-3–producing cell line, CHOmIL-3-3-12M; a gift from T. Sudo, Toray Industry, Inc.), 10% FBS, 10 mU/ml penicillin, and 0.1 mg/ml streptomycin for 4–8 wk. During culture the medium was changed every 3–4 d, and the cells were transferred to new dishes to separate them from adherent cells. After 5 wk, culture BMMCs were ready for in vitro experiments.
RBL-2H3 cells (a gift from T. Matsuda, University of Hokkaido, Hokkaido, Japan) were maintained in DME supplemented with 10% FBS. cDNAs encoding Flag-RhoA (N19) were provided by Y. Ohba and M. Matsuda (Research Institute for Microbial Diseases, Osaka, Japan). A total of 10 μg linearized Flag-RhoA (N19) cDNA in a pcDNA3 vector was transfected into RBL-2H3 cells by electroporation (950 μF, 250V). Clones were selected with 1 mg/ml of G418 (Nacalai Tesque).
Degranulation assay
The degree of degranulation was determined by measuring the release of β-hexosaminidase. Cells (106/ml) were preloaded for 6 h with anti-DNP IgE (1 μg/ml, SPE-7) in medium (without IL-3). To measure β-hexosaminidase release, sensitized cells were stimulated with 100 ng/ml DNP-HSA for 30 min in Tyrode's buffer. Samples were placed on ice and then centrifuged at 1,500 rpm for 5 min. The enzymatic activity of β-hexosaminidase in supernatants and cell pellets solubilized with 1% Triton X-100 in Tyrode's buffer was measured with p-nitrophenyl N-acetyl-β-d-glucosaminide (Sigma-Aldrich) in 0.1 M sodium citrate (pH 4.5) for 60 min at 37°C. The reaction was stopped by the addition of 0.2 M glycine (pH 10.7). The release of the product, 4-p-nitrophenol, was detected by absorbance at 405 nm. The extent of degranulation was calculated by dividing the 4-p-nitrophenol absorbance in the supernatant by the sum of the absorbance in the supernatant and detergent-solubilized cell pellet.
Confocal microscopy
Separate aliquots of 5 × 105 cells were each sensitized with 1 μg/ml SPE-7 for 6 h. Cells were pretreated with different reagents: 1 μM latrunculin for 15 min, 10 μg/ml nocodazole for 30 min, 5 μM jasplakinolide for 3 h, and 10 μM paclitaxel for 30 min. Cells were stimulated with 100 ng/ml DNP-HSA for 5 min at 37°C and fixed with 4% PFA for 30 min at 37°C. Cells were centrifuged at 1,500 rpm for 5 min and permeabilized in Perm Buffer (BD Biosciences) containing 1% BSA for 15 min at RT. Cells were washed with 1 ml of PBS (−) twice and resuspended in 100 μl of PBS (−) and attached to glass slides using cytospin (Thramo Shandon) at 600 rpm for 6 min. Primary and secondary stainings were performed on the glass slides: anti-α-tubulin (B5-1-2; Sigma-Aldrich) at a dilution of 1:50, FITC-conjugated anti–mouse IgG (Molecular Probes, Inc.) at 1:50, Alexa 594–conjugated anti–mouse IgG at a dilution of 1:150, and phalloidin-rhodamine (Molecular Probes, Inc.) at 1:150. Staining was performed for 30 min in the dark; the slides were then washed with PBS (−) and coverslips were mounted with DAKO mounting solution (DakoCytomation). Confocal microscopy was performed using an LSM510 system in conjunction with an Axiovert200 (Carl Zeiss MicroImaging, Inc.).
Polymeric tubulin assay
After the stimulation the cells were suspended in extraction buffer, which contained the following: 0.1 M Pipes, pH 7.1, 1 mM MgSO4, 1 mM EGTA, 2 M glycerol, 0.1% Triton X-100, and protease inhibitors (10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 μg/ml pepstatin A, and 0.5 mM phenylmethysulfonyl fluoride). After incubation on ice for 15 min, the cell lysates were centrifuged at 15,000 rpm for 15 min and the supernatant (containing 0.1% Triton-soluble tubulin) was placed into another 1.5-ml tube. The remaining pellet was resuspended in lysis buffer (25 mM Tris-HCl, pH 7.4, 0.4 M NaCl, and 0.5% SDS) and boiled for 10 min. The sample was centrifuged for 15,000 rpm for 5 min, and the polymeric tubulin-containing supernatant was collected and placed gently into another 1.5-ml tube. The 0.1% Triton-soluble and -insoluble solutions were subjected to SDS-PAGE and detected by immunoblotting with anti-α-tubulin antibodies.
Detection of GTP-bound RhoA and Rac1
GTP-bound RhoA and Rac1 were detected by pull-down assays. In brief, the cleared lysates were incubated with GST-Rhotekin RBD fusion protein (a gift from N. Mochizuki, National Cardiovascular Center Research Institute, Osaka, Japan) and 10 μl of glutathione-sepharose (Amersham Biosciences). The GST-Rhotekin RBD/Rho-GTP complex was collected by incubation with glutathione-sepharose beads and separated on an SDS polyacrylamide gel. The GTP-bound RhoA was visualized by immunoblotting with anti-RhoA antibodies (Santa Cruz Biotechnology, Inc.). To measure the Rac1 activity, cell lysates were incubated with GST-PAK CRIB agarose beads (Upstate Biotechnology). GTP-bound Rac precipitated with the beads was subjected to SDS-PAGE and detected by immunoblotting with anti-Rac1 antibody (Upstate Biotechnology).
Measurement of intracellular calcium
BMMCs were sensitized with 1 μg/ml SPE-7 for 12 h and incubated with the calcium-sensitive dye indo 1-AM (Molecular Probes, Inc.) in the presence of F127 and 0.2% FBS at 37°C for 30 min. Cells were washed twice and resuspended in 1 ml Tyrode's buffer containing 1 mM CaCl2. Cells were analyzed for calcium mobilization in BD-LSR (BD Biosciences). Values were plotted as ratio of fluorescence at FL4 (Ca2+-free indo-1) and FL5 (Ca2+-bound indo-1).
Retroviral transfection
Retroviral transfection was performed as previously described (Itoh et al., 2002). The CD63-GFP plasmid (a gift from M. Nakanishi, Nagoya City University, Nagoya, Japan) was inserted into the retroviral vector pMX (a gift from T. Kitamura, University of Tokyo, Tokyo, Japan). This was then used to transfect the 293T-based packaging cell line phoenix (a gift from G. Nolan, Stanford University, Stanford, CA) with Lipofectamine 2000 (Invitrogen) to generate recombinant retroviruses. Bone marrow cells were infected with the retroviruses in the presence of 10 μg/ml polybrene (Sigma-Aldrich) and IL-3.
Online supplemental material
Fig. S1 shows the GFP fluorescence time-lapse recording of antigen-stimulated BMMCs expressing CD63-GFP. Online supplemental material available at http://www.jcb.org/cgi/content/full/jcb.200501111/DC1.
Acknowledgments
We thank Drs. T. Matsuda, N. Mochizuki, Y. Ohba, M. Matsuda, M. Nakanishi, and T. Kitamura for various reagents. We would like to thank Drs. T. Saito and S. Yamasaki for technical advice and suggestions. We also thank R. Masuda, A. Kubota, and M. Shimura for secretarial assistance.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
K. Nishida and S. Yamasaki contributed equally to this paper.
Abbreviations used in this paper: BMMC, bone marrow–derived mast cell; FcɛRI, Fcɛ receptor I; Gab2, Grb2-associated binder 2; LAT, linker for the activation of T cells; SLP-76, SH2 domain–containing leukocyte protein of 76 kD.
References
- Amano, T., T. Furuno, N. Hirashima, N. Ohyama, and M. Nakanishi. 2001. Dynamics of intracellular granules with CD63-GFP in rat basophilic leukemia cells. J. Biochem. (Tokyo). 129:739–744. [DOI] [PubMed] [Google Scholar]
- Blank, U., and J. Rivera. 2004. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 25:266–273. [DOI] [PubMed] [Google Scholar]
- Cao, M.Y., D. Davidson, J. Yu, S. Latour, and A. Veillette. 1999. Clnk, a novel SLP-76-related adaptor molecule expressed in cytokine-stimulated hemopoietic cells. J. Exp. Med. 190:1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle, J.D., Z. Guo, and L. Liu. 2002. Function of the t-SNARE SNAP-23 and secretory carrier membrane proteins (SCAMPs) in exocytosis in mast cells. Mol. Immunol. 38:1337–1340. [DOI] [PubMed] [Google Scholar]
- Cheek, T.R., and R.D. Burgoyne. 1986. Nicotine-evoked disassembly of cortical actin filaments in adrenal chromaffin cells. FEBS Lett. 207:110–114. [DOI] [PubMed] [Google Scholar]
- Eiseman, E., and J.B. Bolen. 1992. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 355:78–80. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature. 420:629–635. [DOI] [PubMed] [Google Scholar]
- Frigeri, L., and J.R. Apgar. 1999. The role of actin microfilaments in the down-regulation of the degranulation response in RBL-2H3 mast cells. J. Immunol. 162:2243–2250. [PubMed] [Google Scholar]
- Geng, L., S. Pfister, S.K. Kraeft, and C.E. Rudd. 2001. Adaptor FYB (Fyn-binding protein) regulates integrin-mediated adhesion and mediator release: differential involvement of the FYB SH3 domain. Proc. Natl. Acad. Sci. USA. 98:11527–11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitsuka, R., H. Kanazashi, H. Sasanuma, Y. Fujimura, Y. Hidaka, A. Tatsuno, C. Ra, K. Hayashi, and D. Kitamura. 2000. A BASH/SLP-76-related adaptor protein MIST/Clnk involved in IgE receptor-mediated mast cell degranulation. Int. Immunol. 12:573–580. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Espinosa, C., S. Odom, A. Olivera, J.P. Hobson, M.E. Martinez, A. Oliveira-Dos-Santos, L. Barra, S. Spiegel, J.M. Penninger, and J. Rivera. 2003. Preferential signaling and induction of allergy-promoting lymphokines upon weak stimulation of the high affinity IgE receptor on mast cells. J. Exp. Med. 197:1453–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode, B.L., D.G. Drubin, and G. Barnes. 2000. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 12:63–71. [DOI] [PubMed] [Google Scholar]
- Gu, H., and B.G. Neel. 2003. The “Gab” in signal transduction. Trends Cell Biol. 13:122–130. [DOI] [PubMed] [Google Scholar]
- Gu, H., K. Saito, L.D. Klaman, J. Shen, T. Fleming, Y. Wang, J.C. Pratt, G. Lin, B. Lim, J.P. Kinet, and B.G. Neel. 2001. Essential role for Gab2 in the allergic response. Nature. 412:186–190. [DOI] [PubMed] [Google Scholar]
- Guo, Z., C. Turner, and D. Castle. 1998. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 94:537–548. [DOI] [PubMed] [Google Scholar]
- Hamawy, M.M., C. Fischler, J. Zhang, and R.P. Siraganian. 1997. FcɛRI aggregation induces tyrosine phosphorylation of a novel 72 kDa protein downstream of Syk. Biochem. Biophys. Res. Commun. 239:670–675. [DOI] [PubMed] [Google Scholar]
- Hata, D., Y. Kawakami, N. Inagaki, C.S. Lantz, T. Kitamura, W.N. Khan, M. Maeda-Yamamoto, T. Miura, W. Han, S.E. Hartman, et al. 1998. Involvement of Bruton's tyrosine kinase in FcɛRI-dependent mast cell degranulation and cytokine production. J. Exp. Med. 187:1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi, M., and T. Hirano. 2000. Gab-family adapter molecules in signal transduction of cytokine and growth factor receptors, and T and B cell antigen receptors. Leuk. Lymphoma. 37:299–307. [DOI] [PubMed] [Google Scholar]
- Holowka, D., E.D. Sheets, and B. Baird. 2000. Interactions between FcɛRI and lipid raft components are regulated by the actin cytoskeleton. J. Cell Sci. 113:1009–1019. [DOI] [PubMed] [Google Scholar]
- Ishizaki, T., Y. Morishima, M. Okamoto, T. Furuyashiki, T. Kato, and S. Narumiya. 2001. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat. Cell Biol. 3:8–14. [DOI] [PubMed] [Google Scholar]
- Itoh, S., M. Itoh, K. Nishida, S. Yamasaki, Y. Yoshida, M. Narimatsu, S.J. Park, M. Hibi, K. Ishihara, and T. Hirano. 2002. Adapter molecule Grb2-associated binder 1 is specifically expressed in marginal zone B cells and negatively regulates thymus-independent antigen-2 responses. J. Immunol. 168:5110–5116. [DOI] [PubMed] [Google Scholar]
- Kettner, A., V. Pivniouk, L. Kumar, H. Falet, J.S. Lee, R. Mulligan, and R.S. Geha. 2003. Structural requirements of SLP-76 in signaling via the high-affinity immunoglobulin E receptor (FcɛRI) in mast cells. Mol. Cell. Biol. 23:2395–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, T., M. Hisano, Y. Inoue, and M. Adachi. 2001. Tyrosine phosphorylation of the linker for activator of T cells in mast cells by stimulation with the high affinity IgE receptor. Immunol. Lett. 75:123–129. [DOI] [PubMed] [Google Scholar]
- Lin, R.C., and R.H. Scheller. 2000. Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell Dev. Biol. 16:19–49. [DOI] [PubMed] [Google Scholar]
- Liu, Y., and L.R. Rohrschneider. 2002. The gift of Gab. FEBS Lett. 515:1–7. [DOI] [PubMed] [Google Scholar]
- Mariot, P., A.J. O'Sullivan, A.M. Brown, and P.E. Tatham. 1996. Rho guanine nucleotide dissociation inhibitor protein (RhoGDI) inhibits exocytosis in mast cells. EMBO J. 15:6476–6482. [PMC free article] [PubMed] [Google Scholar]
- Martin-Verdeaux, S., I. Pombo, B. Iannascoli, M. Roa, N. Varin-Blank, J. Rivera, and U. Blank. 2003. Evidence of a role for Munc18-2 and microtubules in mast cell granule exocytosis. J. Cell Sci. 116:325–334. [DOI] [PubMed] [Google Scholar]
- Nadler, M.J., and J.P. Kinet. 2002. Uncovering new complexities in mast cell signaling. Nat. Immunol. 3:707–708. [DOI] [PubMed] [Google Scholar]
- Nadler, M.J., S.A. Matthews, H. Turner, and J.P. Kinet. 2000. Signal transduction by the high-affinity immunoglobulin E receptor FcɛRI: coupling form to function. Adv. Immunol. 76:325–355. [DOI] [PubMed] [Google Scholar]
- Nielsen, E.H., and T. Johansen. 1986. Effects of dimethylsulfoxide (DMSO), nocodazole, and taxol on mast cell histamine secretion. Acta Pharmacol. Toxicol. (Copenh.). 59:214–219. [DOI] [PubMed] [Google Scholar]
- Nishida, K., and T. Hirano. 2003. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 94:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, K., Y. Yoshida, M. Itoh, T. Fukada, T. Ohtani, T. Shirogane, T. Atsumi, M. Takahashi-Tezuka, K. Ishihara, M. Hibi, and T. Hirano. 1999. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 93:1809–1816. [PubMed] [Google Scholar]
- Nishida, K., L. Wang, E. Morii, S.J. Park, M. Narimatsu, S. Itoh, S. Yamasaki, M. Fujishima, K. Ishihara, M. Hibi, et al. 2002. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood. 99:1866–1869. [DOI] [PubMed] [Google Scholar]
- Nishikata, H., C. Oliver, S.E. Mergenhagen, and R.P. Siraganian. 1992. The rat mast cell antigen AD1 (homologue to human CD63 or melanoma antigen ME491) is expressed in other cells in culture. J. Immunol. 149:862–870. [PubMed] [Google Scholar]
- Nishizumi, H., and T. Yamamoto. 1997. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J. Immunol. 158:2350–2355. [PubMed] [Google Scholar]
- Nishizumi, H., I. Taniuchi, Y. Yamanashi, D. Kitamura, D. Ilic, S. Mori, T. Watanabe, and T. Yamamoto. 1995. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 3:549–560. [DOI] [PubMed] [Google Scholar]
- Ohnishi, H., S. Yamamori, K. Ono, K. Aoyagi, S. Kondo, and M. Takahashi. 2001. A src family tyrosine kinase inhibits neurotransmitter release from neuronal cells. Proc. Natl. Acad. Sci. USA. 98:10930–10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka, T., K. Sato, M. Hori, H. Ozaki, and H. Karaki. 2002. FcɛRI cross-linking-induced actin assembly mediates calcium signalling in RBL-2H3 mast cells. Br. J. Pharmacol. 136:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo, A.F., T.A. Cook, A.S. Alberts, and G.G. Gundersen. 2001. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3:723–729. [DOI] [PubMed] [Google Scholar]
- Parravicini, V., M. Gadina, M. Kovarova, S. Odom, C. Gonzalez-Espinosa, Y. Furumoto, S. Saitoh, L.E. Samelson, J.J. O'Shea, and J. Rivera. 2002. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 3:741–748. [DOI] [PubMed] [Google Scholar]
- Pfeiffer, J.R., J.C. Seagrave, B.H. Davis, G.G. Deanin, and J.M. Oliver. 1985. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J. Cell Biol. 101:2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivniouk, V.I., T.R. Martin, J.M. Lu-Kuo, H.R. Katz, H.C. Oettgen, and R.S. Geha. 1999. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J. Clin. Invest. 103:1737–1743. [PMC free article] [PubMed] [Google Scholar]
- Price, L.S., J.C. Norman, A.J. Ridley, and A. Koffer. 1995. The small GTPases Rac and Rho as regulators of secretion in mast cells. Curr. Biol. 5:68–73. [DOI] [PubMed] [Google Scholar]
- Rivera, J. 2002. Molecular adapters in FcɛRI signaling and the allergic response. Curr. Opin. Immunol. 14:688–693. [DOI] [PubMed] [Google Scholar]
- Sada, K., S.M. Miah, K. Maeno, S. Kyo, X. Qu, and H. Yamamura. 2002. Regulation of FcɛRI-mediated degranulation by an adaptor protein 3BP2 in rat basophilic leukemia RBL-2H3 cells. Blood. 100:2138–2144. [DOI] [PubMed] [Google Scholar]
- Saitoh, S., R. Arudchandran, T.S. Manetz, W. Zhang, C.L. Sommers, P.E. Love, J. Rivera, and L.E. Samelson. 2000. LAT is essential for FcɛRI-mediated mast cell activation. Immunity. 12:525–535. [DOI] [PubMed] [Google Scholar]
- Saitoh, S., S. Odom, G. Gomez, C.L. Sommers, H.A. Young, J. Rivera, and L.E. Samelson. 2003. The four distal tyrosines are required for LAT-dependent signaling in FcɛRI-mediated mast cell activation. J. Exp. Med. 198:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraganian, R.P., J. Zhang, K. Suzuki, and K. Sada. 2002. Protein tyrosine kinase Syk in mast cell signaling. Mol. Immunol. 38:1229–1233. [DOI] [PubMed] [Google Scholar]
- Smith, A.J., J.R. Pfeiffer, J. Zhang, A.M. Martinez, G.M. Griffiths, and B.S. Wilson. 2003. Microtubule-dependent transport of secretory vesicles in RBL-2H3 cells. Traffic. 4:302–312. [DOI] [PubMed] [Google Scholar]
- Tasaka, K., M. Mio, K. Fujisawa, and I. Aoki. 1991. Role of microtubules on Ca2+ release from the endoplasmic reticulum and associated histamine release from rat peritoneal mast cells. Biochem. Pharmacol. 41:1031–1037. [DOI] [PubMed] [Google Scholar]
- Vitale, M.L., E.P. Seward, and J.M. Trifaro. 1995. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 14:353–363. [DOI] [PubMed] [Google Scholar]
- Xie, Z.H., I. Ambudkar, and R.P. Siraganian. 2002. The adapter molecule Gab2 regulates FcɛRI-mediated signal transduction in mast cells. J. Immunol. 168:4682–4691. [DOI] [PubMed] [Google Scholar]
- Yagi, T., S. Aizawa, T. Tokunaga, Y. Shigetani, N. Takeda, and Y. Ikawa. 1993. A role for Fyn tyrosine kinase in the suckling behaviour of neonatal mice. Nature. 366:742–745. [DOI] [PubMed] [Google Scholar]