Abstract
Musculin/MyoR is a new member of basic helix-loop-helix transcription factors, and its expression is limited to skeletal muscle precursors. Here, we report that musculin/MyoR is expressed in adult kidney side population (SP) cells and can regulate their function. SP phenotype can be used to purify stem cell–rich fractions. Microarray analysis clarified that musculin/MyoR was exclusively expressed in kidney SP cells, and the cells resided in the renal interstitial space. Musculin/MyoR-positive cells were decreased in acute renal failure, but infusion of kidney SP cells increased musculin/MyoR-positive cells and improved renal function. Kidney SP cells in reversible acute renal failure expressed a high level of renoprotective factors and leukemia inhibitory factor (LIF), but not in irreversible chronic renal failure. In cultured kidney SP cells, LIF stimulated gene expression of renoprotective factors, and down-regulation of musculin/MyoR augmented LIF-induced gene expression. Our results suggest that musculin/MyoR may play important roles not only in developmental processes but also in regenerative processes in adult tissue.
Introduction
In 1996, Goodell et al. (1996) reported a new method of obtaining an enriched population of hematopoietic stem cells from adult mouse bone marrow in a single step by using Hoechst 33342 dye staining and FACS. The isolated cells were called side population (SP) cells, and the SP phenotype can be used to purify a stem cell-rich fraction (Zhou et al., 2001; Matsuzaki et al., 2004). Bone marrow–derived SP cells differentiate into cardiomyocytes, endothelial cells (Jackson et al., 2001), and osteoblast precursors (Olmsted-Davis et al., 2003), and skeletal muscle SP cells (Asakura et al., 2002) may differentiate into endothelial cells (Majka et al., 2003). The SP phenotype is determined by BCRP1/ABCG2, and enforced expression of BCRP1/ABCG2 prevents hematopoietic differentiation (Zhou et al., 2001). However, the physiological role of this ATP-binding cassette (ABC) transporter (Gottesman et al., 2002) in SP cells is still unclear.
In this study, we first tried to clarify the gene expression profile of kidney SP cells in healthy control and renal failure models by microarray analysis. The results of microarray and quantitative PCR analysis clarified that musculin/MyoR is exclusively expressed in kidney SP cells but not in non-SP cells, and we further examined the functional role of musulin/MyoR in kidney SP cells. Musculin/MyoR is a newly discovered basic helix-loop-helix (bHLH) protein that represses myogenesis (Robb et al., 1998; Lu et al., 1999) and controls facial muscle development with capsulin (Lu et al., 2002). Musculin/MyoR mRNA is first expressed at 9.8 days postcoitum in the mouse embryo, and is almost exclusively restricted to cells of skeletal muscle lineage, with peak expression at 14–15 days postcoitum. To our knowledge, no study has confirmed the expression of musculin/MyoR in adult kidney tissue.
We then aimed to determine the functional role of BCRP1 and musculin/MyoR in the kidney. We found that kidney SP cells, but not non-SP cells, are protected from cell death by BCRP1. Leukemia inhibitory factor (LIF) is up-regulated in reversible cisplatin-induced acute renal failure (ARF), and is considered to play a role for regeneration process (Yoshino et al., 2003). In this study, we also examined the expression of renoprotective factors such as hepatocyte growth factor (HGF), VEGF, bone morphologic protein (BMP) 7, and LIF in kidney SP cells. In an ARF model, gene expression of renoprotective factors and LIF was up-regulated in kidney SP cells but not in non-SP cells. On the other hand, there was no up-regulation of renoprotective factors and LIF in irreversible chronic renal failure (CRF) models. In cultured kidney SP cells, LIF augmented gene expression of renoprotective factors. Although inhibition of BCRP1 showed no effect on LIF-induced gene expression of renoprotective factors, down-regulation of musculin/MyoR augmented it. These results provide evidence that expression of musculin/MyoR is not limited to the skeletal muscle lineage and the production of renoprotective factors in kidney SP cells is regulated by LIF and musculin/MyoR, but nor by BCRP1.
Results
Gene expression profile of kidney SP cells
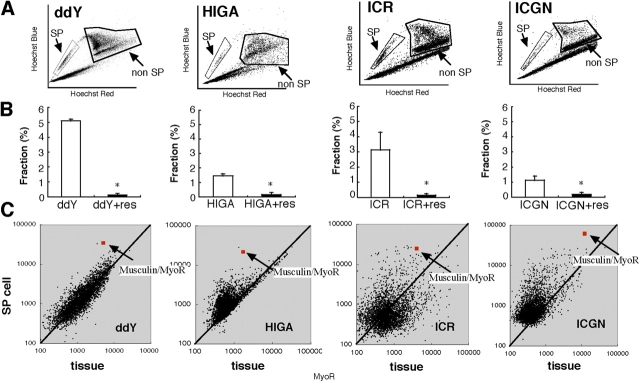
We found a significant increase in blood urea nitrogen (BUN) and IgA in IgA nephropathy (HIGA) mice compared with control mice (ddY; Table I). In nephrotic syndrome mice (ICGN), BUN and total cholesterol were significantly increased as compared with control (ICR). We isolated whole kidney cells from these mice and stained them with Hoechst 33342 dye (Zhou et al., 2001). The isolated cells were subjected to FACS analysis in the presence or absence of reserpine to quantitate the number of cells with a SP and non-SP phenotype (Fig. 1, A and B). As previously reported (Asakura and Rudnicki, 2002), kidney tissue from control mice (ddY and ICR) contained a much higher number of reserpine-sensitive SP cells (5.1 ± 0.1 and 3.1 ± 0.9%, respectively) than did bone marrow (<1%; not depicted). In both renal failure models (HIGA and ICGN), the population of reserpine-sensitive SP cells in the kidney was reduced to ∼30% of control (1.5 ± 0.1 and 1.1 ± 0.2%, respectively). In our preparation, kidney SP cells were Sca-1 positive (>70% on average; n = 20) but CD45 negative (<2% on average; n = 20) in all mice, indicating that they were not part of the hematopoietic system and were not likely to be contaminated with peripheral blood SP cells. To characterize kidney SP cells, gene expression profiling was performed using freshly isolated kidney SP cells from control (ddY and ICR) and renal failure models (HIGA and ICGN), as well as cortical tissue of the kidney from control and renal failure mice. Among the 3,800 genes analyzed, musculin/MyoR (Robb et al., 1998, 1999) was the only gene that was commonly and highly expressed in SP cells of all mice (greater than sixfold higher compared with cortical tissue; Fig. 1 C, red square with arrow). We also performed gene expression profiling using kidney SP cells and non-SP cells, and found that musculin/MyoR was also expressed greater than sixfold higher in SP cells compared with non-SP cells in all mice (unpublished data).
Table I. Biochemical features in control (ddY and ICR) and CRF (HIGA and ICGN) mice.
| BUN | IgA | Total cholesterol | |
|---|---|---|---|
| mg/dl | mg/dl | mg/dl | |
| ddY | 20 ± 2 | 98 ± 12a | ND |
| HIGA | 33 ± 3a | 396 ± 26a | ND |
| ICR | 36 ± 4 | ND | 108 ± 13 |
| ICGN | 96 ± 4a | ND | 188 ± 12 |
BUN, IgA, and total cholesterol levels in serum. Results are presented as the mean ± SEM (n = 12).
P < 0.05 versus normal mice.
Figure 1.
FACS and microarray analysis of kidney SP cells. (A) Representative FACS profile of SP cells isolated from the kidney of normal (ddY and ICR) and renal failure (HIGA and ICGN) mice (total events:1.0 × 105 to 3.0 × 105). (B) Kidney SP cells isolated from ddY, ICR, HIGA, and ICGN mice stained with Hoechst dye (white bars). Total event of all mice were set 1.0 × 105, and uptake of Hoechst dye was significantly blocked in the presence of reserpine (res) in all samples (black bars). Results are presented as the mean ± SEM, n = 15. *, P < 0.05 versus control (without reserpine). (C) Scatter-plot analyses of gene expression (3,800 genes) in SP cells versus kidney tissue prepared from normal (ddY and ICR) and renal failure (HIGA and ICGN) model mice. Average expression levels of each gene were calculated from three independent hybridizations.
Immunohistochemical staining of musculin/MyoR and BCRP1
To confirm the protein expression and localization of musulin/MyoR-positive cells, we performed immunohistochemical analysis. Kidney SP cells were very small, but strongly expressed both musculin/MyoR and BCRP1 protein (Fig. 2, A and B). Although the SP population is a heterogeneous population, musculin/MyoR staining clarified that kidney SP cells were musulin/MyoR positive (>95%), but non-SP cells were negative (<5%; Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200412167/DC1). In other words, the purity of kidney SP cells was >95% with respect to musculin/MyoR staining. As shown in Fig. 2 (C and D), musculin/MyoR-positive cells were localized in the interstitial space of the kidney.
Figure 2.
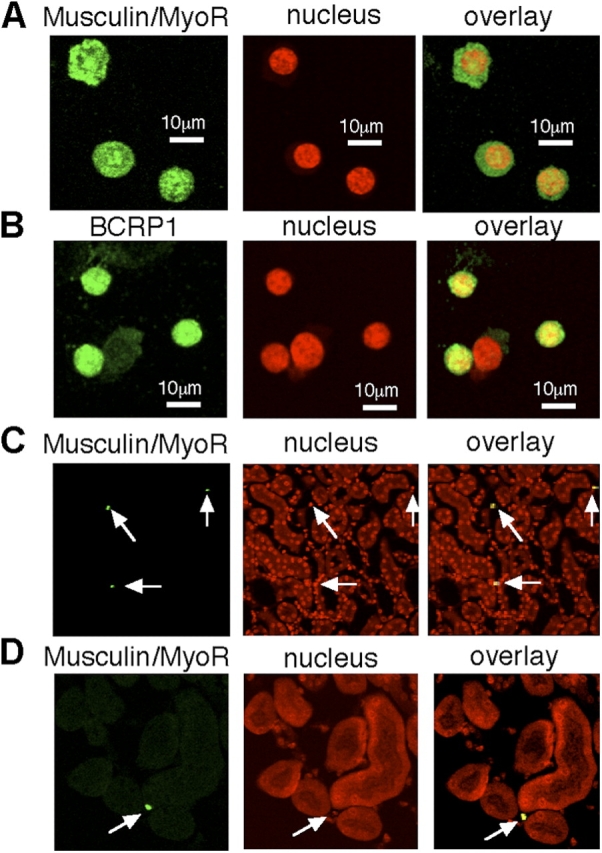
Immunohistochemical analysis of kidney SP cells. (A and B) Representative photomicrographs of fluorescent images of musculin, BCRP1, and nuclear staining (TO-PRO3) in cytospin of kidney SP cells (C57BL/6) just after FACS sorting. Overlay images are shown in the right panels. (C and D) Representative photomicrographs of fluorescent images of musculin and nuclear staining in renal tissue (C57BL/6). Musculin-positive cells (arrows) reside in the interstitial space of the kidney. Overlay images of musculin and nuclear (yellow) images also showed that the cells reside in the interstitial space of the kidney.
BCRP protects SP cells, but not non-SP cells, from cell death
BCRP1/ABCG2 is a member of ABC transporters, which generally protect cells from toxic agents (Gottesman et al., 2002). To clarify the reason why kidney SP cells were decreased in renal failure models (Fig. 1 B) and to examine the protective role of BCRP1 in kidney SP cells, SP and non-SP cells were treated with TNF-α. Treatment with TNF-α (at up to 16 ng/ml) did not reduce cell viability of non-SP cells, but significantly reduced cell viability of SP cells in a concentration-dependent manner (Fig. 3 A). We further examined the effect of an inhibitor of BCRP1/ABCG2 on TNF-α–induced cell death in kidney SP cells. Compared with control, reserpine alone showed no effect (Fig. 3, B and C). TNF-α alone significantly increased apoptosis, and cotreatment with TNF-α and reserpine significantly increased it (Fig. 3, B and C).
Figure 3.
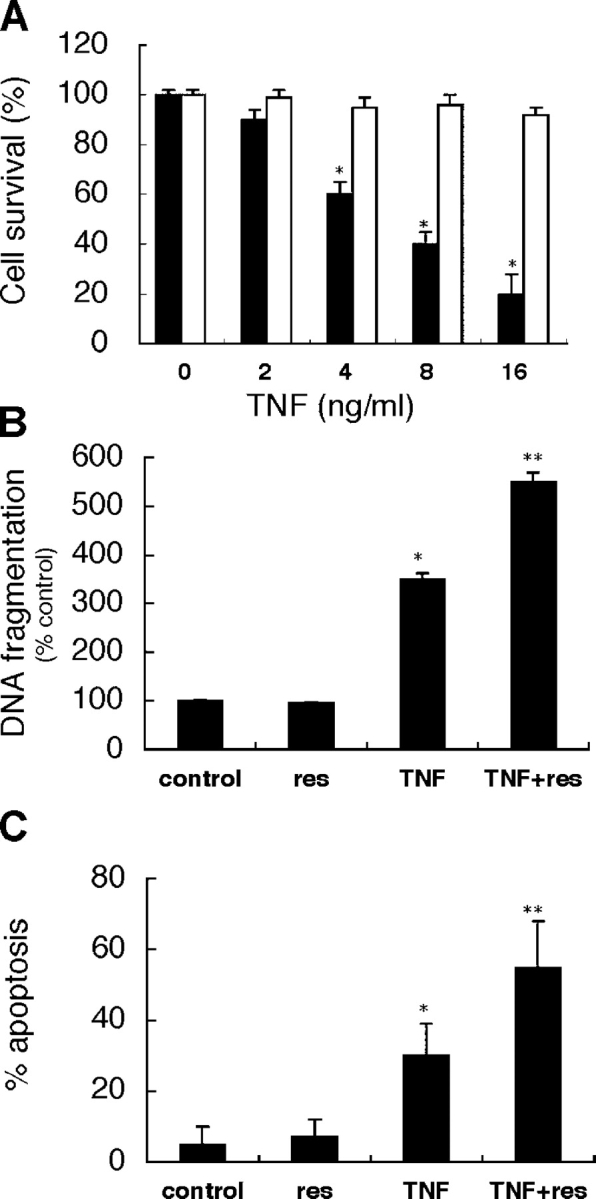
TNF- α induced cell death in kidney SP cells. (A) Kidney SP and non-SP cells just after FACS sorting were incubated with TNF-α at the indicated concentrations for 24 h. TNF-α induced cell death in SP cells (black bars) in a concentration-dependent manner, but not in non-SP cells (white bars). Cell survival was determined by trypan blue exclusion assay. (B and C) Kidney SP cells just after FACS sorting were incubated with 50 μM reserpine (res), 8 ng/ml TNF-α, or both for 24 h. Compared with control (no treatment), TNF-α alone significantly increased DNA fragmentation and apoptosis, and cotreatment with reserpine significantly augmented it. DNA fragmentation assay was performed using an ELISA kit, and percentage of apoptosis was evaluated by nuclear staining with Hoechst 33258. Results are presented as means ± SEM of four independent experiments. *, P < 0.05 compared with control; **, P < 0.05 compared with TNF-α.
Kidney SP cells contribute to regeneration
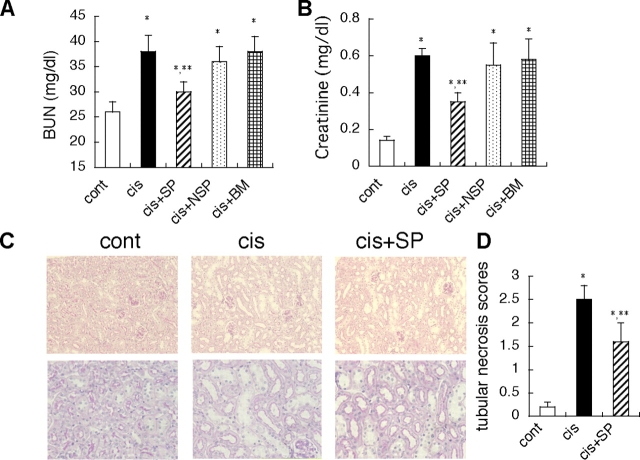
To clarify the functional role of kidney SP cells in vivo, we injected isolated kidney SP cells systemically via the tail vain into mice with ARF induced by cisplatin administration (Ramesh and Reeves, 2002). In this model, i.p. injection of 12 mg/kg cisplatin induced a peaked BUN level on day 7 (Fig. 4 A). On day 7, musculin/MyoR-positive cells in the interstitial space were significantly reduced by cisplatin treatment alone, but kidney SP cell infusion on day 1 significantly increased these cells on day 7 (Fig. 4, B–E). We also compared the effect kidney SP cell infusion on renal function to those of infusion of non-SP cells or bone marrow-derived mononuclear (BM) cells. Infusion of kidney SP cells significantly suppressed the peak BUN and creatinine level on day 7 (Fig. 5, A and B), whereas infusion of non-SP and BM cells had no effect. Tubular necrosis scores were also significantly improved by SP cell infusion (Fig. 5, C and D).
Figure 4.
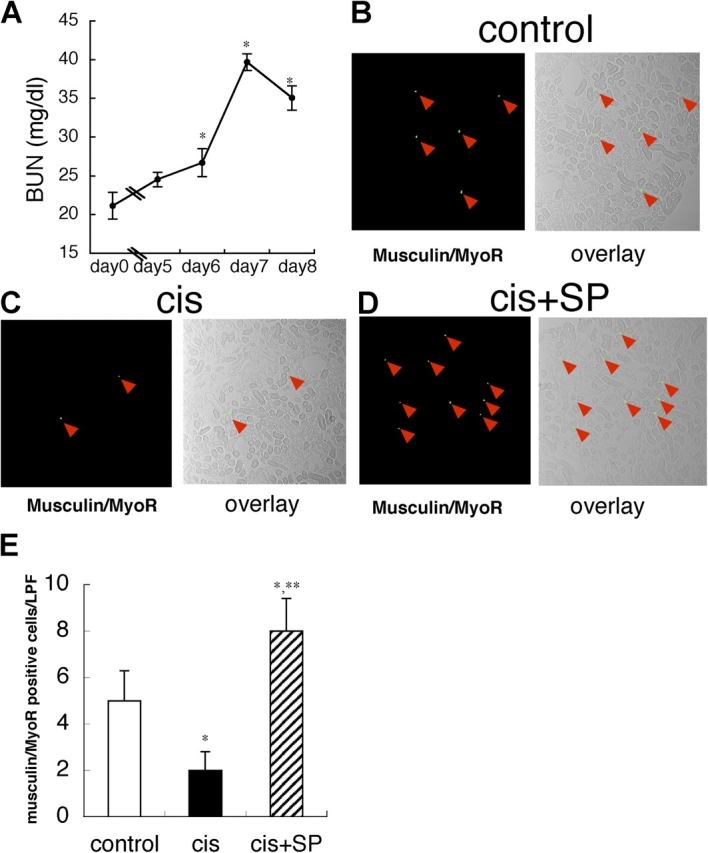
Systemic kidney SP cell infusion increases musculin/MyoR-positive cells in renal interstitial space in ARF model. (A) Time course of plasma BUN level in cisplatin-induced ARF model. Values are the mean ± SEM (n = 8). *, P < 0.05 versus day 0. (B–D) Representative fluorescence photographs and overlay images (fluorescent and phase contrast) of renal tissues of control, cisplatin alone (cis), and infusion of kidney SP cells (cis+SP) on day 7. Green spots with red arrowheads are musculin/MyoR-positive cells. (E) Musculin/MyoR-positive cells were significantly reduced in the cisplatin-induced ARF model (cis) but were markedly increased by systemic kidney SP cell infusion (cis+SP). Values are the average number of musculin-positive cells in 10 randomly selected low power fields (×200) in four independent experiments. Values are the mean ± SEM. *, P < 0.05 versus control; **, P < 0.05 versus cis.
Figure 5.
Systemic kidney SP cell infusion augments recovery of renal function in ARF model. (A and B) Compared with control (cont), cisplatin alone (cis) significantly increased the BUN and creatinine levels, but infusion of kidney SP cells (cis+SP) on day 1 (day after cisplatin injection) significantly reduced the BUN and creatinine levels on day 7. On the other hand, infusion of non-SP cells (cis+NSP) and BM cells (cis+BM) showed no effect. (C and D) Compared with control (cont), cisplatin alone (cis) significantly increased the tubular necrosis scores, but infusion of kidney SP cells (cis+SP) on day 1 (day after cisplatin injection) significantly reduced it on day 7. Values are the mean ± SEM. *, P < 0.05 versus control; **, P < 0.05 versus cis.
Kidney SP cells express growth factors that contribute to tissue regeneration
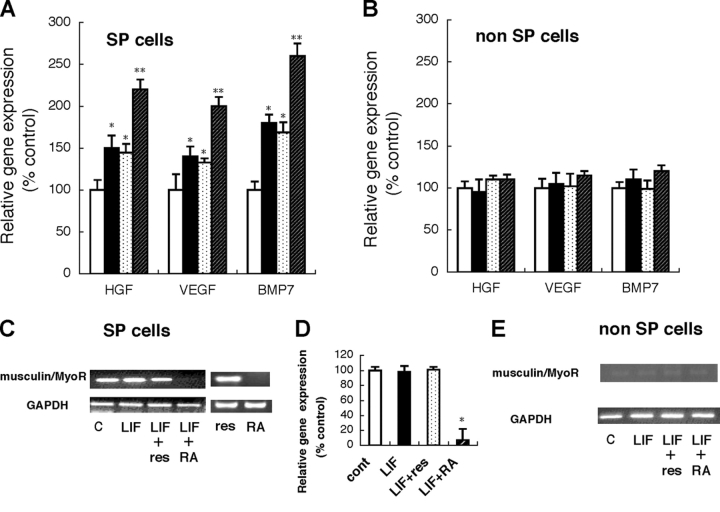
To clarify the role of kidney SP cells in renal failure, we performed quantitative real-time PCR analysis of several factors that contribute to the kidney regeneration process, such as HGF (Mizuno et al., 1998), VEGF (Ostendorf et al., 1999), and BMP7 (Wang and Hirschberg, 2003), in a reversible cisplatin-induced ARF model and two irreversible CRF models (IgA nephropathy, HIGA; nephritic syndrome, ICGN). In ARF mice, kidney SP cells expressed significantly higher levels of HGF, VEGF, and BMP7 compared with the cells in control mice (Fig. 6 A). In contrast, in the CRF models, there was no significant difference between disease mice and control mice (Fig. 6, B and C). Recently, LIF was reported to be up-regulated and to contribute to regeneration processes (Yoshino et al., 2003), so we examined the expression of LIF in kidney SP cells. We also confirmed up-regulation of LIF in the ARF model but not in the CRF models (Fig. 6 A). On the other hand, we could not find significant changes of these factors in kidney non-SP cells of all disease mice as compared with their control mice (unpublished data).
Figure 6.

Quantitative real-time PCR analysis of HGF, VEGF, BMP7, and LIF in kidney SP cells. (A) Cisplatin-induced gg model. White bars show control (no treatment) and black bars show cisplatin treatment. Compared with control, gene expression of HGF, VEGF, BMP7, and LIF was significantly up-regulated in kidney SP cells. (B) IgA nephropathy mice. White bars show control (ddY) and black bars show IgA nephropathy (HIGA). (C) Nephritic syndrome mice. White bars show control (ICR) and black bars show nephritic syndrome (ICGN). Expression was normalized to GAPDH and is shown as the ration to the value of control. Values represent means ± SEM (n = 6). *, P < 0.05 versus control.
Musculin/MyoR but not BCRP1 regulates function of kidney SP cells
As infusion of kidney SP cells improved renal function in an ARF model and LIF is also up-regulated in this model, we examined the effect of LIF on gene expression of renoproptective factors and musculin/MyoR in kidney SP and non-SP cells. Compared with control (without LIF), treatment with LIF significantly up-regulated gene expression of HGF, VEGF, and BMP7 in SP but not in non-SP cells (Fig. 7, A and B). To clarify the role of BCRP1 in LIF-induced gene expression of renoprotective genes, we treated cells with LIF and reserpine, but reserpine showed no effect. Retinoic acid (RA) is reported to down-regulate musculin/MyoR (Yu et al., 2003), and cotreatment with LIF and RA significantly augmented the LIF-induced gene expression in kidney SP cells but not in non-SP cells. Finally, we confirmed the effect of LIF, reserpine, and RA on musculin/MyoR expression in SP and non-SP cells by RT-PCR and real-time PCR. LIF and reserpine alone showed no effect on basal musculin/MyoR gene expression, and cotreatment with reserpine also showed no effect (Fig. 7, C and D). On the other hand, RA significantly down-regulated musculin/MyoR in the presence of LIF (Fig. 7, C and D). In non-SP cells, basal musculin/MyoR expression was almost absent (Fig. 6 E).
Figure 7.
LIF-induced gene expression is regulated by musculin/MyoR. (A and B) Kidney SP cells (A) or non-SP cells (B) just after FACS sorting were incubated with vehicle (white bars), LIF (10 ng/ml; black bars), LIF with reserpine (10 ng/ml and 50 μM, respectively; dotted bars), and LIF with RA (10 ng/ml and 5 × 10−7 mol/l, respectively; hatched bars) for 12 h, and then gene expression of HGF, VEGF, and BMP7 was examined by TaqMan real-time PCR. (C and D) Gene expression of musculin/MyoR in kidney SP cells. An aliquot of RNA in panel A was examined by RT-PCR (C) and TaqMan real-time PCR (D). (E) Gene expression of musculin/MyoR in non-SP cells. Basal expression of musculin/MyoR was almost absent. Values represent means ± SEM (n = 6). *, P < 0.05 versus control; **, P < 0.05 versus LIF.
Discussion
Members of the bHLH family of transcription factors are involved in cell fate determination and tissue differentiation in several developmental processes including hematopoiesis, myogenesis, and neurogenesis. Musculin/MyoR is a newly discovered bHLH protein that acts as a lineage-restricted repressor of embryonic skeletal muscle development. In this study, we clarified that musculin/MyoR is expressed in adult kidney SP cells and regulates LIF-induced gene expression of renoprotective factors such as HGF, VEGF, and BMP7.
Robb et al. (1998) failed to detect expression of musculin/MyoR in adult tissue by Northern blotting but demonstrated expression in the adult brain, spleen, and muscle but not in the kidney, liver, lung, or thymus by RNase protection assay. Yu et al. (2003) detected expression of musculin/MyoR in the adult brain, heart, liver, lung, small intestine, spleen, and thymus by Northern blotting. This discrepancy may be explained by the sensitivity of their assay, but our results also suggest that musculin/MyoR expression is not lineage restricted as has been reported, but is expressed in a relatively broad spectrum of adult tissue. Musculin/MyoR was originally identified as a transcriptional repressor in embryonic skeletal muscle precursors, but its function in adult tissue is not known. In fact, musculin/MyoR knockout mice were not embryonic lethal, and analysis of kidney function in these mice has not been completed (unpublished data). In the present study, we demonstrated that down-regulation of musculin/MyoR augmented LIF-induced gene expression of HGF, VEGF, and BMP7 in kidney SP cells. These results showed that musculin/MyoR plays a role as a transcriptional repressor in adult kidney SP cells as reported in embryonic skeletal muscle. The physiological role of kidney SP cells is still unclear, but systemic administration of RA, which down-regulates musculin/MyoR (Yu et al., 2003), augmented the recovery of ARF (Perez et al., 2004). To down-regulate musculin/MyoR in vivo, we injected histone deacetylase inhibitor trichostatin A (TSA), which down-regulates musculin/MyoR (Yu et al., 2003), in control and CRF model (nephrotoxic serum model, NTN model). As in the case with HIGA and ICGN, kidney SP cells analyzed by FACS were reduced in NTN model. Surprisingly, daily injection (for 21 d) of TSA almost completely abolished musculin/MyoR-positive cells in the kidney of control and NTN model and completely prevented progression of CRF in NTN model. Moreover, regenerative factors were also up-regulated by treatment with TSA (unpublished data). Together, these results suggest that musculin/MyoR-positive cells could be new targets for regeneration in renal failure, and we are currently trying to clarify its role using a renal failure model in musculin/MyoR knockout mice.
Theoretically, an activated transcription factor should be located within the nucleus, but musculin/MyoR was expressed throughout kidney SP cells. Inactive sterol regulatory element binding proteins, another member of the bHLH transcription factor family, are synthesized as precursors in the cytoplasm and bind to the endoplasmic reticulum membrane. Upon activation, the active forms of sterol regulatory element binding proteins enter the nucleus and activate several genes that control the synthesis and uptake of cholesterol and unsaturated fatty acids (Nagoshi and Yoneda, 2001). Pod1, which is also a bHLH transcription factor expressed in kidney stromal cells and is required for glomerulogenesis, is distributed throughout cells located in peritubular interstitial space in the kidney (Cui et al., 2003). These results suggest that localization of a transcription factor such as musculin/MyoR throughout kidney SP cells is not unique, but further characterization may be needed to fully understand its mechanism of translocation.
Infusion of kidney SP cells significantly augmented recovery of ARF and increased musculin/MyoR-positive cells in the interstitial space. Therefore, we speculate that homing of kidney SP cells to the interstitial space is necessary for regeneration in the ARF model induced by cisplatin. Recently, cardiac stem cells were found in the interstitium between well-differentiated myocytes (Beltrami et al., 2003), and myogenic-endothelial progenitor cells with the SP phenotype were also identified in the interstitial spaces of skeletal muscle (Tamaki et al., 2002), suggesting that SP cells commonly reside in the interstitial space. We also systemically injected kidney SP cells from GFP mice and DiI-labeled kidney SP cells, and found that these cells were located in the interstitial spaces of kidney (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200412167/DC1). As the interstitial space of the kidney has a fine microcirculation system, musculin/MyoR-positive kidney SP cells in the interstitial space may provide paracrine growth support for neighboring cells in vivo. Several growth factors such as HGF, VEGF, and BMP7 have been reported to contribute to kidney regeneration. Especially, BMP7 is the only factor that reverses chronic renal injury (Zeisberg et al., 2003) and Lin et al. (2005) recently reported the important role of BMP7 enhancer in CRF. Administration of exogenous LIF ameliorates glomerulonephritis (Tang et al., 1996), and LIF is up-regulated in ARF (Yoshino et al., 2003). Therefore, we examined the expression of these factors in kidney SP cells in renal failure. As shown in Fig. 6 and 7, all of these factors were significantly up-regulated in the reversible ARF model, but not in the irreversible CRF models, and LIF increased the gene expression of HGF, VEGF, and BMP7 in cultured kidney SP cells. Moreover, the number of SP cells was decreased in the irreversible CRF models (Fig. 1 B). We also examined the effect of kidney SP cell infusion on CRF model (unilateral ureteral obstruction model) but found no significant improvement (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200412167/DC1). Recently, we also reported that LIF induces multilineage differentiation of kidney SP cells in culture (Hishikawa et al., 2005), and some other pathways can be involved in the restoration of renal function. Although the precise mechanism regarding the regenerative role of kidney SP cells remains to be clarified, our results suggest that loss of musulin/MyoR-positive kidney SP cells and inadequate expression of key factors such as LIF and BMP7 in CRF may determine the fate of CRF.
BCRP1/ABCG2 is reported to determine the SP phenotype (Zhou et al., 2001). Enforced expression of BCRP1/ABCG2 inhibits hematopoietic development (Zhou et al., 2001), but its mechanism is still unclear. BCRP1/ABCG2 is a member of ABC transporters (Gottesman et al., 2002) and is known to transport doxorubicin, daunorubicin, mitoxantrone, topotecan, and prazocin. Although many ABC transporters have been identified as drug-resistance proteins, they are all expressed in normal tissues. Consistent with their wide distribution, it is becoming clear that in addition to exogenously administered drugs, ABC proteins transport numerous endogenous substrates. It is well know that ABC transporters have an important role in protecting the central nervous system through the blood brain barrier (Schinkel et al., 1996), and BCRP1/ABCG2 in the placenta may play a role in protecting fetal blood from toxic organic anions and excreting glutathione/glucuronide metabolites into maternal circulation (Gottesman et al., 2002). Considering these results, we examined the protective role of BCRP1 in kidney SP cells. The role for TNF-α in cisplatin-induced nephrotoxicity has been well established (Ramesh and Reeves, 2002), and important roles of TNF-α are also reported in IgA nephropathy (Tuglular et al., 2003; Chan et al., 2005) and nephrotic syndrome (Suranyi et al., 1993; Kim et al., 2004; Raveh et al., 2004). As shown in Fig. 3, kidney SP cells were more sensitive to TNF-α than non-SP cells, and BCRP1 protected kidney SP cells from cell death. The precise mechanism of TNF-α–induced cell death in kidney SP cells and direct in vivo evidence linking these effects to progression of renal failure remain to be determined, but our results may explain why the number of musculin/MyoR-positive kidney SP cells was decreased in renal failure in which inflammatory cytokines were overexpressed.
In summary, our study provides evidence for a new role of musculin/MyoR and a protective role of BCRP1 in kidney SP cells. Our results suggest that members of the bHLH family of transcription factors such as musculin/MyoR may play important roles not only in embryonic developmental processes but also in regenerative processes in adult tissue.
Materials and methods
Mice
HIGA mice (Muso et al., 1996) were donated by E. Muso (Kitano Hospital, Osaka, Japan). ICGN mice (Ogura et al., 1989) were donated by J. Matsuda (National Institute of Infectious Diseases, Tokyo, Japan). ICR, ddY, and C57/B6 mice were purchased from Clea Japan. HIGA mice and their control ddY mice were used for analysis at 14 wk of age. ICGN mice and their control ICR mice were used for analysis at 30 wk of age. In Figs. 2, 3, 4, 6 A, and 7, kidney SP and non-SP cells were prepared from C57B/6 (8 wk old). All the procedures described here were approved by the Animal Committee of the University of Tokyo.
Clinical biochemistry
Blood samples were measured using an automated analyzer (model Fuji Dri-Chem 3500V; Fuji Film Co.).
SP cell analysis and cell sorting
Mice were anesthetized and were perfused via the abdominal aorta with normal saline. Kidneys were harvested and the tissue was minced with a razor blade and digested by collagenase. The cell suspensions were filtered through a c ell strainer (Falcon 2350; Becton Dickinson) to remove debris. The filtrates were analyzed as previously described (Matsuzaki et al., 2004). In brief, after filtration by cell strainer, the kidney cells were resuspended at 1 × 106 cells/ml in Hank's balanced salt solution (supplemented with 2% FCS and 10 mM Hepes) and then incubated with 5 μg/ml Hoechst33342 (Sigma-Aldrich) for 60 min for 37°C. A parallel aliquot was stained with Hoechst33342 in the presence of 50 μM of reserpine (Sigma-Aldrich). As the batch of the Hoechst33342 sometimes affected FACS profile, we screened several batches and ultimately chose lot number 31K4028 for use in the experiments. Cell analysis and sorting were performed on a triple laser MoFlo (DakoCytomation). Hoechst33342 was excited at 350 nm, and fluorescence emission was detected by using a 405/BP30 and 570/BP20 optical filter for Hoechst blue and Hoechst red, respectively, and a 550-nm long-pass dichroic mirror to separate the emission wavelengths. Both Hoechst blue and red fluorescence are shown on a linear scale. PI fluorescence was measured through 630BP30 after excitation at 499 nm with an argon laser, and live cell gate was defined as that which excluded the cells positive for PI. After collecting 1 × 105 events, the SP population was defined as reported previously (Matsuzaki et al., 2004).
Microarray analysis
DNA microarray hybridization experiments were performed using Atlas glass Mouse3.8 I microarray (CLONTECH Laboratories, Inc.) according to the manufacturer's protocol. The protocol and the complete list of genes can be viewed at the manufacturer's website. The DNA arrays were scanned using GenePix4000A (Hishikawa et al., 2001).
Histological analysis
Sections were blocked with 1% skimmed milk (Morinaga) in PBS for 60 min at RT, and then incubated with 2 μg/ml of goat anti–mouse musculin polyclonal antibody (Santa Cruz Biotechnology, Inc.) or rabbit anti-BCRP1 antibody (PC-138; Kamiya Biomedical Company) for overnight at 4°C. After washing three times in PBS for 5 min at RT, sections were incubated with Alexa Fluor 488 or 546 donkey anti–goat IgG (Molecular Probes) or Alexa Fluor 594 goat anti–rabbit IgG (Molecular Probes) at a dilution of 1:200 for 30 min at RT. All sections were washed three times in PBS for 5 min and then mounted with Pro-Long Antifade kit (Molecular Probes) and sealed for analysis. For SP cell staining, cells were spun down by cytospin immediately after FACS sorting. Tissues and cells were also stained with TO PRO-3 (T-3605; Molecular Probes) for nuclear staining. Stained sections and cells were observed by a confocal microscope (model TCS SL; Leica). To adjust laser power, we used sections and cells stained with secondary antibody alone as negative controls and confirmed that the fluorescence is due to reactivity with the primary antibody. Tubular injury was assessed in periodic acid Schiff–stained sections using a semiquantitative scale in which the percentage of cortical tubules showing epithelial necrosis was assessed a score: 0, normal; 1, <10%; 2,10–25%; 3, 26–75%; 4, >75% (Ramesh and Reeves, 2002).
Cell death
The kidney SP and non-SP cells (2 × 103 cells/well were seeded in a 96 multi-well plate) were incubated in DME/F12 with 10% FBS for 24 h just after FACS sorting. Cell survival was evaluated by trypan blue exclusion assay, and DNA fragmentation was measured using a cell death detection ELISA (Roche) according to the manufacturer's instructions. The percentage of apoptosis was evaluated based on nuclear morphology. Cells were washed with PBS, fixed with fresh 10% PFA for 30 min, and incubated in Hoechst33258 (Sigma-Aldrich) at RT for 30 min (final concentration, 30 μg/ml; Hishikawa et al., 2001).
Cisplatin-induced ARF and SP cell infusion
Mice (C57BL/6; 8 wk old) were given a single i.p. injection of either vehicle (saline) or cisplatin (12 mg/kg of body weight). Several doses of cisplatin were tested (5–20 mg/kg of body weight), and 12 mg/kg was chosen to produce mild renal failure with the ability for partial or full recovery. Bone marrow mononuclear cells were isolated from the femurs and tibias of mice by density gradient centrifugation with Ficoll-Paque Plus (Amersham Biosciences). Approximately 10,000 freshly isolated bone marrow mononuclear, kidney SP, and non-SP cells prepared from C57BL/6 mice were injected via the tail vein 24 h after cisplatin injection (Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200412167/DC1).
RT-PCR and real-time PCR
The sequences of primers for RT-PCR were as follows. GAPDH forward 5′-TGCTGAGTATGTCGTGGA-3′ and reverse 5′-AGTTGCTGTTGAAGTCGC-3′; musculin/MyoR forward 5′-GGAGGACCGCTACGAGGACA-3′ and reverse 5′-ACCCACAGAAGGCTATGCT-3′. RT-PCR conditions for musculin/MyoR and GAPDH were 35 cycles of 94°C for 30 s, 59°C for 1 min, and 72°C for 1 min. Quantitative real-time PCR was performed using commercially available TaqMan probes (for HGF, VEGF, BMP7, and musculin) and analyzed on a sequence detector system (model ABI PRISM 7000; BD Biosciences). Quantitative values were obtained from the threshold PCR cycle number at which an increase in signal associated with exponential growth of the PCR product started to be detected. The relative mRNA levels in each sample were normalized to its GAPDH content.
Online supplemental material
Fig. S1 shows immunohistochemical analysis of kidney non-SP cells. Fig. S2 shows engraftment of kidney SP cells in interstitial spaces by systemic kidney SP cell infusion. Fig. S3 shows the effect of SP cell injection on CRF model (unilateral ureteral obstruction model). Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200412167/DC1.
Acknowledgments
We thank Dr. Barry S. Oemar for his help with the preparation of this manuscript.
This study was supported by Mochida Pharmaceutical Co., Ltd., Mebiol Inc., and Health and Labor Science Research Grants for Research on Specific Diseases from the Ministry of Health, Labor, and Welfare.
Abbreviations used in this paper: ABC, ATP-binding cassette; ARF, acute renal failure; bHLH, basic helix-loop-helix; BM, bone marrow-derived mononuclear; BMP, bone morphologic protein; BUN, blood urea nitrogen; CRF, chronic renal failure; HGF, hepatocyte growth factor; LIF, leukemia inhibitory factor; RA, retinoic acid; SP, side population; TSA, trichostatin A.
References
- Asakura, A., and M.A. Rudnicki. 2002. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp. Hematol. 30:1339–1345. [DOI] [PubMed] [Google Scholar]
- Asakura, A., P. Seale, A. Girgis-Gabardo, and M.A. Rudnicki. 2002. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 159:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami, A.P., L. Barlucchi, D. Torella, M. Baker, F. Limana, S. Chimenti, H. Kasahara, M. Rota, E. Musso, K. Urbanek, et al. 2003. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 114:763–776. [DOI] [PubMed] [Google Scholar]
- Chan, L.Y., J.C. Leung, A.W. Tsang, S.C. Tang, and K.N. Lai. 2005. Activation of tubular epithelial cells by mesangial-derived TNF-alpha: glomerulotubular communication in IgA nephropathy. Kidney Int. 67:602–612. [DOI] [PubMed] [Google Scholar]
- Cui, S., L. Schwartz, and S.E. Quaggin. 2003. Pod1 is required in stromal cells for glomerulogenesis. Dev. Dyn. 226:512–522. [DOI] [PubMed] [Google Scholar]
- Goodell, M.A., K. Brose, G. Paradis, A.S. Conner, and R.C. Mulligan. 1996. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 183:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman, M.M., T. Fojo, and S.E. Bates. 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2:48–58. [DOI] [PubMed] [Google Scholar]
- Hishikawa, K., B.S. Oemar, and T. Nakaki. 2001. Static pressure regulates connective tissue growth factor expression in human mesangial cells. J. Biol. Chem. 276:16797–16803. [DOI] [PubMed] [Google Scholar]
- Hishikawa, K., T. Marumo, S. Miura, A. Nakanishi, Y. Matsuzaki, K. Shibata, H. Kohike, T. Komori, M. Hayashi, T. Nakaki, et al. 2005. Leukemia inhibitory factor induces multi-lineage differentiation of adult stem-like cells in kidney via kidney-specific cadherin 16. Biochem. Biophys. Res. Commun. 328:288–291. [DOI] [PubMed] [Google Scholar]
- Jackson, K.A., S.M. Majka, H. Wang, J. Pocius, C.J. Hartley, M.W. Majesky, M.L. Entman, L.H. Michael, K.K. Hirschi, and M.A. Goodell. 2001. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 107:1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.D., J.M. Park, I.S. Kim, K.D. Choi, B.C. Lee, S.H. Lee, S.J. Hong, S.Y. Jin, H.J. Lee, M.S. Hong, et al. 2004. Association of IL-1beta, IL-1ra, and TNF-alpha gene polymorphisms in childhood nephrotic syndrome. Pediatr. Nephrol. 19:295–299. [DOI] [PubMed] [Google Scholar]
- Lin, J., S.R. Patel, X. Cheng, E.A. Cho, I. Levitan, M. Ullenbruch, S.H. Phan, J.M. Park, and G.R. Dressler. 2005. Kielin/chordin-like protein, a novel enhancer of BMP signaling, attenuates renal fibrotic disease. Nat. Med. 11:387–393. [DOI] [PubMed] [Google Scholar]
- Lu, J., R. Webb, J.A. Richardson, and E.N. Olson. 1999. MyoR: a muscle-restricted basic helix-loop-helix transcription factor that antagonizes the actions of MyoD. Proc. Natl. Acad. Sci. USA. 96:552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J.R., R. Bassel-Duby, A. Hawkins, P. Chang, R. Valdez, H. Wu, L. Gan, J.M. Shelton, J.A. Richardson, and E.N. Olson. 2002. Control of facial muscle development by MyoR and capsulin. Science. 298:2378–2381. [DOI] [PubMed] [Google Scholar]
- Majka, S.M., K.A. Jackson, K.A. Kienstra, M.W. Majesky, M.A. Goodell, and K.K. Hirschi. 2003. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J. Clin. Invest. 111:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki, Y., K. Kinjo, R.C. Mulligan, and H. Okano. 2004. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity. 20:87–93. [DOI] [PubMed] [Google Scholar]
- Mizuno, S., T. Kurosawa, K. Matsumoto, Y. Mizuno-Horikawa, M. Okamoto, and T. Nakamura. 1998. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J. Clin. Invest. 101:1827–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muso, E., H. Yoshida, E. Takeuchi, M. Yashiro, H. Matsushima, A. Oyama, K. Suyama, T. Kawamura, T. Kamata, S. Miyawaki, et al. 1996. Enhanced production of glomerular extracellular matrix in a new mouse strain of high serum IgA ddY mice. Kidney Int. 50:1946–1957. [DOI] [PubMed] [Google Scholar]
- Nagoshi, E., and Y. Yoneda. 2001. Dimerization of sterol regulatory element-binding protein 2 via the helix-loop-helix-leucine zipper domain is a prerequisite for its nuclear localization mediated by importin beta. Mol. Cell. Biol. 21:2779–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura, A., T. Asano, J. Matsuda, K. Takano, M. Nakagawa, and M. Fukui. 1989. Characteristics of mutant mice (ICGN) with spontaneous renal lesions: a new model for human nephrotic syndrome. Lab. Anim. 23:169–174. [DOI] [PubMed] [Google Scholar]
- Olmsted-Davis, E.A., Z. Gugala, F. Camargo, F.H. Gannon, K. Jackson, K.A. Kienstra, H.D. Shine, R.W. Lindsey, K.K. Hirschi, M.A. Goodell, et al. 2003. Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc. Natl. Acad. Sci. USA. 100:15877–15882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorf, T., U. Kunter, F. Eitner, A. Loos, H. Regele, D. Kerjaschki, D.D. Henninger, N. Janjic, and J. Floege. 1999. VEGF(165) mediates glomerular endothelial repair. J. Clin. Invest. 104:913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez, A., M. Ramirez-Ramos, C. Calleja, D. Martin, M.C. Namorado, G. Sierra, M.E. Ramirez-Ramos, R. Paniagua, Y. Sanchez, L. Arreola, and J.L. Reyes. 2004. Beneficial effect of retinoic acid on the outcome of experimental acute renal failure. Nephrol. Dial. Transplant. 19:2464–2471. [DOI] [PubMed] [Google Scholar]
- Ramesh, G., and W.B. Reeves. 2002. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 110:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveh, D., O. Shemesh, Y.J. Ashkenazi, R. Winkler, and V. Barak. 2004. Tumor necrosis factor-alpha blocking agent as a treatment for nephrotic syndrome. Pediatr. Nephrol. 19:1281–1284. [DOI] [PubMed] [Google Scholar]
- Robb, L., L. Hartley, C.C. Wang, R.P. Harvey, and C.G. Begley. 1998. musculin: a murine basic helix-loop-helix transcription factor gene expressed in embryonic skeletal muscle. Mech. Dev. 76:197–201. [DOI] [PubMed] [Google Scholar]
- Robb, L., T. Brodnicki, N.G. Copeland, D.J. Gilbert, N.A. Jenkins, and R.P. Harvey. 1999. Assignment of the human helix-loop-helix transcription factor gene musculin/activated B-cell factor-1 (MSC) to chromosome 8q21 and its mouse homologue (Msc) to the proximal region of chromosome 1. Genomics. 57:318–319. [DOI] [PubMed] [Google Scholar]
- Schinkel, A.H., E. Wagenaar, C.A. Mol, and L. van Deemter. 1996. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J. Clin. Invest. 97:2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suranyi, M.G., A. Guasch, B.M. Hall, and B.D. Myers. 1993. Elevated levels of tumor necrosis factor-alpha in the nephrotic syndrome in humans. Am. J. Kidney Dis. 21:251–259. [DOI] [PubMed] [Google Scholar]
- Tamaki, T., A. Akatsuka, K. Ando, Y. Nakamura, H. Matsuzawa, T. Hotta, R.R. Roy, and V.R. Edgerton. 2002. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol. 157:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W.W., M. Qi, G.Y. Van, G.P. Wariner, and B. Samal. 1996. Leukemia inhibitory factor ameliorates experimental anti-GBM Ab glomerulonephritis. Kidney Int. 50:1922–1927. [DOI] [PubMed] [Google Scholar]
- Tuglular, S., P. Berthoux, and F. Berthoux. 2003. Polymorphisms of the tumour necrosis factor alpha gene at position -308 and TNFd microsatellite in primary IgA nephropathy. Nephrol. Dial. Transplant. 18:724–731. [DOI] [PubMed] [Google Scholar]
- Wang, S., and R. Hirschberg. 2003. BMP7 antagonizes TGF-beta-dependent fibrogenesis in mesangial cells. Am. J. Physiol. Renal Physiol. 284:F1006–F1013. [DOI] [PubMed] [Google Scholar]
- Yoshino, J., T. Monkawa, M. Tsuji, M. Hayashi, and T. Saruta. 2003. Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J. Am. Soc. Nephrol. 14:3090–3101. [DOI] [PubMed] [Google Scholar]
- Yu, L., J. Mikloucich, N. Sangster, A. Perez, and P.J. McCormick. 2003. MyoR is expressed in nonmyogenic cells and can inhibit their differentiation. Exp. Cell Res. 289:162–173. [DOI] [PubMed] [Google Scholar]
- Zeisberg, M., J. Hanai, H. Sugimoto, T. Mammoto, D. Charytan, F. Strutz, and R. Kalluri. 2003. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9:964–968. [DOI] [PubMed] [Google Scholar]
- Zhou, S., J.D. Schuetz, K.D. Bunting, A.M. Colapietro, J. Sampath, J.J. Morris, I. Lagutina, G.C. Grosveld, M. Osawa, H. Nakauchi, and B.P. Sorrentino. 2001. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 7:1028–1034. [DOI] [PubMed] [Google Scholar]