Abstract
Src-like adaptor protein (SLAP) down-regulates expression of the T cell receptor (TCR)–CD3 complex during a specific stage of thymocyte development when the TCR repertoire is selected. Consequently, SLAP−/− thymocytes display alterations in thymocyte development. Here, we have studied the mechanism of SLAP function. We demonstrate that SLAP-deficient thymocytes have increased TCRζ chain expression as a result of a defect in TCRζ degradation. Failure to degrade TCRζ leads to an increased pool of fully assembled TCR–CD3 complexes that are capable of recycling back to the cell surface. We also provide evidence that SLAP functions in a pathway that requires the phosphorylated TCRζ chain and the Src family kinase Lck, but not ZAP-70 (ζ-associated protein of 70 kD). These studies reveal a unique mechanism by which SLAP contributes to the regulation of TCR expression during a distinct stage of thymocyte development.
Introduction
T cells develop in the thymus, where immature thymocytes undergo a developmental program that ensures the generation of T cells with a diverse repertoire of T cell receptors (TCRs). These TCRs are capable of recognizing foreign antigens that are presented by major histocompatibility complex (MHC) molecules without being autoreactive (Love and Chan, 2003). The αβTCR is part of a multichain complex that is composed of the peptide–MHC-binding TCRα and β chains that are noncovalently associated with the CD3γɛδɛ and TCRζζ chains, which together are referred to as the CD3 complex (Exley et al., 1991). Although αβTCR is responsible for antigen recognition, the remainder of the CD3 complex—the TCRζ chains in particular—is required for coupling the TCR to downstream signaling molecules.
Generation of the αβTCR repertoire is initiated at the most immature stage of thymocyte development (Sebzda et al., 1999). At this early stage of development, thymocytes express neither CD4 nor CD8 coreceptors. Thymocytes that are destined to become αβ T cells stochastically rearrange their TCRβ genes. If rearrangement of TCRβ is successful (in frame), the TCRβ chain is transported together with a nonvariant pre-TCRα chain and the CD3 complex to the cell surface as the pre-TCR complex. Surface expression of the pre-TCR complex induces ligand-independent signals (Irving et al., 1998), which allows for thymocytes to proliferate and up-regulate CD4 and CD8 expression, thereby progressing to the CD4+ CD8+ double-positive (DP) stage of development. At the DP stage of development, thymocytes rearrange their TCRα genes. If the rearrangement of TCRα is successful, low levels of the mature TCR–CD3 complex are expressed on the surface of the developing thymocyte. Expression of the TCR–CD3 complex is crucial at this stage of development, as signals through the TCR are required for the survival (positive selection) or deletion (negative selection) of DP thymocytes (Sebzda et al., 1999; Love and Chan, 2003). If the TCR expressed by a DP thymocyte cannot bind to self-peptide–MHC molecules, the thymocyte fails to receive positively selecting signals and subsequently dies. Conversely, if the TCR interacts too strongly with self-peptide–MHC molecules, the cell is potentially autoreactive and is deleted via apoptosis. Thymocytes that express TCRs with an intermediate affinity for peptide–MHC down-regulate either CD4 or CD8, thereby progressing to the single-positive (SP) stage of development. Progression to this more phenotypically and functionally mature SP stage is also associated with a 10-fold increase in the level of the TCR–CD3 complex to that of mature peripheral T cells. At the SP stage, thymocytes are subjected to further selection and maturation processes before exiting the thymus as mature T cells.
Both positive and negative selection processes are dependent on the strength of signals received through the TCR–CD3 complex. Signal strength is dependent not only on the intrinsic affinity of the TCR for peptide–MHC molecules but also on the number of receptors that interact with peptide–MHC. TCR–CD3 expression on DP thymocytes is only ∼10% of the level observed on SP thymocytes and mature T cells (Finkel et al., 1987; Havran et al., 1987). Previous studies have demonstrated that modulating levels of TCR–CD3 expression in developing thymocytes can lead to alterations in positive selection (Ericsson and Teh, 1995; Naramura et al., 1998; Sosinowski et al., 2001), suggesting that tight regulation of surface TCR–CD3 levels is required for normal TCR repertoire selection. Therefore, proteins that regulate surface TCR–CD3 levels in the thymus are likely to be important determinants of thymocyte development.
Recently, we have shown that Src-like adaptor protein (SLAP) regulates the level of TCR–CD3 expression on DP thymocytes (Sosinowski et al., 2001). SLAP was identified in a yeast two-hybrid screen for proteins that interact with the cytoplasmic domain of the Eck receptor protein tyrosine kinase (Pandey et al., 1995). In particular, SLAP is highly homologous with Src family kinases, which include the T lymphocyte–specific family members of Lck. Like Src family kinases, SLAP has a unique NH2 terminus that is myristolated, thereby targeting SLAP to cellular membranes (Manes et al., 2000). The NH2 terminus of SLAP is followed by Src homology (SH) 3 and 2 domains, which share 55 and 50% amino acid sequence identity with these domains in Lck, respectively. Unlike Src family kinases, however, SLAP lacks a kinase domain and, instead, contains a unique COOH terminus, whose function remains unclear.
Because SLAP is highly homologous to Lck but lacks a kinase domain, it was postulated that SLAP could negatively regulate Src family kinases by functioning in a dominant negative manner. Overexpression and microinjection studies in nonlymphoid cells have shown that SLAP can inhibit Src-mediated signaling through the platelet-derived growth factor receptor (Roche et al., 1998; Manes et al., 2000). In Jurkat T cells, the transient overexpression of SLAP can inhibit signaling downstream of the TCR as measured by nuclear factor of activated T cells, AP-1, or interleukin 2 transcriptional reporter activity (Sosinowski et al., 2000). Maximum inhibition of nuclear factor of activated T cell activity in Jurkat T cells requires both the SH2 and SH3 domains of SLAP. Altogether, these data suggest that SLAP is an inhibitor of Src family kinases. However, the mechanism by which SLAP inhibits signaling remains unclear, as no differences in overall tyrosine phosphorylation were observed in Jurkat T cells that overexpressed SLAP or in SLAP−/− thymocytes (Sosinowski et al., 2000, 2001).
In mice, SLAP protein expression is developmentally restricted and is most highly expressed in DP thymocytes (Sosinowski et al., 2001). Consistent with this restricted pattern of expression, targeted inactivation of the SLAP gene demonstrated that SLAP down-regulates TCR–CD3 expression at the DP stage of thymocyte development. In addition to increased TCR–CD3 expression, SLAP−/− DP thymocytes also display increased levels of CD4, CD5, and CD69. Furthermore, SLAP−/− thymocytes display increases in positive selection in the presence of a transgenic TCR. Finally, SLAP deficiency can partially overcome a developmental block at the DP stage and rescue the development of CD4+ SP thymocytes and peripheral T cells in mice that lack ZAP-70 (ζ-associated protein of 70 kD) tyrosine kinase. These alterations in thymocyte development in the absence of SLAP argue that control of surface TCR–CD3 levels on DP thymocytes is an important regulatory step in the generation of peripheral T cells. Therefore, we set out to elucidate the mechanism of SLAP-mediated TCR–CD3 down-regulation on DP thymocytes. In this study, we show that SLAP-deficient thymocytes have a defect in TCRζ chain degradation, which leads to an increased pool of fully assembled TCR–CD3 complexes that are capable of recycling back to the cell surface.
Results
Total levels of TCRζ are increased in SLAP−/− DP thymocytes
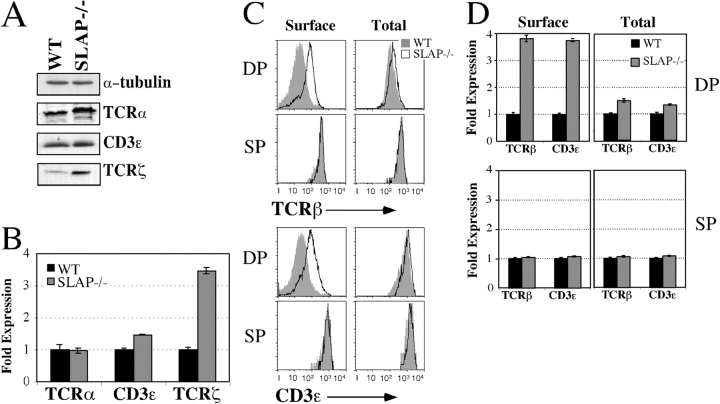
SLAP−/− mice have increased levels of surface TCR–CD3 on DP thymocytes (Fig. 1 C; Sosinowski et al., 2001). We reasoned that if either synthesis or degradation is altered by the absence of SLAP, total levels of TCR–CD3 should also be up-regulated. Total cell lysates were prepared from CD8+ thymocytes, which contained mostly DP thymocytes but also contained small numbers of CD8+ SP thymocytes. Purified cells were >95% DP, as determined by FACS (unpublished data). By Western blot analysis, total levels of the TCRζ chain were consistently increased three- to fourfold in SLAP−/− CD8+ thymocytes as compared with wild-type (WT) controls (Fig. 1, A and B). In contrast, total levels of TCRα and CD3ɛ were not substantially increased. Although lysates contained mostly DP thymocytes, there was a small (<5%) contaminating pool of CD8+ SP thymocytes in lysate preparations. Therefore, we used intracellular FACS staining to analyze total TCR–CD3 levels, specifically in DP thymocytes (Fig. 1, C and D). We were unable to stain for the TCRζ chain by FACS, but intracellular FACS analysis confirmed the results that were obtained by Western blot analysis for CD3ɛ. Although surface levels of CD3ɛ (as well as TCRβ) were consistently three- to fivefold higher on SLAP−/− DP thymocytes, total levels of TCR–CD3 were not significantly increased.
Figure 1.
TCRζ chain expression is increased in SLAP−/− mice. (A) Western blot analysis of TCRα, CD3ɛ, and TCRζ expression in RIPA lysates of purified CD8+ thymocytes from WT and SLAP−/− mice. Data are representative of six independent experiments. (B) Quantitation of Western blots represented in A using quantitative luminescence. Data represent mean expression of TCRα, CD3ɛ, or TCRζ by SLAP−/− CD8+ thymocytes as compared with WT controls ± SEM. TCR–CD3 levels have been normalized to α-tubulin. (C) Surface or total TCRβ and CD3ɛ expression by either surface or intracellular FACS staining. Data are representative of six mice per genotype. (D) Quantitation of FACS staining shown in C. Data represent the mean fluorescence intensity (MFI) ± SEM (error bars) as compared with WT controls for six mice of each genotype.
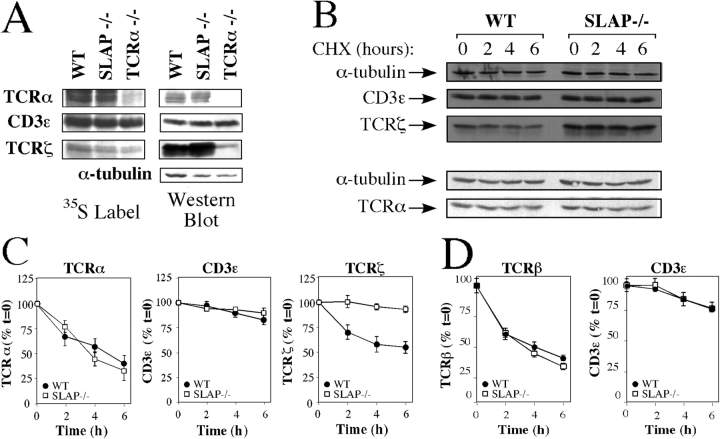
Increased TCRζ chain expression in the absence of SLAP suggests that SLAP modifies the rate of TCRζ synthesis and/or degradation. Metabolic labeling of CD8+ thymocytes demonstrated that the synthesis of TCRζ, TCRα, and CD3ɛ during a 30-min pulse label was similar in WT and SLAP−/− thymocytes (Fig. 2 A). To test whether the rate of TCRζ degradation was altered in the absence of SLAP, thymocyte single-cell suspensions that were enriched for DP thymocytes (CD8+ purified) were treated with cycloheximide to inhibit synthesis of TCR chains, and the stability of CD3ɛ, TCRζ, and TCRα over time was assessed by either Western blot analysis or by intracellular FACS staining. Degradation of TCRζ, but not CD3ɛ, TCRα, or TCRβ, was markedly impaired in SLAP−/− thymocytes as compared with WT controls (Fig. 2, B–D). We were unable to study TCRζ and TCRα degradation using a FACS-based assay. Therefore, we simultaneously investigated CD3ɛ degradation by intracellular FACS staining to determine whether the results obtained by Western blot analysis of CD8+-purified thymocytes were similar to the results obtained by intracellular FACS staining of DP thymocytes. CD3ɛ degradation by Western blot analysis was comparable to the FACS-based assay (Fig. 2, B–D), demonstrating that contamination from SP thymocytes does not significantly contribute to the impaired degradation of TCRζ that was observed in SLAP−/− thymocytes.
Figure 2.
TCRζ degradation is impaired in SLAP−/− thymocytes. (A) TCRα, CD3ɛ, and TCRζ immunoprecipitates from WT and SLAP−/− thymocytes after 30 min of metabolic labeling with radiolabeled (35S) cysteine and methionine. TCRα−/− thymocytes were used as a control for TCRα expression. Data are representative examples of three independent experiments. (B) Degradation of TCRα, CD3ɛ, and TCRζ by WT and SLAP−/− CD8+ thymocytes treated with cycloheximide (CHX), as assessed by Western blot analysis of RIPA lysates. Data are representative of up to four independent experiments. (C) Quantitation of Western blots shown in B. Data are plotted as the mean percent expression relative to time 0 and are the mean of three mice per genotype ± SEM (error bars). (D) Degradation of TCRβ or CD3ɛ in the presence of cycloheximide by DP thymocytes, as assessed by intracellular FACS staining.
Recycling TCR–CD3 complexes are increased in the absence of SLAP
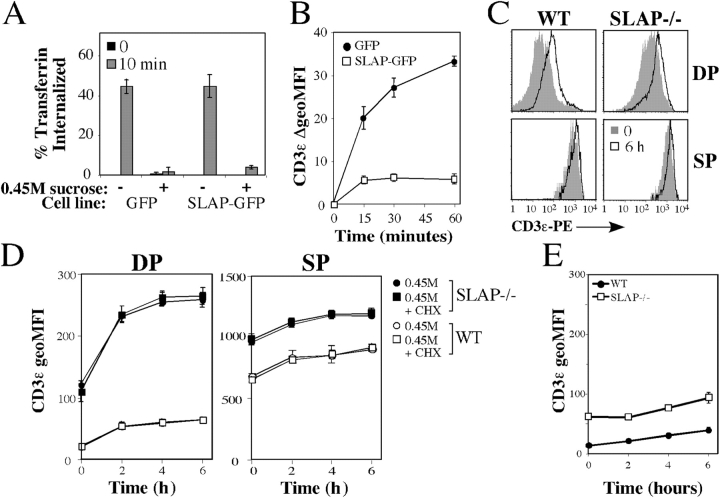
The failure of SLAP−/− thymocytes to degrade TCRζ may be a result of alterations in the dynamics of TCR–CD3 internalization and/or recycling. However, SLAP−/− DP thymocytes that were precoated with anti-CD3ɛ mAbs internalized surface-bound mAb with kinetics similar to WT thymocytes (Fig. 3 A). Next, we tested whether SLAP regulates recycling of the TCR–CD3 complex. Thymocytes were incubated in culture with phycoerythrin (PE)-labeled anti-CD3ɛ mAb for 30 min at 37°C to label an internalized pool of CD3ɛ. Cells were transferred to ice and washed, and surface-bound antibody was removed by using two sequential low pH washes. Thymocytes were then resuspended in cell culture medium and were incubated at 37°C for various time points. Anti-CD3ɛ mAb that recycled back to the cell surface during the course of the assay was removed by a second series of low pH washes. The amount of CD3ɛ–PE fluorescence that was lost as compared with staining of the intracellular pool at time 0 was used to calculate the percentage of CD3ɛ recycled at each time point. SLAP-deficient DP thymocytes recycled CD3ɛ more rapidly than DP thymocytes from WT mice (Fig. 3 B). Furthermore, SLAP-deficient DP thymocytes recycled more CD3ɛ over the course of the assay, suggesting that the size of the CD3ɛ recycling pool is increased in the absence of SLAP. Notably, the increase in CD3ɛ recycling was seen only in DP thymocytes, where SLAP is most highly expressed, because CD3ɛ recycling by SP thymocytes was similar regardless of genotype.
Figure 3.
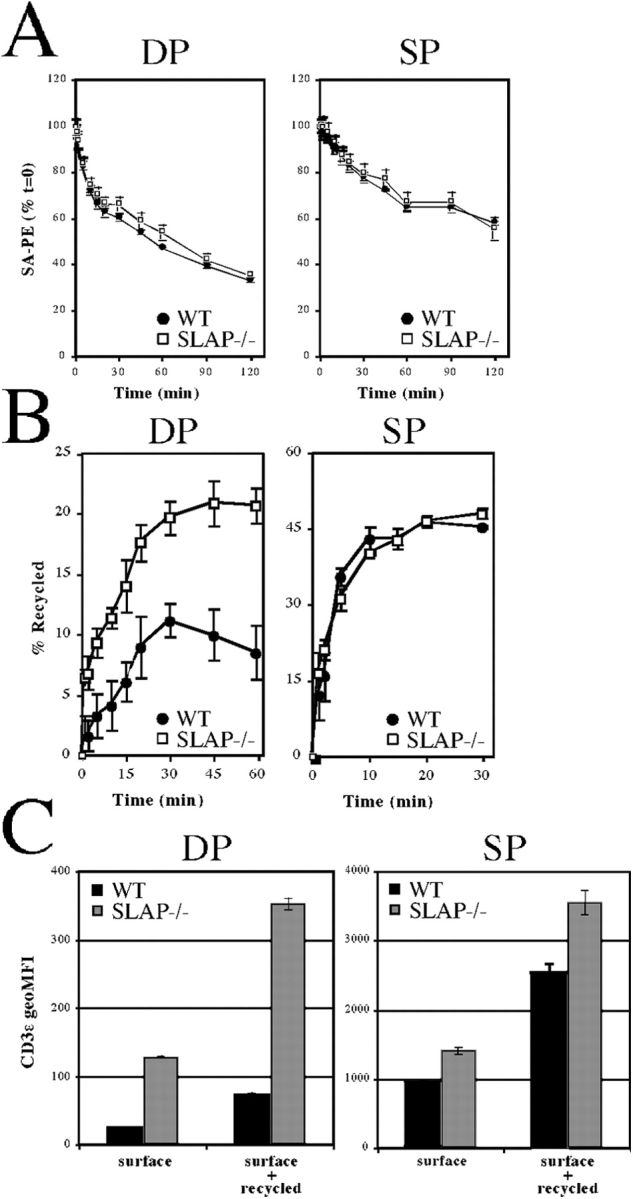
Increased TCR–CD3 recycling in SLAP−/−. (A) Ligand-induced internalization of CD3ɛ from WT or SLAP−/− thymocytes that were precoated with biotinylated anti-CD3ɛ mAb. CD3ɛ remaining on the cell surface was detected by staining with PE-streptavidin (SA-PE). (B) Recycling of previously internalized anti-CD3ɛ antibody by WT and SLAP−/− thymocytes. Data are presented as a percentage of total CD3ɛ internalized at t = 0. (C) Comparison of surface CD3ɛ with the total recycling pool of CD3ɛ. The surface and recycling pools were labeled by incubating thymocytes with a PE anti-CD3ɛ antibody for 60 min on ice or at 37°C, respectively. All data were determined by FACS and represent three mice per genotype ± SEM (error bars).
Next, we tested whether the size of the CD3ɛ recycling pool is altered in the absence of SLAP. We were unable to measure the size of the TCR–CD3 recycling pool by using Fab fragments, presumably as a result of the low level of CD3ɛ expression in DP thymocytes as compared with background staining and lower biotin labeling of the Fab fragments. Therefore, thymocytes were incubated with PE-labeled anti-CD3ɛ mAb either on ice to label only surface CD3ɛ or at 37°C for 60 min to label both surface CD3ɛ and CD3ɛ that recycled to the cell surface during the 60-min incubation. More recycling CD3ɛ was labeled in SLAP−/− DP thymocytes than in WT (Fig. 3 C), indicating that the number of mature TCR–CD3 complexes present in the recycling pool is increased in the absence of SLAP. In contrast, the size of the CD3ɛ recycling pool was more similar to SP thymocytes, suggesting that (consistent with its expression in DP thymocytes) SLAP regulates the size of the recycling pool specifically at the DP stage of thymocyte development.
Our lab has previously shown that SLAP-mediated TCR–CD3 down-regulation on DP thymocytes does not depend on a positively selecting MHC allele (Sosinowski et al., 2001). Therefore, we were interested in studying CD3ɛ recycling in the absence of any TCR ligation. We reasoned that if we could block internalization of the TCR–CD3 complex, any new TCR–CD3 appearing on the cell surface would be caused by newly synthesized or recycled TCR–CD3. To block TCR–CD3 internalization, we exploited the observation that TCR is internalized via clathrin-coated pits (Telerman et al., 1987). Hypertonic medium (e.g., 0.45 M sucrose) blocks clathrin-mediated endocytosis by inducing spontaneous clathrin lattice formation in the absence of cell membranes, thereby depleting the cell of clathrin monomers that would be used in vesicle formation (Daukas and Zigmond, 1985; Heuser and Anderson, 1989). Furthermore, hypertonic medium has been shown to inhibit internalization of the TCR–CD3 complex (Dallanegra et al., 1988).
To validate the use of hypertonic medium to inhibit TCR–CD3 internalization, we first analyzed the uptake of fluorescently labeled transferrin by the transferrin receptor, a process that requires clathrin-mediated endocytosis (Mellman, 1996; Schmid, 1997). Because DP thymocytes do not express the transferrin receptor, we first tested the effect of hypertonic medium on Jurkat T cell lines that stably expressed either SLAP-GFP fusion or GFP alone as a control. Transferrin uptake via the transferrin receptor was completely inhibited by hypertonic medium in both stable cell lines (Fig. 4 A), demonstrating that hypertonic medium blocks clathrin-mediated endocytosis regardless of whether SLAP is present or not. We analyzed the effect of SLAP on CD3ɛ expression while clathrin-mediated endocytosis was blocked in Jurkat T cells. Strikingly, we were able to detect up-regulation of CD3ɛ surface expression over the time course of the experiment in the control cell line. However, up-regulation of CD3ɛ by Jurkat T cells was markedly inhibited in the presence of SLAP (Fig. 4 B).
Figure 4.
Increased TCR–CD3 recycling is revealed in hypertonic medium. (A) Uptake of Alexa647-labeled transferrin by Jurkat T cell lines expressing SLAP-GFP or control (GFP) in the presence or absence of hypertonic medium, as assessed by FACS. (B) Expression of CD3ɛ on Jurkat T cell lines expressing SLAP-GFP or control (GFP) incubated in hypertonic medium, as assessed by FACS. Data are presented as the absolute increase in CD3ɛ MFI expression relative to time 0. Data in A and B are the mean of three experiments ± SEM. (C) CD3ɛ expression on WT or SLAP−/− thymocytes incubated in hypertonic medium for 0 or 6 h, as assessed by FACS. Data are representative of three mice per genotype. (D) MFI of CD3ɛ expression on WT or SLAP−/− thymocytes incubated in 0.45 M hypertonic medium for the indicated time in the presence or absence of cycloheximide (CHX). (E) MFI of CD3ɛ expression on WT or SLAP−/− DP thymocytes incubated for the indicated time in cell culture medium only. Data in D and E are the mean of three mice per genotype ± SEM (error bars).
To investigate whether SLAP expression affects up-regulation of CD3ɛ on DP thymocytes as it did in our model cell line, we incubated thymocyte single-cell suspensions in hypertonic medium and followed CD3ɛ expression over time. Both WT and SLAP−/− DP thymocytes up-regulate CD3ɛ on the cell surface over the course of the assay (Fig. 4 C). However, quantitation demonstrated that CD3ɛ up-regulation was substantially increased in SLAP−/− DP thymocytes as compared with WT controls (Fig. 4 D). Interestingly, the observed up-regulation of CD3ɛ in hypertonic medium is comparable with the estimated amount of CD3ɛ present in the recycling pool (Fig. 3 C), indicating that increases in the size of the TCR–CD3 recycling pool can be revealed upon incubation of thymocytes in hypertonic medium. Notably, the uptake of fluorescently labeled transferrin by double-negative thymocytes (which express low, but detectable, levels of the transferrin receptor) was inhibited in the presence of hypertonic medium (unpublished data), indicating that hypertonic medium inhibited clathrin-mediated endocytosis in our thymocyte cultures.
Previous studies have reported that prolonged culture of DP thymocytes in the absence of TCR–MHC interactions results in up-regulation of the TCR–CD3 complex by the stabilization of newly synthesized TCRα chains (Bonifacino et al., 1990; Kearse et al., 1995). Indeed, both WT and SLAP−/− DP thymocytes up-regulate CD3ɛ when cultured in the absence of 0.45 M sucrose (Fig. 4 E). However, over this time course, the increase in CD3ɛ expression was markedly lower than the increase observed in thymocytes that were incubated in hypertonic medium. In addition, most of the CD3ɛ up-regulation that was observed in hypertonic medium was detected after only 2 h in culture, a time at which little or no CD3ɛ up-regulation had yet occurred in the absence of 0.45 M sucrose. Furthermore, incubation of thymocytes in cycloheximide to inhibit protein synthesis had no effect on the up-regulation of CD3ɛ in hypertonic medium (Fig. 4 D). In contrast, in parallel experiments using thymocytes cultured in media without 0.45 M sucrose, up-regulation of CD3ɛ was sensitive to cycloheximide treatment (unpublished data), demonstrating that cycloheximide effectively inhibited protein synthesis in our cultures.
It is possible that incubation of thymocytes in hypertonic medium has an effect on cell viability. However, Annexin V staining failed to demonstrate a decrease in thymocyte viability for thymocytes incubated in hypertonic medium (unpublished data). In addition, because SLAP protein expression is predominantly restricted to DP thymocytes (Sosinowski et al., 2001), up-regulation of CD3ɛ in hypertonic medium should not be altered in SP thymocytes. Notably, CD3ɛ up-regulation by CD4+ SP thymocytes that were cultured in hypertonic medium was similar regardless of genotype (Fig. 4 D). These data demonstrate that up-regulation of TCR–CD3 expression in hypertonic medium can be used to analyze the pool of recycling TCR–CD3 complexes in the absence of receptor cross-linking.
The TCRζ chain is the target of SLAP
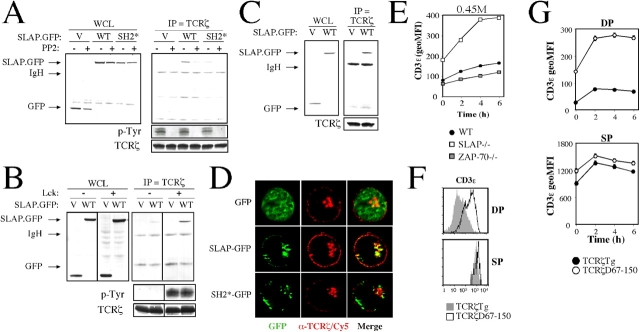
We have demonstrated that expression of TCRζ is increased in SLAP−/− thymocytes as a result of the impaired degradation of TCRζ. A SLAP-GST fusion has previously been shown to interact with several phosphoproteins, including the TCRζ chain (Tang et al., 1999; Sosinowski et al., 2000). Therefore, we postulated that SLAP could bind to TCRζ, leading to its subsequent degradation. To study the interaction between SLAP and TCRζ, we first examined Jurkat T cells that had been transiently transfected with SLAP-GFP to determine whether SLAP can interact with the endogenous TCRζ chain. SLAP-GFP, but not the GFP control, coimmunoprecipitated with TCRζ (Fig. 5 A). Furthermore, SLAP-GFP containing a point mutation in the SH2 domain coimmunoprecipitated only weakly with TCRζ. The cytoplasmic domain of TCRζ is phosphorylated by Lck (Iwashima et al., 1994; van Oers et al., 1996). Therefore, the inhibition of Src family kinase catalytic activity with PP2 caused a loss of basal TCRζ phosphorylation in Jurkat T cells (Fig. 5 A). Interestingly, PP2 treatment also caused a corresponding loss in SLAP-GFP coimmunoprecipitation with TCRζ. The dependence on Src family kinase activity prompted us to study whether SLAP can interact with TCRζ in the Lck-deficient Jurkat T cell line JCaM1. Transiently transfected SLAP-GFP failed to coimmunoprecipitate with TCRζ in JCaM1; however, stable reconstitution of Lck back into the JCaM1 cell line resulted in the restoration of basal phospho-TCRζ as well as the recovery of SLAP-GFP coimmunoprecipitation with TCRζ (Fig. 5 B).
Figure 5.
SLAP interacts with phospho-TCRζ via the SLAP SH2 domain. (A) Immunoprecipitates of endogenous TCRζ from Jurkat T cells transiently transfected with GFP (V), SLAP-GFP (WT), or SH2-GFP (SH2*) as assessed by Western blot analysis. Half of each transfection was pretreated with the Src family kinase inhibitor PP2 before immunoprecipitation (IP). WCL, whole cell lysate. (B) Immunoprecipitates of TCRζ from JCaM1 (Lck−/−) or JCaM1 stably reconstituted with Lck, as assessed by Western blot analysis. Cell lines were transiently transfected with either GFP or WT-GFP. Dividing lines have been placed to indicate where irrelevant lanes were deleted from the figure. Phospho-TCRζ observed in A and B reflects basal TCRζ phosphorylation that is present in unstimulated cells. (C) Immunoprecipitates of TCRζ from the ZAP-70−/− Jurkat T cell line P116 transfected with GFP or SLAP-GFP, as assessed by Western blot analysis. (D) Localization of GFP fluorescence (green) and endogenous TCRζ (red) in Jurkat T cells that were transiently transfected with GFP, SLAP-GFP, or SH2-GFP as analyzed by deconvolution microscopy. Data are representative of ≥10 cells analyzed per transfection for three independent experiments. (E) MFI of CD3ɛ expression on ZAP-70−/− DP thymocytes incubated in hypertonic medium. Data are the mean of three mice of each genotype ± SEM. Thymocytes from one WT and one SLAP−/− mouse were used for comparison. (F) FACS analysis of CD3ɛ expression on DP and SP thymocytes in the presence (TCRζ Tg) or absence (TCRζ D67–150) of most of the TCRζ cytoplasmic domain. Histograms are representative of three mice per genotype. (G) MFI of CD3ɛ expression on DP and SP thymocytes expressing full-length (TCRζ Tg) or a cytoplasmic truncation (TCRζ D67–150) of the TCRζ cytoplasmic domain that was incubated in hypertonic medium (as assessed by FACS). Data are the mean of three mice per genotype ± SEM.
In addition to TCRζ, SLAP-GST has also been shown to interact with phosphorylated ZAP-70 (Tang et al., 1999; Sosinowski et al., 2000). ZAP-70 is also phosphorylated by Lck and can bind to the phosphorylated TCRζ cytoplasmic domain (Iwashima et al., 1994; van Oers et al., 1996). Therefore, SLAP-GFP may indirectly interact with TCRζ via ZAP-70. However, transiently transfected SLAP-GFP also coimmunoprecipitated with TCRζ in the ZAP-70–deficient Jurkat T cell line P116 (Fig. 5 C), indicating that the interaction between phosphorylated TCRζ and SLAP-GFP does not require ZAP-70 and may instead be a direct interaction. Furthermore, neither surface TCR–CD3 expression (Negishi et al., 1995; Kadlecek et al., 1998) nor the up-regulation of CD3ɛ by DP thymocytes that were incubated in hypertonic medium (Fig. 5 E) were increased in the absence of ZAP-70, suggesting that ZAP-70 is not required for SLAP-mediated down-regulation of the TCR–CD3 complex in vivo.
It has previously been shown that upon transient transfection, SLAP localizes to an intracellular compartment and displays partial colocalization with late endosomes (Sosinowski et al., 2000). Therefore, we predicted that SLAP would colocalize with TCRζ in an intracellular compartment. Extensive colocalization of SLAP-GFP with endogenous TCRζ was observed after transient transfection into Jurkat T cells (Fig. 5 D). Colocalization occurred primarily in an intracellular compartment, as very little SLAP-GFP was detected at the plasma membrane. Interestingly, colocalization requires the SH2 domain of SLAP, as an SH2 point mutation of SLAP-GFP displayed very little colocalization with the TCRζ chain. These data suggest that the SH2 domain of SLAP is required for SLAP to interact with phosphorylated TCRζ, thus targeting TCRζ for degradation.
To investigate the requirement of TCRζ cytoplasmic domains for the effects of SLAP on TCR expression in vivo, we obtained TCRζ-deficient mice that were reconstituted with transgenes encoding either full-length TCRζ (TCRζ Tg) or a cytoplasmic truncation of TCRζ (TCRζ D67–150; Shores et al., 1994). TCRζ D67–150 is a deletion of amino acid residues 67–150 of TCRζ, resulting in the loss of five out of six tyrosines that are normally present in the TCRζ cytoplasmic domain. Interestingly, relative to mice expressing TCRζ Tg, mice expressing TCRζ D67–150 displayed increased surface levels of TCRβ and CD3ɛ on DP, but not SP, thymocytes (Fig. 5 F and not depicted). Furthermore, mice expressing the truncated form of TCRζ displayed increased CD3ɛ up-regulation in hypertonic medium on DP, but not SP, thymocytes (Fig. 5 G), suggesting that the TCR–CD3 recycling pool is increased in the absence of the TCRζ cytoplasmic domain. The observed increase was not caused by new synthesis of the complex, as cycloheximide had no effect on TCR–CD3 recycling in hypertonic medium (unpublished data). Additionally, no significant increase in CD3ɛ expression was observed in thymocytes that were incubated in the absence of 0.45 M sucrose (unpublished data). These data indicate that the cytoplasmic domain of TCRζ is required to prevent the accumulation of fully assembled TCR–CD3 complexes in the recycling pool of DP thymocytes.
Discussion
We have studied the mechanism by which TCR–CD3 expression is increased in the absence of SLAP. Our data demonstrate that SLAP−/− thymocytes have increased TCRζ expression as a result of a defect in TCRζ degradation. Failure to degrade TCRζ leads to an accumulation of fully assembled TCR–CD3 complexes that continue to recycle back to the plasma membrane instead of being retained and/or degraded. In addition, we have shown that Lck, but not ZAP-70 activity, is required for SLAP to interact with the phosphorylated form of TCRζ. It has previously been shown that TCRζ is basally phosphorylated in DP thymocytes (Nakayama et al., 1989; van Oers et al., 1994) and that TCRζ phosphorylation is almost undetectable in Lck−/− thymocytes (van Oers et al., 1996). Interestingly, surface TCR–CD3 expression is also up-regulated in Lck−/− DP thymocytes (Molina et al., 1992), suggesting that the inability of SLAP to bind TCRζ may contribute to the increase in TCR–CD3 expression that was observed on Lck−/− DP thymocytes. Thus, SLAP regulates the expression of TCR on DP thymocytes by targeting tyrosine-phosphorylated TCRζ for degradation in an Lck-dependent manner.
In addition to Lck, our studies suggest that regulation of TCR–CD3 expression by SLAP requires the cytoplasmic domain of TCRζ. TCRζ-deficient mice that are transgenic for a truncated form of TCRζ (TCRζ D67–150) express increased levels of surface TCR–CD3 as well as an increased pool of recycling CD3ɛ on DP, but not SP, thymocytes. It is possible that in the absence of the majority of TCRζ cytoplasmic domains, trafficking of the TCR–CD3 complex is altered such that internalization and/or recycling of the complex leads to the phenotype that we have observed in TCRζ D67–150 DP thymocytes via a SLAP-independent mechanism. Notably, the TCRζ cytoplasmic domain contains multiple tyrosine-based motifs that could mediate internalization of the TCR–CD3 complex. However, a recent study demonstrated that the mutation of all six tyrosines present in the TCRζ cytoplasmic domain had no effect on the internalization of the TCR–CD3 complex (Szymczak and Vignali, 2005). An additional study has indicated that a similar deletion of the TCRζ cytoplasmic domain increases the rate of TCR–CD3 internalization (D'Oro et al., 2002). This later study suggests that, if anything, TCR–CD3 expression should be decreased in the absence of the TCRζ cytoplasmic domain. Nonetheless, we cannot exclude the possibility that altered trafficking of the TCR–CD3 complex either contributes to or is responsible for the increased surface TCR–CD3 expression and increased pool of recycling CD3ɛ in TCRζ D67–150 DP thymocytes. However, our findings are also consistent with a role for the phosphorylated TCRζ cytoplasmic domain interacting with SLAP, thereby leading to its accelerated degradation.
Previous data indicate that unassembled TCRζ chains are rapidly degraded in the ER of DP thymocytes (Bonifacino et al., 1990) and that newly synthesized TCRζ chains are stabilized by their incorporation into fully assembled TCR–CD3 complexes (Kearse et al., 1995). This raised the possibility that SLAP could function to prevent assembly of the TCR–CD3 complex. Currently, it is believed that the rate of TCR–CD3 assembly is low in DP thymocytes as a result of the instability of the TCRα chain. However, because we failed to detect a difference in expression, synthesis, or degradation of the TCRα chain in the absence of SLAP, we must conclude that SLAP does not regulate assembly of the TCR–CD3 complex by the mechanism previously described. In addition, our data clearly demonstrate that SLAP interacts with the phosphorylated TCRζ chain, which has been shown to be present only in fully assembled, mature TCR–CD3 complexes (Kearse et al., 1993). Therefore, it is unlikely that SLAP plays a direct role in the assembly of TCR–CD3. We cannot exclude the possibility that SLAP somehow regulates TCR–CD3 assembly through an indirect mechanism that has yet to be described. However, our data suggests that a substantial proportion of TCRζ is present mainly in fully assembled TCR–CD3 complexes, as evidenced by the large increase in TCRζ chain protein expression in WT thymocytes as compared with TCRα−/− thymocytes (Fig. 1 A). Together, these data strongly indicate that the degradation of TCRζ observed in WT DP thymocytes is predominantly caused by the degradation of TCRζ that was derived from fully assembled TCR–CD3 complexes.
The TCR has been shown to undergo constitutive internalization in cell lines and T cells, with an estimate of 0.6–1.2% of TCRs internalized per minute (Liu et al., 2000; Menne et al., 2002). Therefore, subtle modifications to the rate of recycling or internalization could have large effects on the steady-state level of TCR expression on the cell surface. We consistently observed an increase in both the rate as well as the absolute amount of CD3ɛ recycled by SLAP−/− DP thymocytes. Notably, the increased amount of CD3ɛ recycled (5–9% as calculated from Fig. 3 B) is consistent with the 5–7.5% of TCRζ chains that are estimated to be phosphorylated in the thymus (van Oers, N., personal communication). Furthermore, the small difference in CD3ɛ recycling can account for the loss of TCRζ that is observed over time in WT but not for SLAP−/− thymocytes when incubated in cycloheximide. Therefore, SLAP appears to target only a small proportion of the constitutively recycling TCR–CD3 complexes that contain phospho-TCRζ. However, this has a substantial effect on the steady-state level of TCR–CD3 expression in SLAP-deficient thymocytes.
In contrast to TCRζ, we were unable to detect a substantial increase in the level of TCRα, TCRβ, or CD3ɛ in the absence of SLAP. Likewise, degradation of TCRα, TCRβ, and CD3ɛ was not noticeably altered in the absence of SLAP. One possible explanation for these observations is that DP thymocytes typically express a relatively large intracellular pool of unassembled and/or partially assembled TCR–CD3 chains, some of which are rapidly degraded (Chen et al., 1988; Lippincott-Schwartz et al., 1988; Bonifacino et al., 1989, 1990). Therefore, the large pools of unassembled TCR–CD3 chains could mask any differences in TCR–CD3 expression or degradation. Alternatively, our results could also be interpreted to indicate that TCRζ separates from the rest of the mature TCR–CD3 complex and is independently degraded via a SLAP-dependent mechanism. Separation of the TCR–CD3 complex has been previously described (Kishimoto et al., 1995; Ono et al., 1995; Thien et al., 2003; La Gruta et al., 2004). Additional studies will be required to conclusively determine whether the remainder of the recycling TCR–CD3 complex is also degraded via a SLAP-dependent mechanism or is retained in an intracellular compartment.
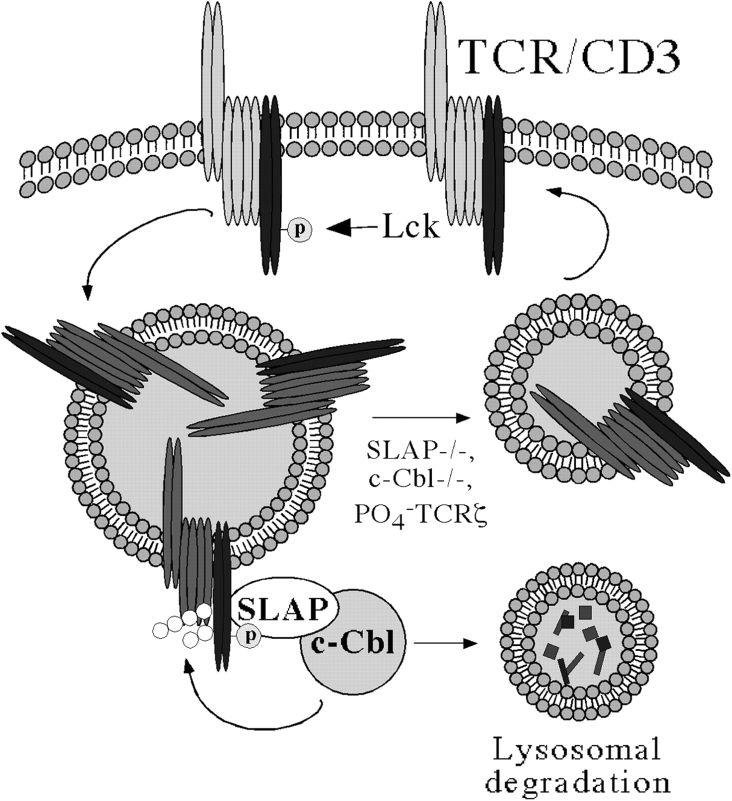
The mechanism by which SLAP targets TCRζ for degradation has yet to be elucidated. Degradation of TCRζ is likely to involve the E3 ubiquitin ligase c-Cbl (Fig. 6). SLAP has been previously reported to interact with the NH2 terminus of c-Cbl (Tang et al., 1999). In addition, c-Cbl–deficient mice have a very similar phenotype to SLAP-deficient mice with regard to TCR–CD3, CD4, and CD5 up-regulation on DP thymocytes and increases in positive selection (Naramura et al., 1998). Finally, TCRζ has been previously shown to be ubiquitinated (Cenciarelli et al., 1992, 1996; Hou et al., 1994), perhaps via c-Cbl (Wang et al., 2001). Therefore, we suspect that the ubiquitin ligase activity of c-Cbl is required to target TCRζ for degradation and subsequently prevent the accumulation of recycling TCR–CD3 complexes. Indeed, our unpublished results indicate that c-Cbl and SLAP function in the same pathway to regulate TCR–CD3 expression on DP thymocytes by targeting the TCRζ chain for degradation (unpublished data). Therefore, we suggest the model shown in Fig. 6. Surface TCR–CD3 complexes in DP thymocytes are constitutively phosphorylated on TCRζ chains by Lck. The TCR–CD3 complex is internalized and transported to an intracellular compartment where SLAP binds to phosphorylated TCRζ, thus targeting TCRζ for ubiquitination and degradation via a c-Cbl–dependent mechanism. In the absence of TCRζ, the remainder of the TCR–CD3 complex is either degraded or retained in an intracellular compartment. Conversely, in the absence of SLAP, the TCRζ is neither ubiquitinated nor degraded, and the TCR–CD3 complex remains intact and continues to recycle back to the plasma membrane.
Figure 6.
TCR–CD3 complexes are endocytosed via clathrin-coated pits and interact with SLAP in an intracellular compartment. TCRζ is ubiquitinated by c-Cbl and degraded. In the absence of TCRζ chains, the remainder of the TCR–CD3 complex is degraded. However, if SLAP fails to interact with TCRζ, the TCR–CD3 complex remains intact and continues to recycle back to the cell surface. p, phosphorylated TCRζ chains.
We have identified a novel mechanism by which TCR–CD3 expression is regulated in a developmentally restricted manner. Together with previous studies (Bonifacino et al., 1989, 1990; Kearse et al., 1995) that have implicated regulation of the TCR–CD3 complex at the level of ER assembly is the current mechanism of SLAP-mediated degradation of phospho-TCRζ–containing complexes. It is interesting to speculate why DP thymocytes possess multiple mechanisms to regulate surface TCR–CD3 expression. We suspect that receptor levels are kept low on DP thymocytes to ensure the selection of developing thymocytes that express TCRs with the appropriate avidity for self-peptide–MHC complexes. As a result of the massive amplification of the TCR signaling cascade, it may be difficult for a thymocyte to differentiate between strong and weak TCR signals if TCR levels are high. Therefore, quantitative differences in signaling may be more easily distinguished if levels of surface TCR–CD3 are kept low. Likewise, the increase in receptor levels after positive selection may be desirable for a heightened sensitivity of thymocytes to self-peptides that lead to negative selection. Such increases in receptor levels could be achieved both by down-regulating SLAP expression as well as by promoting TCR assembly. Thus, SLAP may play a key role in regulating TCR–CD3 levels during thymocyte development to optimize receptor levels for both positive and negative selection.
Materials and methods
Cell lines and plasmids
Jurkat T cells, JCaM1, JCaM1 + Lck, and P116 cell lines were maintained in RPMI containing 5% FBS, 2 mM glutamine, penicillin, and streptomycin. JCaM1 is an Lck−/− Jurkat T cell line (Goldsmith and Weiss, 1987), and JCaM1 + Lck has been stably reconstituted with Lck (Straus and Weiss, 1992). P116 is a ZAP-70−/− Jurkat T cell line (Williams et al., 1998). Plasmids encoding GFP, SLAP-GFP, and SH2-GFP were initially constructed by PCR amplification of WT or SH2 mutated murine SLAP from pEF-BOS (Sosinowski et al., 2000) and ligation into pN1-GFP (CLONTECH Laboratories, Inc.) via EcoRI and BamHI sites. GFP constructs were subcloned into pCDEF3 by using EcoRI and NotI sites.
Mice
SLAP−/− mice have been described previously (Sosinowski et al., 2001) and have been backcrossed to a total of eight generations onto a C57BL/6 background. C57BL/6 mice (Taconic) were used as WT controls. ZAP-70−/− mice have been previously described (Kadlecek et al., 1998). TCRα−/− mice were purchased from the Jackson Laboratory. TCRζ Tg and TCRζ D67–250 were provided by E. Shores (Food and Drug Administration, Bethesda, MD) and have been previously described (Shores et al., 1994).
FACS staining
After washing in PBS, thymocytes were stained with CD4 (RM4-5; eBioscience), CD8α (53-6.7; BD Biosciences), CD3ɛ (145-2C11; eBioscience), and TCRα (H57-197; eBioscience) in FACS buffer (PBS with 1% BSA and 0.01% NaN3) for 30 min at 4°C. For intracellular FACS staining, thymocytes were washed in PBS before fixation in 4% PFA for 20 min at RT. After washing, thymocytes were permeabilized in 0.5% saponin in FACS buffer for 20 min at RT. Thymocytes were stained with the antibodies listed above in 0.5% saponin containing 1% goat serum for 30 min at 4°C. Jurkat T cells were stained with α-CD3ɛ (UCHT1; BD Biosciences) in FACS buffer for 30 min at 4°C. All FACS staining was performed in duplicate.
Internalization and degradation assays
For ligand-induced CD3ɛ internalization, surface CD3ɛ was labeled with biotinylated anti-CD3ɛ antibodies (145-2C11) for 30 min on ice. Thymocytes were washed, resuspended at 5 × 106 ml in primary cell culture medium (RPMI containing 10% FBS, 2 mM glutamine, 50 μM β-mercaptoethanol, penicillin, and streptomycin), and incubated at 37°C for the indicated time points. Anti-CD3ɛ remaining on the cell surface was detected by staining with PE-conjugated streptavidin (Caltag Laboratories). For degradation assays, thymocytes were cultured at 5 × 106 ml in primary cell culture medium containing 100 μg/ml cycloheximide. At each time point, cells were mixed with 100 μl ice-cold PBS containing 1% BSA and 0.1% NaN3. Cells were maintained on ice for the remainder of the assay until FACS staining.
CD3ɛ recycling
20 × 106 ml of freshly isolated thymocyte single-cell suspensions were cultured at 37°C in primary cell culture media containing 5 μg/ml PE-labeled anti-CD3ɛ (145-2C11). After 30 min in culture, thymocytes were washed twice with PBS. Surface-bound antibody was removed by washing thymocytes twice in ice-cold PBS containing 1% BSA, pH 1.5, followed by immediate neutralization in FACS buffer. These conditions removed >97% of cell surface staining. 5 × 106 ml of thymocytes were incubated at 37°C in primary cell culture for the indicated time points. At each time point, cells were mixed with 100 μl of ice-cold PBS containing 1% BSA and 0.1% NaN3 and were maintained on ice for the remainder of the assay. After all time points had been collected, thymocytes were again washed twice in ice-cold PBS containing 1% BSA, pH 1.5, followed by immediate neutralization in FACS buffer to remove anti-CD3ɛ antibody that was bound to recycled CD3ɛ. Thymocytes were stained for CD4 and 8 and were analyzed by FACS. Recycled CD3ɛ was calculated using the following formula:
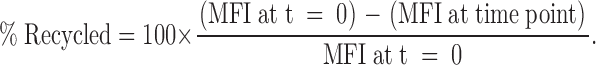 |
Labeling of recycling pool
Freshly isolated thymocyte single-cell suspensions were cultured at 20 × 106 ml in primary cell culture media containing 100 μg/ml cycloheximide and 5 μg/ml PE-labeled anti-CD3ɛ (145-2C11) for 30 min on ice to fully label surface CD3ɛ. This was followed by a subsequent 60-min incubation either on ice or at 37°C to achieve steady-state labeling of the recycling pool. Cells were washed, stained for CD4 and 8 expression, and analyzed by FACS.
Hypertonic recycling assay
106 ml Jurkat T cells (in RPMI) or 5 × 106 ml of freshly isolated thymocyte single-cell suspensions (in primary cell culture media) were incubated with 0.45 M sucrose. At each time point, cells were mixed with 100 μl of ice-cold PBS containing 1% BSA and 0.1% NaN3. Cells were maintained on ice for the remainder of the assay until FACS staining.
Inhibition of clathrin-mediated endocytosis was measured by the inhibition of transferrin uptake. Jurkat T cells were incubated on ice with Alexa647-labeled transferrin (Molecular Probes) for 20 min to allow for transferrin binding. Excess transferrin was washed off with three washes in ice-cold PBS. Cells were resuspended in RPMI ± 0.45 M sucrose and were incubated either on ice or at 37°C for 10 min. After incubation, cells were transferred into ice-cold PBS containing 1% BSA and 0.1% NaN3 and rested for 1 h on ice. Surface-bound transferrin was competed off by using a 50-fold excess of unlabeled transferrin (Sigma-Aldrich) in FACS buffer for 30 min at RT. Internalized transferrin was calculated by using the following formula:
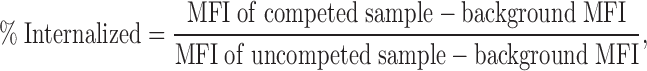 |
where background represents the remaining fluorescence that was present on competed samples incubated on ice.
Western blotting and immunoprecipitations
For total levels of TCRα, CD3ɛ, and TCRζ, CD8+ thymocytes were purified by using magnetic cell sorting (Miltenyi Biotec) according to the manufacturer's protocol. Recovered cells were ≥95% DP, as assessed by FACS. CD8+ thymocytes were lysed at 200 × 106 ml in radioimmunoprecipitation (RIPA) lysis buffer supplemented with protease inhibitors (leupeptin, aprotinin, PMSF, and pepstatin A) for 30 min on ice. Postnuclear supernatants were prepared by centrifuging samples at 16,000 g (4°C) for 30 min. For TCRα, postnuclear supernatants from 50 × 106 cell equivalents were immunoprecipitated with α-TCRα (H-142; Santa Cruz Biotechnology, Inc.) and protein G (GE Healthcare) for 1 h at 4°C. Samples were washed four times with lysis buffer, resuspended in SDS loading buffer, and boiled for 5 min. For CD3ɛ and TCRζ, postnuclear supernatants from 5 × 106 cell equivalents were mixed with SDS loading buffer and were boiled. Samples were electrophoresed in a 12.5% SDS-PAGE gel, transferred to Immobilon, and blotted for α-tubulin (B-5-1-2; Sigma-Aldrich), TCRα (H-142), CD3ɛ (M20; Santa Cruz Biotechnology, Inc.), or TCRζ (8D3; BD Biosciences). Subsequently, membranes were incubated in secondary antibodies coupled to HRP (GE Healthcare) and were detected by using enhanced chemiluminescence (GE Healthcare). For TCR–CD3 degradation experiments, CD8+ thymocytes were cultured in primary cell culture media containing 100 μg/ml cycloheximide. At each time point, thymocytes were lysed in RIPA lysis buffer and were maintained on ice for the remainder of the experiment. Lysates were blotted for α-tubulin, TCRα, CD3ɛ, or TCRζ as described above. Western blots were quantitated on an Image Station (Eastman Kodak Co.) using 1D Image Analysis software version 3.5 (Eastman Kodak Co.).
For SLAP/TCRζ coimmunoprecipitations, cells were transiently transfected with GFP, SLAP-GFP, or SH2-GFP overnight. Cells were washed twice in PBS and were lysed at 50 × 106 ml in RIPA lysis buffer as described above (but supplemented with NaVO4). Postnuclear supernatants from 60 × 106 cells were immunoprecipitated with TCRζ (6B10.2; Santa Cruz Biotechnology, Inc.) and protein G as described above. Samples were electrophoresed in a 12.5% SDS-PAGE gel, transferred to Immobilon, and blotted for GFP (JL8; CLONTECH Laboratories, Inc.) or phosphotyrosine (4G10; Upstate Biotechnology) as described for Western blotting. Subsequently, membranes were stripped (30 min at 55°C in 5% SDS, 100 mM β-mercaptoethanol, and 62.5 mM Tris, pH 6.8) and blotted for TCRζ (8D3; BD Biosciences). In some experiments, transfected cells were incubated overnight with 20 μM PP2 (Calbiochem) to inhibit Src family kinase activity.
Metabolic labeling
80 × 106 freshly isolated thymocytes were cultured for 30 min in 4 ml cysteine and methionine-free media (Biofluids) at 37°C. Thymocytes were labeled with 2 mCi of Tran35SLabel (ICN Biomedicals) for 30 min at 37°C. Cells were washed, lysed in RIPA lysis buffer as described above, and precleared for 30 min at 4°C with protein G. Lysates were immunoprecipitated for TCRα (H-142), CD3ɛ (M-20), or TCRζ (6B10.2), run on an SDS-PAGE gel, and transferred to Immobilon as described above. Half of each immunoprecipitate was used to detect the Tran35SLabel. The other half of the immunoprecipitate was Western blotted (as described above) as a loading control.
Immunofluorescence
Jurkat T cells were transfected with GFP, SLAP-GFP, or SH2-GFP overnight. Transfected cells were washed and allowed to settle onto poly-l-lysine–coated plates. Samples were fixed in 4% PFA (20 min at RT) and were permeabilized with 0.1% Triton X-100 (10 min at RT). Samples were incubated in blocking buffer (PBS with 0.5% BSA, 0.5% milk, and 1% goat serum) for 10 min at RT and were incubated in primary antibody (6B10.2, 1:50) in blocking buffer for 2 h at 37°C. After washing, samples were incubated in secondary antibody (Cy5 goat anti–mouse IgG; Jackson ImmunoResearch Laboratories) in blocking buffer for 20 min at RT. Samples were washed, coverslipped in gel/mount (Biomeda), and visualized on a turnkey inverted digital microscopy system (Marianas; Intelligent Imaging) that was built around an inverted microscope (Axiovert 200M; Carl Zeiss MicroImaging, Inc.) using a plan-Neofluar 40× oil immersion objective (NA 1.3; Carl Zeiss MicroImaging, Inc.). Images were collected at RT with a CCD SensiCam (PCD; Cooke Corp.) using SlideBook software (Intelligent Imaging), were deconvolved using a constrained iterative algorithm (SlideBook), and were exported as TIFF files.
Acknowledgments
We would like to thank Tomasz Sosinowski for advice and generation of SLAP-deficient mice, Elizabeth Shores for providing TCRζ-transgenic mice, Dario Vignali and Jeroen Roose for critical reading of the manuscript, and Frances Brodsky, Mark von Zastrow, and members of the Weiss lab for advice and suggestions.
Abbreviations used in this paper: DP, double-positive; MFI, mean fluorescence intensity; MHC, major histocompatibility complex; PE, phycoerythrin; RIPA, radioimmunoprecipitation; SH, Src homology; SLAP, Src-like adaptor protein; SP, single-positive; TCR, T cell receptor; WT, wild-type.
References
- Bonifacino, J.S., C.K. Suzuki, J. Lippincott-Schwartz, A.M. Weissman, and R.D. Klausner. 1989. Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. J. Cell Biol. 109:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., S.A. McCarthy, J.E. Maguire, T. Nakayama, D.S. Singer, R.D. Klausner, and A. Singer. 1990. Novel post-translational regulation of TCR expression in CD4+CD8+ thymocytes influenced by CD4. Nature. 344:247–251. [DOI] [PubMed] [Google Scholar]
- Cenciarelli, C., D. Hou, K.C. Hsu, B.L. Rellahan, D.L. Wiest, H.T. Smith, V.A. Fried, and A.M. Weissman. 1992. Activation-induced ubiquitination of the T cell antigen receptor. Science. 257:795–797. [DOI] [PubMed] [Google Scholar]
- Cenciarelli, C., K.G. Wilhelm Jr., A. Guo, and A.M. Weissman. 1996. T cell antigen receptor ubiquitination is a consequence of receptor-mediated tyrosine kinase activation. J. Biol. Chem. 271:8709–8713. [DOI] [PubMed] [Google Scholar]
- Chen, C., J.S. Bonifacino, L.C. Yuan, and R.D. Klausner. 1988. Selective degradation of T cell antigen receptor chains retained in a pre-Golgi compartment. J. Cell Biol. 107:2149–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Oro, U., I. Munitic, G. Chacko, T. Karpova, J. McNally, and J.D. Ashwell. 2002. Regulation of constitutive TCR internalization by the zeta-chain. J. Immunol. 169:6269–6278. [DOI] [PubMed] [Google Scholar]
- Dallanegra, A., L. Schaffar, J.P. Breittmayer, J.L. Carpentier, and M. Fehlmann. 1988. Effect of hypertonicity and monensin on CD3/TCR surface expression in human T cells. Immunol. Lett. 19:115–120. [DOI] [PubMed] [Google Scholar]
- Daukas, G., and S.H. Zigmond. 1985. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J. Cell Biol. 101:1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson, P.O., and H.S. Teh. 1995. The protein tyrosine kinase p56lck regulates TCR expression and T cell selection. Int. Immunol. 7:617–624. [DOI] [PubMed] [Google Scholar]
- Exley, M., C. Terhorst, and T. Wileman. 1991. Structure, assembly and intracellular transport of the T cell receptor for antigen. Semin. Immunol. 3:283–297. [PubMed] [Google Scholar]
- Finkel, T.H., M. McDuffie, J.W. Kappler, P. Marrack, and J.C. Cambier. 1987. Both immature and mature T cells mobilize Ca2+ in response to antigen receptor crosslinking. Nature. 330:179–181. [DOI] [PubMed] [Google Scholar]
- Goldsmith, M.A., and A. Weiss. 1987. Isolation and characterization of a T-lymphocyte somatic mutant with altered signal transduction by the antigen receptor. Proc. Natl. Acad. Sci. USA. 84:6879–6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran, W.L., M. Poenie, J. Kimura, R. Tsien, A. Weiss, and J.P. Allison. 1987. Expression and function of the CD3-antigen receptor on murine CD4+8+ thymocytes. Nature. 330:170–173. [DOI] [PubMed] [Google Scholar]
- Heuser, J.E., and R.G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, D., C. Cenciarelli, J.P. Jensen, H.B. Nguygen, and A.M. Weissman. 1994. Activation-dependent ubiquitination of a T cell antigen receptor subunit on multiple intracellular lysines. J. Biol. Chem. 269:14244–14247. [PubMed] [Google Scholar]
- Irving, B.A., F.W. Alt, and N. Killeen. 1998. Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science. 280:905–908. [DOI] [PubMed] [Google Scholar]
- Iwashima, M., B.A. Irving, N.S. van Oers, A.C. Chan, and A. Weiss. 1994. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 263:1136–1139. [DOI] [PubMed] [Google Scholar]
- Kadlecek, T.A., N.S. van Oers, L. Lefrancois, S. Olson, D. Finlay, D.H. Chu, K. Connolly, N. Killeen, and A. Weiss. 1998. Differential requirements for ZAP-70 in TCR signaling and T cell development. J. Immunol. 161:4688–4694. [PubMed] [Google Scholar]
- Kearse, K.P., D.L. Wiest, and A. Singer. 1993. Subcellular localization of T-cell receptor complexes containing tyrosine-phosphorylated zeta proteins in immature CD4+CD8+ thymocytes. Proc. Natl. Acad. Sci. USA. 90:2438–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse, K.P., Y. Takahama, J.A. Punt, S.O. Sharrow, and A. Singer. 1995. Early molecular events induced by T cell receptor (TCR) signaling in immature CD4+ CD8+ thymocytes: increased synthesis of TCR-α protein is an early response to TCR signaling that compensates for TCR-α instability, improves TCR assembly, and parallels other indicators of positive selection. J. Exp. Med. 181:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto, H., R.T. Kubo, H. Yorifuji, T. Nakayama, Y. Asano, and T. Tada. 1995. Physical dissociation of the TCR-CD3 complex accompanies receptor ligation. J. Exp. Med. 182:1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gruta, N.L., H. Liu, S. Dilioglou, M. Rhodes, D.L. Wiest, and D.A. Vignali. 2004. Architectural changes in the TCR:CD3 complex induced by MHC:peptide ligation. J. Immunol. 172:3662–3669. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., J.S. Bonifacino, L.C. Yuan, and R.D. Klausner. 1988. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 54:209–220. [DOI] [PubMed] [Google Scholar]
- Liu, H., M. Rhodes, D.L. Wiest, and D.A. Vignali. 2000. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 13:665–675. [DOI] [PubMed] [Google Scholar]
- Love, P.E., and A.C. Chan. 2003. Regulation of thymocyte development: only the meek survive. Curr. Opin. Immunol. 15:199–203. [DOI] [PubMed] [Google Scholar]
- Manes, G., P. Bello, and S. Roche. 2000. Slap negatively regulates Src mitogenic function but does not revert Src-induced cell morphology changes. Mol. Cell. Biol. 20:3396–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575–625. [DOI] [PubMed] [Google Scholar]
- Menne, C., T. Moller Sorensen, V. Siersma, M. von Essen, N. Odum, and C. Geisler. 2002. Endo- and exocytic rate constants for spontaneous and protein kinase C-activated T cell receptor cycling. Eur. J. Immunol. 32:616–626. [DOI] [PubMed] [Google Scholar]
- Molina, T.J., K. Kishihara, D.P. Siderovski, W. van Ewijk, A. Narendran, E. Timms, A. Wakeham, C.J. Paige, K.U. Hartmann, A. Veillette, et al. 1992. Profound block in thymocyte development in mice lacking p56lck. Nature. 357:161–164. [DOI] [PubMed] [Google Scholar]
- Nakayama, T., A. Singer, E.D. Hsi, and L.E. Samelson. 1989. Intrathymic signalling in immature CD4+CD8+ thymocytes results in tyrosine phosphorylation of the T-cell receptor zeta chain. Nature. 341:651–654. [DOI] [PubMed] [Google Scholar]
- Naramura, M., H.K. Kole, R.J. Hu, and H. Gu. 1998. Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl. Acad. Sci. USA. 95:15547–15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi, I., N. Motoyama, K. Nakayama, S. Senju, S. Hatakeyama, Q. Zhang, A.C. Chan, and D.Y. Loh. 1995. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 376:435–438. [DOI] [PubMed] [Google Scholar]
- Ono, S., H. Ohno, and T. Saito. 1995. Rapid turnover of the CD3 zeta chain independent of the TCR-CD3 complex in normal T cells. Immunity. 2:639–644. [DOI] [PubMed] [Google Scholar]
- Pandey, A., H. Duan, and V.M. Dixit. 1995. Characterization of a novel Src-like adapter protein that associates with the Eck receptor tyrosine kinase. J. Biol. Chem. 270:19201–19204. [DOI] [PubMed] [Google Scholar]
- Roche, S., G. Alonso, A. Kazlauskas, V.M. Dixit, S.A. Courtneidge, and A. Pandey. 1998. Src-like adaptor protein (Slap) is a negative regulator of mitogenesis. Curr. Biol. 8:975–978. [DOI] [PubMed] [Google Scholar]
- Schmid, S.L. 1997. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem. 66:511–548. [DOI] [PubMed] [Google Scholar]
- Sebzda, E., S. Mariathasan, T. Ohteki, R. Jones, M.F. Bachmann, and P.S. Ohashi. 1999. Selection of the T cell repertoire. Annu. Rev. Immunol. 17:829–874. [DOI] [PubMed] [Google Scholar]
- Shores, E.W., K. Huang, T. Tran, E. Lee, A. Grinberg, and P.E. Love. 1994. Role of TCR zeta chain in T cell development and selection. Science. 266:1047–1050. [DOI] [PubMed] [Google Scholar]
- Sosinowski, T., A. Pandey, V.M. Dixit, and A. Weiss. 2000. Src-like adaptor protein (SLAP) is a negative regulator of T cell receptor signaling. J. Exp. Med. 191:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinowski, T., N. Killeen, and A. Weiss. 2001. The Src-like adaptor protein downregulates the T cell receptor on CD4+CD8+ thymocytes and regulates positive selection. Immunity. 15:457–466. [DOI] [PubMed] [Google Scholar]
- Straus, D.B., and A. Weiss. 1992. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 70:585–593. [DOI] [PubMed] [Google Scholar]
- Szymczak, A.L., and D.A. Vignali. 2005. Plasticity and rigidity in adaptor protein-2-mediated internalization of the TCR:CD3 complex. J. Immunol. 174:4153–4160. [DOI] [PubMed] [Google Scholar]
- Tang, J., S. Sawasdikosol, J.H. Chang, and S.J. Burakoff. 1999. SLAP, a dimeric adapter protein, plays a functional role in T cell receptor signaling. Proc. Natl. Acad. Sci. USA. 96:9775–9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telerman, A., R.B. Amson, F. Romasco, J. Wybran, P. Galand, and R. Mosselmans. 1987. Internalization of human T lymphocyte receptors. Eur. J. Immunol. 17:991–997. [DOI] [PubMed] [Google Scholar]
- Thien, C.B., R.M. Scaife, J.M. Papadimitriou, M.A. Murphy, D.D. Bowtell, and W.Y. Langdon. 2003. A mouse with a loss-of-function mutation in the c-Cbl TKB domain shows perturbed thymocyte signaling without enhancing the activity of the ZAP-70 tyrosine kinase. J. Exp. Med. 197:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers, N.S., N. Killeen, and A. Weiss. 1994. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCR zeta in murine thymocytes and lymph node T cells. Immunity. 1:675–685. [DOI] [PubMed] [Google Scholar]
- van Oers, N.S., N. Killeen, and A. Weiss. 1996. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med. 183:1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.Y., Y. Altman, D. Fang, C. Elly, Y. Dai, Y. Shao, and Y.C. Liu. 2001. Cbl promotes ubiquitination of the T cell receptor zeta through an adaptor function of Zap-70. J. Biol. Chem. 276:26004–26011. [DOI] [PubMed] [Google Scholar]
- Williams, B.L., K.L. Schreiber, W. Zhang, R.L. Wange, L.E. Samelson, P.J. Leibson, and R.T. Abraham. 1998. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol. Cell. Biol. 18:1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]