Abstract
Many enveloped viruses exploit the class E vacuolar protein-sorting (VPS) pathway to bud from cells, and use peptide motifs to recruit specific class E VPS factors. Homologous to E6AP COOH terminus (HECT) ubiquitin ligases have been implicated as cofactors for PPXY motif–dependent budding, but precisely which members of this family are responsible, and how they access the VPS pathway is unclear. Here, we show that PPXY-dependent viral budding is unusually sensitive to inhibitory fragments derived from specific HECT ubiquitin ligases, namely WWP1 and WWP2. We also show that WWP1, WWP2, or Itch ubiquitin ligase recruitment promotes PPXY-dependent virion release, and that this function requires that the HECT ubiquitin ligase domain be catalytically active. Finally, we show that several mammalian HECT ubiquitin ligases, including WWP1, WWP2, and Itch are recruited to class E compartments induced by dominant negative forms of the class E VPS ATPase, VPS4. These data indicate that specific HECT ubiquitin ligases can link PPXY motifs to the VPS pathway to induce viral budding.
Introduction
The class E vacuolar protein-sorting (VPS) pathway functions at the limiting membrane of multivesicular bodies (MVBs) during lumenal vesicle formation and is exploited by the late assembly or L-domains of enveloped viruses during budding (Katzmann et al., 2002; Pornillos et al., 2002). Many class E VPS factors are components of three endosomal sorting complexes required for transport (ESCRT-I, -II, or -III; Katzmann et al., 2001; Babst et al., 2002a,b), which are somewhat more elaborate in mammals than in yeast and are linked by protein interactions between ESCRT components and bridging factors (Martin-Serrano et al., 2003a; Strack et al., 2003; von Schwedler et al., 2003). A current model for viral and MVB vesicle budding invokes the sequential recruitment of ESCRT-I, -II, and -III. Thereafter, budding occurs concurrently with the release of ESCRT components from the MVB membrane induced by an AAA-ATPase, Vps4 (Babst et al., 1998; Katzmann et al., 2002). The cascade of recruitment events is initiated by ubiquitin (Hicke, 2001; Katzmann et al., 2001; Bishop et al., 2002; Pornillos et al., 2002) and/or peptide motifs in cellular and viral proteins (see below).
Three viral L-domain types with distinguishable but overlapping requirements for the various VPS factors are defined by PT/SAP (Gottlinger et al., 1991; Huang et al., 1995), YPXL/LXXLF (Puffer et al., 1997; Strack et al., 2003), or PPXY (Wills et al., 1994) peptide motifs. PTAP motifs recruit ESCRT-I by binding to Tsg101 (Garrus et al., 2001; Martin-Serrano et al., 2001; VerPlank et al., 2001; Demirov et al., 2002), whereas YPXL and LXXLF motifs recruit AIP-1/ALIX, a class E VPS factor that binds to both ESCRT-I and -III (Martin-Serrano et al., 2003a; Strack et al., 2003; von Schwedler et al., 2003). All L-domain types require a subset of class E VPS factors and their function is blocked by dominant negative forms of ESCRT-III components (Martin-Serrano et al., 2003a; Strack et al., 2003; von Schwedler et al., 2003) or VPS4 (Garrus et al., 2001; Martin-Serrano et al., 2003b; Tanzi et al., 2003).
Thus, PTAP- and YPDL-type L-domains bind directly to class E VPS factors but how PPXY motifs access the class E VPS pathway is uncertain. PPXY is a consensus sequence for interaction with WW-domains, which are present in homologous to E6AP COOH terminus (HECT) ubiquitin ligases. Although yeast has a single HECT ubiquitin ligase (Rsp5; Huibregtse et al., 1995), mammals have elaborated this family of proteins to ∼10 members (Rotin et al., 2000). Rsp5-mediated ubiquitination of cargo or transacting factors is required for the endocytosis of at least some transmembrane proteins and/or for the sorting of endocytic and biosynthetic cargo into the yeast vacuole (Galan et al., 1996; Dunn and Hicke, 2001; Katzmann et al., 2004). In mammals, the most widely studied member of this family of proteins, Nedd4, is recruited by PPXY motifs in, for example, the cytoplasmic domains of the amiloride-sensitive epithelial Na+ channel (ENaC) and induces its down-regulation (Staub et al., 1996, 2000). Heretofore, the WW and membrane binding (C2) domains are thought to be responsible for directing the localization and substrate recognition of Nedd4/Rsp5 like proteins, whereas the role of the HECT ubiquitin ligase domain has been thought to be confined to modifying cargo or transacting factors with ubiquitin (Dunn and Hicke, 2001; Hicke, 2001).
Several studies suggest that Nedd4-like ubiquitin ligases play roles in viral budding. Overexpression of various HECT ubiquitin ligase-derived WW-domains can block viral budding, and the PPXY motifs in vesicular stomatitis virus (Harty et al., 1999), Ebola virus (Harty et al., 2000; Yasuda et al., 2003), Rous sarcoma virus (RSV; Kikonyogo et al., 2001), human T cell leukemia virus (Bouamr et al., 2003; Blot et al., 2004; Heidecker et al., 2004; Sakurai et al., 2004), and Mason Pfizer monkey virus (Yasuda et al., 2002) have been reported bind to Nedd4, LDI-1, LDI-2, BUL1, or WWP1 HECT ubiquitin ligases. PPXY motifs can also cause retroviral Gag proteins to become ubiquitinated and an ENaC-derived peptide sequence exhibits L-domain activity in the context of a retroviral Gag protein (Strack et al., 2000, 2002). Other observations suggest that ubiquitin itself plays a general role in viral budding, although the details of its participation are not understood. Proteasome inhibitors block the release of certain rhabdoviruses (Harty et al., 2001) and some retroviruses (Patnaik et al., 2000, 2002; Schubert et al., 2000; Strack et al., 2000; Ott et al., 2002, 2003), perhaps due to depletion of free ubiquitin. Importantly, these inhibitors induce a defective viral assembly phenotype that resembles that of L-domain mutants.
Because it remained uncertain as to precisely which HECT ubiquitin ligases mediate PPXY motif–dependent viral budding and how the class E VPS pathway is accessed by these proteins, we surveyed an array of HECT ubiquitin ligases and found that fragments of the HECT ubiquitin ligases WWP1 and WWP2 are unusually potent and specific inhibitors of viral PPXY motif function. The sequence requirements for the binding of the murine leukemia virus (MLV) L-domain motif to WWP1, WWP2, and the closely related ligase, Itch, but not Nedd4 precisely recapitulate those required for virus release. Moreover, WWP1 can be recruited by PPXY motifs to sites of MLV and Ebola virus particle budding from cells. Importantly, we show that these ligases can actively promote PPXY-dependent virus particle release, and that this function requires an enzymatically active HECT domain. Finally, we also show that several HECT ubiquitin ligases display characteristics of class E VPS factors in that they are recruited to endosomal compartments induced by dominant negative VPS4 and, surprisingly, that the HECT domain is largely responsible for this property in WWP1. Together, these data indicate that the enzymatic activity of particular HECT ubiquitin ligases and a physical association with the class E VPS pathway promotes PPXY motif–dependent viral budding.
Results
MLV budding induced by viral and cellular WW-domain binding PPXY motifs
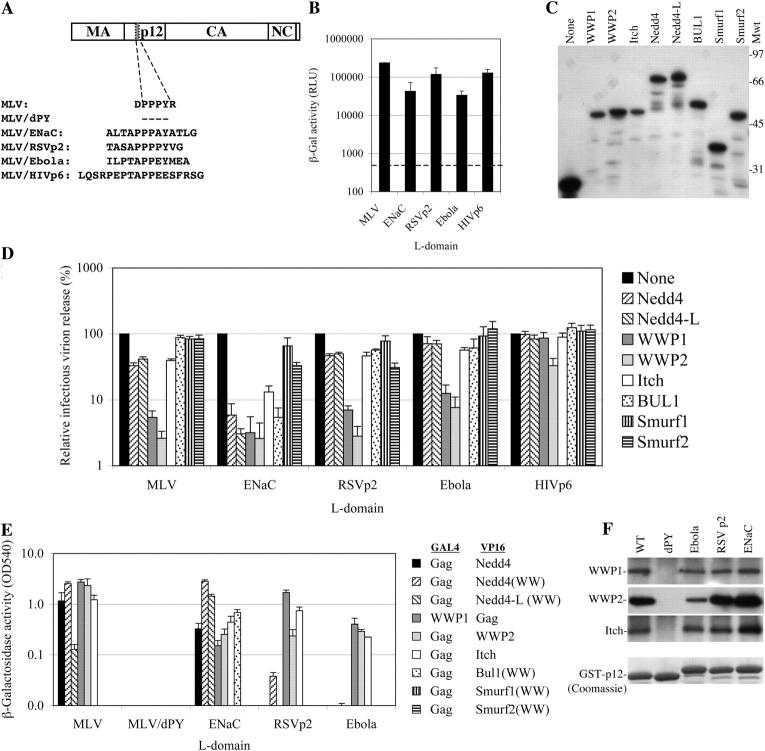
To investigate the cofactor requirements for PPXY-dependent viral budding, we used chimeric MLVs, shown schematically in Fig. 1 A. These contained PPXY motifs and flanking amino acids from the cellular PPXY motif in the cytoplasmic domain of ENaC, or viral L-domain motifs from RSV p2b and Ebola virus VP40 (EbVp40). An MLV provirus containing a PTAP L-domain from HIV-1 p6 in place of the MLV PPPY motif (Yuan et al., 2000), was included for control purposes. Each recombinant MLV provirus generated infectious virion particles upon transfection of 293T cells (Fig. 1 B), at least 100-fold more efficiently than a control construct (MLV/dPY), which lacks the PPPY motif and exhibits a profound late budding defect (Yuan et al., 2000). Thus, each of these cellular and viral PPXY motifs could functionally substitute for the MLV L-domain.
Figure 1.
PPXY motif binding and inhibition of MLV budding by HECT ubiquitin ligase WW-domains. (A) Chimeric MLVs used in this study: the PPPY motif was deleted or replaced by the indicated peptide sequences of viral or cellular origin. (B) Infectious MLV production after transfection of 293T cells with wild-type and chimeric proviral plasmids, measured by chemiluminescent β-galactosidase assay after infection of HeLa P4/R5 indicator cells. The dashed line indicates the background β-galactosidase activity, which was obtained using MLV/dPY. Error bars indicate the SD from the mean of triplicate measurements. (C) Western blot analysis of YFP-WW-domain fusion protein expression in 293T cells. (D) Effect of YFP-WW-domain fusion protein expression on MLV production. The results are plotted as the percentage of the infectious virus production observed in the presence of unfused YFP, which was similar to that shown in B. Error bars indicate the SD from the mean of triplicate measurements. (E) Yeast two-hybrid analysis of the interactions between the indicated PPXY motifs and WW-domains or intact HECT ubiquitin ligases. The mean level of β-galactosidase expression measured as optical density units at 540 nm (OD540) in three pools of Y190 cells, expressing the indicated Gal4 and VP16 fusion proteins is shown. Absence of a bar indicates background levels of β-galactosidase. Error bars indicate the SD from the mean of triplicate measurements. (F) GST-p12 proteins derived from the chimeric MLVs were expressed in 293T cells and samples of proteins that bound to glutathione-Sepharose beads were analyzed by Western blot with WWP1, WWP2, and Itch-specific antibodies (top). Equivalent expression and loading of the GST-p12 fusion proteins was verified by Coomassie blue staining of the glutathione-bound fraction (bottom).
To identify specific HECT ubiquitin ligases that could potentially be important for PPXY-dependent viral budding, we applied two criteria. First, we tested YFP-fused WW-domain fragments from the majority of known mammalian HECT ubiquitin ligases for their ability to inhibit MLV budding, mediated by each of the PPXY motifs. As can be seen in Fig. 1 C, each of the YFP-WW-domain fusion proteins was expressed at similar levels. However, YFP-WW-domain fusion proteins based on WWP1 and WWP2 were unusual in that they inhibited infectious virion production mediated by all of the PPXY motifs tested by 10–50-fold (Fig. 1 D). Other YFP-WW-domain fusion proteins had weak or absent inhibitory activity on budding mediated by the MLV, RSV, and Ebola PPXY motifs. Conversely, most of the YFP-WW-domain fusion proteins, including those based on WWP1, WWP2, Nedd4, Ned4-L, Itch, and BUL1 strongly inhibited ENaC PPXY motif–dependent virion production (Fig. 1 D). Importantly, inhibition by YFP-WW-domain fusion proteins was specific because MLV/HIVp6 release was largely unaffected (Fig. 1 D).
Using yeast 2-hybrid assays, we next tested the ability of each WW-domain to bind to MLV Gag proteins containing the various PPXY motifs. The isolated WW-domains from WWP1, WWP2, and Itch could not be used in this assay, due to toxicity in yeast and constitutive transcriptional activation activity. Therefore, the full-length proteins were used in these cases. As can be seen in Fig. 1 E, the full-length Nedd4 protein and the Nedd4WW fragment exhibited the same specificity for binding to the various PPXY motifs. The intact MLV Gag protein bound to Nedd4, Nedd4WW Nedd4-LWW, WWP1, WWP2, and Itch, and these interactions required the PPXY motif. The MLV/ENaC Gag protein bound to all the proteins tested except Smurf1 and Smurf2. Conversely, MLV/RSVp2 and MLV/Ebola Gag proteins bound only to WWP1, WWP2, and Itch. Overall, the ability of the WW-domains to bind to PPXY motifs in the yeast 2-hybrid assay correlated quite well with the ability of the corresponding YFP-WW-domain fusion proteins to act as dominant inhibitors in the viral budding assay, although there were some WW-domains (Nedd4, Nedd4-L, Itch) that appeared capable of binding to the intact MLV Gag protein that had relatively modest, albeit significant, activity as MLV budding inhibitors (threefold inhibition). That MLV, ENaC, RSVp2, and Ebola PPXY motifs could bind to endogenous WWP1, WWP2, and Itch ubiquitin ligases, was determined using co-precipitation assays. Each of these ubiquitin ligases was co-precipitated with GST-p12 fusion proteins containing MLV, ENaC, RSV, and Ebola PPXY motifs, but not with GST-p12(dPY) (Fig. 1 F). Other ubiquitin ligases were not tested in this assay, either because antibodies were not available or because we were unable to detect them in 293T cell lysates using commercially available antisera.
Sequence requirements for MLV L-domain function and HECT ubiquitin ligase binding
If PPXY motifs indeed mediate viral budding by recruiting specific HECT ubiquitin ligases, then the sequence requirements for viral budding activity should closely match those for binding to the ligases. We tested a series of six, single amino acid to alanine, point mutants scanning through MLV Gag(p12) residues 161–166 (DPPPYR). Each mutation was tested in the context of an MLV proviral plasmid for effects on virus release, and in the context of Gag and GST-p12 proteins for effects on binding to HECT ubiquitin ligases. As can be seen in Fig. 2 A, MLV proviral plasmids carrying the APPPYR, DPPAYR, and DPPPYA mutations generated virions with similar efficiency to wild-type MLV, whereas release of the DAPPYR, DPAPYR, and DPPPAR mutant virions was attenuated. Loss of viral budding was accompanied by a Gag processing defect with accumulation of unprocessed Gag and aberrant Gag fragments in cell lysates and little mature p30 capsid formation (Fig. 2 A). Virion release by the panel of MLV mutants correlated with the ability of Gag to bind to WWP1, WWP2, and Itch, but not to Nedd4 or Nedd4L (Fig. 2 B). In particular, the DPPAYR Gag mutant was devoid of Nedd4 or Nedd4L binding activity (Fig. 2 B), but supported efficient virion release (Fig. 2 A). The DAPPYR, DPAPYR, and DPPPAR mutations inhibited co-precipitation of WWP1, WWP2, and Itch from 293T cell lysates by GST-p12 fusion proteins, whereas the APPPYR, DPPAYR, and DPPPYA mutations had no effect (Fig. 2 C), concordant with the findings in the virus release and yeast 2-hybrid assays (Fig. 2, A and B). A small amount of WWP2 co-precipitation was detected with the DAPPYR mutant (Fig. 2 C) and this mutant supported a low residual level of virion formation (Fig. 2 A). Because the WWP1 and Itch antisera were less efficacious than the WWP2 antiserum, it was unclear whether our inability to detect WWP1 and Itch co-precipitation by GST-p12(DAPPYR) was due to real differences in their binding specificity as compared with WWP2. Similar GST-p12 mutant coprecipitation experiments could not be done with Nedd4. This was because Nedd4 did not bind efficiently to wild-type GST-p12, even when overexpressed, despite interaction with MLV Gag in the yeast 2-hybrid assay. In fact, Nedd4 was only efficiently coprecipitated by the GST-p12(ENaC) protein (unpublished data).
Figure 2.
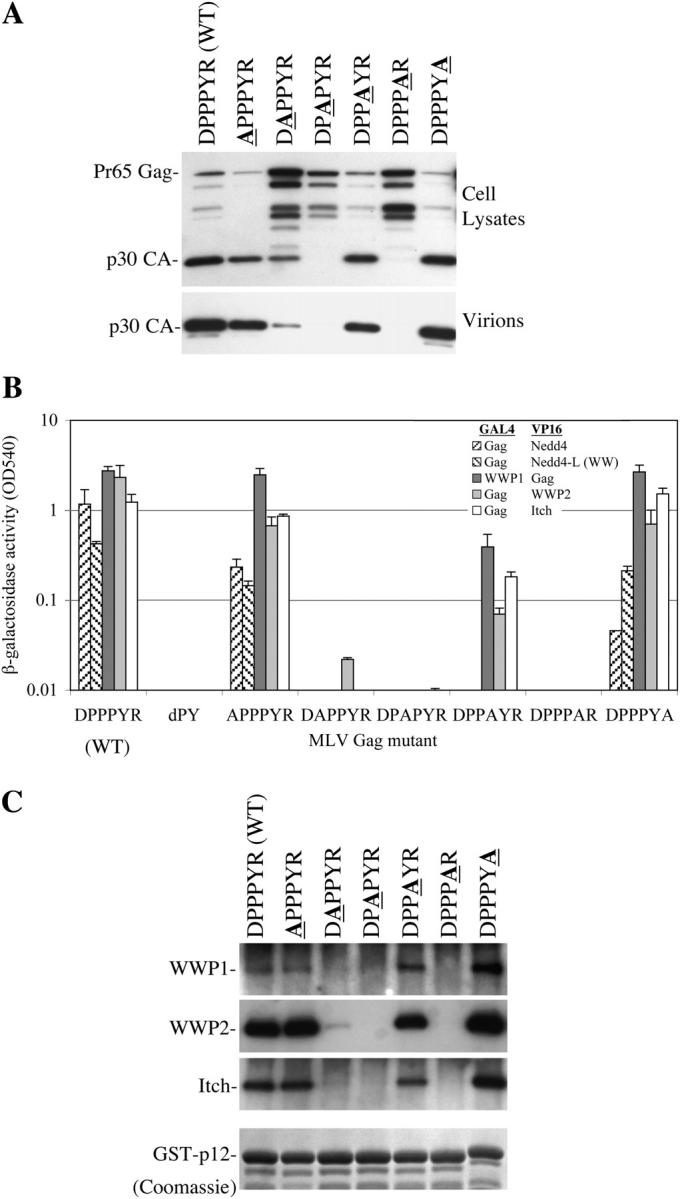
Sequence requirements for MLV L-domain activity and interaction with HECT ubiquitin ligases. (A) Virion production by 293T cells transfected with MLV proviral plasmids bearing the indicated mutations in the DPPPYR sequence. Virion and cell lysates were analyzed by Western blotting with αMLV Gag antibodies. (B) Yeast two-hybrid analysis of interactions between the indicated mutant MLV Gag proteins and HECT ubiquitin ligases. The mean level of β-galactosidase expression in yeast transformed with the indicated Gal4 and VP16 Gag and ubiquitin ligase fusion proteins is shown. Error bars indicate the SD from the mean of triplicate measurements. (C) Binding of endogenous WWP1, WWP2, and Itch to wild-type and mutant GST-p12 proteins expressed in 293T cells. The top panels show Western blots of the glutathione-bound proteins, probed with the indicated specific antibodies and the bottom panel shows Coomassie blue staining of the glutathione-bound GST-p12 proteins, verifying equivalent expression and loading.
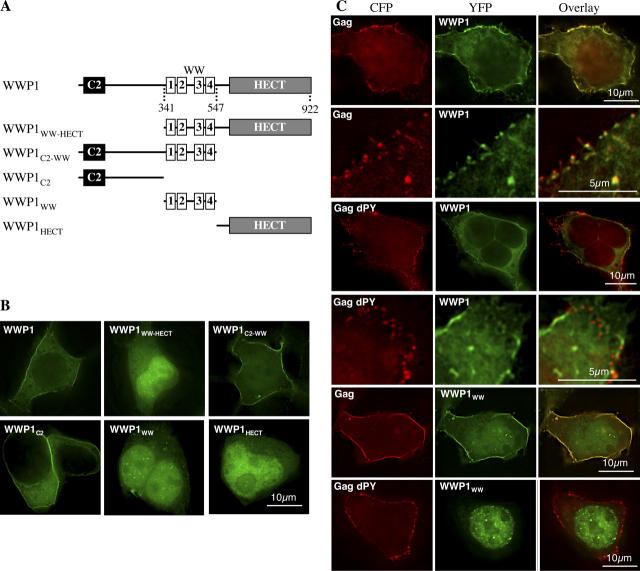
WWP1 recruitment to sites of MLV budding
The aforementioned data suggested WWP1, WWP2, and Itch as the most likely candidates for recruitment by PPXY motifs to support viral budding. We initially selected WWP1 for more detailed analyses because it had the most robust and broad activity in the PPXY binding and budding-inhibition assays (Fig. 1). We constructed a series of truncation mutants containing either one or two of the three WWP1 segments (C2, WW and HECT; Fig. 3 A) and expressed them as YFP-fusion proteins. When expressed alone, a full-length YFP-WWP1 fusion protein localized primarily to the plasma membrane (Fig. 3 B). The C2 domain governed this phenotype, because a YFP-WWP1C2 fusion protein retaining this element exhibited the same localization, whereas truncated YFP-WWP1 fusion proteins lacking the C2 domain exhibited nuclear localization or were distributed throughout the cell. Upon coexpression of YFP-WWP1 with an MLV Gag-CFP fusion protein, the YFP and CFP signals were almost perfectly coincident (Fig. 3 C). This near complete colocalization required that the MLV Gag-CFP protein encoded an intact PPXY motif. Indeed, when YFP-WWP1 and MLV Gag(dPY)-CFP were coexpressed, both YFP and CFP signals were primarily at the plasma membrane, but they did not colocalize to any greater extent than would two signals that were randomly distributed at the plasma membrane (Fig. 3 C). In addition, YFP-WWP1WW that was primarily localized in the nucleus when expressed alone (Fig. 3 B), was relocalized to the plasma membrane upon coexpression with MLV Gag-CFP, in a PPPY motif–dependent manner (Fig. 3 C). YFP-fusion proteins containing the WW domains of WWP2 and Itch also exhibited nuclear localization when expressed alone but were relocalized to the plasma membrane upon expression with MLV Gag-CFP (unpublished data).
Figure 3.
WWP1 recruitment to sites of MLV budding. (A) Domain organization of WWP1 showing the residues at which the protein was truncated to derive the various deletion mutants. (B) Cellular localization of WWP1 and deletion mutants in 293T cells analyzed by deconvolution microscopy. Shown are full-length YFP-WWP1 (residues 1–922) YFP-WWP1WW-HECT (341–922), YFP-WWP1C2-WW (1–547), YFP-WWP1C2 (1–341), YFP-WWP1WW (341–547), and YFP-WWP1HECT (547–922). (C) Analysis of YFP-WWP1 and YFP-WWP1WW localization in 293T cells when coexpressed with wild-type or dPY mutant MLV Gag-CFP as indicated. Images acquired with the CFP filter set (left) are pseudo-colored red for clarity and are combined with those acquired with the YFP filter set (middle, pseudo-colored green) to generate overlaid images (right). For the Gag-CFP + YFP-WWP1 and Gag dPY-CFP + YFP-WWP1 combinations, two sets of images showing a whole cell (top) and an expanded portion of the plasma membrane (bottom) are shown.
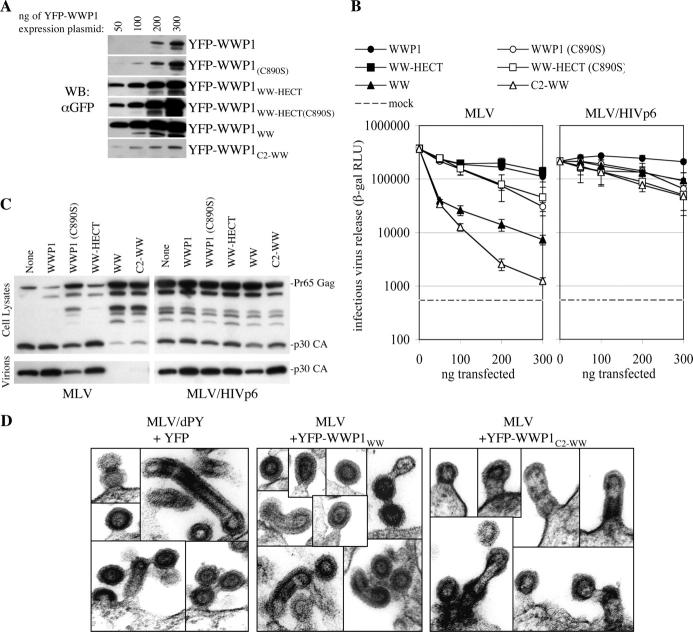
Inhibition of MLV release by WWP1 fragments and mutants suggests that HECT domains serve enzymatic and additional functions
The findings that isolated WW-domains from WWP1 and WWP2 bound to each of the PPXY motifs and acted as potent dominant inhibitors of PPXY-dependent viral budding suggested that they compete with a cellular factor that would ordinarily be recruited by PPXY motifs. However, it has not previously been shown that the competed cellular cofactor is itself a HECT ubiquitin ligase. Moreover, although previous studies have documented that WW-domains can inhibit viral budding, their potency was significantly less than that of YFP-WWP1WW and YFP-WWP2WW (Fig. 1). Therefore, we next determined what protein domains and activities were required to be eliminated in order to render WWP1 a dominant inhibitor of viral budding, and whether the effects induced by these manipulations were specific to PPXY-dependent viral budding. The various truncated YFP-WWP1 fusion proteins are shown schematically in Fig. 3 A. Their expression levels after transfection of varying amounts of plasmid is shown in Fig. 4 A and are important for interpreting the results in Fig. 4 B. As can be seen in Fig. 4 (B and C), neither full-length YFP-WWP1 nor YFP-WWP1WW-HECT exhibited inhibitory or enhancing effects on MLV release, as assessed by infectious particle output or Western blot assays, despite the fact that the YFP-WWP1WW-HECT protein was expressed at a considerably higher level than full-length YFP-WWP1. The YFP-WWP1 and YFP-WWP1WW-HECT proteins carrying an active site mutation (C890S) were expressed at the same level as their wild-type counterparts and modestly inhibited MLV production (Fig. 4, B and C). Interestingly, YFP-WWP1WW was a more potent inhibitor of budding than was YFP-WWP1WW-HECT(C890S), and reduced infectious viral output by nearly 100-fold, even though it was expressed at only a marginally higher level. Note that the only difference between these two proteins is the presence or absence of a catalytically inactive HECT domain. However, by far the most potent inhibitor of MLV budding was the YFP-WWP1C2-WW protein, which decreased infectious MLV release by 500-fold to virtually undetectable levels (Fig. 4 B) even though it was expressed at a considerably lower level than YFP-WWP1WW (Fig. 4 A). We suspect that the reason YFP-WWP1C2-WW was a much more potent inhibitor than YFP-WWP1WW was because it is constitutively localized at the plasma membrane (Fig. 3 A), whereas YFP-WWP1WW is primarily nuclear. YFP-WWP1C2-WW was expressed at approximately the same level as the full-length YFP-WWP1C890S protein (Fig. 4 A) and, therefore, differs from it only in the fact that it lacks a catalytically inactive HECT domain. Nevertheless, it inhibited MLV budding ∼50-fold more potently (Fig. 4 B). Thus, the ability of the WW and C2-WW fragments of WWP1 to act as potent inhibitors of PPXY-dependent MLV budding can be partly reversed by restoring a catalytically inactive HECT domain and completely reversed by restoring an active HECT domain. Importantly, each of these inhibitors had only marginal effects on MLV/HIVp6 release (Fig. 4, B and C). Moreover, electron microscopic analysis of cells coexpressing MLV and either YFP-WWP1WW or YFP-WWP1C2-WW (Fig. 4 D) revealed that the inhibitory WWP1 fragments induced a late budding defect similar to that observed upon mutation of the PPXY L-domain (Yuan et al., 2000), with accumulation of numerous incompletely budded virions, and formation of aberrant budding structures including tubes and chains of linked virions. These budding structures were never observed upon transfection of an MLV proviral plasmid alone (unpublished data).
Figure 4.
Inhibition of MLV release by WWP1 fragments. (A) Increasing amounts of the indicated YFP-WWP1-derived expression plasmids were transfected in 293T cells and fusion protein expression was monitored by Western blotting with an αGFP antibody. (B) Effect of YFP-WWP1 fragments on infectious wild-type MLV or MLV/HIVp6 release, measured by cotransfecting MLV proviral plasmids with increasing amounts of the indicated YFP-WWP1-derived expression plasmids. Infectious MLV virion production was measured as in Fig. 1. The assay background is indicated as a dotted line. Error bars indicate the SD from the mean of triplicate measurements. (C) Western blot analysis, using an αMLV Gag antibody, of cell lysates and extracellular MLV virions, after transfection of 293T cells with wild-type MLV or MLV/HIVp6 proviral plasmids and 200 ng of the indicated YFP-WWP1 expression plasmid derivatives. (D) Galleries of electron microscopic images of arrested and aberrant budding structures observed in 293T cells transfected with the MLV/dPY proviral plasmid or a wild-type MLV proviral plasmid and plasmids expressing the indicated YFP-WWP1 fragments.
EbVP40 can recruit WWP1 and of WWP1 fragments block EbVP40 particle formation
Although the protein sequence of EbVP40 and the morphology of EbVP40 particles is quite different to those of retroviral Gag proteins, EbVP40 particle release also requires L-domains. In this case, overlapping PTAP and PPXY motifs contribute to overall activity (Strack et al., 2000; Martin-Serrano et al., 2001, 2004; Licata et al., 2003). Therefore, we next investigated whether EbVP40 could recruit WWP1. As was found with MLV Gag, EbVP40 bound to WWP1 in yeast 2-hybrid assays (Fig. 5 A), and PPXY motif mutations (P11L or Y13A) that abolish EbVP40 budding (Martin-Serrano et al., 2004) abolished WWP1 binding. Moreover, EbVP40 particle release was reduced to almost undetectable levels by expression of YFP-WWP1WW or YFP-WWP1C2-WW proteins but was unaffected by full-length YFP-WWP1 (Fig. 5 B). As a control, an NH2-terminal fragment (residues 1–157) of Tsg101 that occludes PTAP motifs also inhibited EbVP40 particle release. Finally, coexpression of CFP-EbVP40 with YFP-WWP1WW resulted in a dramatic relocalization of YFP-WWP1WW from the nucleus to filamentous projections, formed at the plasma membrane by CFP-EbVP40, and this property was eliminated by single amino acid substitutions in the EbVP40 PPXY motif (Fig. 5 C). Thus, the EbVP40 protein behaved in much the same way as MLV Gag in terms of its ability to recruit WWP1 and its sensitivity to inhibitors based on WWP1.
Figure 5.
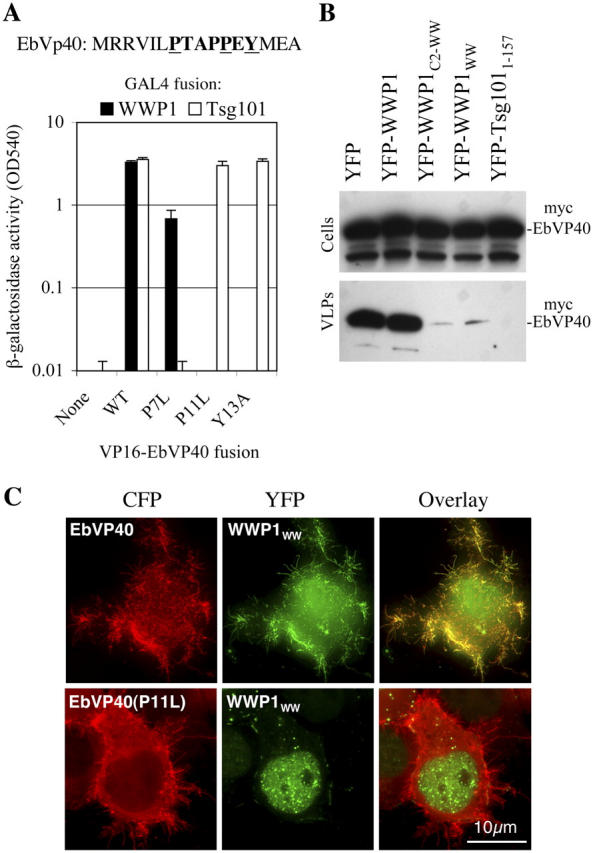
WWP1 is recruited by EbVp40. (A) The NH2-terminal 16 aa of EbVP40 are shown and residues whose mutation selectively ablate the PTAP motif (P7L) or the PPXY motif (P11L and Y13A) are underlined. Shown below is a yeast two-hybrid analysis of wild-type and mutant VP16-EbVP40 binding to Gal4-WWP1 or Gal4-Tsg101. β-galactosidase activity in yeast expressing these fusion proteins was measured as in Fig. 1. Error bars indicate the SD from the mean of triplicate measurements. (B) Effect of WWP1 and Tsg101 fragments on EbVP40 particle release. The indicated YFP-fusion proteins were coexpressed with Myc epitope-tagged EbVP40. Cell and extracellular virus-like particle (VLP) lysates were analyzed by Western blot with an αMyc antibody. (C) EbVP40 recruitment of WWP1WW to sites of particle assembly. 293T cells expressing YFP-WWP1WW and wild-type or P11L mutant forms of CFP-EbVP40 were analyzed by deconvolution microscopy as in Fig. 3 except that a composite of several optical sections is shown to facilitate filament visualization.
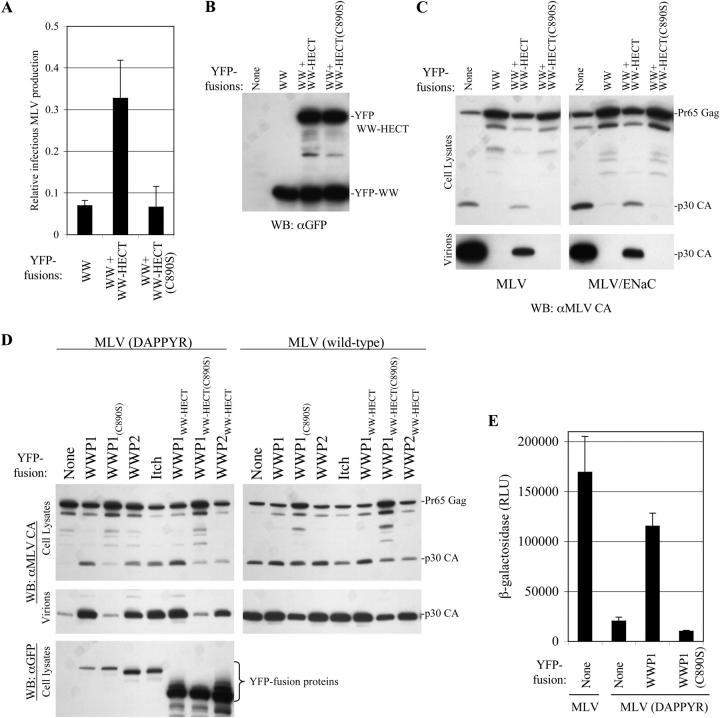
Attenuation of MLV budding by a dominant negative WWP1 fragment or by a PPXY motif mutation is reversed by HECT ubiquitin ligase overexpression
Although fragments of WWP1 and WWP2 were uniquely potent dominant inhibitors of PPXY motif–dependent viral budding, it was possible that they could act by competing with some entirely distinct cellular cofactor for binding to PPXY motifs. Therefore, we sought more direct evidence that HECT ubiquitin ligases are required for PPXY-dependent viral budding. Unfortunately, siRNAs that depleted WWP1 or WWP2 also appeared to reduce cell viability (unpublished data). Therefore, we pursued two alternative strategies. First, we inhibited MLV budding to ∼5–7% of its normal level using a selected dose of YFP-WWP1WW expression plasmid. Thereafter, infectious virion production could be partly restored, to ∼30% of its uninhibited level, by coexpression of a YFP-WWP1WW-HECT protein (Fig. 6 A). This restoration of budding was not due to inhibition of YFP-WWP1WW expression (Fig. 6 B). Rather, we conclude that a functional cofactor (YFP-WWP1WW-HECT) can compete with the inhibitor (YFP-WWP1WW) to restore viral budding. Importantly, YFP-WWP1WW-HECT(C890S) did not restore viral budding, indicating that a catalytically active HECT domain was required (Fig. 6 A). This conclusion held when MLV Gag expression and budding was measured by Western blotting and the experiment was elaborated to include the chimeric MLV/ENaC virus containing the cellular PPXY motif (Fig. 6 C). In both cases, the rescuing YFP-WWP1WW-HECT protein slightly reduced the level of cell associated MLV Gag, corrected the Gag processing defect that was induced by YFP-WWP1WW expression and partly restored particle release. Again, this effect required that the rescuing YFP-WWP1WW-HECT protein had an intact active site (Fig. 6 C), indicating that ubiquitin ligation is required for optimal MLV release.
Figure 6.
HECT ubiquitin ligases mediate PPXY-dependent viral budding. (A) Restoration of infectious MLV release from 293T cells transfected with MLV proviral DNA and an inhibitory YFP-WWP1WW expression plasmids. “Rescuing” plasmids encoded YFP-WWP1WW-HECT or YFP-WWP1WW-HECT(C890S) as indicated. Infectious virus release, measured using HeLa P4R5 indicator cells, is plotted as a proportion of that obtained in the absence of inhibitory YFP-WWP1WW protein expression. Error bars indicate the SD from the mean of triplicate measurements. (B) Western blot analysis using αGFP antibodies showing expression of the inhibitory and “rescuing” YFP-fusion proteins in cell lysates. (C) Western blot analysis of cell and virion lysates using αMLV Gag antibodies upon coexpression of MLV with the inhibitory YFP-WWP1WW and “rescuing” YFP-WWP1WW-HECT fusion proteins. In this experiment, both the wild-type (left) and the chimeric MLV/ENaC (right) proviral plasmids were used. (D) Restoration of Gag processing and virion release by a budding-attenuated MLV mutant (DAPPYR) upon overexpression of the indicated YFP-fusion proteins (left). Wild-type MLV was used as a control (right). Cell and virion lysates were analyzed by Western blotting with αMLV Gag antibodies. The bottom left panel shows Western blot analysis of YFP-fusion protein expression in cell lysates. (E) Rescue of infectious DAPPYR mutant MLV release by WWP1 overexpression. 293T cells were transfected as in D and infectious virus release was measured using HeLa P4R5 indicator cells as in Fig. 1. Error bars indicate the SD from the mean of triplicate measurements.
As was shown in Fig. 2 A, one of the alanine scanning mutants of the MLV L-domain (DAPPYR), exhibited a reduced, but detectable, residual level of virion formation (∼10% of that of wild-type MLV). We reasoned that attenuation was likely due to reduced affinity of the L-domain for an essential cofactor, and could potentially be reversed by overexpression of that cofactor. As can be seen in Fig. 6 D, attenuated DAPPYR mutant MLV release and Gag processing was indeed restored to near wild-type levels by overexpression of YFP-WWP1, YFP-WWP2, or YFP-Itch. Overexpression of these HECT ubiquitin ligases did not enhance wild-type MLV budding (Fig. 6 D). YFP-WWP1-induced MLV (DAPPYR) release resulted in the formation of infectious virions (Fig. 6 E), indicating that authentic virus particle formation occurred. The ability of YFP-WWP1 to restore attenuated MLV (DAPPYR) budding was eliminated by the C890S active site mutation (Fig. 6, D and E) but both WWP1 and WWP2 proteins lacking the C2 membrane-binding domain retained the ability to induce release (Fig. 6 D). It should be noted, however, that YFP-WWP1/2WW-HECT proteins were expressed at a higher level and may not be as active as the full-length ligases.
Recruitment of HECT ubiquitin ligases to VPS4-induced endosomes
These data demonstrate that certain HECT ubiquitin ligases can mediate PPXY-dependent viral budding and that their enzymatic activity is required for this function. However, because WWP1 fragments completely lacking the HECT domain were substantially more potent dominant inhibitors of budding that those containing a catalytically inactive HECT domain (Fig. 4), we investigated the possibility that this protein domain may serve some additional function, perhaps class E VPS factor recruitment. Using yeast 2-hybrid and/or co-precipitation assays (Martin-Serrano et al., 2003a) we tested whether WWP1 or WWP2 could bind to an array of class E VPS factors, including all known components of mammalian ESCRT-I, -II, and -III as well as Hrs, HBP, AIP1/ALIX, LIP-5, VPS4, and the AIP1/ALIX binding proteins CIN85 and CMS. These results were uniformly negative (unpublished data). However, a characteristic property of class E VPS pathway components is that they are often relocalized to aberrant endosomes induced by VPS4 ablation in yeast (Katzmann et al., 2001; Babst et al., 2002a,b) or by expression of catalytically inactive VPS4(DN) mutants in mammalian cells (Bishop and Woodman, 2001). This is probably because most class E VPS factors, including VPS4 itself, cycle on and off the limiting membrane of late endosomes and their disassembly and release requires VPS4 activity. In the absence of VPS4 overexpression (Fig. 3) or in the presence of wild-type CFP-VPS4, which is distributed diffusely in the cytoplasm at steady state (Fig. 7 A), YFP-WWP1 localized primarily at the plasma membrane. However, upon coexpression with CFP-VPS4(DN), YFP-WWP1 accumulated on aberrant CFP-VPS4(DN)-induced endosomes (Fig. 7 A). Surprisingly, this property of WWP1 appeared, at least in part, to be conferred by the HECT domain, because YFP-WWP1HECT also accumulated on VPS4(DN)-induced endosomes, whereas YFP-WWP1C2 remained at the plasma membrane and YFP-WWP1WW exhibited only a marginal tendency to relocalize (Fig. 7 A). Although the YFP-WWP1C2-WW protein exhibited some recruitment to CFP-VPS4(DN)-induced endosomes (unpublished data), this tendency was clearly reduced as compared with full-length YFP-WWP1. Moreover, full-length YFP-WWP1 but not YFP-WWP1C2-WW appeared to enhance the formation of CFP-VPS4(DN)-labeled endosomes (unpublished data).
Figure 7.
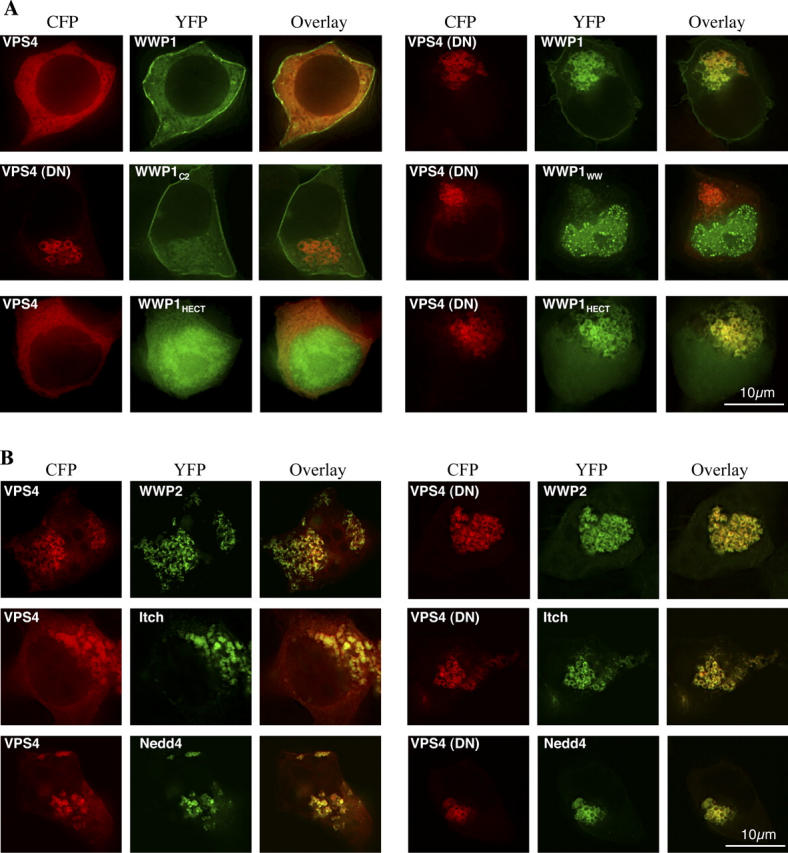
HECT ubiquitin ligases are recruited to VPS4-induced endosomal structures. (A) YFP-fused full-length WWP1 (top), WWP1C2 and WWP1WW (center), and WWP1HECT (bottom) protein localization in 293T cells in the presence of either wild-type CFP-VPS4 or E228Q mutant CFP-VPS4(DN) as indicated. Cells were examined by deconvolution microscopy and each set of three panels shows images acquired using YFP and CFP filter sets alongside overlaid images as in Fig. 3. (B) YFP-WWP2, -Itch, or -Nedd4 fusion protein localization upon coexpression with wild-type (left) or DN mutant (right) CFP-VPS4.
Other HECT ubiquitin ligases, namely WWP2, Itch, and Nedd4, also colocalized with CFP-VPS4(DN) (Fig. 7 B). However, they differed from WWP1 in that they were primarily localized in punctate or vesicular structures when expressed alone or with wild-type CFP-VPS4. Surprisingly, wild-type CFP-VPS4 was partly recruited to the punctate structures containing YFP-WWP2, YFP-Itch, or YFP-Nedd4 (Fig. 7 B). Thus, although we were unable to detect direct interactions between HECT ubiquitin ligases and known class E VPS factors, these findings suggest that HECT ubiquitin ligases might interact with an unknown component of the class E pathway via interactions involving the HECT domain.
Discussion
These data show that particular HECT ubiquitin ligases can act as a functional interface between viral or cellular proteins that contain PPXY motifs and the class E VPS pathway. Previously, HECT ubiquitin ligases were thought to influence the fate of their substrates because ubiquitin modification is responsible for downstream events, including entry into the MVB sorting pathway (Rotin et al., 2000; Dunn and Hicke, 2001; Hicke, 2001; Katzmann et al., 2001, 2004; Bishop et al., 2002; Marchese et al., 2003; Alam et al., 2004). Two lines of evidence presented herein suggest that HECT domains have functions in addition to tagging substrates with ubiquitin. First, WWP1 fragments completely lacking a HECT domain were much more potent inhibitors of PPXY motif function than otherwise equivalent proteins carrying catalytically inactive HECT domains. Second, several HECT ubiquitin ligases were recruited to VPS4(DN)-induced endosomal compartments. Recent studies have shown that the Rsp5, the sole HECT ubiquitin ligase in yeast localizes to the class E compartment induced by Vps4 ablation, but C2 domain–phosphoinositide interactions appear to be responsible for recruitment therein (Dunn et al., 2004). We found that the isolated HECT domain of WWP1, which is not expected to exhibit membrane binding activity, appeared to be sufficient to serve as a signal for recruitment to VPS4(DN) induced compartments. Although we were unable to demonstrate a direct interaction between HECT ubiquitin ligases and any known class E VPS factor, these observations suggest that a physical interaction between HECT domains and some component of the class E pathway exists, perhaps involving some as yet unidentified bridging factor. HECT domains can participate in specific protein–protein interactions (Huibregtse et al., 1993; Huang et al., 1999) but no known interaction would obviously lead to class E VPS factor recruitment.
Although our analyses suggest that HECT domains have unanticipated additional functions, they also clearly demonstrate that the HECT active site, and presumably therefore, ubiquitination of a substrate protein, is required for PPXY-dependent viral budding. What the functional target of ubiquitination is in this context is unclear. Several studies have shown that retroviral Gag proteins are ubiquitinated (Ott et al., 2000; Strack et al., 2000; Blot et al., 2004; Martin-Serrano et al., 2004), and this may stabilize the localization of ubiquitin-binding class E VPS factors at sites of viral budding. Alternatively, other factors, including the class E VPS factor Hrs, are also ubiquitinated during cellular cargo trafficking (Polo et al., 2002) in at least some instances by HECT ubiquitin ligases (Marchese et al., 2003). In addition, there is clear evidence that Rsp5-dependent trafficking in yeast can require ubiquitination of substrates other than the cargo protein (Dunn and Hicke, 2001). In principle, therefore, ubiquitination of factors other than the viral protein could influence their function in viral budding.
Which HECT ubiquitin ligase is actually used during PPXY-dependent viral budding and receptor trafficking? Overexpressed YFP-fusions of WWP1, WWP2, and Itch exhibited distinct patterns of localization, but each of these ligases bound to the MLV L-domain with the sequence specificity expected of a genuine PPXY-type L-domain cofactor. In addition, each of these proteins was recruited to sites of viral budding by MLV Gag and restored the release of a partly defective MLV Gag mutant. All three of these ligases are ubiquitously expressed (Wood et al., 1998) suggesting that some degree of functional redundancy exists, at least for PPXY-dependent viral budding. In addition, these data do not necessarily exclude a role for other HECT ligases such as Nedd4, which bound to a more limited subset of viral PPXY motifs, as viral budding cofactors. However, Nedd4 was not relocalized by MLV Gag or Ebola VP40 (unpublished data) and Nedd4 binding activity was not required for MLV L-domain function. Nonetheless, like other HECT ubiquitin ligases, Nedd4 exhibited evidence of interaction with the class E pathway, because it was recruited to VPS4(DN)-induced compartments. We suppose that multiple HECT ubiquitin ligases can recruit class E VPS factors and precisely which one is used during viral budding likely depends on expression levels in infected tissues, perhaps subcellular localization, and the particular sequence context of the viral PPXY motif. However, at least three PPXY-type L-domains from widely divergent enveloped viruses, as well as the cellular ENaC PPXY motif, proved capable of recruiting WWP1, WWP2, and Itch. Interestingly, based on sequence comparison, these three proteins form a readily distinguishable subgroup of the mammalian HECT ubiquitin ligases.
Although this study focused on the use of HECT ubiquitin ligases and VPS factors during PPXY motif–dependent viral budding, similar considerations likely apply to the recruitment of HECT ubiquitin ligases by cellular proteins to facilitate receptor internalization and entry into the MVB sorting pathway. The elaboration of this family of proteins by mammals as compared with yeast might be to cope with a wider range of substrates. Alternatively, it may be that different ligases act at different points in mammalian trafficking pathways. The cellular PPXY motif from the cellular protein ENaC appeared particularly promiscuous with respect to WW-domain binding, and it may be that multiple ligases are exploited by a single motif at different locations during the trafficking of a protein within a cell. In the absence of PPXY motif–containing viral proteins, the localization of overexpressed WWP1 was governed by the C2-domain that directed the protein to the plasma membrane, and the differential localization of the overexpressed YFP-HECT ubiquitin ligase fusion proteins suggests that endogenous proteins may be located on distinct cellular membranes. In the context of viral budding, constitutive membrane localization of overexpressed HECT ubiquitin ligases appears not to be essential to support viral budding, perhaps because the localized concentration of hundreds or thousands of Gag molecules containing PPXY motifs acts as a potent recruiting influence for WW domains. Nonetheless, the C2 domain potentiates the activity of dominant inhibitory WWP1 fragments, presumably because it would limit the diffusion of the protein to the confines of the plasma membrane, increasing the likelihood of encountering membrane associated viral proteins. Similarly, the C2 domain of Rsp5 has been shown to facilitate the sorting of CPS into the yeast MVB pathway, but mutants lacking this domain retain residual activity (Dunn et al., 2004; Katzmann et al., 2004).
Overall, this study indicates that WWP1, WWP2, and Itch are likely the most frequently used HECT ubiquitin ligases during PPXY motif–dependent viral budding and that their HECT ubiquitin ligase activity is required for this activity. Moreover, these findings also suggest that, in addition to acting as ubiquitin ligases, HECT domains have a previously unappreciated association with the class E pathway. Further work will clarify the relationship between ubiquitin ligases, the sorting of proteins at MVBs and the budding of enveloped viruses.
Materials and methods
Plasmid construction and mutagenesis
The HECT ubiquitin ligases used in this study were of human origin and included Nedd4, Nedd4-L, BUL1, WWP1, WWP2, AIP4/Itch, Smurf1, and Smurf2. The construction of plasmids expressing full-length and/or truncated versions of these proteins, wild-type and mutant VPS4, MLV Gag, GST-p12, and EbVP40 is described in the online supplemental material. The MLV proviral plasmids pNCS (MLV) pNCS D-PY (MLV/dPY) and pNCS P6-PY (MLV/HIVp6) (Yuan et al., 2000) were a gift from S. Goff (Columbia University College of Physicians and Surgeons, New York, NY). The construction of derivatives is described in the online supplemental material.
MLV and EbVP40 viral particle formation assays
293T cells were transfected with MLV proviral plasmids or myc-tagged EbVP40 expression vectors using Lipofectamine Plus (Invitrogen) and supernatants were harvested at 24 h and 48 h after transfection for experiments involving EbVP40 and MLV, respectively. Where the ability of HECT ubiquitin ligases (or fragments thereof) to enhance or inhibit particle release was measured, various amounts of YFP-fusion protein expression plasmid were cotransfected. For both MLV and EbVP40, the culture supernatants were clarified by low speed centrifugation and viral particles were harvested by centrifugation through a 20% sucrose cushion at 100,000 g for 1.5 h. Viral proteins in cell and viral lysates were analyzed by Western blotting.
Protein–protein interaction assays
For two-hybrid assays, yeast cells (Y190) were transformed with Gal4 and VP16 fusion protein expression plasmids and protein–protein interactions were measured by β-galactosidase reporter activity as described previously (Martin-Serrano et al., 2001). For GST-fusion protein co-precipitation assays, 293T cells were transfected with pCAGGS/GST-p12 expression plasmids. 48 h later, GST-p12 and proteins bound to it were precipitated using glutathione-Sepharose beads, eluted, and analyzed by Western blotting as described previously (Martin-Serrano et al., 2003a).
Western blot analyses
Virion and cell lysates or bead eluates were separated on 10 or 12% acrylamide gels and transferred to nitrocellulose membranes. The blots were probed with primary antibodies against WWP1, WWP2, and Itch (Santa Cruz Biotechnology, Inc.) MLV CA, the Myc epitope tag (9E10), or GFP (Roche), followed by peroxidase-conjugated secondary antibodies, and developed with chemiluminescent substrate reagents (Pierce Chemical Co.).
Microscopy
293T cells were plated in 35-mm coverslip dishes (Mattek) and transfected with a variable amount of a pCR3.1/YFP derivative expressing a YFP-HECT ubiquitin ligase fusion protein along with 800 ng of a wild-type or mutant pCAGGS/MLVGag-CFP or pCR3.1/CFP-EbVP40 plasmid. Alternatively, the YFP-fusion protein expression plasmids were cotransfected with 300 ng of pCR3.1/CFP/VPS4. The cells were fixed with PFA 24 h after transfection and images were collected and processed as described in the online supplemental material.
MLV infectivity assays
293T cells were transfected with wild-type, chimeric or mutant MLV proviral plasmids, a vesicular stomatitis virus-G envelope expression plasmid and pMSCV/Tat. HeLa P4/R5 cells, which carry a Tat responsive HIV-1 LTR-LacZ reporter gene, were used as targets to measure infectious MLV production, as described previously (Martin-Serrano et al., 2004). In some experiments, where the ability of ubiquitin ligase fragments to inhibit particle formation was measured, various amounts of YFP-fusion protein expression plasmid were cotransfected with the viral constructs.
Online supplemental material
Details of the plasmid construction, microscopy, and image analysis are contained herein. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200408155/DC1.
Acknowledgments
We thank Trinity Zang for technical assistance, Eleana Sphicas for electron microscopy, Fiona McDonald (Victoria University, Wellington, New Zealand), David Perez-Caballero (Aaron Diamond AIDS Research Center), and Stephen Goff for reagents.
This work was supported by the National Institutes of Health (RO1AI52774, RO1AI50111) and amFAR (02865-31). P.D. Bieniasz is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
J. Martin-Serrano's current address is Dept. of Infectious Diseases, Guy's, King's and St. Thomas' School of Medicine, King's College London, UK.
Abbreviations used in this paper: EbVP40, Ebola virus VP40; ENaC, epithelial Na+ channel; ESCRT, endosomal sorting complex required for transport; HECT, homologous to E6AP COOH terminus; MLV, murine leukemia virus; MVB, multivesicular body; RSV, Rous sarcoma virus; VPS, vacuolar protein-sorting.
References
- Alam, S.L., J. Sun, M. Payne, B.D. Welch, B.K. Blake, D.R. Davis, H.H. Meyer, S.D. Emr, and W.I. Sundquist. 2004. Ubiquitin interactions of NZF zinc fingers. EMBO J. 23:1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst, M., B. Wendland, E.J. Estepa, and S.D. Emr. 1998. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17:2982–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst, M., D.J. Katzmann, E.J. Estepa-Sabal, T. Meerloo, and S.D. Emr. 2002. a. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 3:271–282. [DOI] [PubMed] [Google Scholar]
- Babst, M., D.J. Katzmann, W.B. Snyder, B. Wendland, and S.D. Emr. 2002. b. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 3:283–289. [DOI] [PubMed] [Google Scholar]
- Bishop, N., and P. Woodman. 2001. TSG101/mammalian VPS23 and mammalian VPS28 interact directly and are recruited to VPS4-induced endosomes. J. Biol. Chem. 276:11735–11742. [DOI] [PubMed] [Google Scholar]
- Bishop, N., A. Horman, and P. Woodman. 2002. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J. Cell Biol. 157:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot, V., F. Perugi, B. Gay, M.C. Prevost, L. Briant, F. Tangy, H. Abriel, O. Staub, M.C. Dokhelar, and C. Pique. 2004. Nedd4.1-mediated ubiquitination and subsequent recruitment of Tsg101 ensure HTLV-1 Gag trafficking towards the multivesicular body pathway prior to virus budding. J. Cell Sci. 117:2357–2367. [DOI] [PubMed] [Google Scholar]
- Bouamr, F., J.A. Melillo, M.Q. Wang, K. Nagashima, M. de Los Santos, A. Rein, and S.P. Goff. 2003. PPPYEPTAP motif is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101. J. Virol. 77:11882–11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirov, D.G., A. Ono, J.M. Orenstein, and E.O. Freed. 2002. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. USA. 99:955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, R., and L. Hicke. 2001. Multiple roles for Rsp5p-dependent ubiquitination at the internalization step of endocytosis. J. Biol. Chem. 276:25974–25981. [DOI] [PubMed] [Google Scholar]
- Dunn, R., D.A. Klos, A.S. Adler, and L. Hicke. 2004. The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J. Cell Biol. 165:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan, J.M., V. Moreau, B. Andre, C. Volland, and R. Haguenauer-Tsapis. 1996. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J. Biol. Chem. 271:10946–10952. [DOI] [PubMed] [Google Scholar]
- Garrus, J.E., U.K. von Schwedler, O.W. Pornillos, S.G. Morham, K.H. Zavitz, H.E. Wang, D.A. Wettstein, K.M. Stray, M. Cote, R.L. Rich, et al. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for hiv-1 budding. Cell. 107:55–65. [DOI] [PubMed] [Google Scholar]
- Gottlinger, H.G., T. Dorfman, J.G. Sodroski, and W.A. Haseltine. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA. 88:3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty, R.N., J. Paragas, M. Sudol, and P. Palese. 1999. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J. Virol. 73:2921–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty, R.N., M.E. Brown, G. Wang, J. Huibregtse, and F.P. Hayes. 2000. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. USA. 97:13871–13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty, R.N., M.E. Brown, J.P. McGettigan, G. Wang, H.R. Jayakar, J.M. Huibregtse, M.A. Whitt, and M.J. Schnell. 2001. Rhabdoviruses and the cellular ubiquitin-proteasome system: a budding interaction. J. Virol. 75:10623–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker, G., P.A. Lloyd, K. Fox, K. Nagashima, and D. Derse. 2004. Late assembly motifs of human T-cell leukemia virus type 1 and their relative roles in particle release. J. Virol. 78:6636–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke, L. 2001. A new ticket for entry into budding vesicles-ubiquitin. Cell. 106:527–530. [DOI] [PubMed] [Google Scholar]
- Huang, L., E. Kinnucan, G. Wang, S. Beaudenon, P.M. Howley, J.M. Huibregtse, and N.P. Pavletich. 1999. Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade. Science. 286:1321–1326. [DOI] [PubMed] [Google Scholar]
- Huang, M., J.M. Orenstein, M.A. Martin, and E.O. Freed. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse, J.M., M. Scheffner, and P.M. Howley. 1993. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol. Cell. Biol. 13:4918–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse, J.M., M. Scheffner, S. Beaudenon, and P.M. Howley. 1995. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc. Natl. Acad. Sci. USA. 92:2563–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann, D.J., M. Babst, and S.D. Emr. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 106:145–155. [DOI] [PubMed] [Google Scholar]
- Katzmann, D.J., G. Odorizzi, and S.D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3:893–905. [DOI] [PubMed] [Google Scholar]
- Katzmann, D.J., S. Sarkar, T. Chu, A. Audhya, and S.D. Emr. 2004. Multivesicular body sorting: ubiquitin ligase Rsp5 is required for the modification and sorting of carboxypeptidase S. Mol. Biol. Cell. 15:468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikonyogo, A., F. Bouamr, M.L. Vana, Y. Xiang, A. Aiyar, C. Carter, and J. Leis. 2001. Proteins related to the Nedd4 family of ubiquitin protein ligases intereact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc. Natl. Acad. Sci. USA. 98:11199–11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata, J.M., M. Simpson-Holley, N.T. Wright, Z. Han, J. Paragas, and R.N. Harty. 2003. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J. Virol. 77:1812–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese, A., C. Raiborg, F. Santini, J.H. Keen, H. Stenmark, and J.L. Benovic. 2003. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev. Cell. 5:709–722. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano, J., T. Zang, and P.D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313–1319. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano, J., A. Yaravoy, D. Perez-Caballero, and P.D. Bieniasz. 2003. a. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. USA. 100:12414–12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano, J., T. Zang, and P.D. Bieniasz. 2003. b. Role of ESCRT-I in retroviral budding. J. Virol. 77:4794–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano, J., D. Perez-Caballero, and P.D. Bieniasz. 2004. Context-dependent effects of L domains and ubiquitination on viral budding. J. Virol. 78:5554–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott, D.E., L.V. Coren, E.N. Chertova, T.D. Gagliardi, and U. Schubert. 2000. Ubiquitination of HIV-1 and MuLV Gag. Virology. 278:111–121. [DOI] [PubMed] [Google Scholar]
- Ott, D.E., L.V. Coren, R.C. Sowder II, J. Adams, K. Nagashima, and U. Schubert. 2002. Equine infectious anemia virus and the ubiquitin-proteasome system. J. Virol. 76:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott, D.E., L.V. Coren, R.C. Sowder II, J. Adams, and U. Schubert. 2003. Retroviruses have differing requirements for proteasome function in the budding process. J. Virol. 77:3384–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik, A., V. Chau, and J.W. Wills. 2000. Ubiquitin is part of the retrovirus budding machinery. Proc. Natl. Acad. Sci. USA. 97:13069–13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik, A., V. Chau, F. Li, R.C. Montelaro, and J.W. Wills. 2002. Budding of equine infectious anemia virus is insensitive to proteasome inhibitors. J. Virol. 76:2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo, S., S. Sigismund, M. Faretta, M. Guidi, M.R. Capua, G. Bossi, H. Chen, P. De Camilli, and P.P. Di Fiore. 2002. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 416:451–455. [DOI] [PubMed] [Google Scholar]
- Pornillos, O., J.E. Garrus, and W.I. Sundquist. 2002. Mechanisms of enveloped RNA virus budding. Trends Cell Biol. 12:569–579. [DOI] [PubMed] [Google Scholar]
- Puffer, B.A., L.J. Parent, J.W. Wills, and R.C. Montelaro. 1997. Equine infectious anemia virus utilizes a YXXL motif within the late assembly domain of the Gag p9 protein. J. Virol. 71:6541–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin, D., O. Staub, and R. Haguenauer-Tsapis. 2000. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 176:1–17. [DOI] [PubMed] [Google Scholar]
- Sakurai, A., J. Yasuda, H. Takano, Y. Tanaka, M. Hatakeyama, and H. Shida. 2004. Regulation of human T-cell leukemia virus type 1 (HTLV-1) budding by ubiquitin ligase Nedd4. Microbes Infect. 6:150–156. [DOI] [PubMed] [Google Scholar]
- Schubert, U., D.E. Ott, E.N. Chertova, R. Welker, U. Tessmer, M.F. Princiotta, J.R. Bennink, H.G. Krausslich, and J.W. Yewdell. 2000. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc. Natl. Acad. Sci. USA. 97:13057–13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub, O., S. Dho, P. Henry, J. Correa, T. Ishikawa, J. McGlade, and D. Rotin. 1996. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- Staub, O., H. Abriel, P. Plant, T. Ishikawa, V. Kanelis, R. Saleki, J.D. Horisberger, L. Schild, and D. Rotin. 2000. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int. 57:809–815. [DOI] [PubMed] [Google Scholar]
- Strack, B., A. Calistri, M.A. Accola, G. Palu, and H.G. Gottlinger. 2000. A role for ubiquitin ligase recruitment in retrovirus release. Proc. Natl. Acad. Sci. USA. 97:13063–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack, B., A. Calistri, and H.G. Gottlinger. 2002. Late assembly domain function can exhibit context dependence and involves ubiquitin residues implicated in endocytosis. J. Virol. 76:5472–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack, B., A. Calistri, S. Craig, E. Popova, and H.G. Gottlinger. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 114:689–699. [DOI] [PubMed] [Google Scholar]
- Tanzi, G.O., A.J. Piefer, and P. Bates. 2003. Equine infectious anemia virus utilizes host vesicular protein sorting machinery during particle release. J. Virol. 77:8440–8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VerPlank, L., F. Bouamr, T.J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C.A. Carter. 2001. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag). Proc. Natl. Acad. Sci. USA. 98:7724–7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwedler, U.K., M. Stuchell, B. Muller, D.M. Ward, H.Y. Chung, E. Morita, H.E. Wang, T. Davis, G.P. He, D.M. Cimbora, et al. 2003. The protein network of HIV budding. Cell. 114:701–713. [DOI] [PubMed] [Google Scholar]
- Wills, J.W., C.E. Cameron, C.B. Wilson, Y. Xiang, R.P. Bennett, and J. Leis. 1994. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J. Virol. 68:6605–6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, J.D., J. Yuan, R.L. Margolis, V. Colomer, K. Duan, J. Kushi, Z. Kaminsky, J.J. Kleiderlein, A.H. Sharp, and C.A. Ross. 1998. Atrophin-1, the DRPLA gene product, interacts with two families of WW domain-containing proteins. Mol. Cell. Neurosci. 11:149–160. [DOI] [PubMed] [Google Scholar]
- Yasuda, J., E. Hunter, M. Nakao, and H. Shida. 2002. Functional involvement of a novel Nedd4-like ubiquitin ligase on retrovirus budding. EMBO Rep. 3:636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda, J., M. Nakao, Y. Kawaoka, and H. Shida. 2003. Nedd4 regulates egress of Ebola virus-like particles from host cells. J. Virol. 77:9987–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, B., S. Campbell, E. Bacharach, A. Rein, and S.P. Goff. 2000. Infectivity of Moloney murine leukemia virus defective in late assembly events is restored by late assembly domains of other retroviruses. J. Virol. 74:7250–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]