Abstract
The mitotic checkpoint is the major cell cycle control mechanism for maintaining chromosome content in multicellular organisms. Prevention of premature onset of anaphase requires activation at unattached kinetochores of the BubR1 kinase, which acts with other components to generate a diffusible “stop anaphase” inhibitor. Not only does direct binding of BubR1 to the centromere-associated kinesin family member CENP-E activate its essential kinase, binding of a motorless fragment of CENP-E is shown here to constitutively activate BubR1 bound at kinetochores, producing checkpoint signaling that is not silenced either by spindle microtubule capture or the tension developed at those kinetochores by other components. Using purified BubR1, microtubules, and CENP-E, microtubule capture by the CENP-E motor domain is shown to silence BubR1 kinase activity in a ternary complex of BubR1–CENP-E–microtubule. Together, this reveals that CENP-E is the signal transducing linker responsible for silencing BubR1-dependent mitotic checkpoint signaling through its capture at kinetochores of spindle microtubules.
Introduction
To assure accurate sister chromatid segregation during cell division, eukaryotic cells have evolved a mitotic checkpoint to prevent premature advance to anaphase before successful attachment of every chromosome to microtubules of the mitotic spindle. Chromosome instability leading to an abnormal chromosome number (aneuploidy) is associated with loss of function of the mitotic checkpoint in some human cancers (Cahill et al., 1998; Hanks et al., 2004). Genetics in yeast initially identified seven components of the mitotic checkpoint, Mad1-3 (Li and Murray, 1991), Bub1-3 (Hoyt et al., 1991), and Mps1 (Weiss and Winey, 1996). There are vertebrate homologues of all of these except Bub2 (Chen et al., 1996, 1998; Li and Benezra, 1996; Taylor and McKeon, 1997; Taylor et al., 1998; Abrieu et al., 2001). Additional essential contributors lacking yeast counterparts are known in metazoans. Without the kinetochore-associated microtubule motor protein CENP-E, the checkpoint cannot be established or maintained in vitro (Abrieu et al., 2000) or in mice (Putkey et al., 2002). Inhibition of ZW10 or Rod by mutation (Basto et al., 2000), antibody injection (Chan et al., 2000), or depletion with siRNA (Kops et al., 2005) has revealed both to be required for checkpoint signaling.
As initially shown for Mad2 (Chen et al., 1996), all of these checkpoint proteins are recruited onto unattached kinetochores where they generate a diffusible signal to prevent anaphase onset (for review see Cleveland et al., 2003). Before spindle attachment, a Mad1–Mad2 complex is stably targeted to kinetochores. There it recruits additional Mad2 that is converted into a rapidly released, inhibitory form (Shah et al., 2004), including the possible assembly of a complex with other checkpoint proteins (Sudakin et al., 2001; Fang, 2002). The activated inhibitor(s) binds to and sequesters Cdc20, a specificity factor required for an E3 ubiquitin ligase called the anaphase-promoting complex/cyclosome (APC/C) to recognize substrates (including securin and cyclin B) whose destruction is required for advance to anaphase (Cleveland et al., 2003).
Although the basic plan of the signaling cascade is established, how spindle microtubule binding to kinetochores silences mitotic checkpoint signaling is not known. However, the discovery that CENP-E is associated with BubR1 (Chan et al., 1998; Yao et al., 2000) and stimulates its kinase activity (Mao et al., 2003) had implicated it in activation and maintenance of mitotic checkpoint signaling in Xenopus egg extracts (Abrieu et al., 2000). This is true as well in the mammalian mitotic checkpoint where CENP-E was shown to be required for prevention of premature advance to anaphase in the presence of unattached kinetochores in regenerating liver in mice (Putkey et al., 2002). BubR1, the vertebrate homologue of yeast Mad3, has acquired a kinase domain in every species (including Drosophila, Xenopus, mice, and human) that also has a CENP-E homologue. BubR1 kinase activity is essential for the metazoan mitotic checkpoint and is directly stimulated by CENP-E binding to it (Mao et al., 2003; Weaver et al., 2003).
CENP-E is also one of the components directly responsible for capture and stabilization of spindle microtubules by kinetochores (Lombillo et al., 1995; McEwen et al., 2001; Putkey et al., 2002). Full chromosome alignment is precluded by disruption of CENP-E function in Xenopus egg extracts (Wood et al., 1997), in mammalian cultured cells (Yao et al., 2000; McEwen et al., 2001; Putkey et al., 2002), or in vivo in mice (Putkey et al., 2002). All of this has combined to make CENP-E a candidate for a signal transducing linker responsible for silencing BubR1-dependent checkpoint signaling at each kinetochore through capture by its motor domain of spindle microtubules. We now test this model directly by eliminating CENP-E–mediated microtubule capture in Xenopus egg extracts and mammalian-cultured cells, and by reconstruction with purified components of silencing of BubR1 kinase through CENP-E–mediated microtubule capture.
Results and discussion
Motorless CENP-Etail chronically activates BubR1 kinase and mitotic checkpoint signaling in Xenopus egg extracts
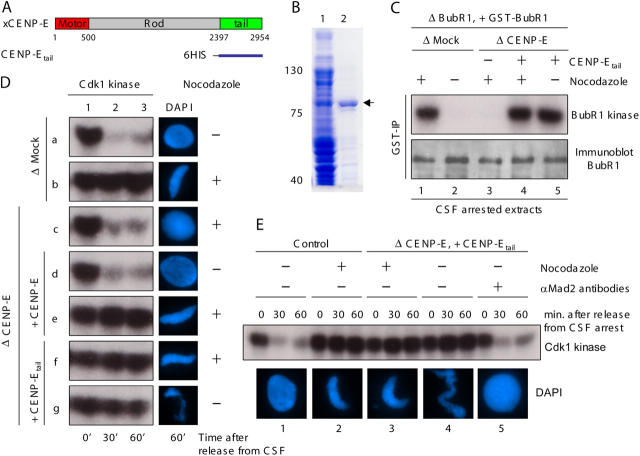
If CENP-E capture of spindle microtubules is required for mitotic checkpoint signal silencing, replacement of CENP-E with a motorless variant sufficient for activating the BubR1 kinase should produce a chronic checkpoint signal independent of kinetochore capture of spindle microtubules. To test this, endogenous CENP-E was immunodepleted from Xenopus egg extracts. Sperm nuclei (as a source of chromosomes) were added, as was a motorless CENP-E containing the carboxyl-terminal 700–amino acid fragment of the 2,954–amino acid Xenopus CENP-E (named CENP-Etail; Fig. 1, A and B). This led to recruitment of both CENP-Etail and BubR1 to kinetochores (Fig. S1 A available at http://www.jcb.org/cgi/content/full/jcb.200505040/DC1). The extracts were then cycled through S phase to generate duplicated sister chromatids. An aliquot of the initial CENP-E–depleted extracts containing both active Cdk1 and cytostatic factor (CSF) was then added to drive the extracts into the subsequent mitosis and to arrest it there. Bipolar spindles with aligned chromosomes formed at high efficiency in undepleted or mock-depleted extracts (Fig. S1, B and C). After removal of CENP-E and replacement of endogenous CENP-E with CENP-Etail, most chromosomes still attached to spindle microtubules, although a proportion were misaligned (Fig. S1, B and C). This mitotic phenotype is indistinguishable with the consequences of inhibition of CENP-E by antibody removal (Schaar et al., 1997; Wood et al., 1997) or gene silencing (Yao et al., 2000; Putkey et al., 2002). It is also consistent with a requirement for the CENP-E motor domain in achieving or maintaining metaphase chromosome alignment.
Figure 1.
Motorless CENP-Etail activates BubR1 kinase activity and mitotic checkpoint signaling in Xenopus egg extracts. (A) Xenopus CENP-E protein structure showing the relative position of the NH2-terminal microtubule motor domain, the rod domain, and the COOH-terminal kinetochore binding tail domain. (B) Purification of recombinant Xenopus CENP-Etail. Initial Escherichia coli lysates encoding 6-His-CENP-Etail (lane 1) and the recombinant protein (arrowhead) after purification over Ni-NTA agarose beads (QIAGEN; lane 2). (C) CENP-Etail activates BubR1 kinase activity in Xenopus egg extracts. After immunodepletion of endogenous BubR1, recombinant GST–BubR1 was added to a molar level comparable to the initial level of endogenous BubR1 in CSF-arrested egg extracts. Sperm nuclei and nocodazole were then added to egg extracts containing either endogenous CENP-E (mock depleted) or CENP-Etail (CENP-E–depleted extracts supplemented with CENP-E tail fragment) as indicated. After 30 min, GST–BubR1 was immunoprecipitated using specific anti-GST antibody and immunoblotted with anti-BubR1 antibody (bottom row) or kinase activity assayed after addition of histone H1 and [γ-32P]ATP (top row). (D) CENP-Etail is sufficient for activating mitotic checkpoint signaling. Xenopus egg extracts were (a and b) mock depleted, or (c) CENP-E depleted, and then supplemented with either (d and e) recombinant CENP-E or (f and g) CENP-Etail. After incubation of sperm nuclei with or without nocodazole, as indicated, CSF activity was inactivated by addition of calcium. Aliquots were taken from each extract at indicated times and then assayed by autoradiography for Cdk1 kinase activity (left) using added histone H1 as a substrate and (right) maintenance of chromatin condensation. (E) CENP-Etail–induced mitotic arrest is dependent on the mitotic checkpoint. CSF-arrested egg extracts were depleted with anti-IgG (1 and 2) or anti-CENP-E antibodies and supplemented with CENP-Etail (3–5) and incubated with sperm nuclei and nocodazole as indicated for 30 min. IgG (1–4) or Mad2 antibodies (5) were then added to reactions as indicated. Subsequently, Cdk1 kinase activity was measured (on histone H1, top) at 0, 30, and 60 min (as indicated) after calcium addition, and chromatin was visualized with DAPI (bottom).
BubR1 kinase activity is CENP-E dependent (Mao et al., 2003; Weaver et al., 2003). In the presence of endogenous full-length CENP-E and a high concentration of kinetochores provided by added sperm nuclei, BubR1 kinase was activated in egg extracts only when microtubule assembly was inhibited (Fig. 1 C, lane 1). This was also true for mitotic checkpoint signaling, as measured by sustained Cdk1 activity and chromosome condensation after calcium-mediated release from CSF arrest (Fig. 1 D, row b). After depletion of endogenous BubR1 and CENP-E, and restoration of normal BubR1 levels with purified GST-tagged BubR1, further addition of CENP-Etail activated the recombinant BubR1 kinase (measured by affinity recovery of BubR1 with GST antibody beads) to the same levels as endogenous BubR1 in mock-depleted extracts (Fig. 1 C, compare lanes 1 and 4). Two specificity controls confirmed that this kinase activity was due to BubR1 and not to a contaminating kinase in the immunoprecipitates. First, there was no detectable activity (Fig. S2 A available at http://www.jcb.org/cgi/content/full/jcb.200505040/DC1) after restoration with a kinase inactive BubR1 (a point mutation in the ATP binding site; KD-BubR1). Second, the most likely contaminant was Cdk1, the major mitotic kinase whose activity is sustained at maximal activity level during either CSF-induced or checkpoint-mediated mitotic arrest. However, the activity was insensitive to roscovitine, a potent inhibitor of Cdk1 (Fig. S2, A and B).
BubR1 kinase activity was silenced as expected (Mao et al., 2003) after spindle assembly and chromosome alignment in CSF-arrested extracts containing full-length CENP-E (Fig. 1 C, lane 2). However, CENP-Etail–stimulated BubR1 kinase activity was undiminished even after spindle assembly (Fig. 1 C, lane 5) producing chronic mitotic arrest, as revealed by continued Cdk1 kinase activity and condensed chromosomes (Fig. 1 D, rows f and g). After addition of function blocking Mad2 antibodies (Chen et al., 1996), extracts rapidly cycled into interphase, with loss of Cdk1 kinase activity and reformation of nuclei with decondensed chromosomes (Fig. 1 E). Thus, the chronic mitotic arrest in extracts reconstituted with motorless CENP-Etail does reflect Mad2-dependent mitotic checkpoint signaling.
Microtubule capture by CENP-E is required for blocking Mad2 recruitment to bioriented sister kinetochores under tension
Mad2 binds preferentially to unattached kinetochores that are producing the stop anaphase inhibitor (Chen et al., 1996; Waters et al., 1998). In cycled egg extracts in which endogenous CENP-E was replaced with full-length recombinant CENP-E, Mad2 was released from kinetochores upon microtubule attachment, whereas BubR1 association remained (Fig. 2 A, insets). After replacement of CENP-E with the microtubule capture–deficient CENP-Etail, a proportion of sister kinetochores were aligned along the spindle axis. Inter-kinetochore spacing of these was stretched to ∼2.5 times their rest length measured in the presence of nocodazole (Maddox et al., 2003). The increased spacing indicated that these kinetochores were attached to spindle microtubules and under tension (Fig. 2, C [insets] and D). Despite this biorientation and generation by spindle-derived forces of tension between sister kinetochores, Mad2 remained associated with these attached kinetochores (Fig. 2, C [inset] and E) at levels very similar to that of unattached kinetochores (Fig. 2, B and C [insets] and E).
Figure 2.
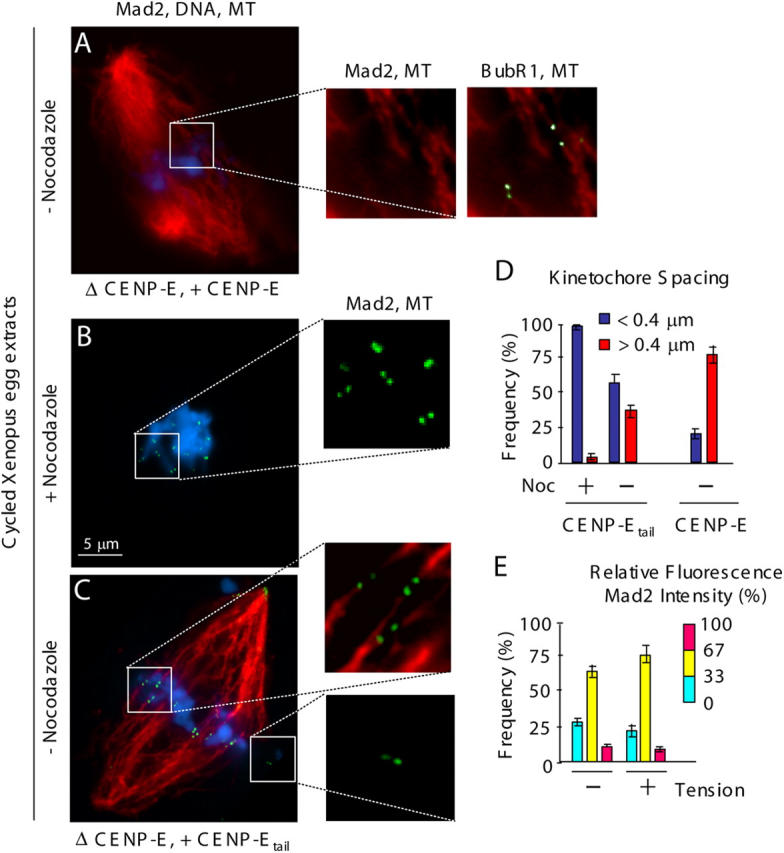
Microtubule capture by CENP-E is required for preventing Mad2 recruitment to sister kinetochores under tension in Xenopus egg extracts. (A–C) CENP-E–depleted egg extracts containing sperm nuclei were cycled through interphase and arrested with CSF activity at the following metaphase. (A) Recombinant CENP-E or (B and C) CENP-Etail were added. (B) Nocodazole or (A and C) no drug was added for 30 min, as indicated, and kinetochore recruitment of Mad2 (green) and spindle microtubules (red) were visualized by indirect immunofluorescence, and chromatin was visualized with DAPI (blue). Paired kinetochores (labeled by [A] Mad2 and BubR1, or [B and C] Mad2 are boxed and shown magnified in insets [right]). (D and E) Quantification of (D) the spacing between paired kinetochores (blue: 0–0.4 μm, red: >0.4 μm); and (E) the normalized integrated intensity of Mad2 signals at kinetochores in D. At least 10 kinetochores from more than three spindles were quantified for each bar. P < 0.05. Error bars represent standard errors.
A similar situation was found for mammalian mitotic checkpoint signaling. When expressed by DNA transfection, human CENP-Etail (Flag:hCENP-Etail, aa 1572–2664) accumulated at kinetochores (Fig. 3 G and J) in human T98G cells. In these cells, Mad2 remained not only on unattached kinetochores (Fig. 3 H), but was also present at comparable levels on both sister kinetochores of chromatid pairs that were bioriented (Fig. 3 K). No Mad2 was detectable at kinetochores of aligned chromosomes in the CENP-E containing nontransfected cells (Fig. 3, B and E). CENP-Etail accumulation at kinetochores was accompanied by a 3.5-fold increase in the mitotic index (Fig. 3 M) as well as a doubling of the proportion of mitotic cells with all chromatid pairs bioriented and aligned in metaphase. These findings, along with a corresponding decrease in the proportion of anaphases (Fig. 3 N), indicated failure of efficient silencing of the mammalian mitotic checkpoint in the presence of kinetochore bound CENP-Etail.
Figure 3.
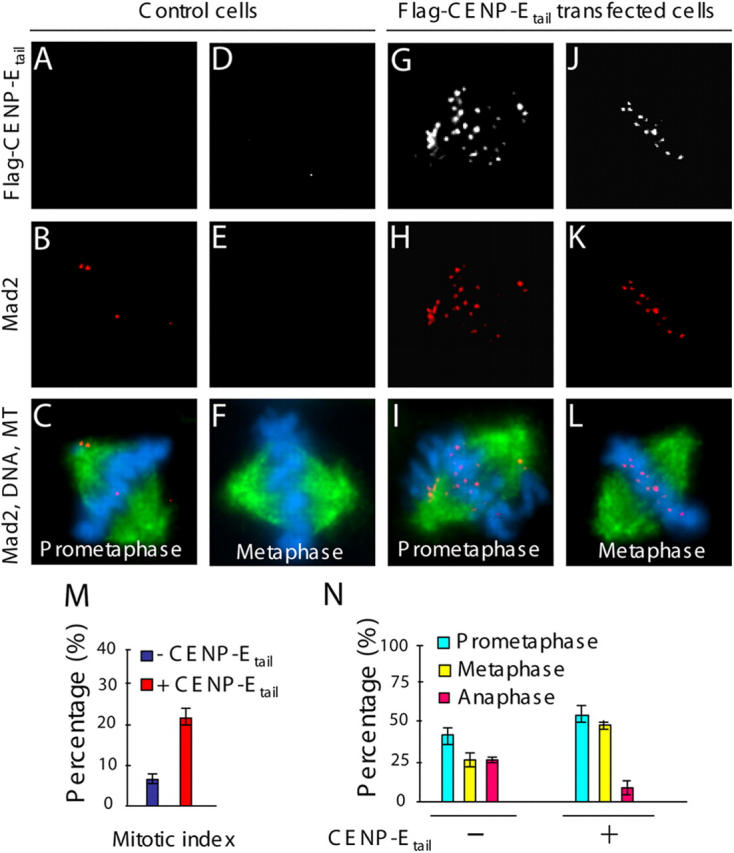
Continued recruitment of mammalian Mad2 to bioriented, sister kinetochores under tension in the presence of CENP-Etail. (A–L) Transient expression of CENP-Etail in cultured human T98B cells. (A, D, G, and J) Flag:hCENP-Etail and (B, C, E, F, H, I, K, and L; red) Mad2 were observed by immunofluorescence with mouse anti-flag, rabbit anti-hMad2, and (C, F, I, and L; green) rat anti-tubulin antibodies, respectively. (C, F, I, and L; blue) Chromatin was visualized with DAPI. (M) The mitotic index and (N) the proportions of mitotic cells exhibiting prometaphase, metaphase, and anaphase chromosome alignments in cells transfected with Flag:hCENP-Etail and control cells. 200 cells were counted in three independent transfections. P < 0.001. Error bars represent standard errors.
Therefore, although other components can mediate spindle microtubule capture at kinetochores and generate tension between sister kinetochores in the absence of CENP-E, silencing of both BubR1 kinase activity and Mad2 recruitment at kinetochores requires an activity of CENP-E beyond its kinetochore binding domain.
Microtubule capture by CENP-E inactivates CENP-E–dependent stimulation of BubR1 kinase activity in a ternary complex in vitro
To directly test whether microtubule capture by CENP-E silences BubR1 kinase–dependent mitotic checkpoint signaling, microtubules were assembled from pure tubulin in the presence of GMPCPP, a slowly hydrolyzable GTP analogue (Weisenberg et al., 1976). GMPCPP stabilizes the GTP conformation of the tubulin lattice (Vale et al., 1994; Hyman et al., 1995; Muller-Reichert et al., 1998; Wang and Nogales, 2005), structurally mimicking GTP-bound microtubule plus ends. Once polymerized, GMPCPP-containing microtubules are stable to dilution driven disassembly (Hyman et al., 1992; Muller-Reichert et al., 1998). Not only has kinesin been shown to move on GMPCPP microtubules in vitro (Vale et al., 1994), reconstituted kinetochores have been shown to bind them preferentially over GDP-containing microtubules (Severin et al., 1997).
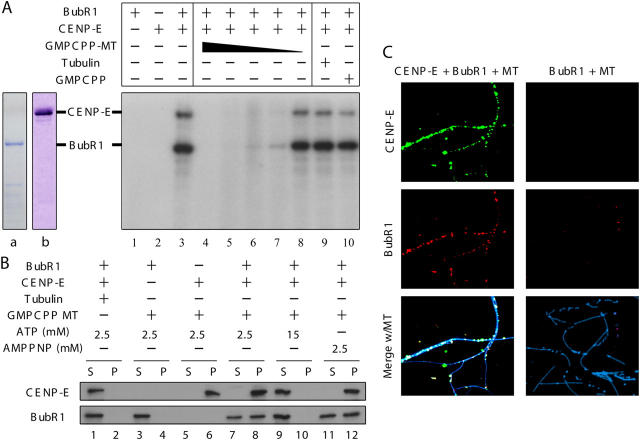
In vitro kinase assays were performed with purified recombinant BubR1, CENP-E, and GMPCPP microtubules to test whether microtubule capture by CENP-E directly silences the BubR1 kinase activity required for mitotic checkpoint signaling. Purified recombinant BubR1 autophosphorylated itself, but only in the presence of CENP-E (Fig. 4 A, compare lanes 1 and 3), as expected (Mao et al., 2003). CENP-E–activated BubR1 also phosphorylated CENP-E (Fig. 4 A, lane 3) or an exogenous substrate such as histone H1 (Fig. 5 A, lane 3). Subsequent addition of GMPCPP microtubules strongly inhibited this CENP-E–dependent BubR1 kinase activity in a dose-dependent manner (Fig. 4 A, lanes 4–8). Kinase inhibition was specific for addition of assembled microtubules, as neither GMPCPP alone nor high concentrations of tubulin affected BubR1 kinase activity (Fig. 4 A, lanes 9 and 10).
Figure 4.
Microtubule capture by CENP-E in vitro produces a ternary complex of microtubule–CENP-E–BubR1 in which CENP-E–activated BubR1 kinase activity is silenced. (A) In vitro kinase assays were performed with combinations of purified GST–BubR1 (lane a), CENP-E (lane b), and 1 μM, 0.1 μM, 10 nM, 1 nM, or 0.1 nM GMPCPP microtubules (lanes 4–8, respectively), 1 μM tubulin (lane 9), or 33 μM GMPCPP (lane 10). (B and C) CENP-E, BubR1, and microtubules form a ternary complex. After centrifugation through a 40% sucrose cushion, BubR1–CENP-E–GMPCPP microtubule complex formation was assayed by (B) immunoblot or (C) triple immunofluorescence for BubR1 (red), CENP-E (green), and microtubules (blue).
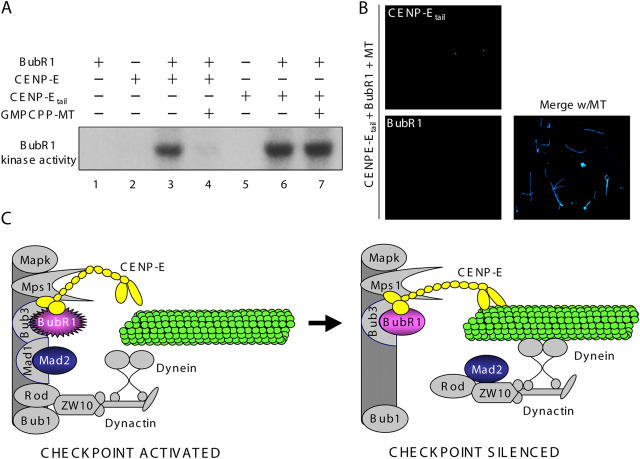
Figure 5.
CENP-Etail activation in vitro of BubR1 kinase cannot be silenced by addition of microtubules. (A) In vitro kinase assays were performed with combinations of purified CENP-E, CENP-Etail, and GST–BubR1 in the presence or absence of GMPCPP microtubules (1 μM), as indicated. Histone H1 was added as a substrate. (B) The CENP-Etail–BubR1 complex cannot bind microtubules. After centrifugation through a 40% sucrose cushion, BubR1–CENP-Etail–GMPCPP microtubule complex formation was assayed by triple immunofluorescence for BubR1 (red), CENP-Etail (green), and microtubules (blue). (C) Schematic of silencing BubR1-dependent, mitotic checkpoint signaling upon microtubule capture by CENP-E. (Left) Activities of four kinetochore associated kinases (MapK, Mps1, Bub1, and BubR1) are essential for generation by unattached kinetochores of a “stop anaphase” mitotic checkpoint inhibitor (for detailed review see Cleveland et al., 2003). CENP-E activates the essential BubR1 kinase activity resulting in rapid cycling of Mad2 onto unattached kinetochores and release in a form that is a “stop anaphase” inhibitor. (Right) Spindle microtubule capture by CENP-E silences CENP-E–dependent BubR1 kinase activity without dissociation from BubR1. In the absence of active BubR1 kinase, Mad2 and its stably bound kinetochore tether, Mad1 (Shah et al., 2004; De Antoni et al., 2005), as well as the Rod–Zw10–dynactin–cytoplasmic dynein complex, are released from kinetochores (Kops et al., 2005), thus silencing mitotic checkpoint signaling at kinetochores.
To determine whether microtubule binding by CENP-E simply dissociates BubR1 from CENP-E, pure CENP-E–BubR1 complex was incubated with unpolymerized tubulin or GMPCPP microtubules, and the microtubules and proteins bound to them were recovered by sedimentation through a sucrose cushion. BubR1 had no microtubule affinity on its own. None copelleted with microtubules (Fig. 4 B, lane 4) and none was found microtubule associated after fixation and visualization using fluorescently tagged BubR1 antibodies (Fig. 4 C, right). In contrast, after addition of full-length CENP-E at low concentrations of ATP or the nonhydrolyzable ATP analogue AMP-PNP, conditions known to produce rigor-like microtubule binding of the CENP-E motor domain (Liao et al., 1994), all CENP-E and more than half of BubR1 cosedimented with microtubules (Fig. 4 B, lanes 8 and 12). CENP-E bound in a dot-like pattern along those microtubules, with BubR1 also targeted only to these CENP-E foci (Fig. 4 C, left). High ATP concentration released both CENP-E and BubR1 from these microtubules (Fig. 4 B, lanes 9 and 10), as expected for binding mediated through the CENP-E motor domain. Thus, microtubule binding by CENP-E produced a ternary complex (CENP-E, BubR1, and microtubule), and this silenced the BubR1 kinase activity known to be essential for mitotic checkpoint signaling (Mao et al., 2003; Kops et al., 2004).
To further confirm that it is microtubule binding by CENP-E through its NH2-terminal motor domain that is responsible for silencing CENP-E–dependent BubR1 kinase activity, purified motorless CENP-Etail was used in in vitro kinase assays. Recombinant CENP-Etail lacking the rod and motor domains was found to activate BubR1 kinase in vitro as efficiently as did full-length CENP-E (Fig. 5 A, compare lanes 3 and 6). Neither CENP-Etail nor BubR1 bound to microtubules (Fig. 5 B). Further, CENP-Etail–stimulated activity was not inhibited by addition of GMPCPP-stabilized microtubules (Fig. 5 A, compare lanes 6 and 7), consistent with silencing of CENP-E–dependent BubR1 kinase activity requiring microtubule binding by the CENP-E NH2-terminal motor domain.
CENP-E as the signal transducing linker that silences the BubR1-dependent mitotic checkpoint
Our findings here with purified proteins have demonstrated the direct role of CENP-E in silencing BubR1 kinase through microtubule capture by the CENP-E motor domain (modeled in Fig. 5 C). We would note that this microtubule binding by CENP-E represents binding to the side of a microtubule structurally similar to the GTP microtubule cap thought to be present at the microtubule end captured by kinetochores in vivo. Although microtubules appear to terminate at kinetochores in many electron-microscopic images and this has lead to the view that kinetochores capture microtubules by end binding, rather than side binding, the kinesin-like motor domain of CENP-E would firmly be expected to bind to the side. This mode of lateral binding of the kinesin family of motors is well established (Vale and Fletterick, 1997; Vale and Milligan, 2000). Further, from consideration of the known crystal structure (Garcia-Saez et al., 2004), the two CENP-E motors in a CENP-E dimer can only attach to one or two of the 13 protofilaments of a microtubule. Thus, the lateral capture on a “GTP-like” microtubule target in vitro is representative of the kind of capture to which CENP-E contributes at kinetochores in vivo.
There is a continuing controversy as to whether the mitotic checkpoint in metazoans is silenced by microtubule attachment (Rieder et al., 1995; Waters et al., 1998; Nicklas et al., 2001) or the subsequent tension generated (Li and Nicklas, 1995). This has lead to suggestions that subsets of the known signaling components are selectively silenced by one or the other (Skoufias et al., 2001; Zhou et al., 2002). This is not unappealing, but is at best a very murky argument. At their normal levels BubR1, Bub1, or Mad2 cannot suppress Cdc20-APC/C activity independently of the others (Tang et al., 2001; Chen, 2002; Mao et al., 2003; Kops et al., 2004; Meraldi et al., 2004). Moreover, attachment and tension are intimately interrelated: generation and maintenance of tension obviously requires attachment. And the converse has long been known: tension stabilizes attachment (Ault and Nicklas, 1989; Nicklas et al., 2001).
For CENP-E, its removal by gene disruption in mammalian cells leads to only half the normal number of kinetochore microtubules attached even on bioriented sister kinetochores that are under some microtubule-dependent tension (Putkey et al., 2002). Nevertheless, our evidence here in Xenopus extracts (Fig. 2) and human cells (Fig. 3) establishes that despite bioriented attachment, alignment and development of levels of tension similar to that in CENP-E containing parental cells, no other microtubule capture component(s) is present that can silence (or diminish) checkpoint signaling at those attached kinetochores. Thus, blocking premature advance to anaphase in order to maintain normal chromosome content is mediated through CENP-E acting as a signal-transducing linker responsible for silencing BubR1-dependent mitotic checkpoint signaling by direct capture by CENP-E of spindle microtubules and a corresponding inactivation of BubR1 kinase in a stable ternary complex comprised of spindle microtubule–CENP–E-BubR1.
Materials and methods
Fusion protein expression
XCENP-Etail (aa 2397–2954) was produced as 6-His fusion protein using the pRSET expression plasmid. After induction with IPTG, recombinant protein was purified over Ni-NTA agarose (QIAGEN).
Immunoblots were blocked with TBST (20 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.1% Tween) containing nonfat dry milk and then probed with affinity-purified primary antibodies in TBST. Primary antibodies were visualized using horseradish peroxidase–labeled secondary antibodies and ECL (GE Healthcare).
Xenopus egg extracts
CSF-arrested extracts were prepared from unfertilized Xenopus eggs as previously described (Murray, 1991). Rhodamine-labeled bovine brain tubulin was added at 1 μl/300 μl (0.05 mg/ml final concentration) of egg extracts. For immunodepletion of BubR1 or CENP-E, affinity purified anti-BubR1 or anti–CENP-E antibodies were used and carried as previously described (Mao et al., 2003). Demembranated sperm were added to a portion of each extracts and exit from metaphase arrest was induced by addition of CaCl2. Progression of extracts through the cell cycle was monitored by fluorescence microscopic examination of 1 μl aliquots squashed under a coverslip (Murray, 1991). At 80 min after exit from metaphase, one half volume of the appropriate extracts were added and reactions were incubated for an additional 60–120 min. M-phase structures accumulating in egg extracts were scored in squashed samples taken after 160 min total elapsed time. Immunofluorescence with Xenopus egg extracts was performed as previous described (Mao et al., 2003). The mitotic checkpoint was activated in the presence of 9,000 sperm head nuclei/μl and 10 μg/ml nocodazole for 30 min before releasing from the CSF arrest.
In vitro kinase assay
For in vitro kinase assays, GST–BubR1 immunoprecipitates were incubated at room temperature for 30 min with 25 mM Hepes (pH 7.5), 10 mM MgCl2, 200 μM ATP, and 1 μCi[γ32P]ATP and 100 μg histone H1 as an exogenous substrates. Recombinant BubR1 was incubated with or without CENP-E, GMPPNP microtubules, and then assayed for kinase activity.
CENP-E–BubR1 microtubule cosedimentation assay
Purified 10 nM CENP-E and/or BubR1 were mixed with GMPCPP-stabilized microtubules or 1 μM unpolymerized tubulin in BRB80 and incubated in room temperature for 30 min. Binding reactions were centrifuged over 40% sucrose/BRB80 10,000 rpm for 15 min at 35°C. Recombinant proteins in the pellet and supernatant were analyzed by immunoblotting.
Cell culture, transfection, and immunofluorescence
T98G cells were grown in DME supplemented with 10% FBS. Transfections were done using Effectene based on the manufacturer's protocol (QIAGEN).
For immunofluorescence, cells grown on poly-l-lysine–coated coverslips were washed once with microtubule stabilizing buffer (MTSB; 100 mM Pipes, 1 mM EGTA, 1 mM MgSO4, and 30% of glycerol), extracted with 0.5% Triton X-100 in MTSB for 5 min, fixed with 4% formaldehyde in MTSB for 10 min, and blocked in TBS containing 0.5% Tween-20 and 1% BSA (Sigma-Aldrich) for 1 h. Coverslips were exposed to primary antibodies diluted in blocking buffer for 1 h, and to secondary antibodies (Jackson ImmunoResearch Laboratories). Images were visualized using a Axiophot microscope (Carl Zeiss MicroImaging, Inc.) at 100× with epifluorescence illumination, and recorded using a cooled CCD camera (model ST138/KAF1400; Princeton Instruments) that was controlled by Metamorph software (Universal Imaging Corp.). All images in each experiment were collected on the same day using identical exposure time.
Online supplemental material
Fig. S1 shows requirement for the motor domain of CENP-E in complete metaphase chromosome alignment. Fig. S2 shows specificity of BubR1 kinase activity from GST–BubR1 immunoprecipitates. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200505040/DC1.
Acknowledgments
We thank B. Weaver for kindly providing rabbit anti-hMad2 antibodies; H. Li for assistance with the kinetochore fluorescence quantification; P. Maddox for advice in spinning disk confocal microscopy; G.J.P.L. Kops, J.V. Shah, D.R. Foltz, I.M. Cheeseman, and P. Maddox for valuable comments on the manuscript; and all members of the Cleveland laboratory for stimulating discussion.
This work has been supported by a grant from the National Institutes of Health (NIH) to D.W. Cleveland (ROI GM29513). Y. Mao is a Leukemia & Lymphoma Society Special Fellow and the recipient of a postdoctoral fellowship from the NIH. A. Desai is a Damon Runyon Scholar supported by the Damon Runyon Cancer Research Foundation (DRS 38-04). D.W. Cleveland and A. Desai receive salary support from the Ludwig Institute for Cancer Research.
Y. Mao's present address is Department of Pathology, Columbia University College of Physicians and Surgeons, New York, NY 10032.
Abbreviations used in this paper: APC/C, anaphase-promoting complex/cyclosome; CSF, cytostatic factor.
References
- Abrieu, A., J.A. Kahana, K.W. Wood, and D.W. Cleveland. 2000. CENP-E as an essential component of the mitotic checkpoint in vitro. Cell. 102:817–826. [DOI] [PubMed] [Google Scholar]
- Abrieu, A., L. Magnaghi-Jaulin, J.A. Kahana, M. Peter, A. Castro, S. Vigneron, T. Lorca, D.W. Cleveland, and J.C. Labbe. 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 106:83–93. [DOI] [PubMed] [Google Scholar]
- Ault, J.G., and R.B. Nicklas. 1989. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma. 98:33–39. [DOI] [PubMed] [Google Scholar]
- Basto, R., R. Gomes, and R.E. Karess. 2000. Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nat. Cell Biol. 2:939–943. [DOI] [PubMed] [Google Scholar]
- Cahill, D.P., C. Lengauer, J. Yu, G.J. Riggins, J.K. Willson, S.D. Markowitz, K.W. Kinzler, and B. Vogelstein. 1998. Mutations of mitotic checkpoint genes in human cancers. Nature. 392:300–303. [DOI] [PubMed] [Google Scholar]
- Chan, G.K., B.T. Schaar, and T.J. Yen. 1998. Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J. Cell Biol. 143:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, G.K., S.A. Jablonski, D.A. Starr, M.L. Goldberg, and T.J. Yen. 2000. Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nat. Cell Biol. 2:944–947. [DOI] [PubMed] [Google Scholar]
- Chen, R.-H. 2002. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 158:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.H., J.C. Waters, E.D. Salmon, and A.W. Murray. 1996. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 274:242–246. [DOI] [PubMed] [Google Scholar]
- Chen, R.H., A. Shevchenko, M. Mann, and A.W. Murray. 1998. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J. Cell Biol. 143:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland, D.W., Y. Mao, and K.F. Sullivan. 2003. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 112:407–421. [DOI] [PubMed] [Google Scholar]
- De Antoni, A., C.G. Pearson, D. Cimini, J.C. Canman, V. Sala, L. Nezi, M. Mapelli, L. Sironi, M. Faretta, E.D. Salmon, and A. Musacchio. 2005. The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol. 15:214–225. [DOI] [PubMed] [Google Scholar]
- Fang, G. 2002. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell. 13:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez, I., T. Yen, R.H. Wade, and F. Kozielski. 2004. Crystal structure of the motor domain of the human kinetochore protein CENP-E. J. Mol. Biol. 340:1107–1116. [DOI] [PubMed] [Google Scholar]
- Hanks, S., K. Coleman, S. Reid, A. Plaja, H. Firth, D. Fitzpatrick, A. Kidd, K. Mehes, R. Nash, N. Robin, et al. 2004. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36:1159–1161. [DOI] [PubMed] [Google Scholar]
- Hoyt, M.A., L. Totis, and B.T. Roberts. 1991. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 66:507–517. [DOI] [PubMed] [Google Scholar]
- Hyman, A.A., S. Salser, D.N. Drechsel, N. Unwin, and T.J. Mitchison. 1992. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol. Biol. Cell. 3:1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman, A.A., D. Chretien, I. Arnal, and R.H. Wade. 1995. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(α,β)-methylene-diphosphonate. J. Cell Biol. 128:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops, G.J., D.R. Foltz, and D.W. Cleveland. 2004. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc. Natl. Acad. Sci. USA. 101:8699–8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops, G.J., Y. Kim, B.A. Weaver, Y. Mao, I. McLeod, J.R. Yates III, M. Tagaya, and D.W. Cleveland. 2005. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J. Cell Biol. 169:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R., and A.W. Murray. 1991. Feedback control of mitosis in budding yeast. Cell. 66:519–531. [DOI] [PubMed] [Google Scholar]
- Li, X., and R.B. Nicklas. 1995. Mitotic forces control a cell-cycle checkpoint. Nature. 373:630–632. [DOI] [PubMed] [Google Scholar]
- Li, Y., and R. Benezra. 1996. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 274:246–248. [DOI] [PubMed] [Google Scholar]
- Liao, H., G. Li, and T.J. Yen. 1994. Mitotic regulation of microtubule cross-linking activity of CENP-E kinetochore protein. Science. 265:394–398. [DOI] [PubMed] [Google Scholar]
- Lombillo, V.A., C. Nislow, T.J. Yen, V.I. Gelfand, and J.R. McIntosh. 1995. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J. Cell Biol. 128:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox, P., A. Straight, P. Coughlin, T.J. Mitchison, and E.D. Salmon. 2003. Direct observation of microtubule dynamics at kinetochores in Xenopus extract spindles: implications for spindle mechanics. J. Cell Biol. 162:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Y., A. Abrieu, and D.W. Cleveland. 2003. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell. 114:87–98. [DOI] [PubMed] [Google Scholar]
- McEwen, B.F., G.K. Chan, B. Zubrowski, M.S. Savoian, M.T. Sauer, and T.J. Yen. 2001. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol. Biol. Cell. 12:2776–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi, P., V.M. Draviam, and P.K. Sorger. 2004. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 7:45–60. [DOI] [PubMed] [Google Scholar]
- Muller-Reichert, T., D. Chretien, F. Severin, and A.A. Hyman. 1998. Structural changes at microtubule ends accompanying GTP hydrolysis: information from a slowly hydrolyzable analogue of GTP, guanylyl (α,β)methylenediphosphonate. Proc. Natl. Acad. Sci. USA. 95:3661–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, A.W. 1991. Cell cycle extracts. Methods Cell Biol. 36:581–605. [PubMed] [Google Scholar]
- Nicklas, R.B., J.C. Waters, E.D. Salmon, and S.C. Ward. 2001. Checkpoint signals in grasshopper meiosis are sensitive to microtubule attachment, but tension is still essential. J. Cell Sci. 114:4173–4183. [DOI] [PubMed] [Google Scholar]
- Putkey, F.R., T. Cramer, M.K. Morphew, A.D. Silk, R.S. Johnson, J.R. McIntosh, and D.W. Cleveland. 2002. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev. Cell. 3:351–365. [DOI] [PubMed] [Google Scholar]
- Rieder, C.L., R.W. Cole, A. Khodjakov, and G. Sluder. 1995. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130:941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar, B.T., G.K. Chan, P. Maddox, E.D. Salmon, and T.J. Yen. 1997. CENP-E function at kinetochores is essential for chromosome alignment. J. Cell Biol. 139:1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin, F.F., P.K. Sorger, and A.A. Hyman. 1997. Kinetochores distinguish GTP from GDP forms of the microtubule lattice. Nature. 388:888–891. [DOI] [PubMed] [Google Scholar]
- Shah, J.V., E. Botvinick, Z. Bonday, F. Furnari, M. Berns, and D.W. Cleveland. 2004. Dynamics of centromere and kinetochore proteins: implications for checkpoint signaling and silencing. Curr. Biol. 14:942–952. [DOI] [PubMed] [Google Scholar]
- Skoufias, D.A., P.R. Andreassen, F.B. Lacroix, L. Wilson, and R.L. Margolis. 2001. Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA. 98:4492–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin, V., G.K. Chan, and T.J. Yen. 2001. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154:925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Z., R. Bharadwaj, B. Li, and H. Yu. 2001. Mad2-Independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell. 1:227–237. [DOI] [PubMed] [Google Scholar]
- Taylor, S.S., and F. McKeon. 1997. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell. 89:727–735. [DOI] [PubMed] [Google Scholar]
- Taylor, S.S., E. Ha, and F. McKeon. 1998. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J. Cell Biol. 142:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, R.D., and R.J. Fletterick. 1997. The design plan of kinesin motors. Annu. Rev. Cell Dev. Biol. 13:745–777. [DOI] [PubMed] [Google Scholar]
- Vale, R.D., and R.A. Milligan. 2000. The way things move: looking under the hood of molecular motor proteins. Science. 288:88–95. [DOI] [PubMed] [Google Scholar]
- Vale, R.D., C.M. Coppin, F. Malik, F.J. Kull, and R.A. Milligan. 1994. Tubulin GTP hydrolysis influences the structure, mechanical properties, and kinesin-driven transport of microtubules. J. Biol. Chem. 269:23769–23775. [PubMed] [Google Scholar]
- Wang, H.W., and E. Nogales. 2005. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature. 435:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, J.C., R.H. Chen, A.W. Murray, and E.D. Salmon. 1998. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141:1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, B.A., Z.Q. Bonday, F.R. Putkey, G.J. Kops, A.D. Silk, and D.W. Cleveland. 2003. Centromere-associated protein-E is essential for the mammalian mitotic checkpoint to prevent aneuploidy due to single chromosome loss. J. Cell Biol. 162:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg, R.C., W.J. Deery, and P.J. Dickinson. 1976. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 15:4248–4254. [DOI] [PubMed] [Google Scholar]
- Weiss, E., and M. Winey. 1996. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, K.W., R. Sakowicz, L.S. Goldstein, and D.W. Cleveland. 1997. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 91:357–366. [DOI] [PubMed] [Google Scholar]
- Yao, X., A. Abrieu, Y. Zheng, K.F. Sullivan, and D.W. Cleveland. 2000. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat. Cell Biol. 2:484–491. [DOI] [PubMed] [Google Scholar]
- Zhou, J., J. Yao, and H.C. Joshi. 2002. Attachment and tension in the spindle assembly checkpoint. J. Cell Sci. 115:3547–3555. [DOI] [PubMed] [Google Scholar]