Abstract
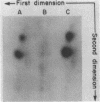
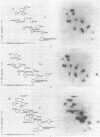
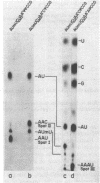
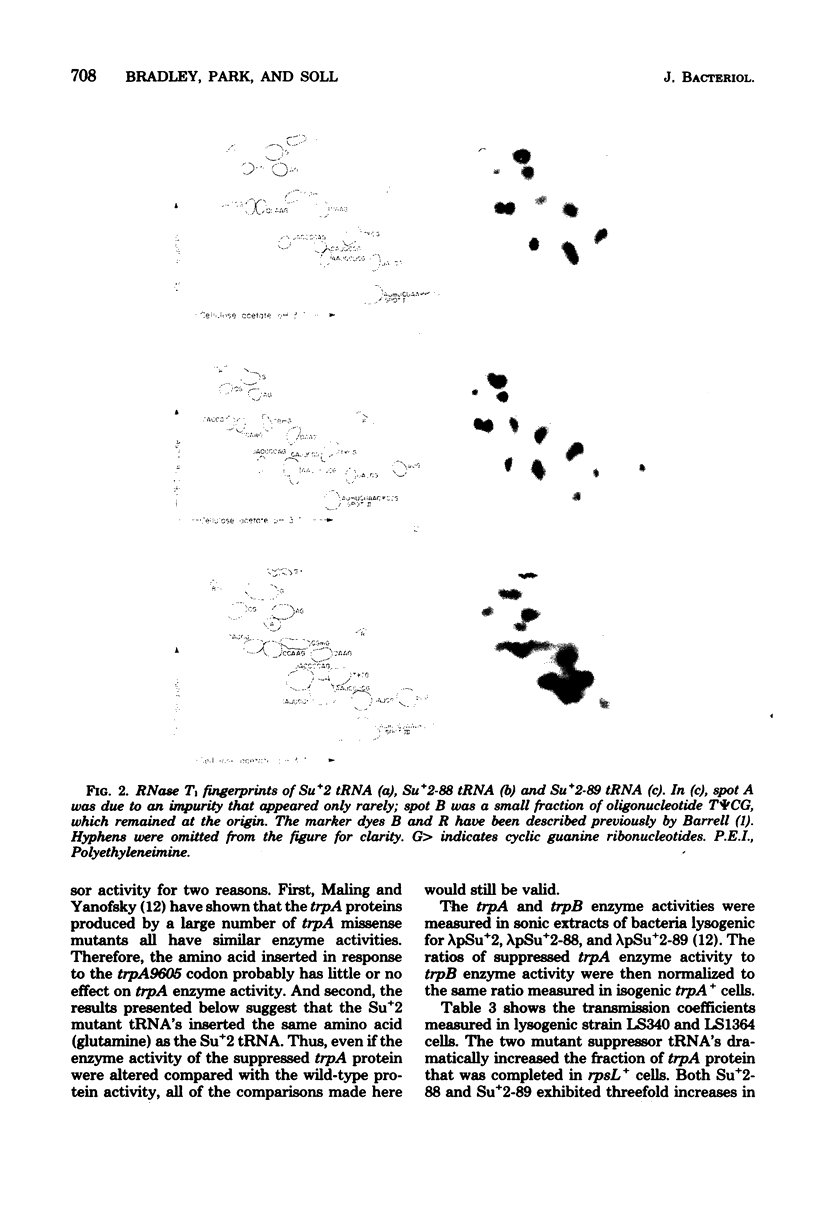
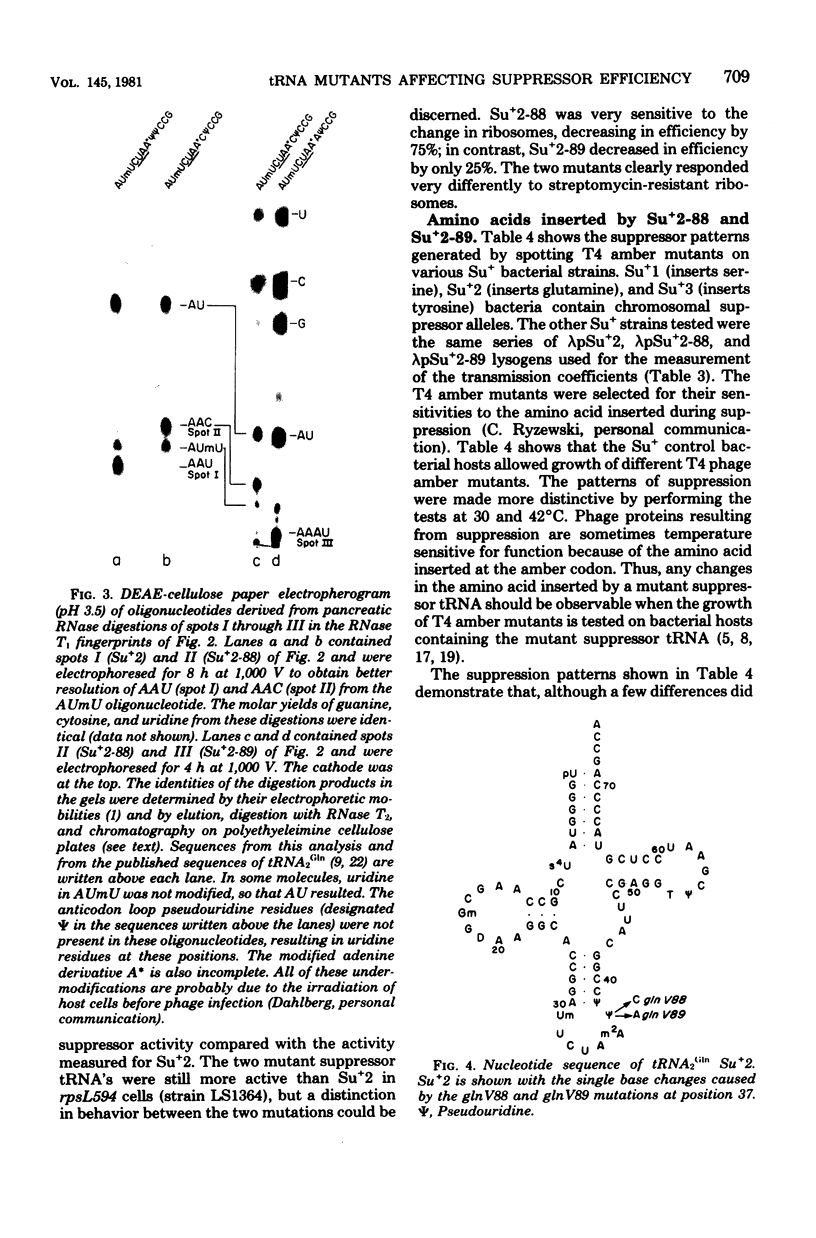
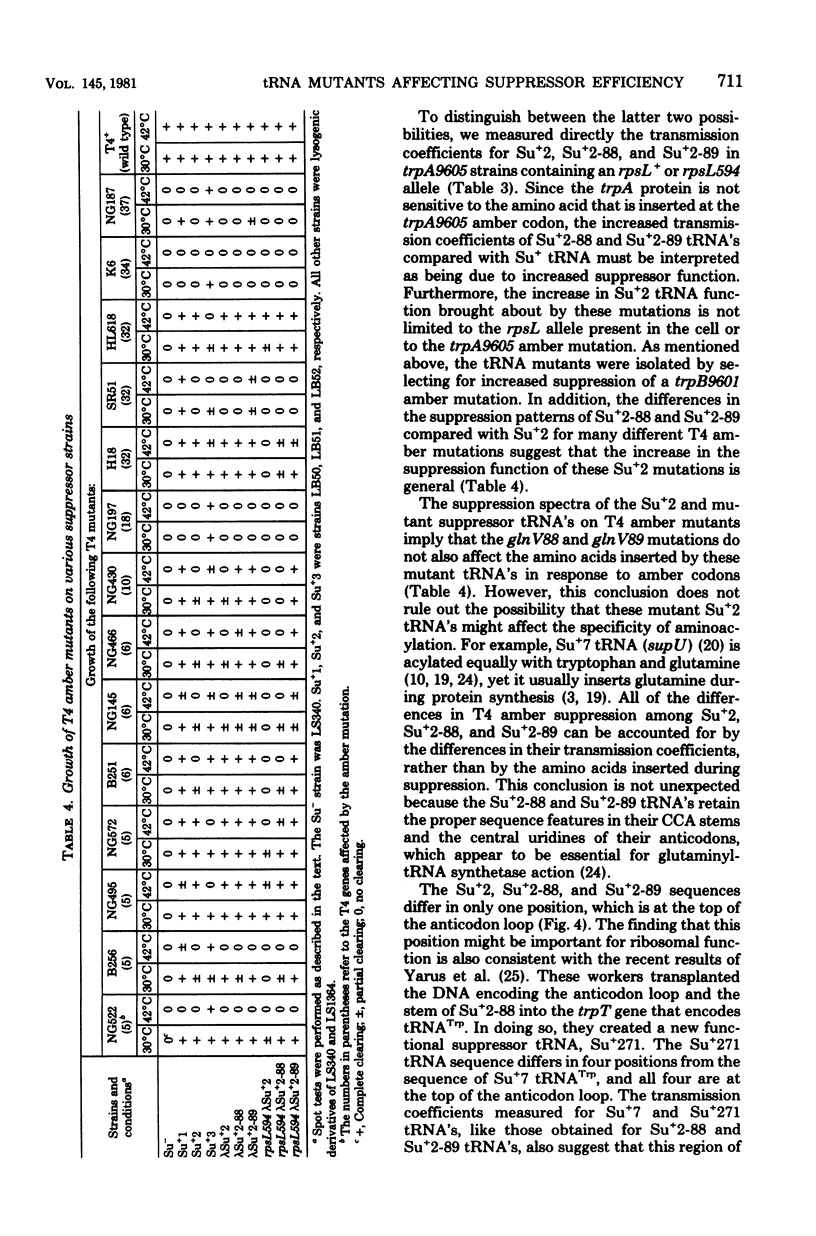
We selected mutants of lambda pSu+2 which had an increased ability to suppress on Escherichia coli trp B9601 amber mutation on translationally stringent rpsL594 streptomycin-resistant ribosomes. tRNA2Gin Su+2 molecules produced from eight independent mutants were purified, and their ribonucleic acid sequences were determined. Two types of mutations were mapped to the tRNA2Gin Su+2(glnV) gene by this method. Both altered the pseudouridine at position 37 of the tRNA anticodon loop. Seven of the isolates were transitions (pseudouridine to cytosine), and one was a transversion (pseudouridine to adenine). These mutations resulted in Su+ transfer ribonucleic acid molecules that exhibited higher transmission coefficients than their parent Su+2 transfer ribonucleic acids. As judged by their suppressor spectra on T4 amber mutants, which were almost identical to that of Su+2, the two mutant Su+ transfer ribonucleic acids inserted glutamine at amber sites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celis J. E., Coulondre C., Miller J. H. Suppressor su+7 inserts tryptophan in addition to glutamine. J Mol Biol. 1976 Jul 5;104(3):729–734. doi: 10.1016/0022-2836(76)90132-7. [DOI] [PubMed] [Google Scholar]
- Couturier M., Desmet L., Thomas R. High pleiotropy of streptomycin mutations in Escherichia coli. Biochem Biophys Res Commun. 1964 Jun 15;16(3):244–248. doi: 10.1016/0006-291x(64)90333-x. [DOI] [PubMed] [Google Scholar]
- Ghysen A., Celis J. E. Mischarging single and double mutants of Escherichia coli sup3 tyrosine transfer RNA. J Mol Biol. 1974 Mar;83(3):333–351. doi: 10.1016/0022-2836(74)90283-6. [DOI] [PubMed] [Google Scholar]
- Griffin B. E. Separation of 32P-labelled ribonucleic acid components. The use of polyethylenimine-cellulose (TLC) as a second dimension in separating oligoribonucleotides of '4.5 S' and 5 S from E. coli. FEBS Lett. 1971 Jun 24;15(3):165–168. doi: 10.1016/0014-5793(71)80304-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Inokuchi H., Celis J. E., Smith J. D. Letter: Mutant tyrosine transfer ribonucleic acids of Escherichia coli: construction by recombination of a double mutant A1G82 chargeable with glutamine. J Mol Biol. 1974 May 5;85(1):187–192. doi: 10.1016/0022-2836(74)90138-7. [DOI] [PubMed] [Google Scholar]
- Inokuchi H., Yamao F., Sakano H., Ozeki H. Identification of transfer RNA suppressors in Escherichia coli. I. Amber suppressor su+2, an anticodon mutant of tRNA2Gln. J Mol Biol. 1979 Aug 25;132(4):649–662. doi: 10.1016/0022-2836(79)90380-2. [DOI] [PubMed] [Google Scholar]
- Knowlton R. G., Soll L., Yarus M. Dual specificity of su+ 7 tRNA. Evidence for translational discrimination. J Mol Biol. 1980 Jun 5;139(4):705–720. doi: 10.1016/0022-2836(80)90056-x. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E., Lindahl L., Jaskunas S. R., Dennis P. P., Nomura M. Transfer RNA genes between 16S and 23S rRNA genes in rRNA transcription units of E. coli. Cell. 1976 Feb;7(2):165–177. doi: 10.1016/0092-8674(76)90016-7. [DOI] [PubMed] [Google Scholar]
- MALING B. D., YANOFSKY C. The properties of altered proteins from mutants bearing one or two lesions in the same gene. Proc Natl Acad Sci U S A. 1961 Apr 15;47:551–566. doi: 10.1073/pnas.47.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethal nonsense suppressor in Escherichia coli which inserts glutamine. Nature. 1969 Sep 27;223(5213):1340–1342. doi: 10.1038/2231340a0. [DOI] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethals: a new class of nonsense suppressors in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jun;63(2):392–399. doi: 10.1073/pnas.63.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini P., Gorini L. Ribosomal mutations affecting efficiency of amber suppression. J Mol Biol. 1970 Feb 14;47(3):517–530. doi: 10.1016/0022-2836(70)90319-0. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Folk W. R. The nucleotide sequences of the two glutamine transfer ribonucleic acids from Escherichia coli. J Biol Chem. 1975 May 10;250(9):3243–3253. [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]
- Yarus M., McMillan C., 3rd, Cline S., Bradley D., Snyder M. Construction of a composite tRNA gene by anticodon loop transplant. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5092–5096. doi: 10.1073/pnas.77.9.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]