Abstract
Nucleostemin (NS) was identified as a stem cell– and cancer cell–enriched nucleolar protein that controls the proliferation of these cells. Here, we report the mechanism that regulates its dynamic shuttling between the nucleolus and nucleoplasm. The nucleolar residence of nucleostemin involves a transient and a long-term binding by the basic and GTP-binding domains, and a dissociation mechanism mediated by the COOH-terminal region. This cycle is propelled by the GTP binding state of nucleostemin. We propose that a rapid nucleostemin cycle is designed to translate extra- and intra-cellular signals into the amount of nucleostemin in the nucleolus in a bidirectional and fast manner.
Introduction
Stem cells are the building blocks during organogenesis. In adults, they are responsible for replacing natural cell loss and may promote recovery after injury. To maintain tissue homeostasis and avoid tumor formation, it is critical to adjust the stem cell division rate based on the supply of nutrients and mitogens, as well as the factors that signal the size of the tissue (Bullough, 1965). However the molecular program regulating the transition of stem cells between the actively dividing and quiescent states remains unclear.
As a step in this direction, we have identified a novel gene nucleostemin (NS) (Tsai and McKay, 2002) that is preferentially expressed in the nucleoli of neural stem cells, embryonic stem cells, and several cancer cell lines. NS is required for stem cells and cancer cells to remain in the cell cycle, suggesting that the rate of proliferation in these cells can be modulated by the activity of NS. Given that NS is localized primarily in the nucleolus where key features of cell growth including ribosome biogenesis occur, partitioning the amount of NS between the nucleolar and nucleoplasmic compartments could provide a way to regulate its activity. This idea is supported by previous reports demonstrating a positive correlation between the size of the nucleolus, the nucleolar activity, and the cell growth rate (Derenzini et al., 1998, 2000). To date, little is known about the molecular machinery that allows the cells to adjust their nucleolar size dynamically and reversibly.
The structure of the nucleolus is complex, and the analysis of nucleolar functions is further complicated by the fact that many of the nucleolar proteins are found both in the nucleolus and the nucleoplasm (Chen and Huang, 2001; Dundr and Misteli, 2002; Fox et al., 2002). In this manuscript, we investigate the mechanism that controls the NS distribution. Our findings suggest a GTP-driven cycling mechanism that modulates the amount of NS in the nucleolus in a fast and bidirectional manner.
Results and discussion
NS shuttles between the nucleolus and the nucleoplasm
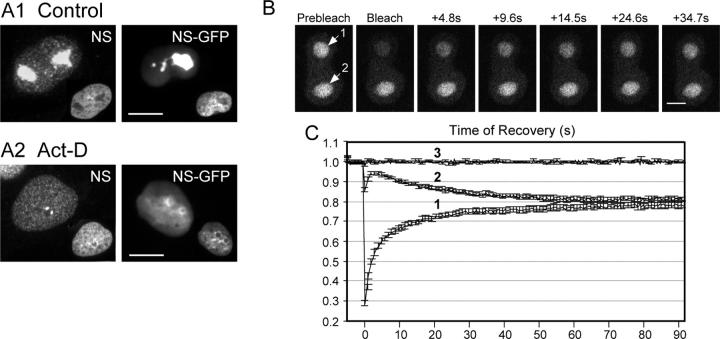
In addition to its predominant nucleolar distribution, NS is also present at low levels in the nucleoplasm (Tsai and McKay, 2002). To determine the mechanism controlling the NS localization, we first examined if there is a dynamic exchange of NS between these two compartments using the FRAP and inverse FRAP (iFRAP) approaches. Photobleaching experiments were conducted on GFP-tagged NS protein (NS-GFP) in living cells using time-lapse confocal microscopy (Phair and Misteli, 2000). U2 OS cells or CHO cells were chosen because of their large, flat-shaped nucleoli, resistance to phototoxicity, and their high levels of endogenous NS expression. In addition to strongly labeled nucleoli, the expressed NS-GFP also gave weak, diffuse signals in the nucleoplasm, similar to the endogenous NS (Fig. 1 A1, NS vs. NS-GFP). After actinomycin D (Act-D) treatment, an RNA polymerase inhibitor that blocks transcription by intercalating between nucleotide base pairs and induces nucleolar reorganization (Recher et al., 1971a,b), both the endogenous NS and NS-GFP were redistributed from the nucleolus to the nucleoplasm, with the strongest signals surrounding the nucleolar region (Fig. 1 A2). The total amount of NS-GFP protein appears higher than the endogenous NS in the Act-D–treated cells, which may be due to the different promoters that drive their expression (cytomegalovirus vs. the endogenous NS promoter). Based on the basal distribution and the parallel changes in localization after Act-D treatment of wild-type NS-GFP, as well as the growth arrest effect seen with the mutated NS-GFP (Tsai and McKay, 2002), we used NS-GFP as a living tracer to track the dynamic distribution of endogenous NS.
Figure 1.
NS cycles between the nucleolus and the nucleoplasm. (A) Endogenous NS and expressed NS-GFP fusion protein display a nucleolar distribution in control cells (A1), and a nucleoplasmic distribution plus intense signals surrounding the nucleolar region in Act-D–treated CHO cells (A2). Inset: DAPI nuclear staining of the same cells. Bars, 10 μm. Time-sequenced images (B) and quantitative analyses (C) of FRAP (label 1) and iFRAP (label 2) on NS-GFP–transfected CHO cells with two nucleoli. After subtraction of the background signal, the relative intensities were normalized to the nonbleached signals in different cells, recorded in parallel, and shown in line 3 (C). Data were averaged over 20 cells in three independent experiments. Y-error bars represent SEM, omitted at every other data point for clarity. Bar, 4 μm.
In the first study, cells with two nucleoli of comparable size and intensity were photobleached in only one of their nucleoli by a short laser pulse that irreversibly quenched the GFP signal. The recovery of fluorescence in the bleached nucleolar area (Fig. 1, B and C; label 1) reached 95% (y axis: 0.758) of the plateau level (y axis: 0.783) within 39 s, reflecting the unbleached nucleoplasmic NS-GFP moving into the nucleolus. The dissociation rate of NS from the nucleolus was measured by recording the signal loss in the nonbleached nucleolus of the same cells (iFRAP). 95% (y axis: 0.822) of the lost signals (y axis: from 1 to 0.813) (Fig. 1 B and C; label 2) were reached within 46 s after photobleaching. Together, these results show that NS moves bidirectionally between the nucleolus and the nucleoplasm. The coordinated changes of fluorescence signals in the bleached and nonbleached nucleoli and the fast kinetics of this process supports that these findings are not caused by protein synthesis or by protein degradation as a result of photobleaching. Finally, the fluorescent recovery rate of NS-GFP relative to the plateau recovery intensity remains constant regardless of the cell types used to express it (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200409053/DC1). These data reveal a bidirectional shuttling of NS between the nucleolus and nucleoplasm at a rate that is fast and cell type independent.
GTP binding regulates the nucleolar targeting of NS
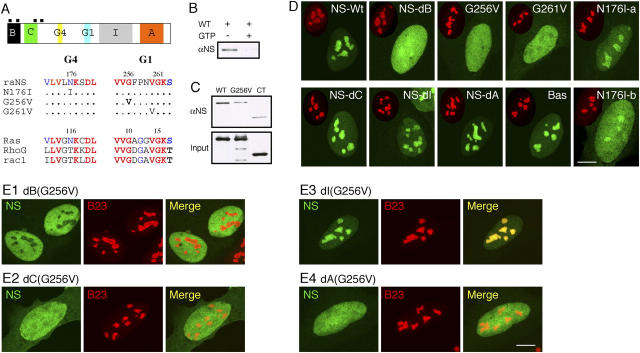
The structure of NS consists of an NH2-terminal basic (B) domain, followed by a coiled-coil (C) domain, two GTP-binding motifs (G4: KXDL; G1: GXXXXGK[S/T]), a COOH-terminal acidic domain (A), and an intermediate (I) region between the G1 motif and the A-domain (Fig. 2 A, top). The GTP-binding motifs of NS allow us to investigate how the GTP-binding state of NS directly influences its nucleolar distribution. To this end, we generated single amino acid mutation on the conserved residues in the G4 or G1 motif (Fig. 2 A, bottom). G256 and G261 are conserved among NS and the Ras family. The N176I mutation mimics the constitutive negative Ras mutant N116I (Huang and Chuang, 2000). GTP-binding assays demonstrated a specific retention of NS by the GTP-conjugated agarose, and this interaction was blocked by preincubating NS with 10 mM free GTP (Fig. 2 B) (Iismaa et al., 1997). This GTP-binding capacity of NS depends on the G1 motif, as single amino acid substitution of the conserved G256 residue (Fig. 2 C, G256V) decreased its GTP-binding ability to the control level (CT, the COOH-terminal 188 aa polypeptide of NS lacking the G4 and G1 motifs). These findings demonstrate a role of the G1 motif in the GTP binding of NS. Just as the NH2-terminal B-domain was required for the nucleolar distribution of NS (Fig. 2 D, NS-dB), mutations in the G1 motif (G256V or G261V) also blocked the nucleolar localization of NS, and created a diffuse distribution in the nucleus. Mutation on the N176 residue, which is not conserved in the small GTPase family, exhibited a partial blocking effect on the nucleolar localization of NS. Cells expressing the N176I mutant, while maintaining a nucleolus-predominant distribution, displayed higher nucleoplasmic intensity than the wild-type NS, with a slight variation from cells to cells (N176I-a, b). Based on these observations, we concluded that N176 is not as critical in mediating the GTP binding as the conserved G256 or G261, and used G256V as the prototypical non-GTP-binding mutant for NS in the following experiments. By contrast, deletions in the C-domain (NS-dC), I-domain (NS-dI), or A-domain (NS-dA) did not affect the static distribution of NS in the nucleolus. These results show that both the B-domain and the wild-type GTP-binding domain are required for the nucleolar targeting of NS.
Figure 2.
Nucleolar targeting of NS requires the B-domain and GTP-binding domain, and is blocked by the I-domain. (A) Top: a schematic diagram of the NS protein structure (B: basic; C: coiled-coil; G4, G1: GTP-binding motifs; I: intermediate; A: acidic domains; black boxes: nuclear localization signals). (Bottom) Sequences of single amino acid substitution on the conserved residues in the G4 and G1 motifs. (B) Purified GST fusion protein of wild-type NS could bind GTP-agarose (left lane). This interaction was abolished by competition with 10 mM free GTP (right lane). (C) The role of the G1 motif in mediating the GTP binding of NS was shown by a mutation in the G256 residue (G256V) that reduced its binding affinity to the background level as the COOH-terminal NS control (CT). (D) The static distribution of NS mutants in U2 OS cells (NS-Wt: wild-type; NS-dB: B-domain deletion; NS-dC: C-domain deletion; NS-dI: I-domain deletion; NS-dA: A-domain deletion; Bas: B-domain alone). Green: GFP-fused mutants; red: anti-B23 immunostaining of the same cell (scaled to 60%). (E) Mapping the inhibitory domain that gates the nucleolus-targeting event mediated by the B-domain. A deletion of the I-domain (E3), but not the B- (E1), C- (E2), or A- (E4) domain, can rescue the nucleolar phenotype of G256V mutation. Bars (D and E), 10 μm.
Notably, despite the requirement of the GTP-binding motifs for the nucleolar localization of NS, B-domain alone was sufficient to target the GFP protein to the nucleolus (Fig. 2 D, Bas). This finding suggests an inhibitory mechanism that gates the nucleolus-targeting activity of the full-length NS, and that it is regulated by the GTP-binding state of NS. To map this region, deletions of the B-, C-, I-, or A-domain were created in the G256V background. Although dB(G256V) (Fig. 2 E1), dC(G256V) (Fig. 2 E2), and dA(G256V) (Fig. 2 E4) remained diffuse in the nucleoplasm, a deletion of the I-domain was able to restore the nucleolar phenotype of the G256V mutant (Fig. 2 E3), indicating that the I-domain acts as the gating mechanism that prevents the non-GTP-bound NS from moving into the nucleolus. These results demonstrate that NS uses its GTP-binding property as a molecular switch to control the transition between the nucleolus- and the nucleoplasm-localized states, and this process involves the interaction between the B-, GTP-binding, and I-domains.
Mechanisms controlling the nucleolar residence of NS
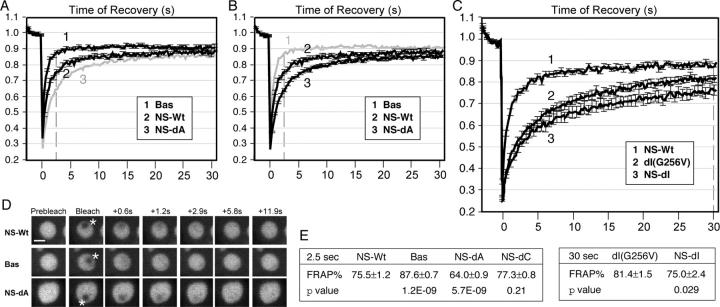
Both the B-domain and the GTP-binding domain contribute to the nucleolar localization of NS, as evidenced by the nucleolus-exclusive distribution of the dB(G256V) double mutant (Fig. 2 E1) and the nucleolus-inclusive diffuse distribution of individual mutation (NS-dB and G256V, Fig. 2 D). To investigate how the B-domain and the full-length NS protein differ in their distribution, we compared the nucleolar residence time of the B-domain alone and the full-length protein using a FRAP paradigm where a 1.5-μm-diameter circle within the nucleolus was bleached. Despite its apparent wild-type static distribution, the B-domain alone has a much faster recovery kinetic than the wild-type protein (Fig. 3, A, D, and E; Bas vs. NS-Wt). 2.5 s after photobleaching, the fluorescent recovery of Bas has reached 87.6% of the prebleach level, compared with the 75.5% recovery of the full-length protein (P < 0.001, n = 20). Notably, a difference exists between the prebleached level and the plateau recovery level. This may be accounted for by a decrease in the amount of fluorescence-active protein due to photobleaching. This notion is supported by a positive correlation between the prebleach-plateau difference and the size of the bleached area, seen by comparing the one-nucleolus (Fig. 1 C, curve 1) and the partial-nucleolus bleaching paradigms (Fig. 3 A, curve 2). This experiment suggests that the B-domain can mediate a short-lived nucleolar targeting, likely of low affinity, and that there is another region in the NS protein required for its long-term residence in the nucleolus.
Figure 3.
The long-term association of NS in the nucleolus is regulated by the I- and A-domains, and the binding of guanine nucleotide. (A and B) Quantitative data of FRAP analyses of the B-domain alone (Bas, trace 1), the wild-type NS (NS-Wt, trace 2), and the A-domain deletion (NS-dA, trace 3) in CHO cells. Y-error bars represent SEM and are omitted at every other data point for clarity. For comparison, Bas and NS-dA were shown in B and A in gray without error bars. (C) FRAP analyses of dI(G256V) (trace 2) and NS-dI (trace 3), compared with the wild-type NS (trace 1). Dotted lines in A–C indicate the points of analysis. (D) Time-sequenced FRAP images of NS-Wt, Bas, and NS-dA mutants before and after photobleaching. Bar, 4 μm. A 1.5-μm-diameter circle (marked by asterisks) within the nucleolus was bleached and the signal recovery was recorded over a period of 31.5 s. (E) T-test analyses of the FRAP results of wild-type and mutant NS-GFP fusion protein (mean ± SEM, n = 20) were conducted at 2.5 s (left) or 30 s (right) after photobleaching.
To dissect the structural requirement for the high affinity binding of NS in the nucleolus, FRAP experiments were conducted on mutants that were deleted of the C-, I-, and A-domains. A deletion in the domain required for the high affinity binding would decrease the retention time, whereas a deletion in the domain necessary for the dissociation from the high affinity binding site would increase the retention time. Our data showed that deletions of the I- and A-domains prolonged the retention time of NS-GFP in the nucleoli. With the NS-dA mutant, only 64.0% recovery of the prebleach intensity was reached at 2.5 s after photobleaching (Fig. 3, B, D, and E; NS-dA, P < 0.001, n = 20). The NS-dI signal in the bleached area had never reached plateau during the 31.5-s recording period (Fig. 3, C and E; NS-dI). A deletion of C-domain had no effect on the nucleolar retention time of NS (Fig. 3 E, left table; NS-dC). These results demonstrate that the I- and A-domains are required for the exit from the high-affinity nucleolar state, and, by exclusion, are consistent with a role of the GTP-binding domain in mediating the high affinity binding of NS to the nucleolus.
The data presented so far demonstrate that GTP binding is required to release the I-domain from blocking the nucleolar targeting of the B-domain, and is involved in the long-term nucleolar engagement of NS. To address the relationship between the dissociation of guanine nucleotides in NS and the dissociation of NS from its high affinity binding site in the nucleolus, we compared the nucleolar retention time of dI(G256V) with that of NS-dI (Fig. 3 C). dI(G256V) harbors the G256V mutation that abolishes the GTP-binding ability of NS and is deleted of the I-domain that would have prevented the nucleolar targeting of the G256V mutant. Lacking the I-domain required for the dissociation step, the FRAP recovery time for both NS-dI and dI(G256V) was delayed compared with the wild-type NS. By comparison, dI(G256V) mutant showed faster recovery than the NS-dI mutant. At 30 s after photobleaching, dI(G256V) recovered 81.4% of the prebleached intensity, whereas the NS-dI mutant recovered 75% (Fig. 3 D, E, right table; P < 0.05, n = 20). These findings are consistent with the idea that guanine nucleotide dissociation favors the dissociation of NS from the nucleolus.
Lowering intracellular GTP level decreases the nucleolar retention of NS
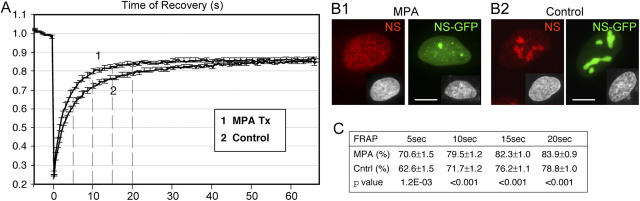
To further support the notion that guanine nucleotide dissociation correlates with the nucleolar exit of NS, we investigated if changing the intracellular GTP level would affect the shuttling kinetics of NS. To lower the intracellular GTP level, mycophenolic acid (MPA), which inhibits the rate-limiting enzyme inositide 5′-monophosphate dehydrogenase (IMPDH) of de novo guanine nucleotide biosynthesis (Majumdar et al., 1995; Olah et al., 1988), was applied to U2 OS cells transfected with NS-GFP. Photobleaching experiments were conducted in which 3-μm-diameter nucleoli were bleached and recorded for signal recovery over a 67-s period. The FRAP results revealed shortened nucleolar residence time of NS in cells treated with 40 μM MPA. At 10 s after photobleaching, the fluorescent signal recovered 80% of the prebleach level in the 40-μM MPA-treated cells, whereas control cells reached only 72% (Fig. 4, A and C; P < 0.001). A clear reduction in the amount of endogenous NS (red) or NS-GFP (green) in the nucleolus was also seen in the MPA-treated cells (Fig. 4 B1) compared with the untreated cells (B2). These MPA treatment results provide consistent evidence that supports our mutagenesis findings, and suggest that the NS cycling receives regulatory inputs from intracellular signals.
Figure 4.
The effect of lowering the intracellular GTP on the static and dynamic distribution of NS. (A) FRAP analyses of NS-GFP in U2 OS cells treated with 40 μM MPA for 24 h (trace 1) or nontreated cells (trace 2). Photobleaching was administered to 3-μm-diameter nucleoli. After subtraction of the background signal, the relative intensities were normalized to the nonbleached signals in different cells, and averaged over 20 cells in three independent experiments. Y-error bars were omitted at every other data points for clarity. Dotted lines indicate the points of analysis. (B) The static distribution of endogenous NS (red), NS-GFP (green), and DAPI nuclear staining (inset) in cells treated with MPA (B1) or untreated cells (B2). Bar, 10 μm. (C) T-tests were conducted at 5, 10, 15, and 20 s after photobleaching to compare between the FRAP rates of MPA-treated cells and the nontreated cells (mean ± SEM).
The FRAP rate of NS is distinctly different from a non-GTP-binding nucleolar protein, B23
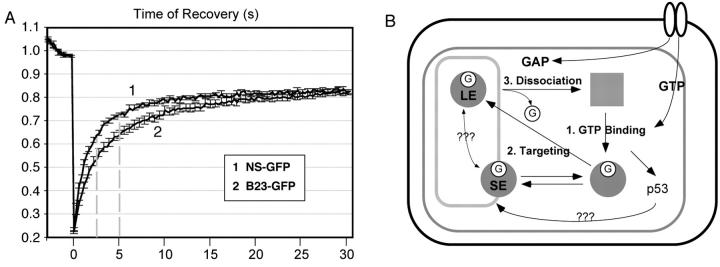
Previous work has shown that lowering the GTP level could alter the distribution of a non-GTP-binding nucleolar protein, B23, which also displayed a dynamic property to shuttle between the nucleolus and nucleoplasm (Finch et al., 1993; Chen and Huang, 2001). In the next experiment, we investigate the difference in the cycling kinetics between NS and B23. FRAP results demonstrated that the exchange rate for B23 was distinctively slower than that of NS (Fig. 5 A). At 2.5 and 5 s after photobleaching, B23 reached only 53.0% (± 1.8; mean ± SEM) and 63.6% (± 1.8) of its prebleach level, whereas the wild-type NS returned to 63.0% (± 1.1) and 72.2% (± 0.9) of the prebleach intensity at the same time points (P < 0.001, n = 24). The slower FRAP rate of the non-GTP-binding B23 compared with the GTP-binding NS may reflect different regulatory mechanisms controlling the dynamic distribution of these two proteins, or suggest that NS acts upstream of B23.
Figure 5.
FRAP comparison of NS and B23, and a regulatory model for the dynamic distribution of NS. (A) The nucleolar residence time of NS is shorter than the non-GTP-binding B23. Photobleaching experiments were conducted as described in Fig. 3. Dotted lines indicate the points of analysis. (B) A multistep, GTP-driven model for the regulation of NS distribution. The three rectangular boxes indicate the cell membrane, the nuclear membrane, and the nucleolus (outside-in). The filled circles and square represent the GTP (G)-bound and unbound NS. In the nucleoplasm, the GTP-bound NS is released of the inhibitory activity mediated by the I-domain and can be targeted to the nucleolus. Binding to the nucleolus through the B-domain alone is short lived (SE). The long-term engagement (LE) of NS in the nucleolus involves the GTP-binding domain. Its release requires the I- and A-domains, and may be triggered by the dissociation of guanine nucleotides.
In this manuscript, we show that NS shuttles between four different states in the nucleus (Fig. 5 B). In the nucleoplasm, the non-GTP-bound state of NS is prevented from being localized to the nucleolus by the I-domain, and this inhibitory mechanism is released upon GTP binding. The nucleolus-bound NS exhibits a fast FRAP rate when mediated by the B-domain and a slow FRAP rate when mediated by the GTP-binding domain. It is unclear if these two nucleolus-engaged states take place sequentially or cooperatively. Finally, the dissociation of the nucleolus-engaged NS is mediated by the I- and A-domains, and may be triggered by the dissociation of guanine nucleotide. The transition of NS between these four different molecular states in the nucleus constitutes a cycling machinery that operates in a rapid and dynamic manner. With the identification of several putative GTPases in the nucleolus (Racevskis et al., 1996; Jensen et al., 2003; Kallstrom et al., 2003) and the GTP effect on the non-GTP-binding B23 (Finch et al., 1993), this proposed GTP-driven mechanism provides a molecular framework that may explain how some nucleolar proteins move between the nucleolar and nucleoplasmic compartments dynamically.
Such a dynamic system also generates a bidirectional, fast, and sensitive measurement to control the amount of NS engaged in the nucleolus. By monitoring the targeting probability and dissociation kinetics, this mechanism can be used to translate the intra- and extra-cellular growth signals into the amount of NS in the nucleolus as a way to dynamically control cell proliferation (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200409053/DC1). In addition, it also allows nucleolar proteins, such as Werner's syndrome protein, DNA topoisomerase, and NS (Blander et al., 1999; Gobert et al., 1999; Tsai and McKay, 2002; Horn and Vousden, 2004), the opportunity to interact with nucleoplasmic proteins such as p53 under physiological conditions. Our findings that the FRAP kinetics of NS remains the same in both p53-wild-type and p53-null cell lines (Fig. S1) indicate that the dynamic distribution of NS is not controlled by p53, but suggest a possibility that it may be involved in controlling the amount of p53 in the active transcriptional sites within the nucleolus (Rubbi and Milner, 2000). Further understanding of the NS machinery and the signaling pathways that control its activity will help elucidate the molecular mechanism that gates the transition of stem cells between the mitotically active and quiescent states in a fast and reversible way.
Materials and methods
Deletion and point mutation of NS-GFP fusion constructs
Deletions and point mutations were introduced by stitching PCR reactions. Both strands of mutagenic primers were synthesized using NS-GFP as the template and paired with respective flanking primers for PCR reactions. The resulting PCR fragments were used as the template for the next round of PCR with only the flanking primers. The final products were restricted, subcloned into expression vector (pCIS), and confirmed by sequencing.
FRAP and iFRAP
Cells grown in LabTek II chamber slides (Nalgene) were transfected with 0.6 μg plasmid DNA complexed with 3 μl LipofectAMINE 2000 reagent in 1.2 ml Opti-MEM 1 d before the measurement. The photobleaching experiments were performed on a confocal microscope (model 510; Carl Zeiss MicroImaging, Inc.) with a 63× plan-apochromat oil objective (Phair and Misteli, 2000). The GFP signal was excited with the 488-nm argon laser and emission was monitored above 505 nm. Cells were maintained at 35°C with a heat blower. The whole nucleolar region or a small area of the nucleolus was bleached using a short laser pulse administered at 95% of the power for three iterations, with all experiments ensured to achieve 70–80% bleaching of the original intensity. The recovery of signal in the bleached area (FRAP) was monitored. The relative fluorescence intensity was normalized to the nonbleached signal after subtraction of the background signal. Values are averages of at least 20 cells from three independent experiments. The iFRAP paradigm was designed where cells with two nucleoli of comparable size and intensity were chosen and one nucleolus was bleached. The loss of fluorescence from the unbleached nucleoli was determined as described for the FRAP paradigms.
GTP-binding assay
The GTP-binding assays were conducted as described in Iismaa et al. (1997) with 1 μg purified proteins and 80 μl GTP-agarose (2.2 μmol/ml). After extensive wash procedure, the amount of bound protein was detected by Western blot.
Online supplemental material
Fig. S1: FRAP rate comparison of the wild-type NS-GFP protein in different cell lines. Fig. S2: serum deprivation has a mild effect on the static distribution of NS and its FRAP recovery rate relative to the recovery plateau. Fig. S3: the distribution of NS in response to aluminum fluoride (AlF4 −) treatments. Online supplemental material available at http://www.jcb.org/cgi/content/full/jcb.200409053/DC1.
Acknowledgments
We thank Carolyn Smith and Cem Elbi for their helpful advice in the photobleaching experiments, and Dave Owens for his critical comments on this manuscript.
Abbreviations used in this paper: Act-D, actinomycin D; iFRAP, inverse FRAP; MPA, mycophenolic acid; NS, nucleostemin.
References
- Blander, G., J. Kipnis, J.F. Leal, C.E. Yu, G.D. Schellenberg, and M. Oren. 1999. Physical and functional interaction between p53 and the Werner's syndrome protein. J. Biol. Chem. 274:29463–29469. [DOI] [PubMed] [Google Scholar]
- Bullough, W.S. 1965. Mitotic and functional homeostasis: a speculative review. Cancer Res. 25:1683–1727. [PubMed] [Google Scholar]
- Chen, D., and S. Huang. 2001. Nucleolar components involved in ribosome biogenesis cycle between the nucleolus and nucleoplasm in interphase cells. J. Cell Biol. 153:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenzini, M., D. Trere, A. Pession, L. Montanaro, V. Sirri, and R.L. Ochs. 1998. Nucleolar function and size in cancer cells. Am. J. Pathol. 152:1291–1297. [PMC free article] [PubMed] [Google Scholar]
- Derenzini, M., D. Trere, A. Pession, M. Govoni, V. Sirri, and P. Chieco. 2000. Nucleolar size indicates the rapidity of cell proliferation in cancer tissues. J. Pathol. 191:181–186. [DOI] [PubMed] [Google Scholar]
- Dundr, M., and T. Misteli. 2002. Nucleolomics: an inventory of the nucleolus. Mol. Cell. 9:5–7. [DOI] [PubMed] [Google Scholar]
- Finch, R.A., G.R. Revankar, and P.K. Chan. 1993. Nucleolar localization of nucleophosmin/B23 requires GTP. J. Biol. Chem. 268:5823–5827. [PubMed] [Google Scholar]
- Fox, A.H., Y.W. Lam, A.K. Leung, C.E. Lyon, J. Andersen, M. Mann, and A.I. Lamond. 2002. Paraspeckles: a novel nuclear domain. Curr. Biol. 12:13–25. [DOI] [PubMed] [Google Scholar]
- Gobert, C., A. Skladanowski, and A.K. Larsen. 1999. The interaction between p53 and DNA topoisomerase I is regulated differently in cells with wild-type and mutant p53. Proc. Natl. Acad. Sci. USA. 96:10355–10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, H.F., and K.H. Vousden. 2004. Cancer: guarding the guardian? Nature. 427:110–111. [DOI] [PubMed] [Google Scholar]
- Huang, C.F., and N.N. Chuang. 2000. Disrupting the geranylgeranylation at the C-termini of the shrimp Ras by depriving guanine nucleotide binding at the N-terminal. J. Exp. Zool. 286:441–449. [DOI] [PubMed] [Google Scholar]
- Iismaa, S.E., L. Chung, M.J. Wu, D.C. Teller, V.C. Yee, and R.M. Graham. 1997. The core domain of the tissue transglutaminase Gh hydrolyzes GTP and ATP. Biochemistry. 36:11655–11664. [DOI] [PubMed] [Google Scholar]
- Jensen, B.C., Q. Wang, C.T. Kifer, and M. Parsons. 2003. The NOG1 GTP-binding protein is required for biogenesis of the 60 S ribosomal subunit. J. Biol. Chem. 278:32204–32211. [DOI] [PubMed] [Google Scholar]
- Kallstrom, G., J. Hedges, and A. Johnson. 2003. The putative GTPases Nog1p and Lsg1p are required for 60S ribosomal subunit biogenesis and are localized to the nucleus and cytoplasm, respectively. Mol. Cell. Biol. 23:4344–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar, A., S. Kerby, B. Mullikin, J.H. Beckstead, P.E. Stenberg, and M.M. Seidman. 1995. IL-3 and ribavirin induce differentiation and growth suppression during long-term treatment of a megakaryocytic leukemia cell line. J. Cell. Physiol. 165:530–537. [DOI] [PubMed] [Google Scholar]
- Olah, E., Y. Natsumeda, T. Ikegami, Z. Kote, M. Horanyi, J. Szelenyi, E. Paulik, T. Kremmer, S.R. Hollan, J. Sugar, et al. 1988. Induction of erythroid differentiation and modulation of gene expression by tiazofurin in K-562 leukemia cells. Proc. Natl. Acad. Sci. USA. 85:6533–6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair, R.D., and T. Misteli. 2000. High mobility of proteins in the mammalian cell nucleus. Nature. 404:604–609. [DOI] [PubMed] [Google Scholar]
- Racevskis, J., A. Dill, R. Stockert, and S.A. Fineberg. 1996. Cloning of a novel nucleolar guanosine 5′-triphosphate binding protein autoantigen from a breast tumor. Cell Growth Differ. 7:271–280. [PubMed] [Google Scholar]
- Recher, L., L.G. Briggs, and N.T. Parry. 1971. a. A reevaluation of nuclear and nucleolar changes induced in vitro by actinomycin D. Cancer Res. 31:140–151. [PubMed] [Google Scholar]
- Recher, L., N.T. Parry, L.G. Briggs, and J. Whitescarver. 1971. b. Difference in effects of proflavine and actinomycin D on mammalian cell nucleoli. Cancer Res. 31:1915–1922. [PubMed] [Google Scholar]
- Rubbi, C.P., and J. Milner. 2000. Non-activated p53 co-localizes with sites of transcription within both the nucleoplasm and the nucleolus. Oncogene. 19:85–96. [DOI] [PubMed] [Google Scholar]
- Tsai, R.Y., and R.D. McKay. 2002. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 16:2991–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]