Abstract
The cell surface heparan sulfate proteoglycan (HSPG) glypican-1 is up-regulated by pancreatic and breast cancer cells, and its removal renders such cells insensitive to many growth factors. We sought to explain why the cell surface HSPG syndecan-1, which is also up-regulated by these cells and is a known growth factor coreceptor, does not compensate for glypican-1 loss. We show that the initial responses of these cells to the growth factor FGF2 are not glypican dependent, but they become so over time as FGF2 induces shedding of syndecan-1. Manipulations that retain syndecan-1 on the cell surface make long-term FGF2 responses glypican independent, whereas those that trigger syndecan-1 shedding make initial FGF2 responses glypican dependent. We further show that syndecan-1 shedding is mediated by matrix metalloproteinase-7 (MMP7), which, being anchored to cells by HSPGs, also causes its own release in a complex with syndecan-1 ectodomains. These results support a specific role for shed syndecan-1 or MMP7–syndecan-1 complexes in tumor progression and add to accumulating evidence that syndecans and glypicans have nonequivalent functions in vivo.
Introduction
Many growth factors use heparan sulfate proteoglycans (HSPGs) as cofactors in receptor binding and/or signaling. Dependence on HSPGs has been demonstrated for FGFs, heparin-binding members of the EGF family, such as heparin-binding EGF-like growth factor (HB-EGF) and the heregulins, hepatocyte growth factor (HGF), Wnts, hedgehogs, and at least some members of the transforming growth factor β superfamily (Rapraeger et al., 1991; Aviezer and Yayon, 1994; Zioncheck et al., 1995; Bellaiche et al., 1998; Tsuda et al., 1999; Li and Loeb, 2001; Fujise et al., 2003).
The major HSPGs of the cell surface are the syndecans and glypicans (Lander and Selleck, 2000; Perrimon and Bernfield, 2000). The syndecans are four related transmembrane proteins that sometimes also carry chondroitin sulfate. The glypicans are six glycosylphosphatidylinositol (GPI)-anchored proteins that exclusively carry heparan sulfate. We previously reported that the expression of glypican-1 (but not other glypicans) is induced in human pancreatic and breast cancer cells and that the ability of heparin-binding growth factors to drive the proliferation of these cells is blocked by phosphoinositide-specific PLC (PIPLC), an enzyme that releases GPI-anchored proteins from the cell surface (Kleeff et al., 1998; Matsuda et al., 2001). Responsiveness can be restored with a transmembrane variant of glypican-1 (i.e., one that cannot be cleaved by PIPLC). Conversely, the loss of proliferative response results when antisense RNA is used to decrease levels of endogenous glypican-1. Manipulation of glypican-1 levels with either PIPLC or antisense RNA affects mitogenic responses to growth factors that are HSPG dependent, such as FGF2 and HB-EGF, but not other growth factors such as insulin-like growth factor-1 and EGF (Kleeff et al., 1998; Matsuda et al., 2001). Inhibition of glypican-1 expression also causes pancreatic carcinoma cell lines to form tumors that grow more slowly in vivo (Kleeff et al., 1999).
These studies suggest that glypican-1 plays an important role in the development of at least some cancers. Such a strong dependence on a glypican is surprising given that most cells have both glypicans and syndecans and that both HSPG families function efficiently as growth factor coreceptors (Steinfeld et al., 1996; Zhang et al., 2001). Indeed, substantial syndecan-1 is made by both the pancreatic and breast cancer cells that are dependent on glypican-1 for their growth factor responses (Conejo et al., 2000; Matsuda et al., 2001). These data suggest that, in some circumstances at least, glypicans and syndecans do not function equivalently. In this study, we take up the question of why this is.
Results
Previous studies uncovered a requirement for glypican-1 in the responses of pancreatic and breast cancer cells to several polypeptide mitogens, including FGF2, HB-EGF, HGF, heregulin-α, and heregulin-β (Kleeff et al., 1998, 1999; Matsuda et al., 2001). In those studies, mitogenesis was quantified as an increased cell number 48 h after growth factor addition. As this endpoint is far downstream of initial growth factor signaling, we tested for glypican dependence at earlier times. Fig. 1 A shows that when PANC-1 pancreatic carcinoma cells are treated with FGF2, the increase in incorporation of [3H]thymidine during the first 24 h is dramatically reduced by pretreatment with PIPLC, just as it is by pretreatment with heparinase III (which removes heparan sulfate from the cell surface). This effect of PIPLC was completely rescued by the expression of a transmembrane glypican-1 variant (Fig. 1 B).
Figure 1.
Glypican dependence of pancreatic carcinoma cell responses to FGF2. (A) PANC-1 cells maintained in serum-free medium were treated with or without 10 ng/ml FGF2 along with [3H]thymidine for 24 h. Cultures received 1 U/ml PIPLC (third bar) or 8 mU/ml heparinase III (fourth bar) 1 h before FGF addition and throughout the remainder of the assay. DNA was precipitated, and 3H incorporation was measured. Data are means ± SD of triplicates. (B) Glyp-TM+ samples were two independent clones of PANC-1 cells stably transfected with a transmembrane variant of glypican-1 (Kleeff et al., 1998). Sham-transfected samples were from two independent control PANC-1 clones transfected with vector only. Both types of clones were assayed as in A except that the concentration of FGF2 was 1 ng/ml. (A and B) Y axis is measured in counts per minute. (C) One of the Glyp-TM+ and one of the control clones from B were tested for MAPK activation after exposure to 2 ng/ml FGF2 for 1 h. For the right two lanes (PIPLC), 1 U/ml enzyme was added 1 h before FGF2 addition as well as during FGF2 incubation. Cells were lysed and subjected to Western blotting for p42/44ERK. (D) Mean values ± SD (error bars) of band intensities for each of the duplicate determinations shown in C; a similar picture is obtained if the data are expressed normalized to a loading control (not depicted). The reduction in MAPK activation by PIPLC in sham-transfected cells is statistically significant (P < 0.05; t test). Y axis is measured in arbitrary units. The results in A–C were also confirmed with FGF2 isolated from bovine brain (not depicted).
The inhibitory effect of PIPLC on the FGF2 response was also evident at the level of MAPK activation and there, too, could be rescued by a transmembrane glypican-1 (Fig. 1, C and D). In these experiments, MAPK induction was measured as the level of phosphorylation of p42/44ERK 1 h after FGF exposure.
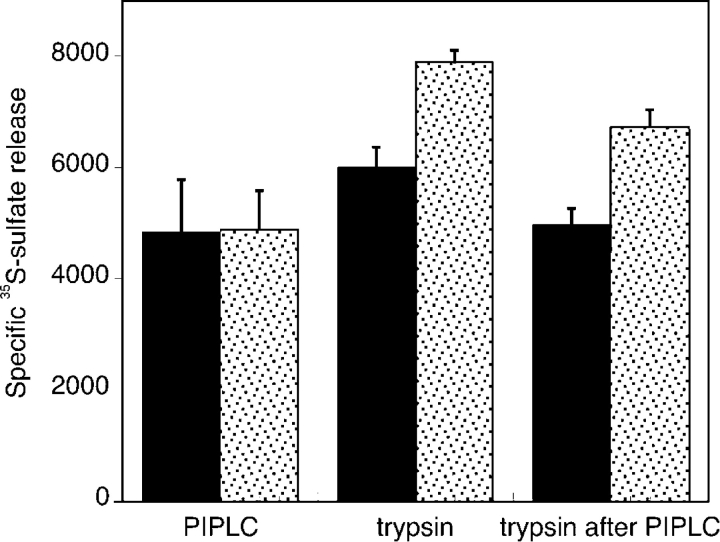
The ability of HSPGs to act as FGF coreceptors is thought to be a function of heparan sulfate chains and independent of core protein structure. The fact that PANC-1 cells express syndecan-1 (Conejo et al., 2000) but PIPLC (which removes only GPI-anchored molecules) blocks the responsiveness to FGF2 strongly suggests that the syndecan-1 on these cells is not an FGF coreceptor. Trivial explanations for this could be that there is not enough syndecan-1, it is not localized to the cell surface, or it lacks heparan sulfate. A variety of observations argue against these possibilities, the most general of which is shown in Fig. 2. Cells were cultured in the presence of [35S]sulfate, and the release of sulfated glycosaminoglycans (GAGs) was measured in response to either PIPLC or to a mild trypsin treatment that selectively cleaves cell surface syndecan-1, which has a juxtamembrane protease-sensitive site, to release an intact ectodomain (Subramanian et al., 1997). Released GAG-containing polypeptides were digested and precipitated to separately quantify protein-bound heparan and chondroitin sulfates.
Figure 2.
PIPLC releases only a fraction of cell surface heparan sulfate. PANC-1 cells metabolically labeled with [35S]sulfate were treated with or without PIPLC, and the supernatant was collected. Cells of each type were then incubated with or without TPCK-treated trypsin for 10 min on ice followed by the addition of trypsin inhibitor and collection of the supernatant. In this way, labeled fractions released by no enzyme, PIPLC, trypsin, and trypsin after PIPLC were obtained. To quantify protein-associated GAGs in these fractions, aliquots were digested with heparinase III, chondroitinase ABC, or no enzyme followed by TCA precipitation. Specific release of heparan sulfate (solid bars) by PIPLC was defined as the difference between chondroitinase-resistant radioactivity released from cells exposed and cells not exposed to PIPLC. The analogous calculations were performed to measure the specific release of heparan sulfate by trypsin or by trypsin after pretreatment with PIPLC. Similarly, the specific release of chondroitin sulfate (dotted bars) was calculated as heparinase-resistant radioactivity in the same fractions. Data are means ± SD (error bars) for duplicate experiments. Similar overall results were obtained when heparan sulfate was calculated by subtracting the amount of radioactivity precipitated by TCA after heparinase digestion from that precipitated from undigested supernatants. Likewise, similar results were obtained when chondroitin sulfate was calculated by subtracting the amount of radioactivity precipitated by TCA after chondroitinase digestion from that precipitated from undigested supernatants.
The results (Fig. 2) show that the amount of heparan sulfate released by PIPLC is approximately the same as that released by mild trypsinization. Moreover, after PIPLC treatment, trypsin released almost as much heparan sulfate as from cells that were not PIPLC treated (Fig. 2, second and third set of bars). Thus, PIPLC and trypsin release nearly nonoverlapping pools of cell surface heparan sulfate, with PIPLC-resistant heparan sulfate making up at least half of the total. From this, we conclude that on the surface of PANC-1 cells, HSPGs that are not glypicans and that possess the trypsin sensitivity of syndecan-1 are at least as abundant a carrier of heparan sulfate as are glypicans.
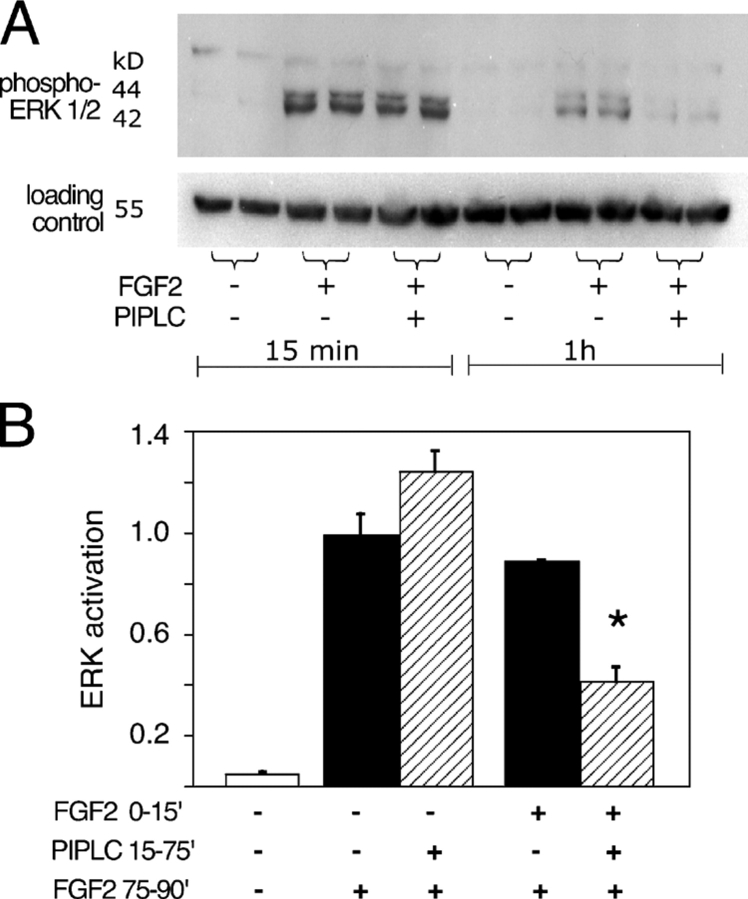
Although these results raised the possibility that differences in the structure of heparan sulfate on syndecan-1 versus glypican-1 might explain their differential use as FGF coreceptors, an alternate explanation came to mind after we examined the responses of PANC-1 cells to very brief FGF2 exposures. As shown in Fig. 3, when we measured MAPK activation 15 min after FGF2 addition (a time at which p42/44ERK phosphorylation is maximal), we found that prior treatment of cells with PIPLC had no significant effect (Fig. 3 A). However, if cells were first exposed to FGF2, treated with or without PIPLC for 1 h, and reexposed to FGF2 for 15 min, MAPK activation was substantially lower in the PIPLC-treated cells (Fig. 3 B).
Figure 3.
PIPLC blocks long-term but not short-term MAPK activation. (A) Where indicated, PANC-1 cells were incubated with 1 U/ml PIPLC for 1 h. Serum-free medium containing 1 ng/ml FGF2 (with 1 U/ml PIPLC where used) was added, and incubation continued for either 15 min or 1 h. Cells were lysed, and activated MAPK (p42/44ERK) was detected by immunoblotting. As a control for protein loading, immunoblotting was performed with an anti–β-tubulin mAb. Results from duplicate cultures are shown. (B) Cells were treated with FGF2 and analyzed as in A except that some samples were also exposed to FGF2 during the 15 min before the 1-h PIPLC incubation. When such cells were subsequently reexposed to FGF, short-term (15 min) MAPK activation was substantially PIPLC sensitive (asterisk; P < 0.02; t test). Data are duplicates ± SEM (error bars). Y axis is measured in arbitrary units.
These results suggest that when cells are first exposed to FGF2, glypican-1 is not the only HSPG that can serve as an FGF coreceptor, but FGF2 triggers an event that causes it to become so later on. An obvious candidate for such an event would be loss of syndecan-1 from the cell surface. Indeed, syndecan-1 (as well as other syndecans) is known to be shed from cell surfaces through cleavage by endogenous proteases. Moreover, a variety of ligands can trigger such shedding, including EGF and HB-EGF (Subramanian et al., 1997; Fitzgerald et al., 2000), although in the only case in which FGF2 was examined (a lymph node endothelial cell line), induced shedding of syndecan-1 was not seen (Subramanian et al., 1997).
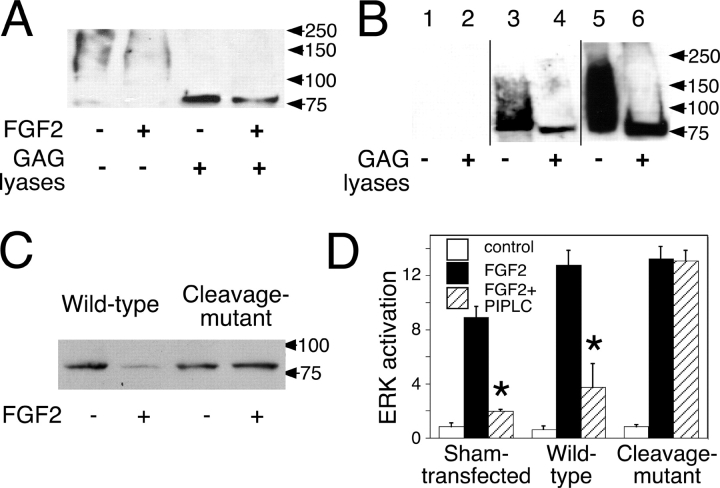
To test whether FGF2 might cause PANC-1 cells to shed their syndecan, we exposed cells to FGF2 for 30 min, washed, and then used mild trypsin to release the ectodomains of whatever syndecan-1 remained on the cells; these were then quantified by immunoblotting. As shown in Fig. 4 A, FGF2 caused a substantial reduction in syndecan-1 remaining on cell surfaces. In three independent experiments, we observed a mean decrease of 59 ± 11%. This value is likely to be an underestimate because the pool of trypsin-released syndecan-1 probably includes some molecules derived from dead cells, cell fragments, or substratum-attached material that would not be expected to respond to FGF2.
Figure 4.
FGF2 induces shedding of syndecan-1, and an “unsheddable” syndecan-1 makes FGF2 responses PIPLC resistant. (A) PANC-1 cells were treated with or without 2 ng/ml FGF2 for 30 min. After washing, trypsin was used to specifically release syndecan-1 ectodomains. Half of each sample was digested with 8 mU/ml heparinase III and 0.1 U/ml chondroitinase ABC. Syndecan-1 ectodomains were detected by Western blotting with mAb B-B4. Exposure times for the first and second lanes were longer than for the third and fourth lanes. (B) PANC-1 cells were stably transfected with expression constructs for wild-type mouse syndecan-1, an engineered variant of mouse syndecan-1 that replaces the cleavage sequence required for shedding with a heterologous one, or empty expression vector. Multiple clones of each type were expanded and examined by immunocytochemistry (not depicted) and Western blotting using mouse-specific syndecan-1 mAb 281.2. Western blot results from three representative clones are shown. Lanes 1 and 2, sham transfected; lanes 3 and 4, wild-type syndecan-1; lanes 5 and 6, cleavage mutant syndecan-1. (C) PANC-1 cells stably transfected with wild-type or cleavage mutant mouse syndecan-1 (from B) were treated with 2 ng/ml FGF2 for 30 min. After rinsing, cells were treated with trypsin as in A, and the released material was digested with 8 mU/ml heparinase III and 0.1 U/ml chondroitinase ABC. Syndecan-1 core protein was measured as in B. (A–C) Arrowheads show positions of molecular mass standards (in kD). (D) The three clones shown in B were tested for MAPK activation 1 h after the addition of 1 ng/ml FGF2. For both the sham-transfected and wild-type syndecan-1–transfected clones, pretreatment with 1 U/ml PIPLC (for 1 h) dramatically reduced FGF signaling (P < 0.02 in both cases; asterisks), whereas in the clone-expressing cleavage mutant syndecan-1, no significant reduction was seen. Data are from triplicate cultures for each condition and are normalized to loading controls. Error bars represent SEM. Y axis is measured in arbitrary units.
To test whether shedding of syndecan-1 plays a causal role in its failure to act as a long-term FGF coreceptor, we transfected PANC-1 cells with a mouse syndecan-1 in which the juxtamembrane protease-sensitive site had been replaced with a heterologous sequence. This form is known to resist shedding by endogenous proteases (Fitzgerald et al., 2000). As a control, we used wild-type mouse syndecan-1. As shown in Fig. 4 B, substantial expression of both wild-type and cleavage site–modified syndecan-1 could be detected in stably transfected cell clones. As was the case with the endogenous sydecan-1, we could readily detect FGF2-induced shedding of exogenous wild-type mouse syndecan-1 but saw no evidence for shedding of the cleavage mutant form (Fig. 4 C). When cells expressing these constructs were tested for MAPK activation 1 h after FGF2 addition, cells expressing cleavage-resistant syndecan-1 showed no inhibition by PIPLC, whereas the responses of cells expressing wild-type syndecan-1, like sham-transfected cells, were highly PIPLC sensitive (Fig. 4 D). Thus, when cells express a form of syndecan-1 that cannot be shed, long-term responses to FGF2 lose their glypican dependence.
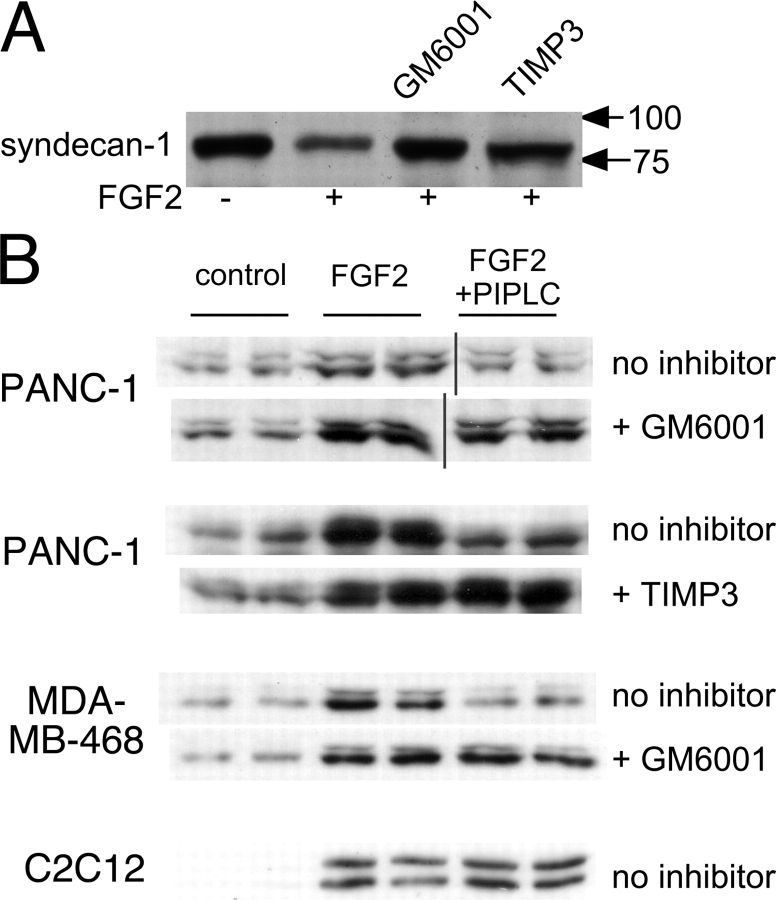
Studies in rodent cells suggest that the enzymes responsible for syndecan-1 shedding are members of the matrix metalloproteinase (MMP) family (Subramanian et al., 1997; Fitzgerald et al., 2000; Li et al., 2002; Asundi et al., 2003). If such enzymes also shed syndecan-1 in pancreatic carcinoma cells, one might expect inhibitors of MMPs to cause FGF2 responses to lose glypican dependence. This is indeed the case. As shown in Fig. 5 A, in PANC-1 cells, shedding of syndecan-1 can be blocked by pretreatment with either the broad spectrum MMP inhibitor GM6001 or TIMP-3, which is an endogenous polypeptide inhibitor of MMPs. At the same time, both GM6001 and TIMP-3 make the long-term FGF2 response of PANC-1 cells insensitive to PIPLC (Fig. 5 B, top two sets of blots). Results with GM6001 were not caused by any direct inhibitory effect of this drug on PIPLC itself, as such a possibility was assayed directly (using the PIPLC-mediated release of alkaline phosphatase from appropriate cell lines; not depicted).
Figure 5.
Inhibition of metalloproteinases protects cells from PIPLC inhibition of the FGF2 response. (A) Metalloproteinase inhibitors block FGF2-induced shedding of syndecan-1. PANC-1 cells were treated with or without 1μM GM6001 or 500 ng/ml TIMP-3 for 1 h and with 2 ng/ml FGF2 for 30 min. Syndecan-1 remaining on cell surfaces was released with trypsin, concentrated, digested with heparinase and chondroitinase, and quantified by Western blotting with mAb B-B4 as in Fig. 4. Arrows show molecular mass markers. (B) Metalloproteinase inhibition makes long-term FGF2 responses of tumor cells PIPLC insensitive, whereas the FGF2 responses of a nontumor cell line are already insensitive to PIPLC. PANC-1 cells, MDA-MB-468 breast carcinoma cells, and C2C12 mouse myoblasts were treated for 1 h with 1 μM GM6001, 500 ng/ml TIMP-3, or no protease inhibitor as indicated. Cells were then cultured for 1 h in the presence of 1 ng/ml FGF2 or no growth factor (control). FGF2 + PIPLC cells were also exposed to 1 U/ml PIPLC during both the first and second hours of incubation. Cell lysates were probed for p42/44ERK activation as in Figs. 1–4.
Pancreatic carcinoma cells are not the only cells that display strong glypican dependence of growth factor signaling; we reported similar behavior in several breast carcinoma cell lines (Matsuda et al., 2001). Like PANC-1 cells, such breast cancer cells express syndecan-1 (Matsuda et al., 2001). To determine whether syndecan-1 shedding might underlie the glypican dependence of these cells, we tested the effects of MMP inhibition on the ability of PIPLC to abrogate FGF2-mediated signaling. As shown in Fig. 5 B, MDA-MB-468 breast carcinoma cells behaved much like PANC-1 cells: long-term (1 h) MAPK activation was blocked by prior PIPLC exposure but could be completely rescued by GM6001. Fig. 5 B also shows results from a nontransformed mouse myoblast cell line (C2C12), which is known to express both syndecan-1 and glypican-1 and to respond to FGF2 (Brandan et al., 1996; Larrain et al., 1997). Unlike the tumor-derived cell lines, the long-term FGF2 responses of these cells were not diminished by pretreatment with PIPLC, suggesting, perhaps, that they do not undergo FGF2-induced syndecan shedding.
Recent studies have implicated MMP7 (matrilysin) as the protease that mediates stimulated shedding of syndecan-1 (Li et al., 2002), but some have argued that membrane-type (MT) MMPs (MT1-MMP and MT3-MMP; Endo et al., 2003) or nonmatrix-type metalloproteinases, such as members of the ADAM (a disintegrin and metalloproteinase domain) family (Holen et al., 2001), are actually responsible. Interestingly, MMP7 is frequently overexpressed by pancreatic cancer cells (Fukushima et al., 2001; Yamamoto et al., 2001; Crawford et al., 2002; Nakamura et al., 2002). To determine whether MMP7 mediates syndecan-1 shedding by PANC-1 cells and, thereby, accounts for the glypican-1 dependence of growth factor signaling, we first treated PANC-1 cells with exogenous active MMP7 and demonstrated that it is capable of releasing syndecan-1 into the medium (Fig. 6 A). Significantly, MMP7 did not release detectable glypican-1 (not depicted). Next, we showed that after treatment with exogenous MMP7, even short-term (15 min) responses of PANC-1 cells to FGF2 become PIPLC sensitive (Fig. 6 B). Thus, exposure to active MMP7 mimics the effects of prior exposure to FGF2 (Fig. 3 B). Control experiments verified that MMP7 does not itself degrade FGF2 (not depicted).
Figure 6.
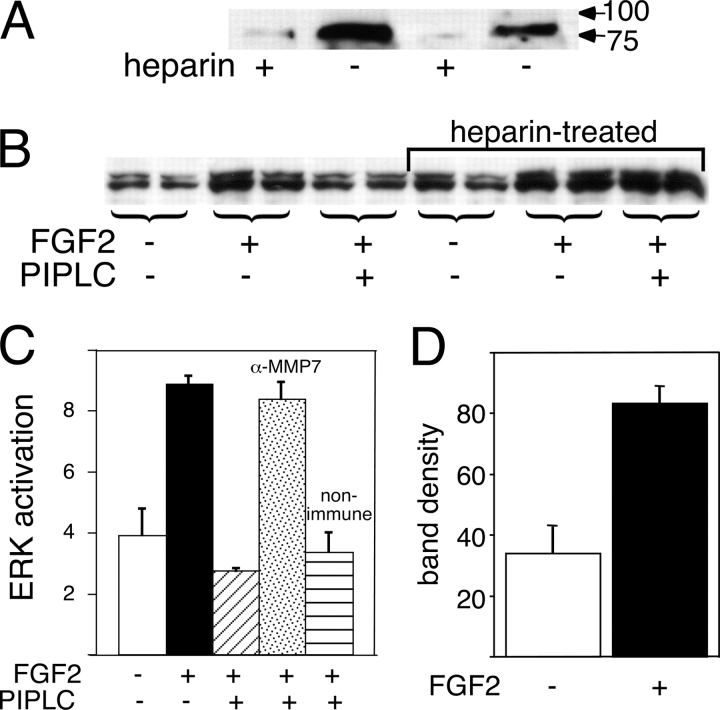
MMP7 is activated by FGF2, causes syndecan-1 shedding, and is sufficient to make FGF responses PIPLC sensitive. (A) Exogenous MMP7 causes shedding of syndecan-1. PANC-1 cells were treated with or without 1 μg/ml MMP7 for 30 min. The media were collected, concentrated, and digested with 8 mU/ml heparinase III and 0.1 U/ml chondroitinase ABC followed by SDS-PAGE and Western blotting with mAb B-B4. Syndecan-1 is seen as a high molecular mass smear that is converted by GAGases to a band of ∼80 kD apparent size. (B) When PANC-1 cells are pretreated with MMP7, even short-term responses to FGF2 become PIPLC sensitive. The responses of PANC-1 cells to a 15-min treatment with FGF2 were measured as in Fig. 3 A except that where indicated, cells were treated with 1 μg/ml MMP7 30 min before the addition of PIPLC (if used) and throughout the 15-min exposure to FGF2. In MMP7-treated cells, MAPK activation was strongly decreased by PIPLC (P < 0.01). (C) FGF2 induces MMP7 activation and release from the cell surface. PANC-1 cells were exposed to 2 ng/ml FGF2 for 30 min. The medium was removed, and cell surface MMP7 was released with 0.3 mg/ml heparin (in PBS). After concentrating as in A, samples were subjected to SDS-PAGE and Western blotting using a mixture of antibodies specific for human pro-MMP7 and activated MMP7. The asterisk shows the increase in released active MMP7 in response to FGF2 treatment. (A and C) Arrows on the right show positions of molecular mass standards (in kD). (D) FGF2 releases complexes of syndecan-1 and active MMP7. PANC-1 cells were treated with 5 ng/ml FGF2 for 30 min. Medium was collected and immunoprecipitated with antisyndecan-1 antibody, and precipitates were subjected to SDS-PAGE and Western blotting for activated MMP7. Controls consisted of immunoprecipitates from cells not treated with FGF2 or precipitation omitting antisyndecan antibody. The top two bands (asterisks) in the antibody-containing samples are IgG heavy and light chains. The lowest band is active MMP7 (arrow).
To test whether FGF2 causes the activation of endogenous cell surface MMP7, we took advantage of the fact that MMP7 associates with cell surfaces by binding to GAGs and can be specifically released by heparin (Yu and Woessner, 2000). Therefore, after treatment of PANC-1 cells with FGF2, we probed heparin washes of cells with antibodies specific for the precursor and active forms of MMP7. We detected both pro- and active MMP7 on the surface of untreated cells and found that the levels of both forms were lower after FGF2 treatment (Fig. 6 C). In contrast, when we examined what was released by cells during growth factor exposure, we saw more MMP7, especially the active form, in the material released from FGF2-treated as opposed to untreated cells (Fig. 6 C).
These results suggest that FGF2 not only activates MMP7 but that it causes newly activated MMP7 and some pro-MMP7 to be released from the cell surface. This makes sense if one recalls that cell surface MMP7 is associated with heparan sulfate, that about half of the heparan sulfate on PANC-1 cells exhibits the protease sensitivity of a syndecan (Fig. 2), and that MMP7 cleaves syndecan-1. Newly activated MMP7 molecules would thus be expected to induce their own shedding in association with syndecan-1 ectodomains. We can show that this occurs by collecting the material released by FGF2-treated PANC-1 cells, immunoprecipitating syndecan-1, and probing the immunoprecipitate with antibody to activated MMP7 (Fig. 6 D). The results confirm that MMP7–syndecan-1 ectodomain complexes are specifically released when PANC-1 cells are treated with FGF2.
Although these results demonstrate that FGF2 is sufficient to activate MMP7 and that activated MMP7 is sufficient to account for both the shedding of syndecan-1 and the subsequent glypican dependence of cell growth, they do not prove that MMP7 is solely responsible for syndecan-1 shedding. Other proposed syndecan-1 shedding enzymes are MT1-MMP, MT3-MMP (Endo et al., 2003), and ADAM metalloproteinases (Holen et al., 2001). One distinguishing feature of MMP7 is that it associates with cell surfaces through binding to GAGs (Yu and Woessner, 2000), whereas MT-MMPs and ADAMs are integral membrane proteins. Accordingly, MMP7—but not MT-MMPs or ADAMS—can be released by heparin. Therefore, we pretreated PANC-1 cells with heparin, washed, exposed the cells to FGF2, and tested for the release of syndecan-1. As shown in Fig. 7 A, heparin pretreatment blocked the ability of cells to shed syndecan-1. FGF2 signaling was not itself inhibited by the heparin pretreatment; in fact, such signaling became PIPLC insensitive (i.e., glypican-1 independent), as would be expected if syndecan-1 were remaining on the cell surface (Fig. 7 B). These data argue that the molecule responsible for syndecan-1 shedding and glypican-1–dependent mitogenesis is heparin displaceable, as would be expected for MMP7.
Figure 7.
MMP7 is required for syndecan-1 shedding. (A) Syndecan-1 shedding activity can be removed from the cell surface by treatment with heparin. PANC-1 cells were treated with or without 0.3 mg/ml heparin for 30 min. After washing twice with PBS, cells were exposed to 5 ng/ml FGF2 for 30 min. Medium was collected and subjected to heparinase and chondroitinase digestion as in Fig. 6 A. Samples were concentrated and subjected to SDS-PAGE and Western blotting for syndecan-1, the core protein of which appears at ∼80 kD (arrows show positions of molecular mass markers in kD). Results from two independent tests are shown. (B) Pretreatment with heparin rescues the PIPLC dependence of long-term FGF signaling. PANC-1 cells were treated with heparin as in A and were tested for the PIPLC dependence of long-term (1 h) FGF2 signaling as in Fig. 3 A. Whereas PIPLC greatly diminished the MAPK response of control cells (P < 0.01; t test), the responses of heparin-treated cells were unchanged (P > 0.4). (C) Pretreatment with a neutralizing antibody to MMP7 renders long-term FGF signaling PIPLC insensitive. PANC-1 cells were tested for the PIPLC dependence of long-term (1 h) FGF2 signaling as in Fig. 3 A, with antiactivated MMP7 or nonimmune antibody (both at 4 μg/ml) added 1 h before FGF2. (D) Activation of MMP7 by FGF2 does not require the action of a GM6001-sensitive metalloproteinase. PANC-1 cells were exposed to 1 μM GM600l for 1 h. Then, either 5 ng/ml FGF2 or control medium was added along with 1 μM GM6001 for 30 min. Cell surface MMP7 was harvested by extraction with 0.3 mg/ml heparin (in PBS), and samples were concentrated as in A. The presence of activated MMP7 was detected by Western blotting as in Fig. 6 D. Band densities are averaged from triplicate determinations ± SD (error bars) from equal numbers of cells and reveal a greater than twofold increase in activated MMP7 (P < 0.05; t test). (C and D) Y axis is measured in arbitrary units.
To establish definitively that this molecule is MMP7, we took advantage of the fact that a mAb specific for mature MMP7 has been shown to be function blocking (Wroblewski et al., 2003). As shown in Fig. 7 C, pretreatment of PANC-1 cells with this antibody abrogated the ability of PIPLC to inhibit the long-term FGF2 response, whereas pretreatment with control antibody had no effect.
The mechanisms by which growth factors activate pro-MMP7 are diverse and still poorly understood, although they all ultimately result in cleavage of a propeptide (Crabbe et al., 1992). Interestingly, when one treats PANC-1 cells with FGF2 in the presence of GM6001, one can see an increase in the level of activated MMP7 (using an antibody specific for this form of the enzyme) on the cell surface (Fig. 7 D). It makes sense that the newly activated MMP7 remains on the cell surface as opposed to being shed (Fig. 6 C) given that GM6001 should prevent syndecan-tethered MMP7 from releasing itself. That MMP7 becomes activated at all in the presence of GM6001 tells us that its activation by FGF2 is not mediated by another GM6001-sensitive metalloproteinase. Thus, MMP7 appears to be the sole metalloproteinase that is either directly or indirectly required for syndecan-1 shedding in these cells.
Discussion
The aforementioned results argue that in PANC-1 pancreatic carcinoma cells, both glypican-1 and syndecan-1 can act as coreceptors for FGF2, but because FGF2 activates MMP7, which, in turn, induces the shedding of syndecan-1, a sustained mitogenic response requires glypican-1. This conclusion is based on several observations. The FGF2 response of these cells is not initially sensitive to the removal of GPI-anchored proteins by PIPLC but becomes so after exposure to FGF2. FGF2 induces syndecan-1 shedding and also induces the activation of MMP7. Active MMP7 causes syndecan-1 shedding and by itself will render initial responses to FGF2 glypican dependent. When syndecan shedding is blocked with GM6001, TIMP-3, expression of a cleavage-resistant syndecan-1, depletion of cell surface MMP7 with heparin, or immunological blockade of MMP7, FGF2 responses no longer become glypican dependent.
Although this study focuses on the FGF2 responses of pancreatic carcinoma cells, it is likely that the phenomenon described here has wider relevance. First, glypican dependence is observed in the growth factor responses of breast cancer cells, and it is abrogated by the metalloproteinase inhibitor GM6001 (Fig. 5 D). Second, long-term responses to multiple heparin-binding growth factors (e.g., HB-EGF, HGF, and heregulins) are glypican dependent in pancreatic and breast cancer cells, and at least one of these factors (HB-EGF) is known to induce syndecan-1 shedding in some cells (Subramanian et al., 1997). Third, all four syndecans (not just syndecan-1) undergo shedding, with metalloproteinase dependence established for the shedding of syndecans-3 and -4 (Subramanian et al., 1997; Asundi et al., 2003). Indeed, although syndecan-1 is the major syndecan on PANC-1 cells, we can detect in these cells small amounts of mRNA for syndecans-2, -3, and -4 (unpublished data), raising the possibility that shedding of these syndecans could be of quantitative significance.
It is interesting that even before exposure to FGF2, substantial mature (activated) MMP7 is detected on PANC-1 cells (Fig. 6 C). The fact that substantial syndecan-1 is also present on the cell surface suggests that most of this MMP7 is nonfunctional, which is presumably a result of complex formation with an endogenous inhibitor (e.g., TIMP-1, which pancreatic carcinoma cells frequently overexpress; Zhou et al., 1998). Accordingly, when pro-MMP7 molecules are activated by exposure to FGF2, one might expect them to eventually become inhibited too. In agreement with this prediction, when we treat PANC-1 cells with FGF2 in the presence of GM6001 (Fig. 7 D) so that newly activated MMP7 is reversibly blocked and then remove GM6001 after 1 h, we do not observe an onset of syndecan-1 shedding (unpublished data). This suggests that over the course of an hour, activated MMP7 molecules do become stably inactivated.
It is provocative that the one nontransformed cell type studied here (C2C12 myoblasts) showed no evidence for glypican dependence of long-term FGF2 signaling, suggesting that growth factor–mediated syndecan shedding may be a phenomenon that is more common in tumor cells than in normal cells. If so, it increases the likelihood that the inhibition of glypican function could be of therapeutic value in selectively inhibiting tumor cell growth.
Our results add to an expanding list of studies on the functions of cell surface HSPGs (Bernfield et al., 1999; Lander and Selleck, 2000; Park et al., 2000; Perrimon and Bernfield, 2000). The degree to which syndecans and glypicans have similar versus different functions is a long-standing question. Comparisons among heparan sulfate structures on glypicans and syndecans on the same cells have failed to identify functionally relevant differences (Liu et al., 1996; Tumova et al., 2000; Zako et al., 2003), yet in a variety of in vitro systems, syndecans have been seen to carry out functions that glypicans cannot. For example, syndecan-1, but not glypican-1, inhibits the invasive behavior of myeloma cells, which is a function that maps to parts of the syndecan-1 core protein (Liu et al., 1996; Langford et al., 2005). In contrast, there are few direct examples of glypicans performing functions that syndecans cannot. Recently, one group reported that glypicans, but not syndecans, can support growth factor responses of glioma-associated brain endothelial cells (Qiao et al., 2003). It will be interesting to see whether, as in this study, such specificity arises as a consequence of induced syndecan shedding and, if so, whether MMP7 (which is not commonly found at high levels in gliomas; Vince et al., 1999) or a different protease is the culprit.
From a more general perspective, it is remarkable that the in vivo effects of the loss of function of glypicans in man, mice, frogs, and flies almost universally are abnormalities in growth or growth factor signaling (Pilia et al., 1996; Jackson et al., 1997; Tsuda et al., 1999; Grisaru et al., 2001; Desbordes and Sanson, 2003; Galli et al., 2003), whereas syndecan loss-of-function mutations influence cell adhesion, migration, axon guidance, neuropeptide activities, and synaptic function (Woods and Couchman, 2001; Bellin et al., 2002; Bhanot and Nussenzweig, 2002; Ishiguro et al., 2002; Kaksonen et al., 2002; Reizes et al., 2003; Steigemann et al., 2004) but only rarely influence cell growth (Alexander et al., 2000). As more genetic studies are undertaken, it will be interesting to see the extent to which this dichotomy holds up and whether growth factor–induced syndecan shedding explains some or all of it.
This study raises a number of questions concerning the roles of HSPGs in tumor formation and progression. Although it is widely accepted that overexpression of growth factors and their receptors by cancer cells plays a pivotal role in tumor progression (Friess et al., 1996; Mendelsohn and Baselga, 2000; Haddad et al., 2001; LeRoith and Roberts, 2003; Yu et al., 2003), the up-regulation of HSPG coreceptors by at least some tumor cells has only recently been appreciated (Kleeff et al., 1998; Matsuda et al., 2001; Zhu et al., 2001; Nakatsura et al., 2004). In part, this is because most early studies of HSPGs in cancer focused on syndecan-1 and generally reported no increase or even a decrease in its expression (Nackaerts et al., 1997; Pulkkinen et al., 1997; Fujimoto and Kohgo, 1998; Wiksten et al., 2000, 2001; Harada et al., 2003). However, in pancreatic carcinoma cells, both syndecan-1 and glypican-1 are highly up-regulated (Kleeff et al., 1998; Conejo et al., 2000; Barbareschi et al., 2003). In view of the present data, it seems likely that whatever advantage is afforded such cells by expressing syndecan-1, it comes from the shed, not the cell surface, form of the molecule. Interestingly, Yang et al. (2002) reported that the growth of human myeloma cells in vivo is strongly enhanced when the cells are engineered to express a constitutively shed form of syndecan-1. How shed syndecan-1 enhances tumor growth is unclear, but an attractive hypothesis is that syndecan ectodomains influence the extracellular proteolysis that is essential for tumor invasion and metastasis. Kainulainen et al. (1998) found that syndecan-1 ectodomains enhance proteolytic activities in wound fluids by protecting proteases from their endogenous inhibitors. Moreover, the activity of MMP7, which is strongly implicated in tumor progression (Yamamoto et al., 2001) and metastasis (Wilson et al., 1997), is stimulated by heparin (Yu and Woessner, 2000) and, therefore, may be greater when MMP7 is complexed with syndecan-1.
Another intriguing possibility, suggested by the present data, is that syndecan-1 shedding aids in transporting activated MMP7 away from tumor cells and into surrounding stroma. Ordinarily, one might expect that MMP7, which so strongly binds heparan sulfate, would not readily diffuse away from tumor cells as a result of trapping by cell surface and extracellular matrix HSPGs. By associating with syndecan-1 ectodomains (Fig. 6 D), MMP7 could avoid such trapping and, thereby, act at a greater distance. Such a mechanism of action is strikingly analogous to that demonstrated by Li et al. (2002) for syndecan-1 ectodomains as promoters of the diffusion of chemokines away from injured lung epithelial cells, an essential step in leukocyte recruitment.
Materials and methods
Materials
Materials were purchased from the following companies: DME and Leibovitz' L-15 medium (MediaTech, Inc.); OptiMEM (Invitrogen); HBSS (Irvine Scientific); FBS (Hyclone); penicillin–streptomycin solution and l-glutamine (Invitrogen); LipofectAMINE and Geneticin (G418; GIBCO BRL); methyl-[3H]thymidine and [35S]sulfate (PerkinElmer); DEAE-Sephacel, PD-10 desalting columns, ECL blotting reagents, and HRP-conjugated anti–rabbit antibodies (GE Healthcare); and Immobilon P (Millipore). Heparinase III and chondroitinase ABC were purchased from Seikagaku; GM6001 (Galardin) was obtained from BIOMOL Research Laboratories, Inc.; Texas red–conjugated goat anti–mouse IgG antibodies were obtained from Jackson ImmunoResearch Laboratories; anti-p42/44ERK (anti-ERK1/ERK2) was purchased from Promega or Cell Signaling Technology (similar results were obtained with either reagent); PIPLC was obtained from Invitrogen; recombinant human FGF2 was purchased from R&D Systems; bovine FGF2 was isolated from cow brain (Lobb and Fett, 1984); mouse anti–human syndecan-1 mAb (B-B4) was purchased from Serotec; anti–β-tubulin (D-10) was obtained from Santa Cruz Biotechnology, Inc.; and monoclonal anti-myc (9E10) was purchased from Covance, Inc. Active human MMP7 was obtained from Calbiochem, and antibodies to precursor and active forms of MMP7 (mAbs 3315 and 3322, respectively) as well as to TIMP-3 were obtained from Chemicon. A full-length mouse syndecan-1 expression plasmid and anti–mouse syndecan-1 mAb 281.2 were gifts from A. Rapraeger (University of Wisconsin, Madison, WI) and R.D. Sanderson (University of Arkansas for Medical Sciences, Little Rock, AR). A cleavage-resistant mouse syndecan-1 expression plasmid (Fitzgerald et al., 2000) was obtained from the research group of the late M. Bernfield (Harvard Medical School, Cambridge, MA). A construct encoding full-length placental alkaline phosphatase was a gift from J.L. Millan (Burnham Institute, La Jolla, CA). All other reagents were obtained from Sigma-Aldrich.
Cell culture and transfection
PANC-1 cells were cultured in DME with 6% (vol/vol) FBS. MDA-MB-468 breast carcinoma cells were cultured in L-15 medium with 10% (vol/vol) FBS. C2C12 myoblasts were cultured in DME with 15% FBS. Antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin sulfate) were added to all media. Cells were maintained at 37°C in a 5% CO2 atmosphere. Stable transfection of full-length mouse syndecan-1, cleavage-resistant mouse syndecan-1 (Fitzgerald et al., 2000), and pCDNA3.1/glyp1-VSVGTMR (Kleeff et al., 1998) into PANC-1 cells was performed using LipofectAMINE (Kleeff et al., 1998). After reaching confluence, cells were split into complete medium with 1 mg/ml G418. 2–3 wk later, independent clones were isolated. The expression of glyp1-VSVGTMR was evaluated by immunostaining using anti-myc antibodies. The expression of syndecan-1 was evaluated by immunostaining using anti–mouse syndecan-1 mAb 281.2 and by immunoblotting of detergent extractable proteoglycans. For the latter measurements, cells were extracted with 2% Triton X-100, 0.15 M NaCl, 10 mM EDTA, 10 mM KH2PO4, pH 7.5, along with 5 μg/ml BSA, 100 μg/ml PMSF, and 25 μg/ml N-ethylmaleimide (NEM). After centrifugation, supernatants were subjected to DEAE-Sephacel purification and were eluted with 150, 250, and 750 mM NaCl in 50 mM Tris-HCl, pH 8.0, at 4°C. The pool eluted by 750 mM NaCl was desalted on PD-10 followed by lyophilization. The resulting material was digested with 10 mU/ml heparinase III (37°C overnight in 3 mM Ca(OAc)2, 50 mM NaOAc, 50 mM Hepes, and 10 mM EDTA, pH 6.5) and chondroitinase ABC (20 mU/ml in 0.1 M Tris-HCl, 10 mM EDTA, pH 7.3) and subjected to 4–20% gradient SDS-PAGE and immunoblotting using anti–mouse syndecan-1.
DNA synthesis assay
PANC-1 cells were cultured in 24-well plates (30,000 cells/well) and allowed to attach for 24 h. After washing with HBSS, cells were switched to serum-free medium containing 0.2% BSA (RIA grade; Sigma-Aldrich) for 48 h. Where indicated, cells were then treated with 8 mU/ml heparinase III or 1 U/ml PIPLC for 1 h. 40 μCi/ml methyl-[3H]thymidine was added together with FGF2 at the indicated concentrations in fresh serum-free medium (containing fresh heparinase III and/or PIPLC, where used), and incubation continued for 24 h. Monolayers were washed with PBS, fixed with methanol, and washed with water. DNA was precipitated with 6% (wt/vol) trichloroactic acid and, following a water wash, was extracted with 0.3 M NaOH (Tanaka et al., 1992). Radioactivity was measured by scintillation counting. By this assay, the EC50 for the PANC-1 response to FGF2 was ∼0.7 ng/ml.
MAPK assay
5 × 105 PANC-1 or MDA-MB-468 cells were plated in 100-mm plates, cultured until confluent, and switched to serum-free medium containing 0.1% BSA for 48 h. In studies with C2C12 cells, cultures were switched to serum-free medium at 70% confluence for 16 h. Where indicated, cells were exposed to reagents such as 1 U/ml PIPLC, 1 μM GM6001, or 0.3 mg/ml heparin in serum-free growth medium for 1 h (or as indicated), and the medium was replaced with fresh serum-free medium containing FGF2 (plus PIPLC and/or GM6001 as indicated) for an additional period of either 15 min or 1 h at 37°C. Cells were washed twice with cold HBSS and lysed with PBS containing 0.2% (wt/vol) SDS, 0.5% (vol/vol) Triton X-100, 0.5% (wt/vol) sodium deoxycholate, 100 μg/ml PMSF, 1 μg/ml pepstatin A, 25 μg/ml NEM, 1 μg/ml aprotonin, 1 μg/ml leupeptin, 5 mM EDTA, 50 mM NaF, 50 mM Na4P2O7, and 100 mM Na3VO4. Samples were mixed with 0.25 vol of fivefold concentrated SDS-PAGE sample buffer and heated to 95°C for 8 min. After centrifugation, samples were subjected to 12% SDS-PAGE and transferred to Immobilon P. Membranes were incubated for 1 h or overnight with polyclonal rabbit anti–human p42/44ERK antibody, washed, and probed using HRP-conjugated anti–rabbit IgG. Visualization of bands by ECL was performed according to manufacturer's instructions. To verify equal protein loading, blots were washed and reprobed with anti–β-tubulin mAb (Leask and Stearns, 1998). In cases in which statistical tests were applied to the data, p42/44ERK band intensities were always first normalized to β-tubulin band intensities from the same samples.
Measurement of trypsin- and PIPLC-sensitive pools of cell surface GAGs
Confluent cultures of PANC-1 cells were incubated in growth medium with 12.25 μM reduced sulfate for 1 h and were cultured for 24 h in reduced sulfate medium containing 50 μCi/ml [35S]sulfate. After washing with ice-cold 0.5 mM EDTA–TBS (Subramanian et al., 1997), cells were incubated for 30 min at RT in the presence or absence of 2 U/ml PIPLC (in 0.5 mM EDTA–TBS), and the supernatant was collected. Cells were then incubated for 10 min on ice with or without 10 μg/ml tosylphenylchloroketone (TPCK)-treated trypsin (in 0.5 mM EDTA–TBS) followed by the addition of soybean trypsin inhibitor (50 μg/ml final concentration). Supernatants were subjected to digestion with either 8 mU/ml heparinase III, 0.1 U/ml chondroitinase ABC, both enzymes, or no enzyme for 2 h at 37°C followed by TCA precipitation and scintillation counting.
Measurement of cell surface, shed syndecan-1, and MMP7
To quantify endogenous cell surface syndecan-1, PANC-1 cells were incubated with 10 μg/ml TPCK-treated trypsin for 10 min on ice (Subramanian et al., 1997) followed by the addition of 50 μg/ml soybean trypsin inhibitor. Supernatants containing syndecan-1 ectodomains were digested with a mixture of 8 mU/ml heparinase III and 100 mU/ml chondroitinase ABC, subjected to SDS-PAGE, blotted, and probed using anti–human syndecan-1 (B-B4) mAb. To quantify shed syndecan-1, conditioned media were desalted on a PD-10 column, concentrated by lyophilization, and subjected to 4–20% SDS-PAGE. To quantify cell surface MMP7, cells were treated with 0.3 mg/ml heparin (in PBS) for 30 min at 4°C, and the supernatant was collected, desalted on a PD-10 column, concentrated by lyophilization, subjected to SDS-PAGE, blotted, and probed using mAbs specific for either the precursor or active forms of human MMP7 as indicated. To quantify shed MMP7, conditioned media were desalted, concentrated, subjected to SDS-PAGE, and analyzed by immunoblotting. To detect MMP7 that is complexed with shed syndecan-1, conditioned media were treated with 100 μg/ml PMSF, 2.5 μg/ml NEM, and antisyndecan-1 antibody B-B4 (1:200) overnight at 4°C. Syndecan-1 antibody complexes were precipitated with protein G–agarose beads, which were then washed, boiled in SDS sample buffer containing 2% β-mercaptoethanol, and subjected to SDS-PAGE and immunoblotting for active MMP7.
Antibody blockade of MMP7
The proteolytic activity of MMP7 was inhibited using mAb 3322 (Chemicon) as described previously (Wroblewski et al., 2003). In brief, cells were exposed to antibody or control nonimmune antibody (purified mouse IgG) at a final concentration of 4 μg/ml in culture medium beginning 1 h before exposure to FGF2.
Acknowledgments
We thank Dr. Alan Rapraeger, Dr. Ralph D. Sanderson, and Dr. Jose Luis Millan for gifts of reagents.
This work was supported by National Institutes of Health grants NS26862 (to A.D. Lander) and CA101306 (to M. Korc and A.D. Lander).
Abbreviations used in this paper: GAG, glycosaminoglycan; GPI, glycosylphosphatidylinositol; HB-EGF, heparin-binding EGF-like growth factor; HGF, hepatocyte growth factor; HSPG, heparan sulfate proteoglycan; MMP, matrix metalloproteinase; PIPLC, phosphoinositide-specific PLC.
References
- Alexander, C.M., F. Reichsman, M.T. Hinkes, J. Lincecum, K.A. Becker, S. Cumberledge, and M. Bernfield. 2000. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat. Genet. 25:329–332. [DOI] [PubMed] [Google Scholar]
- Asundi, V.K., R. Erdman, R.C. Stahl, and D.J. Carey. 2003. Matrix metalloproteinase-dependent shedding of syndecan-3, a transmembrane heparan sulfate proteoglycan, in Schwann cells. J. Neurosci. Res. 73:593–602. [DOI] [PubMed] [Google Scholar]
- Aviezer, D., and A. Yayon. 1994. Heparin-dependent binding and autophosphorylation of epidermal growth factor (EGF) receptor by heparin-binding EGF-like growth factor but not by EGF. Proc. Natl. Acad. Sci. USA. 91:12173–12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbareschi, M., P. Maisonneuve, D. Aldovini, M.G. Cangi, L. Pecciarini, F. Angelo Mauri, S. Veronese, O. Caffo, A. Lucenti, P.D. Palma, et al. 2003. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 98:474–483. [DOI] [PubMed] [Google Scholar]
- Bellaiche, Y., I. The, and N. Perrimon. 1998. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature. 394:85–88. [DOI] [PubMed] [Google Scholar]
- Bellin, R., I. Capila, J. Lincecum, P.W. Park, O. Reizes, and M.R. Bernfield. 2002. Unlocking the secrets of syndecans: transgenic organisms as a potential key. Glycoconj. J. 19:295–304. [DOI] [PubMed] [Google Scholar]
- Bernfield, M., M. Götte, P.W. Park, O. Reizes, M.L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729–777. [DOI] [PubMed] [Google Scholar]
- Bhanot, P., and V. Nussenzweig. 2002. Plasmodium yoelii sporozoites infect syndecan-1 deficient mice. Mol. Biochem. Parasitol. 123:143–144. [DOI] [PubMed] [Google Scholar]
- Brandan, E., D.J. Carey, J. Larrain, F. Melo, and A. Campos. 1996. Synthesis and processing of glypican during differentiation of skeletal muscle cells. Eur. J. Cell Biol. 71:170–176. [PubMed] [Google Scholar]
- Conejo, J.R., J. Kleeff, A. Koliopanos, K. Matsuda, Z.W. Zhu, H. Goecke, N. Bicheng, A. Zimmermann, M. Korc, H. Friess, and M.W. Buchler. 2000. Syndecan-1 expression is up-regulated in pancreatic but not in other gastrointestinal cancers. Int. J. Cancer. 88:12–20. [DOI] [PubMed] [Google Scholar]
- Crabbe, T., F. Willenbrock, D. Eaton, P. Hynds, A.F. Carne, G. Murphy, and A.J. Docherty. 1992. Biochemical characterization of matrilysin. Activation conforms to the stepwise mechanisms proposed for other matrix metalloproteinases. Biochemistry. 31:8500–8507. [DOI] [PubMed] [Google Scholar]
- Crawford, H.C., C.R. Scoggins, M.K. Washington, L.M. Matrisian, and S.D. Leach. 2002. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J. Clin. Invest. 109:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbordes, S.C., and B. Sanson. 2003. The glypican Dally-like is required for Hedgehog signalling in the embryonic epidermis of Drosophila. Development. 130:6245–6255. [DOI] [PubMed] [Google Scholar]
- Endo, K., T. Takino, H. Miyamori, H. Kinsen, T. Yoshizaki, M. Furukawa, and H. Sato. 2003. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 278:40764–40770. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M.L., Z. Wang, P.W. Park, G. Murphy, and M. Bernfield. 2000. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J. Cell Biol. 148:811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess, H., P. Berberat, M. Schilling, J. Kunz, M. Korc, and M.W. Buchler. 1996. Pancreatic cancer: the potential clinical relevance of alterations in growth factors and their receptors. J. Mol. Med. 74:35–42. [DOI] [PubMed] [Google Scholar]
- Fujimoto, Y., and Y. Kohgo. 1998. Alteration of genomic structure and/or expression of cancer associated genes in hepatocellular carcinoma. Rinsho Byori. 46:9–14. [PubMed] [Google Scholar]
- Fujise, M., S. Takeo, K. Kamimura, T. Matsuo, T. Aigaki, S. Izumi, and H. Nakato. 2003. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development. 130:1515–1522. [DOI] [PubMed] [Google Scholar]
- Fukushima, H., H. Yamamoto, F. Itoh, H. Nakamura, Y. Min, S. Horiuchi, S. Iku, S. Sasaki, and K. Imai. 2001. Association of matrilysin mRNA expression with K-ras mutations and progression in pancreatic ductal adenocarcinomas. Carcinogenesis. 22:1049–1052. [DOI] [PubMed] [Google Scholar]
- Galli, A., A. Roure, R. Zeller, and R. Dono. 2003. Glypican 4 modulates FGF signalling and regulates dorsoventral forebrain patterning in Xenopus embryos. Development. 130:4919–4929. [DOI] [PubMed] [Google Scholar]
- Grisaru, S., D. Cano-Gauci, J. Tee, J. Filmus, and N.D. Rosenblum. 2001. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev. Biol. 231:31–46. [DOI] [PubMed] [Google Scholar]
- Haddad, R., K.E. Lipson, and C.P. Webb. 2001. Hepatocyte growth factor expression in human cancer and therapy with specific inhibitors. Anticancer Res. 21:4243–4252. [PubMed] [Google Scholar]
- Harada, K., S. Masuda, M. Hirano, and Y. Nakanuma. 2003. Reduced expression of syndecan-1 correlates with histologic dedifferentiation, lymph node metastasis, and poor prognosis in intrahepatic cholangiocarcinoma. Hum. Pathol. 34:857–863. [DOI] [PubMed] [Google Scholar]
- Holen, I., N.L. Drury, P.G. Hargreaves, and P.I. Croucher. 2001. Evidence of a role for a non-matrix-type metalloproteinase activity in the shedding of syndecan-1 from human myeloma cells. Br. J. Haematol. 114:414–421. [DOI] [PubMed] [Google Scholar]
- Ishiguro, K., T. Kojima, and T. Muramatsu. 2002. Syndecan-4 as a molecule involved in defense mechanisms. Glycoconj. J. 19:315–318. [DOI] [PubMed] [Google Scholar]
- Jackson, S.M., H. Nakato, M. Sugiura, A. Jannuzi, R. Oakes, V. Kaluza, C. Golden, and S.B. Selleck. 1997. dally, a Drosophila glypican, controls cellular responses to the TGF-β-related morphogen, Dpp. Development. 124:4113–4120. [DOI] [PubMed] [Google Scholar]
- Kainulainen, V., H. Wang, C. Schick, and M. Bernfield. 1998. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J. Biol. Chem. 273:11563–11569. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M., I. Pavlov, V. Voikar, S.E. Lauri, A. Hienola, R. Riekki, M. Lakso, T. Taira, and H. Rauvala. 2002. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell. Neurosci. 21:158–172. [DOI] [PubMed] [Google Scholar]
- Kleeff, J., T. Ishiwata, A. Kumbasar, H. Friess, M.W. Büchler, A.D. Lander, and M. Korc. 1998. The cell surface heparan sulfate proteoglycan glypican-1 is an essential regulator of growth factor action in pancreatic carcinoma cells, and is overexpressed in human pancreatic cancer. J. Clin. Invest. 102:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeff, J., S. Wildi, A. Kumbasar, H. Friess, A.D. Lander, and M. Korc. 1999. Stable transfection of a glypican-1 antisense construct decreases tumorigenicity in PANC-1 pancreatic carcinoma cells. Pancreas. 19:281–288. [DOI] [PubMed] [Google Scholar]
- Lander, A.D., and S.B. Selleck. 2000. The elusive functions of proteoglycans: in vivo veritas. J. Cell Biol. 148:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford, J.K., Y. Yang, T. Kieber-Emmons, and R.D. Sanderson. 2005. Identification of an invasion regulatory domain within the core protein of syndecan-1. J. Biol. Chem. 280:3467–3473. [DOI] [PubMed] [Google Scholar]
- Larrain, J., J. Alvarez, J.R. Hassell, and E. Brandan. 1997. Expression of perlecan, a proteoglycan that binds myogenic inhibitory basic fibroblast growth factor, is down regulated during skeletal muscle differentiation. Exp. Cell Res. 234:405–412. [DOI] [PubMed] [Google Scholar]
- Leask, A., and T. Stearns. 1998. Expression of amino- and carboxyl-terminal gamma- and alpha-tubulin mutants in cultured epithelial cells. J. Biol. Chem. 273:2661–2668. [DOI] [PubMed] [Google Scholar]
- LeRoith, D., and C.T. Roberts Jr. 2003. The insulin-like growth factor system and cancer. Cancer Lett. 195:127–137. [DOI] [PubMed] [Google Scholar]
- Li, Q., and J.A. Loeb. 2001. Neuregulin-heparan-sulfate proteoglycan interactions produce sustained erbB receptor activation required for the induction of acetylcholine receptors in muscle. J. Biol. Chem. 276:38068–38075. [DOI] [PubMed] [Google Scholar]
- Li, Q., P.W. Park, C.L. Wilson, and W.C. Parks. 2002. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 111:635–646. [DOI] [PubMed] [Google Scholar]
- Liu, W., E.D. Litwack, M.J. Stanley, J.K. Langford, A.D. Lander, and R.D. Sanderson. 1996. Heparan sulfate proteoglycans as anti-invasive molecules. Syndecans and glypican have distinct functions. J. Biol. Chem. 273:22825–22832. [DOI] [PubMed] [Google Scholar]
- Lobb, R.R., and J.W. Fett. 1984. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 23:6295–6299. [DOI] [PubMed] [Google Scholar]
- Matsuda, K., H. Maruyama, F. Guo, J. Kleeff, J. Itakura, Y. Matsumoto, A.D. Lander, and M. Korc. 2001. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 61:5562–5569. [PubMed] [Google Scholar]
- Mendelsohn, J., and J. Baselga. 2000. The EGF receptor family as targets for cancer therapy. Oncogene. 19:6550–6565. [DOI] [PubMed] [Google Scholar]
- Nackaerts, K., E. Verbeken, G. Deneffe, B. Vanderschueren, M. Demedts, and G. David. 1997. Heparan sulfate proteoglycan expression in human lung-cancer cells. Int. J. Cancer. 74:335–345. [DOI] [PubMed] [Google Scholar]
- Nakamura, H., S. Horita, N. Senmaru, Y. Miyasaka, T. Gohda, Y. Inoue, M. Fujita, T. Meguro, T. Morita, and K. Nagashima. 2002. Association of matrilysin expression with progression and poor prognosis in human pancreatic adenocarcinoma. Oncol. Rep. 9:751–755. [PubMed] [Google Scholar]
- Nakatsura, T., T. Kageshita, S. Ito, K. Wakamatsu, M. Monji, Y. Ikuta, S. Senju, T. Ono, and Y. Nishimura. 2004. Identification of glypican-3 as a novel tumor marker for melanoma. Clin. Cancer Res. 10:6612–6621. [DOI] [PubMed] [Google Scholar]
- Park, P.W., O. Reizes, and M. Bernfield. 2000. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J. Biol. Chem. 275:29923–29926. [DOI] [PubMed] [Google Scholar]
- Perrimon, N., and M. Bernfield. 2000. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 404:725–728. [DOI] [PubMed] [Google Scholar]
- Pilia, G., R.M. Hughes-Benzie, A. MacKenzie, P. Baybayan, E.Y. Chen, R. Huber, G. Neri, A. Cao, A. Foarabosco, and D. Schlessinger. 1996. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat. Genet. 12:241–247. [DOI] [PubMed] [Google Scholar]
- Pulkkinen, J.O., M. Penttinen, M. Jalkanen, P. Klemi, and R. Grenman. 1997. Syndecan-1: a new prognostic marker in laryngeal cancer. Acta Otolaryngol. 117:312–315. [DOI] [PubMed] [Google Scholar]
- Qiao, D., K. Meyer, C. Mundhenke, S.A. Drew, and A. Friedl. 2003. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J. Biol. Chem. 278:16045–16053. [DOI] [PubMed] [Google Scholar]
- Rapraeger, A., A. Krufka, and B.B. Olwin. 1991. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 252:1705–1708. [DOI] [PubMed] [Google Scholar]
- Reizes, O., S.C. Benoit, A.D. Strader, D.J. Clegg, S. Akunuru, and R.J. Seeley. 2003. Syndecan-3 modulates food intake by interacting with the melanocortin/AgRP pathway. Ann. NY Acad. Sci. 994:66–73. [DOI] [PubMed] [Google Scholar]
- Steigemann, P., A. Molitor, S. Fellert, H. Jackle, and G. Vorbruggen. 2004. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr. Biol. 14:225–230. [DOI] [PubMed] [Google Scholar]
- Steinfeld, R., H. Van Den Berghe, and G. David. 1996. Stimulation of fibroblast growth factor receptor-1 occupancy and signaling by cell surface-associated syndecans and glypican. J. Cell Biol. 133:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, S.V., M.L. Fitzgerald, and M. Bernfield. 1997. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J. Biol. Chem. 272:14713–14720. [DOI] [PubMed] [Google Scholar]
- Tanaka, A., K. Miyamoto, N. Minamino, M. Takeda, B. Sato, H. Matsuo, and K. Matsumoto. 1992. Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc. Natl. Acad. Sci. USA. 89:8928–8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda, M., K. Kamimura, H. Nakato, M. Archer, W. Staatz, B. Fox, M. Humphrey, S. Olson, T. Futch, V. Kaluza, et al. 1999. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature. 400:276–280. [DOI] [PubMed] [Google Scholar]
- Tumova, S., A. Woods, and J.R. Couchman. 2000. Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences. J. Biol. Chem. 275:9410–9417. [DOI] [PubMed] [Google Scholar]
- Vince, G.H., S. Wagner, T. Pietsch, R. Klein, R.H. Goldbrunner, K. Roosen, and J.C. Tonn. 1999. Heterogeneous regional expression patterns of matrix metalloproteinases in human malignant gliomas. Int. J. Dev. Neurosci. 17:437–445. [DOI] [PubMed] [Google Scholar]
- Wiksten, J.P., J. Lundin, S. Nordling, A. Kokkola, and C. Haglund. 2000. A prognostic value of syndecan-1 in gastric cancer. Anticancer Res. 20:4905–4907. [PubMed] [Google Scholar]
- Wiksten, J.P., J. Lundin, S. Nordling, M. Lundin, A. Kokkola, K. von Boguslawski, and C. Haglund. 2001. Epithelial and stromal syndecan-1 expression as predictor of outcome in patients with gastric cancer. Int. J. Cancer. 95:1–6. [DOI] [PubMed] [Google Scholar]
- Wilson, C.L., K.J. Heppner, P.A. Labosky, B.L. Hogan, and L.M. Matrisian. 1997. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc. Natl. Acad. Sci. USA. 94:1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A., and J.R. Couchman. 2001. Syndecan-4 and focal adhesion function. Curr. Opin. Cell Biol. 13:578–583. [DOI] [PubMed] [Google Scholar]
- Wroblewski, L.E., P.J. Noble, A. Pagliocca, D.M. Pritchard, C.A. Hart, F. Campbell, A.R. Dodson, G.J. Dockray, and A. Varro. 2003. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J. Cell Sci. 116:3017–3026. [DOI] [PubMed] [Google Scholar]
- Yamamoto, H., F. Itoh, S. Iku, Y. Adachi, H. Fukushima, S. Sasaki, M. Mukaiya, K. Hirata, and K. Imai. 2001. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J. Clin. Oncol. 19:1118–1127. [DOI] [PubMed] [Google Scholar]
- Yang, Y., S. Yaccoby, W. Liu, J.K. Langford, C.Y. Pumphrey, A. Theus, J. Epstein, and R.D. Sanderson. 2002. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 100:610–617. [DOI] [PubMed] [Google Scholar]
- Yu, J., C. Ustach, and H.R. Kim. 2003. Platelet-derived growth factor signaling and human cancer. J. Biochem. Mol. Biol. 36:49–59. [DOI] [PubMed] [Google Scholar]
- Yu, W.H., and J.F. Woessner Jr. 2000. Heparan sulfate proteoglycans as extracellular docking molecules for matrilysin (matrix metalloproteinase 7). J. Biol. Chem. 275:4183–4191. [DOI] [PubMed] [Google Scholar]
- Zako, M., J. Dong, O. Goldberger, M. Bernfield, J.T. Gallagher, and J.A. Deakin. 2003. Syndecan-1 and -4 synthesized simultaneously by mouse mammary gland epithelial cells bear heparan sulfate chains that are apparently structurally indistinguishable. J. Biol. Chem. 278:13561–13569. [DOI] [PubMed] [Google Scholar]
- Zhang, Z., C. Coomans, and G. David. 2001. Membrane heparan sulfate proteoglycan-supported FGF2-FGFR1 signaling: evidence in support of the “cooperative end structures” model. J. Biol. Chem. 276:41921–41929. [DOI] [PubMed] [Google Scholar]
- Zhou, W., L.J. Sokoll, D.J. Bruzek, L. Zhang, V.E. Velculescu, S.B. Goldin, R.H. Hruban, S.E. Kern, S.R. Hamilton, D.W. Chan, et al. 1998. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol. Biomarkers Prev. 7:109–112. [PubMed] [Google Scholar]
- Zhu, Z.W., H. Friess, L. Wang, M. Abou-Shady, A. Zimmermann, A.D. Lander, M. Korc, J. Kleeff, and M.W. Buchler. 2001. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 48:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zioncheck, T.F., L. Richardson, J. Liu, L. Chang, K.L. King, G.L. Bennett, P. Fugedi, S.M. Chamow, R.H. Schwall, and R.J. Stack. 1995. Sulfated oligosaccharides promote hepatocyte growth factor association and govern its mitogenic activity. J. Biol. Chem. 270:16871–16878. [DOI] [PubMed] [Google Scholar]