Abstract
In Drosophila, myoblasts are subdivided into founders and fusion-competent myoblasts (fcm) with myotubes forming through fusion of one founder and several fcm. Duf and rolling pebbles 7 (Rols7; also known as antisocial) are expressed in founders, whereas sticks and stones (SNS) is present in fcm. Duf attracts fcm toward founders and also causes translocation of Rols7 from the cytoplasm to the fusion site. We show that Duf is a type 1 transmembrane protein that induces Rols7 translocation specifically when present intact and engaged in homophilic or Duf–SNS adhesion. Although its membrane-anchored extracellular domain functions as an attractant and is sufficient for the initial round of fusion, subsequent fusions require replenishment of Duf through cotranslocation with Rols7 tetratricopeptide repeat/coiled-coil domain-containing vesicles to the founder/myotube surface, causing both Duf and Rols7 to be at fusion sites between founders/myotubes and fcm. This implicates the Duf–Rols7 positive feedback loop to the occurrence of fusion at specific sites along the membrane and provides a mechanism by which the rate of fusion is controlled.
Introduction
Skeletal muscle fibers are syncytia that form from the fusion of mono-nucleated myoblasts during embryogenesis (Hauschka, 1994). Growth and repair of the muscles in the adult also require myoblast fusion. In Drosophila, the somatic muscles (analogous to the vertebrate skeletal muscles) comprise ∼30 distinct muscles that are segmentally reiterated in a stereotypical pattern. Each of these muscles arises through fusion between two types of myoblasts, the founders and fusion-competent myoblasts (fcm), with the largest muscles containing ∼25 nuclei, whereas the smallest is made up of approximately four (Baylies et al., 1998; Frasch and Leptin, 2000; Dworak and Sink, 2002). Parallels at the cellular, subcellular, and molecular levels between the fusion process here and in vertebrates, and the ability to genetically dissect the process render the former an attractive model to study the cues that control myotube formation and enlargement (Abmayr et al., 2003).
During embryogenesis in Drosophila, mesodermal cells expressing high levels of the transcription factor twist acquire a myoblast cell fate, and from within this population, a subset of lethal-of-scute–expressing myoblasts is selected through Notch-mediated lateral inhibition to become founders; the remaining twist-expressing cells differentiate into fcm. This partitioning leads to the conferment of distinct properties upon each population of myoblasts. Soon after birth, each founder initiates expression of a unique combination of transcription factors (e.g., Even-skipped [Eve]), collectively referred to as muscle identity genes, which direct differentiation toward a particular muscle fate. The fcm, in comparison, are relatively homogenous and do not appear to be preassigned to any muscle fate. Second, founders produce myoblast attractants, whereas fcm express ligands for these. This results in movement of fcm toward founders and eventually contact between several fcm with one founder, thus establishing directionality to the fusion process. Upon fusion, the newly incorporated nuclei within the syncytium are “entrained” by the founder to express its identity genes, directing the entire myotube toward a specific differentiation program. By being the cellular entity at the center of the fusion process, founders seed myotube formation. In addition, as bearers of identity cues, the seeding event ensures that each founder develops into a muscle with unique characteristics, which includes having a defined size.
Although a large number of fcm cluster in the vicinity of founders, adhesion usually occurs between a founder and just one or two fcm. The fusing partners align against one another and the closely apposed cell membranes breakdown to give cytoplasmic continuity. Hence, fusion first yields a bi- or tri-nucleated myotube called the syncytial precursor (Bate, 1990). The precursor attracts and fuses with more fcm, enlarging in size until it reaches its final size as defined by its founder specification program. Intriguingly, although the larger surface area of a nascent myotube membrane could support multiple simultaneous fusions, this is not seen. Instead, the growth of the myotube occurs in stages, each time through fusion with two or three fcm at discrete regions along its surface. Analyses using EM reveal an aggregate of electron dense vesicles lining the membranes of founders/nascent myotubes and fcm at points of contact (Doberstein et al., 1997). Subsequently, electron-dense plaques form along these sites, possibly arising from the deposition of the contents from the vesicles. The nature of the vesicular content and its role in fusion are not known.
Genetic studies have now identified a number of molecules that mediate myoblast fusion. dumbfounded (duf; also known as kin-of-irre) is expressed specifically in founders just before the onset of fusion and encodes a myoblast attractant (Ruiz-Gomez et al., 2000; Strunkelnberg et al., 2001). Its transcript is thought to encode a transmembrane (TM) protein with a single membrane-spanning region. This, together with its role as a myoblast attractant, has led to two alternative proposals concerning its mode of action. First, Duf may be cleaved to release its putative extracellular (EC) region. Fcm move toward founders by “sensing” gradients of the secreted chemoattractant. On the other hand, the intact duf protein may anchor to the cell surface where fcm detect its presence through random contacts with founders. roughest (rst; also known as irregular chiasm-C) appears to have a similar function because embryos lacking both duf and rst exhibit disrupted attraction, and consequently fusion, whereas single mutants of either have a wild-type (WT) musculature (Strunkelnberg et al., 2001). Consistently, reintroduction of either of these proteins in double mutants restores fusion. The distribution of the sticks and stones (sns) and hibris (hbs) proteins at the fcm cell surface, and loss of myoblast attraction in sns mutant embryos suggest that these molecules actively participate in or modulate this process (Bour et al., 2000; Artero et al., 2001; Dworak et al., 2001). The scaffold-like rolling pebbles 7 (rols7; also known as antisocial) protein is another component of the fusion machinery that shows a differential distribution pattern (Chen and Olson, 2001; Menon and Chia, 2001; Rau et al., 2001). Like duf/rst, Rols7 is expressed in founder myoblasts before fusion and is seen to translocate from the cytosol to sites of fcm adhesion in a Duf-dependent manner. In doing so, it recruits an element of the cytoskeleton, D-Titin, to the fusion site. As Rols7 translocation and D-Titin recruitment occur even in the absence of sns, where random contact between founders and fcm is rarely seen, it has been postulated that these events might be constitutive thereby implicating the presence of preformed sites specialized for fusion on the founder cell membrane (Menon and Chia, 2001). Similarly, Duf causes translocation of the guanine nucleotide exchange factor Loner, which then recruits and activates the ARF6 and D-Rac GTPases at the fusion site (Chen et al., 2003). The involvement of both these small GTPases, kette (and thus, actin; Schroter et al., 2004) and D-Titin in fusion highlight the need for cytoskeletal reorganization during fusion. It is conceivable that the role of Duf as a translocator of various cytoplasmic fusion effectors could be mediated through the intact protein or a part thereof, as suggested by coimmunoprecipitation with Rols7 (Chen and Olson, 2001).
In this paper, we show that Duf is a rate-limiting factor in myoblast fusion. Its expression on the surface of founders and actively fusing myotubes is tightly regulated. In addition, Rols7 translocation is not constitutive but induced by founder/myotube-fcm adhesion (or founder–founder adhesion in sns and hbs;sns mutants) mediated through the intact Duf receptor. With the translocation of Rols7-associated vesicles, the level of Duf at the precursor surface is replenished and this promotes myotube enlargement through more rounds of myoblast fusion.
Results
Duf encodes a type 1 TM protein that must remain intact for Rols7 to translocate
The duf-dependent translocation of Rols7 can be reproduced in nonmesodermal tissues that do not normally express these proteins. When Rols7 alone is expressed in the embryonic epidermal epithelium or salivary gland, it is seen throughout the cytoplasm in distinct puncta (Fig. 1, A and C, Rols7, green). In the presence of Duf, the Rols7 puncta become enriched along the apical membrane at adherence junctions identified by Crumbs expression, suggesting conservation of the translocation mechanism seen in the mesoderm (Fig. 1, B and D, Crumbs, red).
Figure 1.
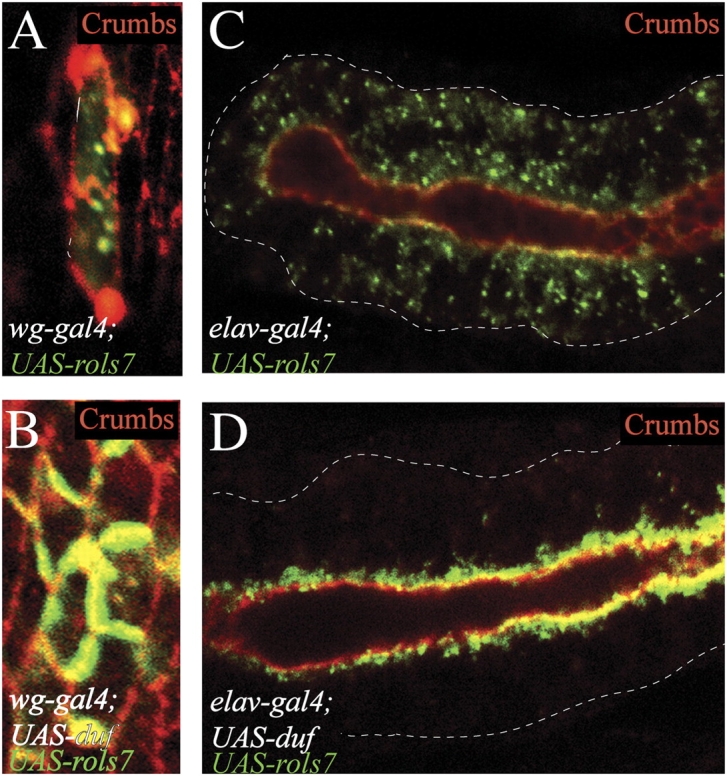
Rols7 translocates to adherence junctions in polarized nonmesodermal tissues when Duf is present. Rols7 was expressed in epidermal (A and B) or salivary gland (C and D) epithelium using the gal4-UAS system. Embryos were stained with antibodies against Rols7 (green) and Crumbs, a marker for adherence junctions (red). Dashes outline epidermal cell or salivary gland.
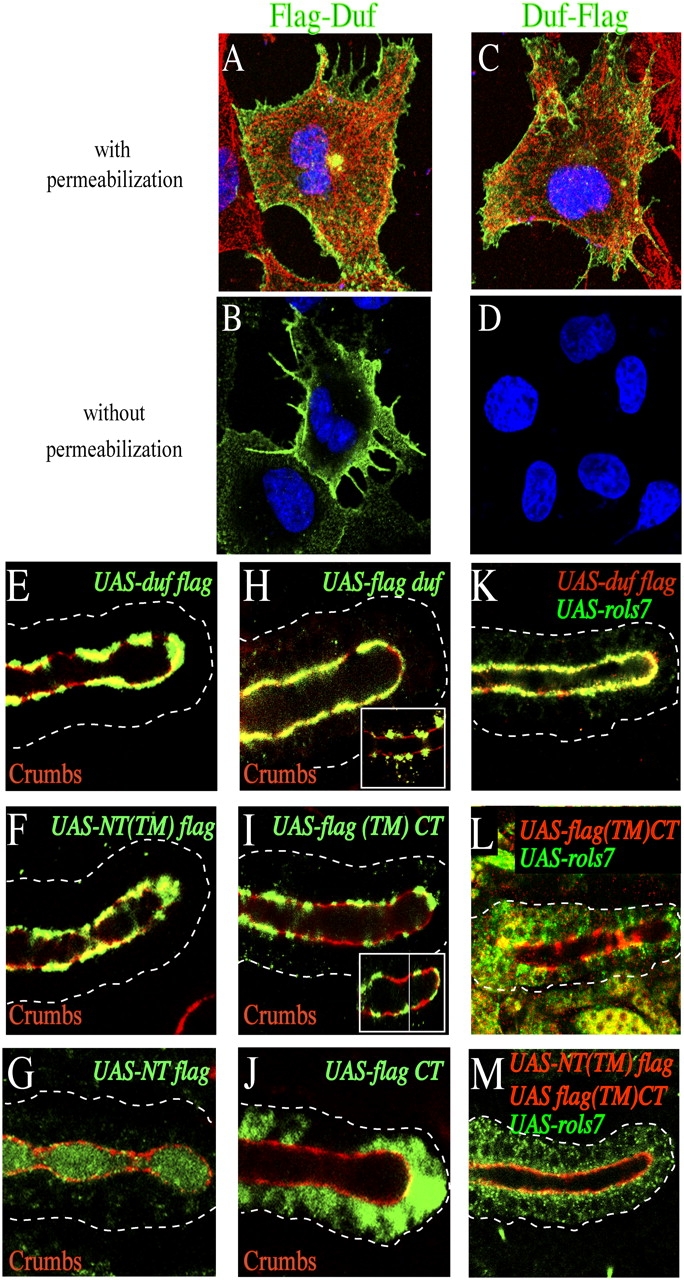
To analyze domains of Duf necessary for the translocation event, we first verified the topology of this putative TM protein. Cos cells were transfected with plasmids that express an NH2- or COOH-terminal Flag epitope-tagged Duf and then stained with anti-Flag antibodies. In permeabilized cells, the staining pattern using either tagged construct is similar, and Duf is seen along the cell surface (Fig. 2, A and C). In contrast in cells that are not permeabilized and thus impenetrable to antibodies, only NH2-terminal–tagged Duf (Flag-Duf) is detectable at the cell periphery, whereas cells expressing COOH-terminal–tagged Duf (Duf-Flag) show no staining at all (Fig. 2, compare B with D). Together, these results show that Duf is located at the cell surface as a type 1 TM protein, i.e., with an EC NH2-terminal region.
Figure 2.
The intact Duf type 1 TM protein induces Rols7 to translocate. (A–D) Duf localization and topology. Full-length Duf Flag-tagged at its NH2 (A and B) or COOH terminus (C and D) was expressed in Cos cells. Cells were stained with anti-Flag antibodies (green), anti-tubulin antibodies (red), and Hoechst (blue). (E–J) Full-length or truncated Duf was expressed in the salivary gland and detected using antibodies against Flag (green, constructs shown schematically in Fig. 4 M). Crumbs marks adherence junctions (red). (K–M) Coexpression of Flag-tagged Duf constructs (red) and Rols7 (green) in the salivary gland. NT, NH2-terminal/EC; CT, COOH-terminal/IC. Position of tag in construct indicated by where “Flag” is placed in nomenclature. Dashes outline salivary gland.
We created Flag-tagged truncations of Duf and examined where these, in comparison to Flag-tagged full-length Duf, localize to in polarized cells. We also ascertained if the constructs retained the ability to recapitulate Rols7 translocation (see Fig. 4 M for schematic structure of Duf constructs. All constructs were sequenced in their entirety and express similar levels of protein in whole extracts from embryos as detected by Western blot; unpublished data). Full-length Duf tagged at its COOH terminus is seen at the apical surface, including the adherence junctions (Duf-Flag; Fig. 2 E, overlap between Crumbs and Flag, yellow). A construct retaining the EC and putative TM regions but with the intracellular (IC) region replaced by a Flag tag, NT(TM)-Flag, is also clearly seen at the apical cell surface (Fig. 2 F). However, a deletion that extends further from the COOH-terminal into the construct, thus removing the putative TM sequence no longer anchors to the cell membrane and NT-Flag is secreted into the lumen (Fig. 2 G).
Flag-Duf, where the tag is positioned at the NH2 terminus of full-length Duf, is detected along the apical surface, particularly the junctions, in all embryos examined using antibodies raised against the Duf COOH-terminal region (Fig. 2 H). However, using anti-Flag antibodies, this construct is sometimes seen in small patches along the apical membrane that alternate rather than overlap with Crumbs, suggesting that it is present at the apical surface other than the adherence junctions (Fig. 2 H, inset). In most embryos, it cannot be detected using anti-Flag antibodies. It is likely that this difference in detection arises from masking of the Flag epitope (see Discussion). Flag-(TM)CT, where most of the EC region is deleted and replaced by an NH2-terminal Flag tag followed by the putative TM and IC regions, is also detected in small patches along the apical membrane that alternate rather than overlap with Crumbs using anti-Flag antibodies (Fig. 2 I). Detection using antibodies raised against the Duf IC region reveals a similar staining pattern, suggesting that this construct is largely excluded from adherence junctions (Fig. 2 I, inset). A deletion that extends further from the NH2 terminus, thereby removing the putative TM region, no longer anchors to the cell membrane and Flag-CT appears throughout the cytoplasm (Fig. 2 J). Together, these results show that the putative TM region of Duf indeed functions as a membrane anchor. In addition, localization at adherence junctions requires its EC region to be present.
When coexpressed with Rols7, only full-length Duf causes Rols7 to translocate (Fig. 2 K). Notably, the membrane-anchored Duf IC region does not bring about translocation, although it has been reported to interact with Rols7 in immunoprecipitation assays when both Duf and Rols7 are coexpressed in Drosophila S2 cells (Fig. 2 L; Chen and Olson, 2001). Concurrent expression of membrane-anchored EC and IC regions of Duf from two separate plasmids also does not induce Rols7 to translocate, suggesting that translocation requires the Duf receptor to remain intact (Fig. 2 M).
Translocation occurs only where Duf is engaged in homophilic or Duf:SNS–directed adhesion
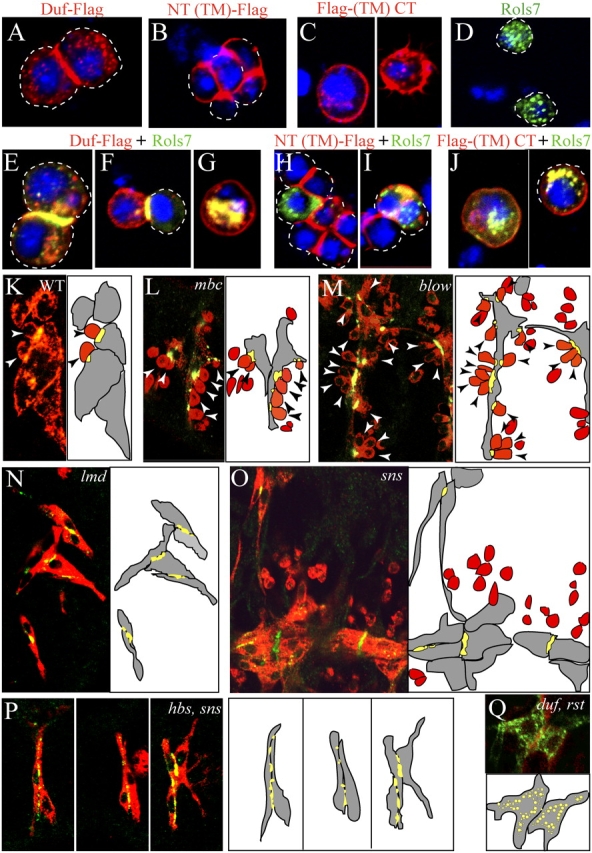
We next examined if cell type–specific differences might contribute to the interaction between the Duf IC region and Rols7 reported in S2 cells. In these nonpolarized cells, full-length and membrane-anchored truncations of Duf localize at the cell surface (Fig. 3, A–C). Expression of full-length Duf or its membrane-anchored EC region NT(TM)-Flag causes transfected cells to cluster and these proteins are enriched at the adhesion site (Fig. 3, A and B). This indicates that the EC region of Duf is sufficient to bring about homophilic interaction in trans. Rols7 when expressed alone, is seen in distinct cytoplasmic puncta and the transfected cells do not cluster (Fig. 3 D).
Figure 3.
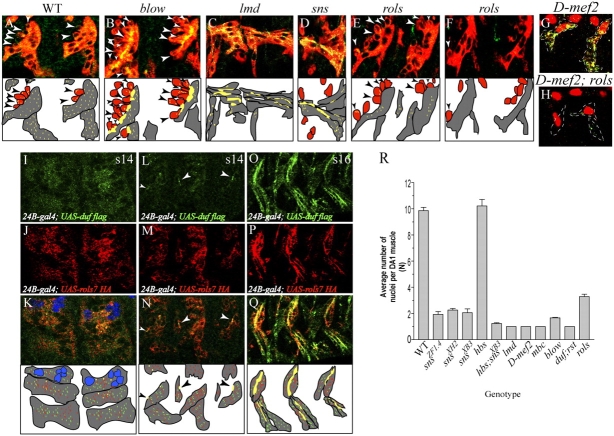
Engagement of the Duf receptor through homophilic or Duf–SNS adhesion initiates and directs translocation of Rols7. (A–J) Full-length Duf, membrane-anchored Duf EC or IC regions (red) were expressed alone or together with Rols7 (green) in S2 cells. Dashes outline cell surface. Hoechst-labeled DNA in blue. (K–Q) WT (early stage 15) and mutant embryos (stage 16) were stained with anti-Rols7 (green) and anti-myosin heavy chain (MHC, red) to determine Rols7 distribution in relation to myoblast adhesion. Schematics show founders/myotubes in gray, fcm in red, and Rols7 in green (or yellow when it overlaps with MHC); arrowheads highlight fcm that adhere to founders/myotubes.
The Rols7 puncta aggregate at the cell surface only in the presence of full-length Duf (Fig. 3, E, F, H, and J). Importantly, translocation is restricted to sites where Duf engages in cell–cell contact and not elsewhere along the periphery where Duf is also present (Fig. 3 F, cell on the left shows Duf all around the cell surface but Rols7 enrichment is exclusively at site of adhesion). Translocation also occurs when cells coexpressing full-length Duf and Rols7 adhere to SNS-expressing cells, with the Rols7 puncta aggregating along adhesion sites (unpublished data). Cells that express both Duf and Rols7 but are isolated from other Duf/SNS-expressing cells or in close proximity to cells that only express Rols7 also fail to show Rols7 translocation (Fig. 3 G). From this, it appears that Rols7 translocation does not arise through Duf-mediated targeting of preassembled Duf–Rols7 complex or constitutive delivery of nascent Rols7 to sites where Duf is surface localized. Rather, the engagement of the EC region of the intact Duf receptor precedes and is necessary to direct movement of Rols7 puncta to where Duf is located.
If either homo- or heterophilic engagement of the Duf receptor can induce Rols7 to translocate, why does translocation during myoblast fusion occur at sites of adhesion between founders/myotubes and fcm and not at sites of contact between neighboring founders and/or myotubes (Fig. 3 K)? Analyses of mutants such as myoblast city (mbc; Erickson et al., 1997), blownfuse (blow; Doberstein et al., 1997), Drosophila-myocyte enhancer factor 2 (D-mef2; Bour et al., 1995; Lilly et al., 1995), and hbs show that translocation of Rols7 remains unperturbed and Rols7 is present where fcm contact the founders (Fig. 3, L and M; Menon and Chia, 2001; unpublished data).
In contrast, in lame duck (lmd; also known as gleeful or myoblasts incompetent) mutants (Duan et al., 2001; Furlong et al., 2001; Ruiz-Gomez et al., 2002) where fcm are absent and fusion does not occur, Rols7 translocates to founder–founder contact sites instead (Fig. 3 N). Similarly, when fcm fail to express both SNS and Hbs or SNS alone, and are no longer attracted toward the founders, Rols7 becomes enriched at the adhesion sites between founders (PFig. 3, O and P). Examination of Duf distribution reveals that it parallels that of Rols7 in each of these mutants (see next section). We conclude that in myoblasts, heterophilic interaction between Duf and SNS is favored over homophilic Duf interaction, thus targeting Duf and Rols7 to sites of adhesion between founder/myotube and fcm. However, in the absence of SNS, Duf is free to engage itself and, in doing so, directs Rols7 there.
Early and later myoblast fusions differ in their requirements of the Duf receptor
To understand how Duf promotes fusion, we introduced various Duf constructs into myoblasts lacking Duf and its paralogue Rst (henceforth, referred to as duf, rst mutants) and assessed the extent to which fusion occurred (see Fig. 4 M for summary of results). In duf, rst double mutant embryos, the founders fail to attract fcm, Rols7 does not translocate and fusion stalls altogether (Fig. 3 Q). With the reintroduction of either untagged or Flag-tagged full-length Duf, the ability of founders and myotubes to attract fcm is restored, Rols7 translocates to fcm-founder/myotube adhesion sites and fusion occurs to a significant extent. This culminates in a WT muscle pattern in every segment (Fig. 4, compare A with C). Quantification of the Eve-expressing nuclei shows that a WT DA1 myotube contains an average of 9.9 ± 0.3 nuclei at stage 15, whereas its equivalent in mutants expressing full-length Duf comprises at least 6.6 ± 0.8 nuclei (Fig. 4, compare B with D; Fig. 4 L).
Figure 4.
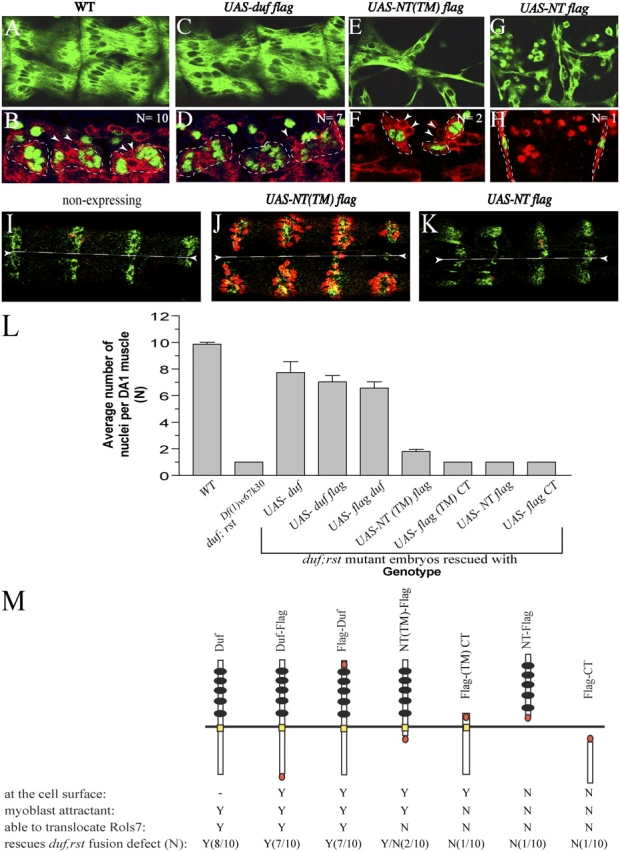
Functional analyses of Duf. (A–H) Full-length and Duf deletions were introduced into duf, rst mutants using the 24B-gal4 driver. Embryos were stained with anti-MHC antibodies (A, C, E, and G, showing dorsal muscles) or double labeled with antibodies against Eve (green nuclear stain) and either D-Titin (B, red cytoplasmic stain) or MHC (D, F, and H). Founders and myotubes outlined with dashes; arrowheads indicate adherent fcm. (I–K) Restoration of myoblast attraction as gauged by clustering of somatic fcm at epidermal sites from ectopic expression of constructs using the wingless (wg)-gal4 driver. Panels show ventral view of late stage 14 embryos with ventral midline demarcated by dashes. Anti-MHC stain (red) marks myoblasts and anti-Wg stain (green) marks the alternating strips of wg-gal4-driven expression domains in the epidermis. Mutant embryos expressing NT(TM)-Flag show fcm extending filopodia toward and adhering to the Wg-expressing epidermal regions (J) in contrast to nonexpressing or NT-Flag–expressing mutant embryos where fcm are not seen (I and K). (L) Extent of fusion in the somatic muscles was assessed based on the average number of Eve-positive nuclei within DA1 muscles at stage 15, denoted as “N” ± SD, from three independent experiments where 20–30 abdominal hemisegments from two to three embryos were analyzed at a time. A WT DA1 contains an average of 10 nuclei (n = 9.9 ± 0.3, whereas those in duf;rst Df(1)w67k30 mutants are mono-nucleated (n = 1.0 ± 0.0). Embryos were also stained with an antibody against Rols7 to ascertain its localization. Results are summarized in M. Yellow bar, TM region; orange oval, Flag tag; black circles, immunoglobulin repeats; Y (yes), N (no), Y/N (partial). As expression levels of Duf constructs in myoblasts was beyond the sensitivity of detection, its localization in M is as observed when constructs are ectopically expressed in the epidermis or salivary gland.
Expression of the membrane-anchored Duf EC region NT(TM)-Flag restores fcm attraction and adhesion (Fig. 4, compare I with J) but does not trigger Rols7 translocation (unpublished data). Nevertheless, a small degree of fusion occurs and most of the DA1 muscles develop into bi-nucleated precursors (n = 1.8 ± 0.3), a phenotype analogous to that seen in embryos lacking rols (Fig. 4, E, F, and L). On the other hand, embryos expressing the soluble Duf EC region NT-Flag fail to exhibit any myoblast attraction and there is neither translocation nor rescue of the aberration (Fig. 4, compare K with I–J and L). Expression of the Duf IC region either as the membrane-anchored or soluble form also fails to confer attraction or induce Rols7 translocation, and fusion remains completely disrupted (Fig. 4 L). Concurrent expression of the membrane-anchored Duf EC and IC regions does not reconstitute functions of the intact receptor and fusion stalls after precursors form (unpublished data). Collectively, these data show that the attractant properties of Duf reside wholly in a membrane-anchored EC region and that this region is sufficient to initiate the first round of fusion leading to the development of the precursor. However, fusion beyond this stage requires both the membrane-anchored EC and IC regions to be present as a single entity. The inability of precursors to fuse, despite conditions that sustain expression of the attractant NT(TM)-Flag and maintain adhesion between fcm and precursors (Fig. 4 F, arrowheads), underscores the need for IC events such as the translocation of Rols7 to take place before fusion can proceed. None of the expressed mutants showed dominant negative effects.
Distinct regions of Rols7 perform different functions: ankyrin repeats confer Duf responsiveness, whereas the TPR/coiled-coil region confers myoblast fusion competence
To delineate the functional region(s) of Rols7, we introduced HA-tagged Rols7 constructs deleted progressively from the NH2 or COOH terminus into the salivary gland epithelium and tested if these translocated in the presence of Duf (Fig. 5, A–F; Fig. 5 H shows schematic structure of constructs. All constructs were sequenced in their entirety. Transcripts were detectable by RNA in situ hybridization and, with the exception of HA-Rols7 (Δ1-863) and (Δ1-1150), proteins were produced at comparable levels, as seen on Western blots; Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200501126/DC1). We also introduced the constructs into rols mutant myoblasts to ascertain the effect on fusion (Fig. 5 G and Fig. 5 H, summary of results). Full-length Rols7 tagged at either its NH2 or COOH terminus associates with distinct puncta, just like the untagged protein, translocates to the apical surface in polarized cells or the fcm-founder/myotube adhesion site and restores fusion (Fig. 5, A, B, and G). Analyses of DA1 muscles reveal an average of at least 9.3 ± 1.3 Eve-expressing nuclei per muscle in embryos rescued using full-length Rols7, within the limits of the “N” value for WT muscles, and a marked increase from 3.3 ± 0.2 in rols mutants. Surprisingly, removal of the first 309 aa unique to Rols7 and absent in the alternatively spliced Rols6 (Menon and Chia, 2001) or point mutations of the catalytic residues of a putative lipolytic enzyme signature sequence encompassed within this region, does not lead to any deleterious effects. Similarly, construct HA-Rols7 (Δ1-439), where the deletion extends to remove the RING finger, is functionally equivalent to full-length Rols7. On the other hand, constructs HA-Rols7 (Δ1-863) and (Δ1-1150) that lack the other unique region encoded by exon 6 in rols7 cannot be detected, suggesting that this region is likely to confer protein stability. Consistently, Rols6 is not detected when ectopically expressed in the salivary gland or rols mutant myoblasts and fusion is also not restored (Fig. 5 G).
Figure 5.
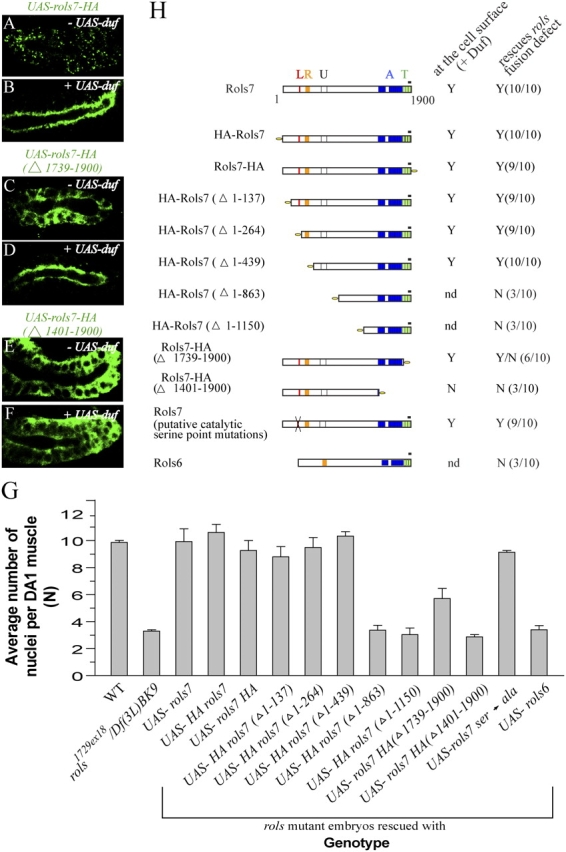
Distinct regions of Rols7 perform different functions. (A–F) HA-tagged full-length Rols7, deletions, and point mutations of catalytic residues in the putative lipolytic enzyme signature sequence were expressed alone or with Duf in the salivary gland. Glands were stained with an antibody against HA to ascertain Rols7 localization (green, constructs shown schematically in H). (G) Constructs were introduced into rols P1729ex18/Df(3L)BK9 mutant myoblasts using the 24B-gal4 driver and extent of fusion assessed by the average number of Eve-positive nuclei, “N” ± SD, within a DA1 muscle at stage 15 in comparison to 9.9 ± 0.3 in WT and 3.3 ± 0.2 in rols mutants. Data represent results from three independent experiments in which 20–30 abdominal hemisegments from two to three embryos were analyzed each time. (H) Summary of data obtained in G. Rols7 subcellular distribution was ascertained as described before. L (red), putative lipolytic enzyme sequence; R (orange), RING finger; U (white box), a region in Rols7 that is absent in Rols6; A (blue), ankyrin repeats; T (green), TPR repeats; black dash, coled-coil region; yellow oval, HA tag; Y (yes), N (no), Y/N (partial); nd, not detected. Position of tag in construct indicated by where “HA” is placed in nomenclature.
A COOH-terminal deletion construct lacking the tetratricopeptide repeats (TPRs) and coiled-coil region, Rols7-HA (Δ1739-1900), no longer associates with discrete puncta and instead appears diffused in the cytoplasm (Fig. 5, compare C with A). However, this truncation remains fully Duf responsive and translocates efficiently (Fig. 5, compare C with D). When introduced into rols mutant myoblasts, fusion occurs to a greater extent (n = 5.7 ± 1.3) but not to a degree that produces a functional musculature—most of these embryos are unable to hatch and eventually die, like rols mutant embryos (Fig. 5 G). A deletion that also removes the ankyrin repeats, Rols7-HA (Δ1401-1900), results in a truncated protein that fails to translocate in the presence of Duf and is unable to rescue the fusion defect (Fig. 5, compare E with F and G). Hence, Rols7 comprises regions that perform distinct functions—the ankyrin repeats are essential for Duf-dependent translocation, whereas the TPR/coiled-coil region functions after translocation. Although both these regions are present in Rols6, Rols6 is not stable when ectopically expressed and cannot substitute for Rols7. None of the constructs showed dominant negative effects.
Rols7 sustains myoblast fusion by maintaining Duf expression at the precursor surface
How does Rols7 sustain fusion? Our observation that fusion beyond the precursor stage requires intact Duf (and Rols7) prompted us to analyze Duf expression during fusion. In situ indirect immunofluorescent stains using antibodies raised against the bulk of the EC region of Duf (Galletta et al., 2004) as well as an antibody raised against an IC region that is unique to Duf detect ectopically expressed Duf at the surface in a variety of tissues such as the salivary glands, epidermis, central and peripheral nervous systems, amnioscerosa, and developed muscles (Fig. 2; Fig. 6, O–Q; Fig. S2, F–H, available at http://www.jcb.org/cgi/content/full/jcb.200501126/DC1). In contrast, endogenous Duf or that which is driven with gal4 drivers is largely seen as puncta within the cytoplasm of actively fusing myotubes at stage 13/14 and is rarely detected at the myotube surface using either of the two anti-Duf antibodies or anti-Flag antibodies, despite an abundance in duf mRNA (Fig. 6, A, I–N; Fig. S2, A and F–H). Nevertheless, in fusion mutants such as blow, lmd, D-mef2, mbc, sns, and hbs;sns, endogenous Duf or ectopically expressed Duf-Flag becomes clearly visible at the founder/myotube surface (Fig. 6, B–D, and G; Fig. S2, B–D; unpublished data). Where fcm differentiate and express SNS, Duf is seen enriched at adhesion sites between fcm and founders (Fig. 6 B). On the other hand, absence of the entire fcm population or just the lack of SNS on fcm changes this distribution pattern; Duf now localizes at adhesion sites between neighboring founders in lmd and sns single mutants and hbs;sns double mutants (Fig. 6, C and D; unpublished data). It thus appears that the level of Duf at the surface of WT myoblasts and enlarging myotubes is tightly controlled posttranscriptionally. In mutants where fusion is disrupted or in WT embryos where fusion is over, this control is overcome thereby elevating the level of Duf to a point that allows its detection at the myoblast/myotube/muscle surface.
Figure 6.
Rols7 sustains Duf expression and allows fusion to progress. (A–F) WT and mutant embryos were double labeled with an antibody raised against the Duf EC region (green) and another against MHC (red). Schematics depict founders/myotubes in gray, fcm in red and Duf in green (or yellow where it overlaps with MHC); arrowheads show adherent fcm. (G and H) Embryos carrying the D-mef2 mutation alone (G) or in combination with the rols mutation (H) were stained with antibodies raised against Eve (red nuclear stain), Rols7 (red cytoplasmic stain), and the Duf IC region (green cytoplasmic stain). Dashes outline founders. (I–Q) Indirect immunofluorescent analyses of ectopically expressed epitope-tagged Duf and Rols7. (I–K) Actively fusing dorsal myotubes at stage 13/14 with DA1 labeled with anti-Eve (blue nuclei). (L–N) Ventral myotubes at stage 13/14. (O–Q) After fusion ventral muscles at stage 16. Schematics show myotubes/muscles in gray and overlapping Duf and Rols7 expression in yellow. When ectopically expressed in actively fusing myotubes, both Duf and Rols7 are visible as cytoplasmic puncta and rarely seen as “dashes” or “lines” at the myotube surface (L and N, arrowheads). At stage 16, both proteins clearly localize at the surface of the ventral muscles, particularly at muscle-muscle adhesion sites. (R) The extent of fusion in various mutants gauged by the average number of Eve-positive nuclei “N” ± SD within a DA1 muscle at stage 15. Data represent results from three independent experiments in which 20–30 abdominal hemisegments from two to three embryos were analyzed at a time.
Of the mutants tested, rols is an exception in that Duf could not be detected at fcm-precursor adhesion sites and was also seen at relatively lower levels within the precursor cytoplasm (Fig. 6, E and F; Fig. S2 E). The inability to detect Duf at the surface here cannot be accounted for on the basis of myotube size because Duf is clearly seen in the equally small tri-nucleated precursors that sometimes develop in blow and sns mutants (Fig. 6, compare E with B and D; Fig. 6 R, “N” value of fusion mutants). Strikingly, D-mef2;rols double mutant embryos no longer exhibit the elevated levels of Duf seen in D-mef2 single mutants (Fig. 6, compare G with H). This and the failure to sustain myoblast attraction and adhesion in the absence of Rols7 show that Rols7 plays a role in maintaining Duf at the surface of precursors through directional transport (Fig. 6, compare E with F, fcm initially cluster and adhere to rols mutant precursors but this process is not sustained). The manifestation of a similar phenotype in rols mutant embryos expressing Rols7-HA (Δ1739-1900), a truncation that lacks the TPR/coiled-coiled region but nevertheless translocates efficiently, suggests that precursors do not maintain expression of surface-localized Duf here as well.
Attempts to bypass the need for Rols7 through gal4-UAS–driven Duf expression in the rols null background neither enables detection of Duf at the precursor surface nor restores the attraction and adhesion between fcm and precursors (unpublished data). Similarly, overexpression of Rols7 alone or Rols7 and Duf in WT myoblasts and actively fusing myotubes does not lead to elevated Duf expression at the surface (Fig. 6, I–N). Hence, Rols7 is essential but not sufficient for the maintenance of surface levels of Duf in precursors.
Discussion
The formation of the myotube is a fundamental part of myogenesis in many organisms. In Drosophila, the advent of a bi- or tri-nucleated syncytial precursor marks the earliest stage in the formation of a myotube and subsequent fusions lead to a gradual increase in myotube size (Bate, 1990). Our results indicate that Duf is a key component in both the initial and subsequent fusions and its expression at the founder/enlarging myotube cell surface is under tight regulation. Functionally active Duf requires molecular integrity because its IC region-dependent Rols7 translocation is regulated in cis by the homo/heterophilic interactions of its chemoattractive EC region. Adhesion between Duf and fcm-expressed SNS (and Hbs to a lesser extent) triggers and directs movement of Rols7-vesicles to the fusion site, replenishing Duf at the cell/myotube surface. This Duf–Rols7 positive feedback loop enables the progression of fusion at specific sites and highlights a means of gating the growth of the myotube.
From myoblast adhesion to Rols7 translocation: the intact Duf receptor as a transducer of an EC event into an IC response
A salient feature of the Duf-dependent Rols7 translocation is most evident in S2 cells. In these cells, sites where Rols7 translocates to clearly coincide with regions along the cell membrane where Duf mediates homo- or heterophilic adhesion between cells, where Duf itself is seen to be enriched, and not elsewhere along the cell membrane where Duf may also be present (Fig. 3, E and F). Hence, association with itself or SNS in trans confers upon Duf the ability to trigger the relocation of Rols7 puncta. In epithelial cells where Duf is targeted to the apical cell surface, Rols7 translocates only to the adherence junctions suggesting that Duf–Duf interaction at contact points between neighboring cells is required for translocation here as well (Fig. 1, B and D). Consistently, a membrane-anchored Duf truncation lacking the EC region no longer accumulates at epithelial adherence junctions and fails to bring about Rols7 translocation (Fig. 2 L). In view of this requirement for Rols7 translocation, it is plausible that the inability to detect Duf with an NH2-terminal Flag tag at adherence junctions (although this construct can be seen elsewhere along the apical surface using anti-Flag antibodies and is detected at adherence junctions using antibodies against its IC region) is due to masking of the Flag epitope by homophilic engagement, rather than cleavage and shedding of the Duf EC region (Fig. 2 H). In myoblasts, sites of Rols7 translocation also overlap with points of adhesion, where Duf itself is seen to localize (Fig. 3, K–P; Fig. 6, B–D). However in the presence of SNS-expressing fcm, there appears to be a preference for Duf–SNS interaction over Duf–Duf interaction and Rols7 translocates specifically to sites of heterophilic adhesion. The observation that Duf–SNS-induced S2 cell adhesions form more rapidly and to a greater extent than Duf–Duf adhesions supports our findings (Galletta et al., 2004). Although fusion is not perturbed to any significant extent in hbs mutants, Duf-Hbs heterophilic adhesion could play a minor role in mediating the initial fusion as suggested by the presence of largely bi-nucleated precursors in sns mutants in contrast to hbs;sns double mutants where founders are mostly mono-nucleated (Fig. 6 R). Hence, Rols7 translocation does not arise as a result of constitutive Duf-dependent targeting of preformed Duf–Rols7 complex to the cell surface or movement of newly synthesized Rols7 to where Duf is surface localized and unengaged. As the TM and/or cytoplasmic tail of SNS is also crucial for membrane fusion, it is possible that analogous adhesion-induced IC events occur in the fcm in parallel (Galletta et al., 2004).
Perpetuating the cycle of myoblast adhesion and fusion through Rols7 translocation during enlargement of the precursor
The duf mRNA is detectable in myotubes so long as they are increasing in size through fusion with fcm, after which expression declines and is eventually lost altogether (Ruiz-Gomez et al., 2000). Although the duf protein can be detected within cytoplasmic puncta, its level at the founder/fusing myotube surface is not commensurate with its transcript level and we are rarely able to detect Duf here, even at a time when fusion with fcm is at its peak. Importantly, attempts to boost its levels at the surface of myoblasts/fusing myotubes using various gal4-UAS combinations were unsuccessful, despite enhancement of its transcript level over that in WT embryos (Fig. 6, I–N; Fig. S2, F–H). As translocation of Rols7 to the cell surface is dependent on surface-localized Duf, we also analyzed surface levels of Rols7 in embryos overexpressing both Rols7 and Duf as an indirect measure of Duf localization. Although cytoplasmic levels of Rols7 were markedly elevated over that in WT stage 13/14 embryos, there was no significant increase in surface-localized Rols7, thus reflecting a lack of enrichment of Duf at the myoblast/enlarging myotube surface. This pattern changes at stage 16, when surface-localized Rols7 (and Duf) clearly outline the muscle surface, particularly at muscle-muscle adhesion sites (Fig. 6, O–Q).
Duf also becomes detectable when myoblast fusion is disrupted (Fig. 6, B–D). In most fusion mutants, its expression is up-regulated to a point where it is clearly seen at sites of myoblast adhesion. At the same time, these mutants display enhanced levels of Rols7 expression and Rols7 is also seen at myoblast adhesion sites. This, the loss of attraction between fcm and rols - precursors, and failure to detect Duf specifically in rols - embryos show that Rols7 is required for accumulation of Duf at the founder/precursor surface. The observation that D-mef2 mutants no longer exhibit increased Duf levels when Rols7 is absent further supports this conclusion (Fig. 6, G and H). Of note, surface levels of the paralogue Rst may also be tightly regulated as analyses using antibodies raised against the EC region of Duf, a region that is highly conserved in Rst, yielded data similar to that using antibodies against an IC region unique to Duf (Strunkelnberg et al., 2001; Galletta et al., 2004).
How might Rols7 affect Duf/Rst expression level? The concurrent loss of Duf and its paralogue Rst leads to a complete block in myoblast fusion, demonstrating that these molecules must be present for the fusion process to start (Fig. 6 R). In contrast in the rols null allele, fusion is not abrogated altogether. Here, an initial round of fusion occurs and generates bi- and tri-nucleated syncytial precursors (Fig. 6 R; Menon and Chia, 2001; Rau et al., 2001). Thereafter, fcm attraction and adhesion to precursors sharply declines and myotube enlargement stalls (Fig. 6, E and F). Hence, the first round of fusion is clearly Duf/Rst dependent but Rols7 independent, whereas sustenance of the fusion cycle needs Rols7. Given that the punctate staining of Rols7 in all cell types tested is indicative of its association with an endosomal compartment, and that Duf can colocalize with these Rols7-positive vesicles, it is plausible that translocation of such vesicles to the myoblast adhesion site followed by its fusion with the cell membrane replenishes Duf/Rst at the cell surface (colocalization of Duf and Rols7 within vesicles most apparent in S2 cells; Fig. 3, G and J). It is likely that other components of the fusion machinery are replenished at the same time through translocation of Rols7- and/or Loner-associated vesicles because duf, rst double mutant founders expressing NT(TM)-Flag, a membrane-anchored Duf truncation lacking the IC region, undergo one cycle of fusion but fail to enlarge any further (Fig. 4, E, F, and L). For Rols7 to respond to the Duf/Rst-induced changes within the cell through translocation, its ankyrin repeat region must be intact (Fig. 5, E–G). However, translocation per se is insufficient for the sustenance of surface-localized Duf/Rst and progression of fusion because only partially formed myotubes are generated when rols mutant founders express the translocation-effective Rols7-HA (Δ1739-1900), a construct lacking the TPR/coiled-coiled motifs (Fig. 5, C, D, and G). Based on the precedence of the coiled-coiled motif in promoting IC membrane fusion, we deduce that the Rols7 COOH-terminal region mediates fusion between the membranes of its host vesicle and the founder/precursor surface (Skehel and Wiley, 1998).
These data emphasize the complex nature of myoblast fusion. Although components of the fusion machinery, such as Duf and Rols7, are both expressed in founder myoblasts at the onset of fusion, the dynamics of their subcellular distribution differs. In our model (Fig. 7), Duf is first targeted to the founder cell surface. Its localization at this time is independent of Rols7. Contact between fcm and founders lead to the relatively stable Duf–SNS interaction, culminating in fcm-founder adhesion and fusion between these closely juxtaposed cell membranes. At the same time, Duf-mediated cell adhesion initiates changes within the cell that lead to cell polarization, such that translocation of Rols7-vesicles is targeted only to sites along the membrane where the first lot of Duf engages in adhesion. With the fusion of these vesicles to the precursor membrane, another batch of Duf (and other fusion machinery) becomes deposited at the cell/precursor surface, this time in a Rols7-dependent manner, and fuels the next round of myoblast fusion. On the surface of the vesicle, Rols7 may interact with the IC region of Duf because significant colocalization is observed in puncta between Rols7 and full-length Duf-Flag or Flag-(TM)CT but not with NT(TM)-Flag (Fig. 3, compare G and J with H and I). This point is exemplified by coimmunoprecipitation of Rols7 with either full-length Duf or a fragment encompassing the IC region (Chen and Olson, 2001).
Figure 7.
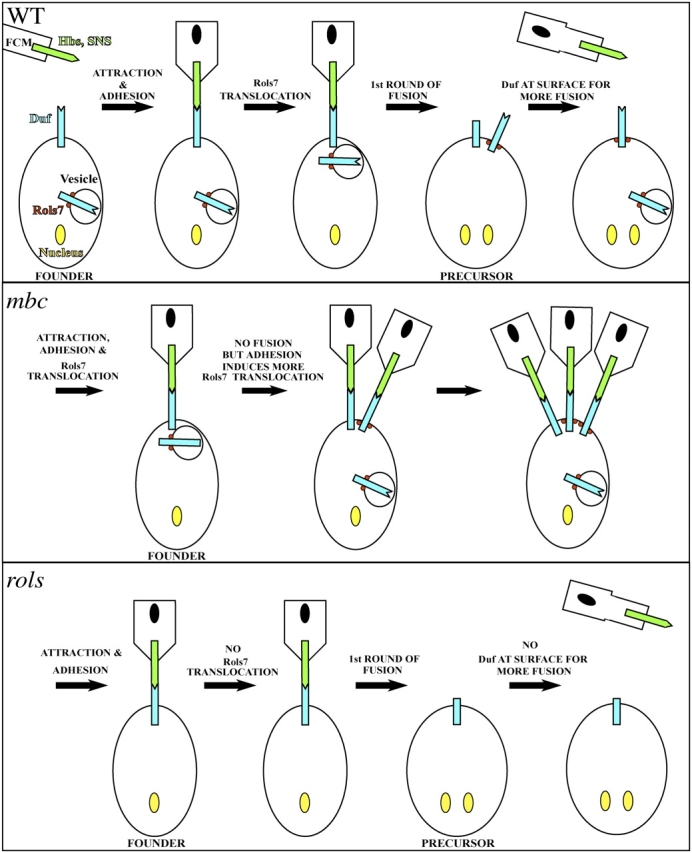
Schematic of events envisaged to occur in WT and mutant myoblasts. (Top) Duf localizes at the WT founder cell surface. Its EC region serves as an attractant, binds Hbs and SNS and, in doing so, brings about adhesion between founder and fcm. The first round of intercellular fusion ensues, culminating in the formation of a precursor, and this may result in the cleavage/removal of the Duf receptor from the cell surface. At the same time, adhesion between the founder and fcm initiates IC events in the founder that lead to translocation (and possibly fusion) of Duf-loaded Rols7 vesicles to the adhesion site. The new batch of Duf at the surface initiates another round of myoblast fusion. (Middle) In mbc or D-mef2 mutants, engagement of Duf induces Rols7 vesicles to translocate and deposit the next batch of Duf at the founder cell surface. However, in these mutants, fusion does not occur and the persistent adhesion between fcm and the founder leads to additional Rols7 and Duf being deposited at the founder surface. In lmd and sns mutants, similar events triggered by homophilic Duf adhesion causes Rols7 and Duf to be targeted to sites of contact between founders. (Bottom) In the rols mutant, the first round of fusion occurs undisrupted. However, without Rols7, Duf levels at the precursor surface is not replenished and fusion stalls. Surface levels of Rst may be similar regulated.
By maintaining low levels of key components of the fusion machinery such as Duf and regulating its localization at fusion sites through Rols7, the enlargement of the myotube is made to occur in stages, irregardless of the final size of the muscle. Restriction of myoblast fusion to specific sites along the membrane and the stepwise size increase may be critical for proper coordination between the multiple events occurring at this time and prevent loss of myotube structural integrity, as seen when its enlargement is artificially accelerated in cultured myoblasts (Yagami-Hiromasa et al., 1995).
Materials and methods
Drosophila strains
The following strains were used: Canton-S, rols Df(3L)BK9, da-gal4, and elav-gal4 (from the Flybase consortium); D-mef2 22-21 (Bour et al., 1995), duf;rst Df(1)w67k30, and UAS-duf transgenic flies (Ruiz-Gomez et al., 2000); sns ZF1.4, snsXH2, snsXB3 (Bour et al., 2000); mbc D11.2 (Erickson et al., 1997); lmd 1 (Duan et al., 2001); hbs 459 and hbs 2593;snsXB3 (Artero et al., 2001); blow 2 (Doberstein et al., 1997); the rols allele P1729ex18, rP298-gal4, UAS-rols6, and UAS-rols7 transgenic flies (Menon and Chia, 2001); 24B-gal4 (Zaffran et al., 1997); and wg-gal4 (Glise and Noselli, 1997).
Epitope-tagged duf and rols7 constructs
Full-length Duf with a Flag tag at its NH2-terminal (Flag-Duf) was made by inserting the epitope tag downstream of the signal sequence after amino acid 75. Flag-(TM)CT contains Flag in a similar position but lacks the segment encoding amino acids 103–553. Flag-CT carries the epitope tag just after the translation start site but lacks amino acids 2–611. To create full-length Duf with Flag at its COOH-terminal (Duf-Flag), we inserted the epitope tag just before the translation stop site. NT(TM)-Flag and NT-Flag carry the epitope tag at the end of the COOH-terminal as well but lack amino acids 610–959 and 586–959, respectively.
HA-tagged full-length Rols7 constructs were created by placing the epitope tag just after amino acid 130 (HA-Rols7) or before the translation stop site (Rols7-HA). HA-Rols7 (Δ1-137) was made by deleting the Rols7 NH2-terminal up to amino acid 137 and replacing it with a PCR product carrying the HA epitope immediately after the translational start site. This construct was used to produce the NH2-terminal truncations HA-Rols7 (Δ1-264), (Δ1-439), (Δ1-863), and (Δ1-1150) by deleting the regions depicted in the nomenclature for each construct. The Rols7 COOH-terminal deletion constructs Rols7-HA (Δ1739-1900) and (Δ1401-1900) carry the HA epitope at the end of the COOH-terminal and lack the regions shown in the construct nomenclature. Point mutations of the putative lipolytic sequence were made by converting serine residues 241 and 243 to alanine.
Expression of constructs in Cos and S2 cells
Cos cells were grown at 37°C in Dulbecco's minimum essential medium (GIBCO BRL); S2 cells were cultured at 25°C in M3 insect media (Sigma-Aldrich). Both media were supplemented with 10% heat-inactivated FBS (Hyclone). For immunostaining, S2 cells were made to adhere by culturing on poly-l-lysine–coated coverslips (Iwaki). Constructs were subcloned into pCIneo (Promega) or pAc5.1 vectors (Invitrogen) and then transfected into Cos or S2 cells, respectively, using Effectene (QIAGEN). Cells were processed for staining 24 h (Cos) or 48 h (S2) after transfection (Singh et al., 1993).
Germline transformation and gal4-UAS–driven expression studies
Transgenic flies were generated as described previously (Spradling, 1986). Constructs were cloned into the pUAST vector and expressed using gal4-UAS (Brand and Perrimon, 1993). Results are representative of data obtained using at least two independent insertions for each transgene. Ectopic expression studies were done using a copy each of the elav-gal4 or wg-gal4 drivers and the UAS-controlled construct(s) of interest. Expression in embryonic myoblasts was performed using single copies of 24B-gal4 and UAS-controlled gene(s) of interest in either the duf, rst double mutant Df(1)w 67k30, the rols mutant 1729ex18/Df(3L)BK9, or WT embryos.
Immunostaining
Polyclonal rat antiserum was prepared against a COOH-terminal histidine-tagged fusion peptide encompassing the amino acid residues 942–955 of Duf, which are absent from the structurally related Rst protein.
Immunostains were performed as described previously (Menon and Chia, 2001). The following primary antibodies were used: mouse anti-MHC (Kiehart and Feghali, 1986), anti-Wg, and anti-Crumbs (Developmental Studies Hybridoma Bank) and anti-tubulin (Sigma-Aldrich); rabbit anti-MHC (a gift from D. Kiehart, Duke University, Durham, NC), anti–β-galactosidase (Cappel), anti-Eve (Frasch et al., 1987), anti-FLAG (Affinity BioReagents, Inc.), and anti-Rols (ANTS antibody; Chen and Olson, 2001); rat anti-Crumbs (Tepass et al., 1990), anti-HA (Roche), rat or mouse anti-Rols7 (this work; Menon and Chia, 2001); and guinea pig anti-Duf/Rst antibody (Galletta et al., 2004). Secondary antibodies were conjugated to Cy3 or FITC (Jackson ImmunoResearch Laboratories). DNA was labeled using Hoechst (Sigma-Aldrich). Samples were mounted in Vectashield. Images were acquired using a LSM 510 Meta confocal microscope (40× Plan Neofluar lens, NA = 1.3; Carl Zeiss MicroImaging, Inc.) or a digital camera (model DXM 1200F; Nikon) mounted onto a Axioplan 2 microscope (10× Plan Neofluar lens, NA = 0.3, 40× Plan Neofluar lens, NA = 1.3 [Carl Zeiss MicroImaging, Inc.], acquisition software [Nikon ACT-1]) at ∼22°C.
Online supplemental material
Fig. S1 shows RNA in situ and Western blot analyses of HA-tagged Rols7 constructs. Fig. S2 shows indirect immunofluorescent analyses of embryos using anti-Duf specific antibodies. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200501126/DC1.
Acknowledgments
We would like to thank Karen Wong and Thangaperakasam Veronica Mary for technical assistance. We are extremely grateful to Susan Abmayr, Malabika Chakravarti, Kiranmai Kocherlakota (all from Stowers Institute for Medical Research, Kansas City, MO), Mar Ruiz-Gomez (Universidad Autonoma de Madrid, Cantoblanco, Madrid, Spain), Michael Bate (University of Cambridge, Cambridge, UK), Michael Taylor (Cardiff University, Cardiff, UK), Mary Baylies (Memorial Sloan-Kettering Cancer Center, NY, NY), Hanh Nguyen (Albert Einstein College of Medicine, Bronx, NY), Stephen Doberstein (Exelisis Pharmaceuticals, Inc., South San Francisco, CA), Dan Kiehart, K. Vijayraghavan, Devkanya Dutta (both from Tata Institute of Fundamental Research, Bangalore, India), Manfred Frasch (Mout Sinai School of Medicine, NY, NY), Eric Olson (University of Texas Southwestern Medical Center, Dallas, TX), Elizabeth Chen, Deborah Andrew (both from Johns Hopkins University School of Medicine, Baltimore, MD), Uli Tepass (University of Toronto, Toronto, Ontario, Canada), and the Bloomington Stock Centre (Indiana University, Bloomington, IN) for providing critical materials and fly strains. We are very grateful to Alan Michelson, K Vijayraghavan, Veronica Rodrigues, Mar Ruiz-Gomez, and Paramjeet Singh for comments on the manuscript.
This work is supported by the Temasek Lifesciences Laboratory. W. Chia was supported by a Wellcome Trust Principal Research fellowship during part of this work.
Abbreviations used in this paper: blow, blownfuse; D-mef2, Drosophila-myocyte enhancer factor 2; duf, dumbfounded; EC, extracellular; Eve, Even-skipped; fcm, fusion-competent myoblasts; hbs, hibris; IC, intracellular; lmd, lame duck; mbc, myoblast city; rols7, rolling pebbles 7; rst, roughest; sns, sticks and stones; TM, transmembrane; TPR, tetratricopeptide repeat; WT, wild-type.
References
- Abmayr, S.M., L. Balagopalan, B.J. Galletta, and S.J. Hong. 2003. Cell and molecular biology of myoblast fusion. Int. Rev. Cytol. 225:33–89. [DOI] [PubMed] [Google Scholar]
- Artero, R.D., I. Castanon, and M.K. Baylies. 2001. The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development. 128:4251–4264. [DOI] [PubMed] [Google Scholar]
- Bate, M. 1990. The embryonic development of larval muscles in Drosophila. Development. 110:791–804. [DOI] [PubMed] [Google Scholar]
- Baylies, M.K., M. Bate, and G.M. Ruiz. 1998. Myogenesis: a view from Drosophila. Cell. 93:921–927. [DOI] [PubMed] [Google Scholar]
- Bour, B.A., M.A. O'Brien, W.L. Lockwood, E.S. Goldstein, R. Bodmer, P.H. Taghert, S.M. Abmayr, and H.T. Nguyen. 1995. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 9:730–741. [DOI] [PubMed] [Google Scholar]
- Bour, B.A., M. Chakravarti, J.M. West, and S.M. Abmayr. 2000. Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 14:1498–1511. [PMC free article] [PubMed] [Google Scholar]
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. [DOI] [PubMed] [Google Scholar]
- Chen, E.H., and E.N. Olson. 2001. Antisocial, an intracellular adaptor protein, is required for myoblast fusion in Drosophila. Dev. Cell. 1:705–715. [DOI] [PubMed] [Google Scholar]
- Chen, E.H., B.A. Pryce, J.A. Tzeng, G.A. Gonzalez, and E.N. Olson. 2003. Control of myoblast fusion by a guanine nucleotide exchange factor, loner, and its effector ARF6. Cell. 114:751–762. [DOI] [PubMed] [Google Scholar]
- Doberstein, S.K., R.D. Fetter, A.Y. Mehta, and C.S. Goodman. 1997. Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J. Cell Biol. 136:1249–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, H., J.B. Skeath, and H.T. Nguyen. 2001. Drosophila Lame duck, a novel member of the Gli superfamily, acts as a key regulator of myogenesis by controlling fusion-competent myoblast development. Development. 128:4489–4500. [DOI] [PubMed] [Google Scholar]
- Dworak, H.A., and H. Sink. 2002. Myoblast fusion in Drosophila. Bioessays. 24:591–601. [DOI] [PubMed] [Google Scholar]
- Dworak, H.A., M.A. Charles, L.B. Pellerano, and H. Sink. 2001. Characterization of Drosophila hibris, a gene related to human nephrin. Development. 128:4265–4276. [DOI] [PubMed] [Google Scholar]
- Erickson, M.R., B.J. Galletta, and S.M. Abmayr. 1997. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J. Cell Biol. 138:589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch, M., and M. Leptin. 2000. Mergers and acquisitions: unequal partnerships in Drosophila myoblast fusion. Cell. 102:127–129. [DOI] [PubMed] [Google Scholar]
- Frasch, M., T. Hoey, C. Rushlow, H. Doyle, and M. Levine. 1987. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 6:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong, E.E., E.C. Andersen, B. Null, K.P. White, and M.P. Scott. 2001. Patterns of gene expression during Drosophila mesoderm development. Science. 293:1629–1633. [DOI] [PubMed] [Google Scholar]
- Galletta, B.J., M. Chakravarti, R. Banerjee, and S.M. Abmayr. 2004. SNS: Adhesive properties, localization requirements and ectodomain dependence in S2 cells and embryonic myoblasts. Mech. Dev. 121:1455–1468. [DOI] [PubMed] [Google Scholar]
- Glise, B., and S. Noselli. 1997. Coupling of Jun amino-terminal kinase and decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 11:1738–1747. [DOI] [PubMed] [Google Scholar]
- Hauschka, S.D. 1994. The embryonic origin of muscle. Myology. A.G. Engel and C. Franzini-Armstrong, editors. McGraw Hill, New York. 3–73.
- Kiehart, D.P., and R. Feghali. 1986. Cytoplasmic myosin from Drosophila melanogaster. J. Cell Biol. 103:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly, B., B. Zhao, G. Ranganayakulu, B.M. Paterson, R.A. Schulz, and E.N. Olson. 1995. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 267:688–693. [DOI] [PubMed] [Google Scholar]
- Menon, S.D., and W. Chia. 2001. Drosophila rolling pebbles: a multidomain protein required for myoblast fusion that recruits D-Titin in response to the myoblast attractant Dumbfounded. Dev. Cell. 1:691–703. [DOI] [PubMed] [Google Scholar]
- Rau, A., D. Buttgereit, A. Holz, R. Fetter, S.K. Doberstein, A. Paululat, N. Staudt, J. Skeath, A.M. Michelson, and R. Renkawitz-Pohl. 2001. rolling pebbles (rols) is required in Drosophila muscle precursors for recruitment of myoblasts for fusion. Development. 128:5061–5073. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez, M., N. Coutts, A. Price, M.V. Taylor, and M. Bate. 2000. Drosophila dumbfounded: a myoblast attractant essential for fusion. Cell. 102:189–198. [DOI] [PubMed] [Google Scholar]
- Ruiz-Gomez, M., N. Coutts, M.L. Suster, M. Landgraf, and M. Bate. 2002. myoblasts incompetent encodes a zinc finger transcription factor required to specify fusion-competent myoblasts in Drosophila. Development. 129:133–141. [DOI] [PubMed] [Google Scholar]
- Schroter, R.H., S. Lier, A. Holz, S. Bogdan, C. Klambt, L. Beck, and R. Renkawitz-Pohl. 2004. kette and blown fuse interact genetically during the second fusion step of myogenesis in Drosophila. Development. 131:4501–4509. [DOI] [PubMed] [Google Scholar]
- Singh, P., B.L. Tang, S.H. Wong, and W. Hong. 1993. Transmembrane topology of the mammalian KDEL receptor. Mol. Cell. Biol. 13:6435–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel, J.J., and D.C. Wiley. 1998. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell. 95:871–874. [DOI] [PubMed] [Google Scholar]
- Spradling, A.C. 1986. P element-mediated transformation. Drosophila: A Practical Approach. D.B. Roberts, editor. IRL Press, 175–197.
- Strunkelnberg, M., B. Bonengel, L.M. Moda, A. Hertenstein, H.G. de Couet, R.G. Ramos, and K.F. Fischbach. 2001. rst and its paralogue kirre act redundantly during embryonic muscle development in Drosophila. Development. 128:4229–4239. [DOI] [PubMed] [Google Scholar]
- Tepass, U., C. Theres, and E. Knust. 1990. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 61:787–799. [DOI] [PubMed] [Google Scholar]
- Yagami-Hiromasa, T., T. Sato, T. Kurisaki, K. Kamijo, Y. Nabeshima, and A. Fujisawa-Sehara. 1995. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 377:652–656. [DOI] [PubMed] [Google Scholar]
- Zaffran, S., M. Astier, D. Gratecos, and M. Semeriva. 1997. The held out wings (how) Drosophila gene encodes a putative RNA-binding protein involved in the control of muscular and cardiac activity. Development. 124:2087–2098. [DOI] [PubMed] [Google Scholar]