Abstract
The Sec61 translocon of the endoplasmic reticulum membrane forms an aqueous pore that is gated by the lumenal Hsp70 chaperone BiP. We have explored the molecular mechanisms governing BiP-mediated gating activity, including the coupling between gating and the BiP ATPase cycle, and the involvement of the substrate-binding and J domain–binding regions of BiP. Translocon gating was assayed by measuring the collisional quenching of fluorescent probes incorporated into nascent chains of translocation intermediates engaged with microsomes containing various BiP mutants and BiP substrate. Our results indicate that BiP must assume the ADP-bound conformation to seal the translocon, and that the reopening of the pore requires an ATP binding–induced conformational change. Further, pore closure requires functional interactions between both the substrate-binding region and the J domain–binding region of BiP and membrane proteins. The mechanism by which BiP mediates translocon pore closure and opening is therefore similar to that in which Hsp70 chaperones associate with and dissociate from substrates.
Introduction
In the initial stages of the eukaryotic secretory pathway, ribosome-nascent chain complexes (RNCs) are targeted to the membrane of the ER in a signal recognition particle (SRP)–dependent manner, and substrates are cotranslationally translocated through the membrane at sites termed translocons (Johnson and van Waes, 1999). The translocon consists of the core heterotrimeric Sec61 complex (Sec61αβγ) and associated proteins, forming a cylindrical channel that aligns with the large subunit of the ribosome during translocation (Beckmann et al., 1997). Electrophysiological studies demonstrated that the Sec61 translocon forms an aqueous, ion-conducting channel (Simon and Blobel, 1991; Wirth et al., 2003). Moreover, the selective positioning of water-sensitive fluorescent probes into the nascent chains of secretory protein translocation intermediates directly demonstrated that the nascent chain is in an aqueous environment as it passes through the membrane; therefore, the nascent chain occupies an aqueous pore in the mammalian translocon that spans the bilayer (Crowley et al., 1993, 1994). The translocon is structurally dynamic, expanding from an inner diameter of 9–15 Å in the ribosome-free state (Hamman et al., 1998) to a diameter of 40–60 Å in the active, ribosome-bound state (Hamman et al., 1997; Wirth et al., 2003).
Because translocons form aqueous pores of this magnitude, gating mechanisms must preserve the permeability barrier that maintains critical ion gradients (e.g., Ca2+ stores) across the ER membrane during translocation. These mechanisms have been elucidated experimentally by incorporating fluorescent probes into the nascent chains of translocation intermediates and assessing their accessibility to externally added quenching agents. The ER permeability barrier is maintained from the cytosolic side of the membrane by the ion-tight binding of the ribosome to the translocon during translocation (Crowley et al., 1994). The lumenal side of the translocation pore is sealed either directly or indirectly by BiP, an ER lumenal Hsp70 chaperone that is the only soluble protein necessary and sufficient to effect closure of ribosome-free translocons and those engaged in the early stages of translocation (Hamman et al., 1998). During secretory protein translocation, the BiP-mediated seal opens after the nascent chain reaches a threshold length of ∼70 amino acids (Crowley et al., 1994; Hamman et al., 1998). During membrane protein integration, the ribosome and translocon alternately gate the cytosolic and lumenal ends of the pore through a tightly regulated series of conformational changes (Liao et al., 1997; Haigh and Johnson, 2002; Alder and Johnson, 2004; Woolhead et al., 2004). The BiP-mediated gate operates in a stoichiometric (noncatalytic) fashion, and requires the presence of nucleotide (Hamman et al., 1998; Haigh and Johnson, 2002).
All Hsp70 chaperones contain a conserved NH2-terminal 44-kD nucleotide-binding domain, a 15-kD substrate-binding domain (SBD), and a variable COOH-terminal domain (Bukau and Horwich, 1998). The polypeptide binding affinity of the SBD is allosterically modulated by cycles of ATP binding and hydrolysis at the nucleotide-binding region, which mediates the opening and closure of an α-helical lid over the substrate-binding cavity (Zhu et al., 1996). ATP-bound Hsp70 has low substrate affinity, with fast rates of peptide binding and release, and ADP-bound Hsp70 has high substrate affinity, with stable peptide association. The low intrinsic Hsp70 ATPase rates are regulated in the cell by cochaperones such as DnaJ-type proteins, which contain a conserved 70-aa J domain and increase ATP hydrolysis rates, and nucleotide exchange factors that promote ATP/ADP exchange. Several mammalian ER-resident cochaperones have been characterized, including translocon-associated J domain proteins Sec63 (Meyer et al., 2000; Tyedmers et al., 2000) and Mtj1 (Chavalier et al., 2000; Dudek et al., 2002), and the nucleotide exchange factor BAP (Chung et al., 2002).
In this work, we addressed several questions regarding the molecular mechanism of BiP-mediated translocon pore gating. First, how might the ATP catalytic cycle modulate BiP gating activity? Second, does pore gating involve the SBD of BiP? Finally, does BiP-mediated gating require an interaction with a membrane-bound J domain protein? We used a well-established fluorescence quenching assay to measure the gating activity of recombinant hamster BiP (rBiP) reconstituted into mammalian ER microsomes. Our results elucidate key steps of the translocon gating cycle, and strongly suggest that the mechanism governing BiP gating is analogous to that in which Hsp70 chaperones bind and release substrate polypeptide.
Results
Experimental design
The lumenal translocon gating activity of BiP was monitored by creating fluorescent translocation intermediates functionally engaged with ER translocons and then measuring the exposure of the nascent polypeptide to externally added collisional quenching agents. In vitro translation reactions containing SRP and ER microsomes were programmed with mRNA truncated in the coding region to generate homogeneous populations of fully assembled intermediates with a nascent chain of a defined length. The absence of a stop codon on the transcript prevents translation termination, and the nascent chain remains ribosome bound as a peptidyl-tRNA.
Cotranslational incorporation of fluorescent-labeled lysine residues into nascent chains was achieved by inclusion of Lys-tRNA analogues in which the fluorescent probe NBD is covalently attached to the Nɛ-amino group of the lysyl side chain. The position of in-frame lysine codons on the mRNA transcript then dictates the location of fluorescent probes on the nascent chain. Two nascent secretory proteins were used: pPL64, a 64-residue intermediate of preprolactin (pPL) with Lys (probes) at residues 4 and 9 in the signal sequence, and pPL-sK78, a 78-residue derivative of pPL with probes at residues 64 and 70. Parallel translations containing ɛNBD-Lys-tRNA and unmodified Lys-tRNA were prepared and compared spectroscopically to correct for the significant scattering and background signal of the samples. Importantly, ɛNBD-Lys incorporates as efficiently into nascent chains as unmodified Lys, and does not affect targeting or translocation (Crowley et al., 1993).
The exposure of NBD-labeled nascent chains to the external medium or ER lumen was measured by the extent of collisional quenching by iodide ions (I−). Such quenching agents reduce sample fluorescence by colliding with excited dyes and returning them to the ground state without photon emission. The ratio of fluorescence intensity in the absence of quencher Q to that in its presence (F0/F) increases linearly with [Q], as described by the Stern-Volmer equation: (F0/F) − 1 = KSV[Q]. Thus, the Stern-Volmer constant (KSV) provides a measure of the accessibility of added I− to the NBD probes on the nascent chain. Charged I− do not penetrate the nonpolar ER membrane and will quench nascent chain probes only upon direct contact (Crowley et al., 1994). To introduce I− into the lumenal space we used melittin, a 26-residue cytolytic peptide that oligomerizes to create pores in the membrane with an inner diameter of 25–30 Å (Ladokhin et al., 1997). At the concentrations used here, melittin had no effect on targeting, translocation, or signal peptidase activity, nor did the presence of melittin affect the spectral characteristics of fluorescent translocation intermediates (unpublished data).
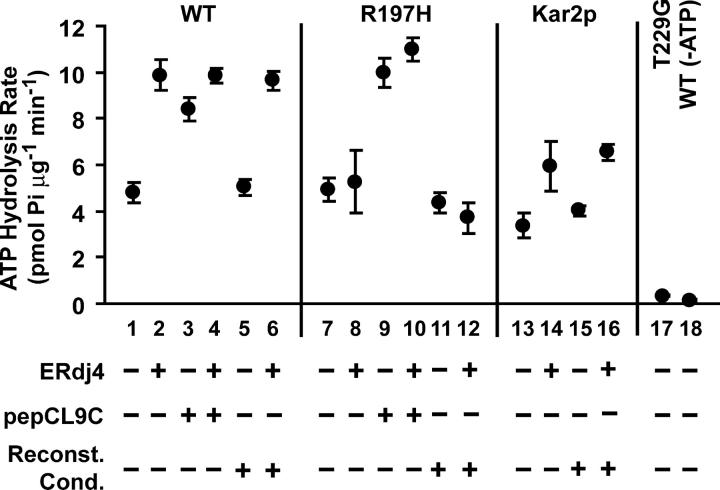
To determine the ability of different rBiP derivatives to gate the lumenal end of the translocon, microsomes were extracted of soluble lumenal proteins and selectively reconstituted with the desired rBiP derivative (Bulleid and Freedman, 1988; Nicchitta and Blobel, 1993; Hamman et al., 1998). Salt-washed ER microsomes (KRMs) were disrupted by high pH to release their lumenal contents. Upon return to neutral pH, the vesicles resealed in the native orientation to yield lumen-extracted microsomes (XRMs). XRMs may be incubated with purified protein(s) at high pH before resealing to produce reconstituted microsomes (RRMs). The reconstitution procedure had no effect on basal rBiP ATPase activity (Fig. 1, compare lanes 1, 7, and 13 with 5, 11, and 15, respectively) or on the functional interaction of rBiP with a J domain protein (Fig. 1, compare lanes 2, 8, and 14 with 6, 12, and 16, respectively); therefore, the conformation and activity of rBiP were unaffected by the high pH treatment.
Figure 1.
ATP hydrolysis rates of rBiP derivatives. Inorganic phosphate production was measured spectroscopically to determine rates of steady-state ATP hydrolysis by purified rBiP variants (WT, R197H, T229G) or Kar2p. Measurements were conducted under basal conditions (lanes 1, 7, 13, and 17) or in the presence of purified ERdj4 J domain (fourfold molar excess, lanes 2, 8, and 14), pepCL9C (200-fold molar excess, lanes 3 and 9), or both (lanes 4 and 10), as indicated. Control reactions contained the ATPase mutant rBiP T229G (lane 17) or rBiP WT in the absence of ATP (lane 18). To determine the effect of reconstitution conditions on BiP ATPase activity, rBiP (and ERdj4, where indicated) was exposed to high pH (lanes 5, 6, 11, 12, 15, and 16) before being returned to neutral pH. The mean values from 3–5 independent experiments are shown ± SD.
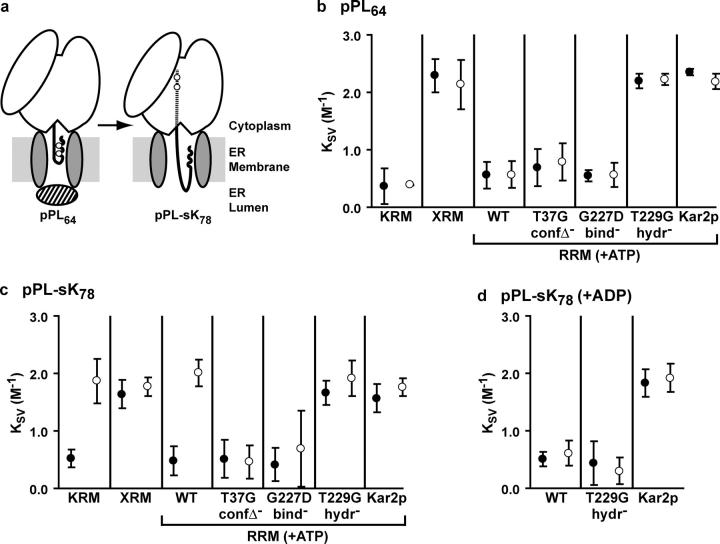
Using this approach, BiP was previously found to be the only soluble lumenal protein required to effect closure of inactive (ribosome-free) translocons, and also to gate translocons engaged in the early steps of translocation (Hamman et al., 1998) or in membrane protein integration (Haigh and Johnson, 2002) (Fig. 2 a). Immediately after SRP-dependent RNC targeting to the translocon, the nascent chain is sealed off from both the cytosol and the lumen. The BiP-mediated seal then opens once the nascent chain reaches ∼70 amino acids (Crowley et al., 1994; Hamman et al., 1998) (Fig. 2 a). Thus, NBD-pPL64 and NBD-pPL-sK78 will be inaccessible to externally added I− when functionally engaged with KRMs or RRMs containing sealing-competent rBiP, yielding low KSV values. I− introduced into the lumen by melittin will remain inaccessible to NBD-pPL64 due to the BiP-mediated lumenal seal. In contrast, lumenal I− will quench NBD-pPL-sK78 probes because the lumenal seal has been opened, thereby yielding a high KSV value. When associated with XRMs or RRMs containing sealing-incompetent rBiP, the probes in both NBD-pPL64 and NBD-pPL-sK78 will be accessible to externally added I− because I− (hydrated diameter 9 Å) are small enough to pass though unsealed translocon pores that are ribosome free (∼50% of total translocons; Hamman et al., 1998) and enter the lumen. The ER translocon has been shown to be the sole conduit through which I− passed through the membrane in such experiments, because affinity-purified Sec61α antibodies blocked I− transit (Hamman et al., 1998; Haigh and Johnson, 2002).
Figure 2.
NBD-labeled translocation intermediates engaged with KRMs, XRMs, or RRMs. (a) Short nascent chains (e.g., pPL64) are sealed off from the lumen by the BiP-mediated gate (hatched oval), whereas longer nascent chains (e.g., pPL-sK78) are exposed to the lumen. Probe positions are indicated by open circles and the nascent chain within the ribosome tunnel is depicted as a dashed line. (b–d) Mean KSV values (n = 3–12 ± SD) are shown for NBD-pPL64 (b) or NBD-pPL-sK78 (c and d) translocation intermediates functionally engaged with KRMs, XRMs, or RRMs as indicated. For RRMs, reconstitutions were performed either in the presence of ATP (b and c) or ADP (d). Measurements were made in the absence (•, I− on cytosolic side of sealed membranes only) or presence (○, I− both on cytosolic side and in lumen) of melittin.
To examine the mechanistic details governing BiP gating we used this fluorescence quenching approach to monitor the gating activity of rBiP impaired at different stages of the ATPase cycle, rBiP impaired in J domain interactions, or rBiP with excess peptide substrate. The rBiP ATPase mutants used include: T37G, which binds and hydrolyzes ATP, but does not undergo an ATP-induced conformational change in the SBD; G227D, which has reduced binding affinity for nucleotide; T229G, which binds, but does not hydrolyze, ATP; and 44K, which lacks the entire SBD. Each of these mutants has been biochemically characterized (Wei et al., 1995). The synthesis and characterization of a J domain–binding mutant (rBiP R197H) and a peptide substrate (pepCL9C) used in the present work are described below.
BiP must be in the ADP-bound form to seal the translocon pore
Consistent with previous KRM results (Hamman et al., 1998), the probes in pPL64 were inaccessible to both external (= cytosolic) and lumenal I−, whereas those in pPL-sK78 were accessible to I− introduced into the lumen after melittin incubation (Fig. 2, b and c; compare open and closed circles). When bound to lumen-extracted XRMs, both intermediates were quenched by added I−, confirming the extraction of BiP and the absence of the lumenal seal in these microsomes (Fig. 2, b and c). These KSV values thus provided the expected values for “quenched” and “unquenched” cases when evaluating the ability of different rBiP mutants to seal translocons.
The quenching patterns obtained for both pPL64 and pPL-sK78 bound to RRMs reconstituted with wild-type rBiP (rBiP WT) in the presence of ATP were identical to those obtained with KRMs (Fig. 2, b and c). The ability of hamster rBiP to seal canine microsomes in this heterologous system was also observed when integration intermediates were examined (Haigh and Johnson, 2002), and these results confirm that reconstitution does not impair RNC targeting or compromise the ion permeability barrier of resealed microsomes.
Next, we tested the sealing competence of rBiP ATPase mutants. When pPL64 RNCs were bound to RRMs containing ATP and either rBiP T37G or rBiP G227D, the nascent chain was inaccessible to I−, as seen also with KRMs (Fig. 2 b, closed circles). Thus, neither the lack of an ATP-dependent conformational change in the SBD nor a weaker binding affinity for ATP, respectively, detectably affected the ability of BiP to mediate pore closure. On the other hand, the pPL64 probes were fully exposed to I− when RRMs were prepared with rBiP T229G, a mutant that can bind but not hydrolyze ATP (Fig. 2 b, closed circles). A similar pattern of nascent chain quenching was observed with pPL-sK78 intermediates (Fig. 2 c, closed circles). These results suggest that a BiP–ATP complex is not sealing competent, and that BiP must assume the ADP-bound conformation to seal the translocon pore. This result is consistent with the previous demonstration that wild-type BiP in the presence of a nonhydrolyzable ATP analogue cannot seal translocon pores (Hamman et al., 1998).
To further explore the nucleotide state of BiP required for pore sealing, microsome reconstitutions were performed in the presence of ADP. When exclusively in the ADP-bound state, rBiP WT was sealing competent (Fig. 2 d, closed circles). Interestingly, when forced into the ADP-bound state, rBiP T229G also formed a lumenal seal (Fig. 2 d, closed circles). These results confirm that BiP must assume an ADP-bound conformation to seal the translocon, and that ATP hydrolysis is not, per se, a requisite step in pore sealing.
We also found that yeast BiP (Kar2p) reconstituted into mammalian microsomes in either the presence of ATP (Fig. 2, b and c; closed circles) or ADP (Fig. 2 d, closed circles) was unable to seal translocons. Given that the ATPase activity of Kar2p was not impaired under our experimental conditions (Fig. 1), some feature of this homologue other than the nucleotide-bound state must block its ability to gate translocons in this heterologous system.
Translocon pore opening by BiP requires an ATP binding–coupled conformational change
Once targeted secretory nascent chains are ∼70 aa in length the BiP-mediated gate opens, exposing the nascent chain to the lumen (Fig. 2 a; Crowley et al., 1994; Hamman et al., 1998). A mutation in BiP that blocks the ability of the BiP-mediated gate to open would be manifested as a lack of exposure of long nascent chains (pPL-sK78) to lumenal I−. We unexpectedly found that, in contrast to rBiP WT, both rBiP T37G and rBiP G227D maintained the translocon seal even at a nascent chain length that normally induces the opening of the native lumenal gate (Fig. 2 c, open circles). This suggests that an ATP binding–induced conformational change by BiP opens the translocon channel to the lumen, at least for intermediates just beyond the threshold length of 70 aa. To further explore this seal reversal, we measured the exposure of pPL-sK78 intermediates to lumenal I− when associated with RRMs containing rBiP WT in the presence of ADP (Fig. 2 d). Interestingly, when no ATP was present, rBiP WT sealed the translocons, but the seal was not reversed with longer nascent chains (Fig. 2 d, compare open and closed circles). These results indicate that opening of the BiP-mediated gate requires that BiP–ADP must bind ATP and undergo a coupled opening of the substrate-binding pocket.
The BiP SBD is involved in translocon gating
Next, we explored the possible involvement of the SBD of BiP in the establishment of the translocon seal. First, rBiP 44K, a mutant lacking the SBD and COOH terminus entirely, did not seal translocons when reconstituted into RRMs with ATP (Fig. 3 b), yielding KSV values similar to unsealed microsomes (Fig. 2 c, “XRMs”). This indicates that the NH2-terminal ATPase domain alone cannot gate the translocon pore. However, this result does not necessarily implicate BiP SBD interactions in the gating process because the molecular mass of the rBiP 44K mutant is substantially reduced relative to full-length BiP, and this may abrogate sealing, particularly if BiP is the physical translocon seal.
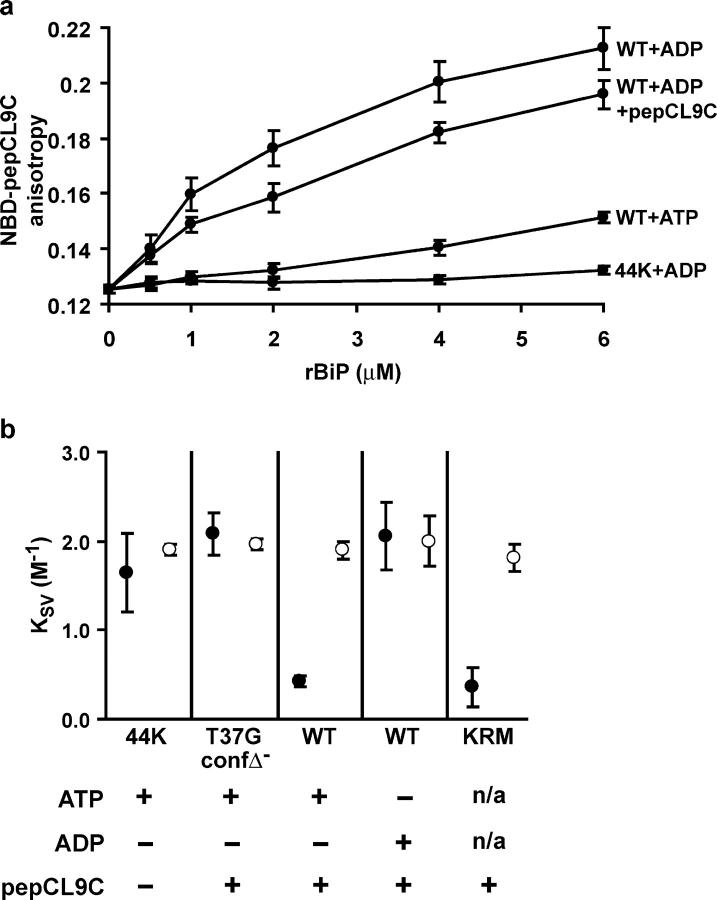
Figure 3.
Dependence of translocon gating on the SBD of BiP. (a) Mean fluorescence anisotropy values of NBD-pepCL9C (n = 3–5 ± SD) preincubated with increasing concentrations of rBiP (WT or 44K) with ATP, ADP, and unlabeled pepCL9C (fourfold molar excess) as indicated. (b) Mean KSV values (n = 3 ± SD) for NBD-pPL-sK78 associated with RRMs reconstituted with rBiP 44K or full-length rBiP (WT or T37G) measured in the absence (•) or presence (○) of melittin. RRMs contained pepCL9C (20-fold molar excess over rBiP) and ATP or ADP as indicated. In the final lane, KRMs were incubated with pepCL9C before fluorescence measurements (n/a, not applicable).
To analyze the role of the BiP SBD in gating more directly, we examined the effects of substrate-binding pocket occupancy on sealing-competent rBiP. This was done by reconstitution of microsomes with rBiP in the presence of a variant of peptide C, a well-characterized 13-residue Hsp70 substrate with high affinity (submicromolar Kd) (Flynn et al., 1989). Our variant introduced a cysteine at position 9 (pepCL9C) for modification with a thiol-reactive fluorescent probe to monitor peptide–BiP interactions (see below). We reasoned that if the presence of excess pepCL9C impaired the ability of otherwise sealing-competent rBiP to seal translocons, then gating must involve direct interaction between the BiP SBD and a translocon (or translocon-associated) component.
Functional interaction between rBiP and pepCL9C was confirmed by two independent means. First, the ATP hydrolysis rate of rBiP WT increased approximately twofold in the presence of pepCL9C (Fig. 1, compare lanes 1 and 3), consistent with previous results (Wei et al., 1995). Second, we monitored the fluorescence anisotropy of NBD-labeled pepCL9C in the presence of increasing rBiP concentrations (Fig. 3 a). ADP-bound rBiP WT showed high affinity pepCL9C binding that was reduced by competition with unlabeled pepCL9C, indicating that the fluorescent peptide interacted with the authentic peptide-binding site. In contrast, rBiP WT in the presence of ATP showed a marked reduction in affinity, and rBiP 44K displayed no detectable binding. Together, these results confirm that pepCL9C is a bona fide rBiP substrate.
To assay BiP translocon gating activity in the presence of pepCL9C, we first chose conditions in which BiP would maintain a high affinity interaction with the peptide throughout the analysis: either by reconstitution in the presence of ADP, or by using the rBiP T37G mutant. Indeed, when incubated with pepCL9C at a 20-fold molar excess before reconstitution, neither rBiP T37G in the presence of ATP nor rBiP WT in the presence of ADP sealed translocons when reconstituted into microsomes (Fig. 3 b, closed circles). In contrast, rBiP WT reconstituted under the same conditions except in the presence of ATP formed a lumenal seal. We interpret the latter result to indicate that rBiP WT undergoes cycles of pepCL9C binding and release in the presence of ATP, and over the course of the experiment sufficient rBiP is available (non-pepCL9C associated) to effect translocon pore closure. To ensure that the peptide itself did not render ER membranes permeable to I−, control experiments were conducted by analyzing KRMs that had been incubated with pepCL9C before quenching measurements. The presence of pepCL9C did not make KRMs permeable to I− (compare Fig. 3 b with Fig. 1 c), and therefore the peptide does not have cytolytic properties that would artificially induce quenching in the above experiments. These results provide strong evidence that BiP binds to one or more translocon components through its substrate-binding pocket during sealing.
BiP functionally interacts with a membrane-bound J domain–containing protein to seal translocon pores
Next, we addressed whether BiP interacts with a J domain–containing protein during translocon gating. Because BiP is the only soluble lumenal protein required for translocon sealing, such an interaction could only be with a membrane-bound protein with a lumen-exposed J domain.
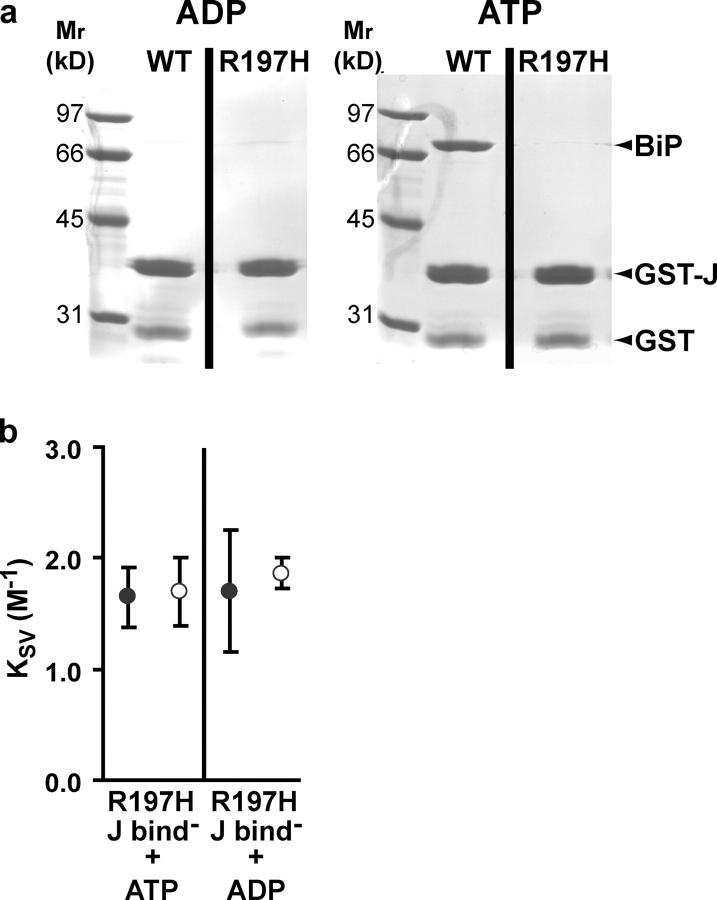
To this end, we sought to create a hamster rBiP mutant impaired in J domain interactions. Genetic and biochemical approaches have shown that DnaK, the prokaryotic Hsp70 homologue, interacts, at least in part, with the flexible loop of the DnaJ J-domain via a cleft between the IA and IIA subdomains of the ATPase domain (Greene et al., 1998; Suh et al., 1998). We generated multiple hamster rBiP constructs with mutations in this site that were homologous with DnaK point mutations known to abrogate DnaK–DnaJ interaction. Among these, the R197H point mutation completely abolished the interaction between rBiP and J domain proteins. First, the steady-state ATPase rate of rBiP R197H did not show a J domain–mediated increase, in contrast with rBiP WT (Fig. 1, compare lanes 1 and 2 with lanes 7 and 8). Second, rBiP WT stably interacted with ERdj4 in an ATP-dependent manner in GST binding experiments, but rBiP R197H did not (Fig. 4 a). The homologous DnaK R167H mutant was shown to be a strong allele-specific suppressor of the binding-defective dnaJD35N mutant, and to block interaction with wild-type DnaJ (Suh et al., 1998). Importantly, rBiP R197H is impaired only in its J domain interaction, and does not show global functional defects, as indicated by its wild-type basal ATPase rate (Fig. 1, lane 7), its ability to undergo peptide-stimulated ATP hydrolysis (Fig. 1, lane 9), its ATPase rate after reconstitution conditions (Fig. 1, lane 11), and its wild-type proteolytic pattern when digested with proteinase K in the presence of ADP or ATP (not depicted).
Figure 4.
Dependence of translocon gating on the J domain–binding region of BiP. (a) GST-J recombinant protein was immobilized on glutathione agarose and incubated with purified rBiP (WT or R197H) in the presence of ADP (left) or ATP (right). Unbound protein was removed by extensive washing, and the bound protein was released from the glutathione agarose by SDS sample buffer, subjected to SDS-PAGE, and visualized by Coomassie blue staining. (b) Mean KSV values (n = 3 ± SD) for NBD-pPL-sK78 RNCs bound to RRMs reconstituted with rBiP R197H and ATP or ADP measured in the absence (•) or presence (○) of melittin.
Our collisional quenching analyses revealed that rBiP R197H was not sealing competent, irrespective of the nucleotide present during reconstitution (Fig. 4 b). These data suggest that BiP must interact with a membrane-bound J domain protein at or near the translocon to effect pore closure. Moreover, the requirement of this interaction is not due solely to the J domain–mediated stimulation of the BiP ATPase rate (both ADP- and ATP-bound forms of the mutant are sealing incompetent); rather, it appears that this BiP–J protein interaction may have other functional consequences during gating (see Discussion).
Translocon gating by BiP is competitively inhibited by yeast Kar2, but not by sealing-incompetent hamster BiP
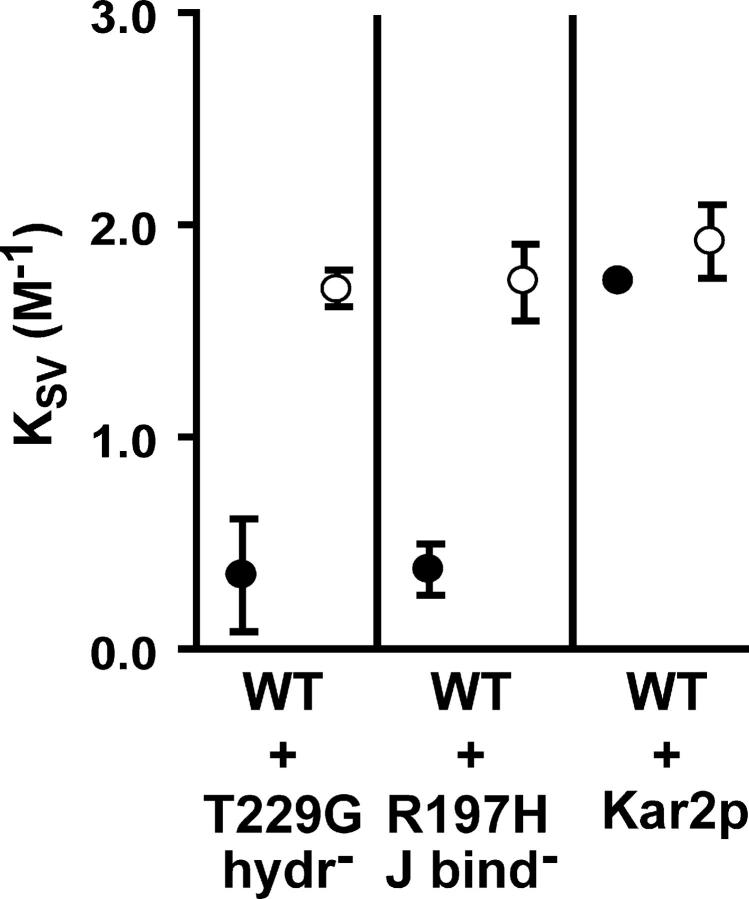
Next, we addressed whether sealing-incompetent rBiP displayed a dominant-negative effect for gating in the presence of sealing-competent rBiP. rBiP WT was co-reconstituted into microsomes in equimolar concentrations with sealing-incompetent rBiP T229G, rBiP R197H, or yeast Kar2p in the presence of ATP (Fig. 5). Co-reconstitution of rBiP WT with rBiP T229G or with rBiP R197H yielded a mean KSV value similar to that measured with rBiP WT alone (Fig. 5); therefore, neither the presence of rBiP T229G nor rBiP R197H had any effect on the ability of rBiP WT to seal translocon pores. We conclude that these mutants (ATP-bound rBiP T229G, and ATP- or ADP-bound rBiP R197H) did not interfere with the ability of rBiP WT to seal the translocon. In contrast, the presence of Kar2p resulted in a high KSV value, and therefore blocked the ability of rBiP WT to seal ER translocons (Fig. 5). The nature of the Kar2p dominant-negative effect is discussed below.
Figure 5.
Co-reconstitution of rBiP WT and sealing-incompetent rBiP. Mean KSV values (n = 3 ± SD) for NBD-pPL-sK78 RNCs bound to RRMs reconstituted with ATP and equimolar concentrations of rBiP WT and either rBiP T229G, rBiP R197H, or Kar2p measured in the absence (•) or presence (○) of melittin.
Discussion
In this work, we have analyzed the molecular mechanisms by which BiP mediates the gating of the Sec61 translocon. Four major conclusions can be drawn from our results. First, BiP-mediated gating of the translocon pore is tightly coupled to the ATPase catalytic cycle of BiP. Second, BiP binds directly, via its substrate-binding cleft, to a membrane protein that is part of or associated with the translocon during sealing. Third, BiP must functionally interact with a membrane-bound J domain protein to affect pore closure. Finally, whereas sealing-incompetent hamster BiP variants did not abrogate the gating activity of rBiP WT, Kar2p completely blocked pore sealing by rBiP WT.
BiP translocon gating is controlled by the ATPase cycle
Our results indicate that BiP seals the translocon pore while in the ADP-bound conformation and must undergo an ATP binding–induced conformational change to open the pore once the translocating polypeptide reaches a threshold length. Cycles of ATP binding, hydrolysis, and nucleotide exchange in the Hsp70 ATPase domain regulate substrate binding through interdomain communication (Bukau and Horwich, 1998). An ATP-dependent conformational change in the substrate-binding pocket results in low substrate affinity. This step of the cycle is blocked in the rBiP T37G mutant, which binds ATP but does not undergo a coupled allosteric change in the binding pocket, and in the rBiP G227D mutant, which has a significantly reduced nucleotide binding affinity (Wei et al., 1995). ATP hydrolysis converts the binding pocket to a high affinity state with low rates of polypeptide binding and release. This step is blocked in the rBiP T229G mutant, which binds ATP and undergoes an opening of the binding pocket, but does not catalyze hydrolysis and therefore remains in the low affinity state in the presence of ATP (Wei et al., 1995).
It has been demonstrated that BiP requires nucleotide (ATP or ADP) to effect translocon pore closure during secretory protein translocation (Hamman et al., 1998) and during membrane protein integration (Haigh and Johnson, 2002); however, the coupling between the ATPase cycle and gating events has remained unknown. When reconstituted into microsomes in the presence of ATP, rBiP T229G was the only full-length hamster rBiP mutant unable to seal the translocon (Fig. 2, b and c; closed circles), suggesting that BiP must assume the high substrate affinity ADP-bound state for pore closure. This was independently verified by reconstituting this mutant in the presence of ADP: rBiP T229G binds ADP with submicromolar affinity, and in the presence of ADP will bind peptide substrate and generate a proteolytic pattern identical to that of ADP-bound rBiP WT (Wei et al., 1995). In our analysis, rBiP T229G was capable of sealing translocon pores when forced into the ADP-bound conformation (Fig. 2 d). Other hamster rBiP constructs reconstituted in the presence of ATP presumably then attained the ADP-bound state either through basal ATP hydrolysis, or perhaps by J domain–stimulated ATP hydrolysis at the translocon. Notably, a steady-state basal ATPase rate of 2–5 pmol μg BiP−1 min−1 (Wei et al., 1995; Y. Shen et al., 2002; Mayer et al., 2003; Fig. 1, this paper) translates into one ATP turnover per BiP molecule every 2–6 min; therefore, there was sufficient time for BiP to attain the high affinity ADP state during the course of our experiments.
Two separate lines of evidence in the present paper indicate that the opening of the lumenal gate requires BiP to bind ATP and undergo a coupled conformational change in the binding pocket. First, the rBiP T37G and G227D mutants in the presence of ATP sealed translocon pores but were unable to mediate pore opening, as evidenced by the lack of exposure of pPL-sK78 nascent chains to lumenal quencher (Fig. 2 c, open circles). In the presence of ATP, neither mutant released bound peptide or generated a proteolytic pattern characteristic of the ATP-bound state, due to either blocked interdomain coupling (rBiP T37G) or reduced ATP affinity (rBiP G227D) (Wei et al., 1995). Second, when reconstituted in the presence of ADP (i.e, no ATP available), rBiP WT did not allow pore opening (Fig. 2 d).
BiP binds to a translocon-associated membrane protein via its substrate-binding pocket during pore closure
BiP binds nascent, misfolded, or unassembled proteins in the ER and mediates the translocation of newly synthesized precursors and the retrotranslocation of misfolded protein across the ER membrane (Matlack et al., 1999; Kabani et al., 2003). Our results show that the SBD of BiP is also involved in translocon gating, given that the binding pocket must be unoccupied for pore closure to occur (Fig. 3 b). Together with the ATPase mutant data, this suggests that ADP-bound BiP binds to a membrane protein at, or associated with, the translocon via its SBD during gating.
There are several other examples in which Hsp70 chaperones interact through their SBD with functional (i.e., mature and folded) proteins such as transcription factors and signaling proteins to regulate their activity. For example, BiP negatively regulates membrane-bound kinases (PERK and IRE1) and a transcription factor (ATF6) involved in the unfolded protein response by binding to their lumenal domains (Bertolotti et al., 2000; Ma et al., 2002; J. Shen et al., 2002; Kimata et al., 2003). Upon ER stress, these proteins are released and activated as BiP preferentially binds to accumulating unfolded proteins. Interestingly, BiP ATPase mutants (e.g., rBiP T37G) that bind but do not release peptides (Wei et al., 1995) or substrates such as Ig light chain (Hendershot et al., 1996) constitutively bind ATF6 (J. Shen et al., 2002) and IRE1 (Kimata et al., 2003), blocking their release even during ER stress. These observations are consistent with our conclusion that BiP-mediated translocon pore sealing entails an interaction between the BiP SBD and a membrane protein. ATPase mutants whose substrate dissociation is blocked (rBiP T37G and rBiP G227D), or rBiP WT in the presence of ADP only, remain bound to the putative site and keep the translocon sealed from the lumenal side, even when associated with intermediates beyond the 70-residue threshold (Fig. 2).
We speculate that this putative binding site at or near the translocon bears similarity to Hsp70 substrates (e.g., an unstructured loop). Given that BiP binds peptides with extensive sequence diversity (Bukau and Horwich, 1998), and that its substrate range becomes broader still in the presence of a J domain protein (Misselwitz et al., 1998), many candidate binding sites are possible. Identification of such sites at the translocon awaits further investigation.
Like all Hsp70s, BiP preferentially releases peptide substrates in the presence of ATP (Wei et al., 1995; Theyssen et al., 1996). However, our results suggest that the ADP–BiP interaction with the translocon is prolonged, even when microsomes contain ATP. The protracted interaction between BiP and the lumenal domains of other proteins (e.g., ATF6) must be equally stable, dissociating only after the proper stimulus. One mechanism to account for this sustained association may entail suppression of BiP ATPase activity until a specific stimulus (the presence of a growing polypeptide chain in this case) is encountered. Experiments designed to measure the rate of BiP cycling from Ig heavy chains in vivo failed to detect reiterative cycles of BiP binding, whereas the association of Ig light chains readily triggered the release of BiP from heavy chains (Vanhove et al., 2001). Similarly, Mayer et al. (2003) have shown that peptides stimulate the ATPase rate of BiP by increasing the rate-limiting step of ATP hydrolysis, whereas interaction with an unfolded antibody domain decelerates ATPase activity. Interaction of a translocon protein with BiP, perhaps on a regulatory site, could have a similar effect on ATPase rate. Alternatively, stabilization of the BiP–ADP complex at the translocon might involve a cochaperone with properties similar to those observed for the Hsp70 cofactor Hip, which has been shown to stabilize the ADP-bound form of Hsp70 (Höhfeld et al., 1995). Regardless of the mechanism, we propose that pore opening involves the de-repression of BiP ATPase activity and promotion of BiP nucleotide exchange (see below).
BiP pore sealing requires a functional interaction with a membrane J domain protein
Several mammalian ER-resident J domain proteins have been identified and characterized, including the transmembrane Sec63 (Meyer et al., 2000; Tyedmers et al., 2000) and Mtj1 (Chavalier et al., 2000; Dudek et al., 2002), both of which are associated with the Sec61 translocon. The functional interaction between BiP/Kar2p and Sec63 has been demonstrated (Corsi and Schekman, 1997; Misselwitz et al., 1999), as has the interaction between BiP and Mtj1 (Dudek et al., 2002). Moreover, both BiP/Kar2 and Sec63 are required for both post-translational translocation (Matlack et al., 1999) and cotranslational translocation (Brodsky et al., 1995; Young et al., 2001; Miller et al., 2003). Therefore, it was not unexpected that the establishment of the BiP-mediated seal should depend on a functional interaction between BiP and a membrane-bound J domain protein (Fig. 4).
What is intriguing is the nature of this interaction. If the role of the J domain protein in translocon gating were limited to a transient interaction with BiP and stimulation of its ATPase activity (e.g., to induce SBD binding to a putative membrane protein), then no such requisite J protein association would be seen for BiP reconstituted in the presence of ADP. This was not the case (Fig. 4). It appears instead that the BiP–J domain interaction is important for something other than stimulating the ATPase rate: perhaps recruitment to the translocon and/or proper positioning of BiP, or perhaps BiP binding induces a conformational change on the J domain protein itself that is required to seal the pore. Notably, we cannot discount the potential importance of a kinetic requirement for a J domain protein in vivo (i.e, to facilitate rapid ATP hydrolysis at the translocon for gating), as it would not be detected on the time scale of our assay.
Our data suggest that BiP-mediated pore closure requires a functional interaction of BiP with a J domain while BiP is in the ADP conformation. Although many Hsp70s require an ATP-induced conformational change to stably bind J domain proteins (e.g., Sec63 [Corsi and Schekman, 1997; McClellan et al., 1998]; ERdj4, this paper [Fig. 4 a]), there is evidence that J domain proteins can form stable complexes with Hsp70 chaperones in both the ADP- and ATP-bound states (e.g., Greene et al., 1998). Importantly, BiP has been shown to interact stably with Mtj1 in both nucleotide-bound states (Chavalier et al., 2000).
Kar2p displays a dominant-negative effect with respect to gating the mammalian translocon
Kar2p displayed basal, J domain–activated, and peptide-activated ATPase activity similar to hamster rBiP (Fig. 1), and yet did not gate translocon pores when reconstituted into mammalian microsomes (Fig. 2). One possible explanation is a difference in substrate-binding specificity between BiP and Kar2p. Alternatively, Kar2p may be unable to form a specific chaperone–cochaperone interaction required for pore gating. Given that both SBD and J domain interactions are required for BiP gating (Figs. 3 and 4, respectively), either of these explanations is possible. Importantly, several reports have shown that many Hsp70 proteins are not functionally interchangeable (Brodsky et al., 1998; McClellan et al., 1998). Future experiments to ascertain which regions of BiP are critical for gating may include domain swaps between Kar2p and rBiP.
In the presence of rBiP WT, Kar2p displayed a dominant-negative effect with respect to pore sealing (Fig. 5). Similarly, the presence of Kar2p ATPase mutants such as G247D (analogous to rBiP G227D) strongly reduced the ability of wild-type Kar2p to drive post-translational translocation when reconstituted into yeast proteoliposomes (McClellan et al., 1998). With regard to gating, the dominance of Kar2p could be explained, for example, if it bound irreversibly to a putative translocon binding site (without effecting closure) and thereby blocked interaction with gating-competent BiP. Alternatively, if BiP dimerization were required for sealing, a mixture of sealing-competent and sealing-incompetent BiP/Kar2p may form nonproductive dimers. The sealing-incompetent rBiP T229G and R197H, in contrast, appear not to engage the translocon in such a way that they inhibit gating by wild-type rBiP (Fig. 5).
BiP-mediated translocon gating involves multiple binding sites
Our model for the molecular mechanism by which BiP gates the translocon pore involves functional interactions between the SBD- and J domain–binding sites of BiP and translocon-associated proteins (Fig. 6). Because BiP must attain the ADP-bound state to seal the pore (Fig. 6, step 1), this step requires at least one ATP turnover that does not necessarily occur at the translocon. Simultaneous interaction with both an SBD-associated site and with a J protein during the closure of inactive translocons may be facilitated, for example, by the regulated accessibility of such sites to BiP. Similarly, pore opening when the nascent chain reaches a threshold length (Fig. 6, step 3) may be facilitated by conformational changes that promote nucleotide exchange, perhaps by alleviating some steric hindrance. Given the dynamic conformational changes observed as the translocon cycles through different functional states (Johnson and van Waes, 1999), such a scenario is possible. We note that BiP is depicted here as the physical seal, although BiP may effect pore gating indirectly through interactions with other proteins. Further, our data cannot distinguish whether one BiP molecule leaves the translocon after opening and is replaced by a separate molecule, or if BiP operates processively, remaining associated with a given translocon over multiple gating cycles.
Figure 6.
Proposed mechanism by which BiP gates the ER translocon pore. Step 1: ADP-bound BiP seals the lumenal end of the translocon via an interaction both between the SBD and a translocon-associated component (represented here as a lumenal loop) and also between BiP and a J protein (J). Step 2: after SRP-dependent targeting of a RNC, the BiP-mediated gate continues to seal the translocon from the lumen for short nascent chains. Step 3: opening of the BiP-mediated gate after the nascent chain reaches a threshold length of ∼70 residues requires that BiP assumes the ATP-bound, open-binding pocket conformation. Step 4: after translation of substrate, ribosome-free translocons are resealed by the BiP-mediated gate. Steps blocked by BiP mutations are indicated.
How might the BiP gating cycle be regulated by other components of the translocation machinery? During the integration of a signal-cleaved membrane protein, the presence of a transmembrane segment in the nascent chain deep within the ribosome tunnel elicits BiP-mediated pore closure, followed by opening of the ribosome–translocon junction (Liao et al., 1997; Haigh and Johnson, 2002). Such a nascent transmembrane segment folds into a conformation compatible with an α-helix, and is adjacent to ribosomal proteins that are not adjacent to secretory nascent chains (Woolhead et al., 2004). Thus, BiP-mediated translocon gating is regulated by communication between the ribosome and the ER lumen that likely involves both ribosomal and transmembrane proteins (Alder and Johnson, 2004; Woolhead et al., 2004). Such communication may involve translocon components that bind to ribosomes (e.g., Sec61α [Raden et al., 2000] or Sec61β [Levy et al., 2001]), to BiP (e.g., Sec63 [Corsi and Schekman, 1997]), or to both (e.g., Mtj1 [Dudek et al., 2002]). In light of the present work, this intricate communication may include the sites to which BiP interacts via its SBD- or J domain–binding region.
The ATPase-regulated substrate and J domain interactions shown here indicate that BiP-mediated translocon gating has the same underlying mechanism as BiP chaperone activity. This demonstrates how the myriad functions of BiP in the ER lumen could be governed by the same fundamental processes, yet fine-tuned for specific tasks by association with different cochaperones and/or substrate. Further elucidation of the mechanism of BiP gating will come with the identification of the BiP-binding sites and J protein partners at the translocon that are required for gating, and with further understanding of how the ribosome and nascent chain may regulate BiP binding and release.
Materials and methods
Translocation intermediates
Plasmids pSPBP4 and pVW1, encoding secretory substrates pPL and pPL-sK, respectively, were cut with restriction endonucleases or used for PCR amplification of DNA fragments and transcribed with SP6 RNA polymerase to obtain mRNAs truncated in the coding region as described previously (Crowley et al., 1994). In vitro translations (at 26°C for 35 min, 500 μl) contained wheat germ extract, excess mRNA, 40 nM canine SRP, 80 equivalents (Walter and Blobel, 1983) of canine ER microsomes (KRM, XRM, or RRM; see below), and 300 pmol of either ɛNBD-[14C]Lys tRNALys or unmodified [14C]Lys tRNALys (Crowley et al., 1993). After 10 min on ice, translocation intermediates were purified by gel filtration using a Sepharose CL-2B column (0.7 cm i.d. × 50 cm) equilibrated and eluted in buffer A (50 mM Hepes, 40 mM KOAc, and 5 mM MgCl2 at pH 7.5). After spectral measurements, the extent of RNC association with microsomes was determined by scintillation counting (Crowley et al., 1993); pellet-associated 14C counts typically totaled >90%.
Protein and peptide preparation
WT and mutant rBiP and J protein constructs with NH2-terminal hexahistidine tags in QE10 vectors (QIAGEN) were expressed in Escherichia coli M15 cells by IPTG induction as described previously (Gaut and Hendershot, 1993). Proteins were purified on Ni2+-NTA agarose (QIAGEN) as before (Gaut and Hendershot, 1993), except that protein was eluted with a stepwise imidazole gradient (25–40 mM for wash steps and 100 mM for elution). Kar2p was purified as described previously (McClellan et al., 1998). Purified protein concentration, determined by the BCA assay (Pierce Chemical Co.), ranged from 100 to 200 μM. The rBiP R197H mutant was produced by site-directed mutagenesis (QuikChange; Stratagene) and was verified by sequencing.
Peptide C L9C (N-KLIGVLSSCFRPK-C) was synthesized by solid phase chemistry and purified by HPLC (Bio-Synthesis, Inc.). Lyophilized peptide was reconstituted in 50 mM Hepes (pH 7.5) and stored at −20°C. To prepare fluorescent-labeled PepCL9C, 1.8 μmol N-((2-(Iodoacetoxy)ethyl)-N-methyl)-amino-7-nitrobenz-2-oxa-1,3-diazole (I-ANBD) in dimethylformamide was added to 0.7 μmol PepCL9C (2.5 molar excess dye/peptide), incubated (at 37°C for 60 min), and quenched by adding 25 mM DTT. NBD-PepCL9C was purified from free dye and unlabeled peptide by HPLC (Chromasil 5-μm C18 column; Whatman) using a 90-ml linear gradient (2 ml/min) from 0.1% (vol/vol) trifluoroacetic acid to 80% (vol/vol) acetonitrile, 0.085% (vol/vol) trifluoroacetic acid. NBD-PepCL9C eluted at 29.3 min and was lyophilized.
Extraction of soluble lumenal proteins and rBiP reconstitution
KRMs (100 equivalents) in 100 μl buffer C (50 mM Hepes, pH 7.5, 1 mM DTT, and 200 mM sucrose) were added to 100 μl buffer B (500 mM Hepes/500 mM Caps, pH titrated from 9.5 to 10.0) and 800 μl H2O and were incubated at 0°C for 20 min (Nicchitta and Blobel, 1993). For isolation of XRMs, samples were sedimented through a 200-μl 0.5 M sucrose cushion in buffer A (at 60,000 rpm and 4°C for 20 min; TLA 100.2 rotor; Beckman Coulter) and resuspended in 100 μl of buffer C.
XRMs were reconstituted with purified protein as before (Bulleid and Freedman, 1988; Hamman et al., 1998) by sedimentation through a 0.5 M sucrose cushion in 0.1× buffer B and resuspension in a 240-μl solution containing 200 mM sucrose, 0.2× buffer B (pH 9.5), and 20 μM rBiP. After a 5-min incubation on ice, ATP or ADP was added to 5 mM (and, where relevant, pepCL9C was added to 0.4 mM), and the solution immediately adjusted to pH 7.5 by the addition of 1.0 M Hepes (pH 6.8). After a second incubation (at 0°C for 5 min), RRMs were diluted to 1 ml with buffer C and sedimented through a 0.5 M sucrose cushion in buffer A as above. By comparison with rBiP standards on a Coomassie-stained SDS-polyacrylamide gel, RRMs contained ∼1.5 pmol rBiP per equivalent of microsomes. Assuming 0.4 pmol ER translocons per microsome equivalent (Hanein et al., 1996), the RRMs contained about a 3.5-molar excess of rBiP over translocons.
Fluorescence spectroscopy
Steady-state fluorescence measurements were made on an SLM-8100 photon-counting spectrofluorometer at 4°C as described previously (Crowley et al., 1993). Fluorescent (ɛNBD-Lys-containing) and nonfluorescent (Lys-containing) samples were split into four 250-μl aliquots and added to quartz cuvettes (4 × 4 mm). NBD was excited at 468 nm (4 nm bandpass) and emission was monitored at 530 nm (4 nm bandpass). Collisional quenching was measured after the addition of 1 M KI and 2 mM Na2S2O3 to a final KI concentration of 0, 10, 20, or 30 mM, and the ionic strength was kept constant by the addition of 1 M KCl and 2 mM Na2S2O3 to a final KCl concentration of 30, 20, 10, or 0 mM, respectively (Crowley et al., 1993). Mean KSV values are from a minimum of three independent experiments conducted before and after incubation with 5 μM melittin (at 25°C for 20 min). Anisotropy measurements were done as before (Crowley et al., 1993) using 1 μM NBD-PepCL9C and up to 6 μM rBiP (WT/44K) in buffer A supplemented with 1 mM DTT and 1 mM ADP or ATP (25°C).
BiP ATPase activity and GST pull-down assay
BiP steady-state ATPase activity was measured by a variation of the Fiske-Subbarow technique for monitoring inorganic phosphate production (Fiske and Subbarow, 1925). Reactions (100 μl) with 8 μM BiP in the presence or absence of 32 μM ERdj4 J domain and/or 1.6 mM pepCL9C in an ATPase assay buffer (20 mM Hepes, 25 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, and 0.5 mM DTT at pH 7.0) with 1 mM ATP were incubated at 37°C. At appropriate time points (up to 1 h), 20-μl aliquots were removed and added to 10 μl of 5% (wt/vol) TCA to quench the reaction. After dilution of each sample with 300 μl H2O, 90 μl of 2.5% (wt/vol) ammonium molybdate in 5 N HCl and 60 μl of 0.6% (wt/vol) SnCl2 in 0.2 N HCl were added, and samples were mixed and incubated (at 23°C for 20 min). The phosphomolybdate concentration was measured spectroscopically (absorbance at 800 nm) against a phosphate standard curve.
GST pull-down assays were performed as described previously (Yu et al., 2000). In brief, 20 μg GST-J proteins were added to 20 μl glutathione agarose (Amersham Biosciences) and equilibrated with assay buffer containing 1 mM of either ATP or ADP before 20 μg of rBiP WT or rBiP R197H were added in a final volume of 400 μl. After 1 h incubation while rotating at 4°C, the beads were sedimented and washed five times with 400 μl assay buffer in the presence of 0.1 mM ATP or ADP. The beads were boiled in SDS sample buffer and proteins were subjected to SDS-PAGE and Coomassie blue staining.
Acknowledgments
We thank Yuanlong Shao and Yiwei Miao for excellent technical assistance and Mehdi Kabani for assistance with protein purification.
This work was supported by National Institutes of Health grants R01 GM26494 (to A.E. Johnson) and GM54068 (to L.M. Hendershot), by National Science Foundation grant MCB-0110331 (to J.L. Brodsky), and by the Robert A. Welch Foundation (to A.E. Johnson).
Abbreviations used in this paper: I−, iodide ion; KRM, salt-washed ER microsome; pPL, preprolactin; rBiP, recombinant hamster BiP; RNC, ribosome-nascent chain complex; RRM, reconstituted microsome; SBD, substrate-binding domain; SRP, signal recognition particle; XRM, lumen-extracted microsome.
References
- Alder, N.N., and A.E. Johnson. 2004. Cotranslational membrane protein biogenesis at the endoplasmic reticulum. J. Biol. Chem. 279:22787–22790. [DOI] [PubMed] [Google Scholar]
- Beckmann, R., D. Bubeck, R. Grassucci, P. Penczek, A. Verschoor, G. Blobel, and J. Frank. 1997. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 278:2123–2126. [DOI] [PubMed] [Google Scholar]
- Bertolotti, A., Y. Zhang, L.M. Hendershot, H.P. Harding, and D. Ron. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326–332. [DOI] [PubMed] [Google Scholar]
- Brodsky, J.L., J. Goeckeler, and R. Schekman. 1995. BiP and Sec63p are required for both co- and post-translational protein translocation into the yeast endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 92:9643–9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky, J.L., M. Bäuerle, M. Horst, and A.J. McClellan. 1998. Mitochondrial Hsp70 cannot replace BiP in driving protein translocation into the yeast endoplasmic reticulum. FEBS Lett. 435:183–186. [DOI] [PubMed] [Google Scholar]
- Bukau, B., and A.L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell. 92:351–366. [DOI] [PubMed] [Google Scholar]
- Bulleid, N.J., and R.B. Freedman. 1988. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 335:649–651. [DOI] [PubMed] [Google Scholar]
- Chavalier, M., H. Rhee, E.C. Elguindi, and S.Y. Blond. 2000. Interaction of murine BiP/GRP78 with the DnaJ homologue MTJ1. J. Biol. Chem. 275:19620–19627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, K.T., Y. Shen, and L.M. Hendershot. 2002. BAP, a mammalian BiP-associated protein, is a nucleotide exchange factor that regulates the ATPase activity of BiP. J. Biol. Chem. 277:47557–47563. [DOI] [PubMed] [Google Scholar]
- Corsi, A.K., and R. Schekman. 1997. The lumenal domain of Sec63p stimulates the ATPase activity of BiP and mediates BiP recruitment to the translocon in Saccharomyces cerevisiae. J. Cell Biol. 137:1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley, K.S., G.D. Reinhart, and A.E. Johnson. 1993. The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell. 73:1101–1115. [DOI] [PubMed] [Google Scholar]
- Crowley, K.S., S. Liao, V.E. Worrell, G.D. Reinhart, and A.E. Johnson. 1994. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 78:461–471. [DOI] [PubMed] [Google Scholar]
- Dudek, J., J. Volkmer, C. Bies, S. Guth, A. Müller, M. Lerner, P. Feick, K.-H. Schäfer, E. Morgenstern, F. Hennessy, et al. 2002. A novel type of co-chaperone mediates transmembrane recruitment of DnaK-like chaperones to ribosomes. EMBO J. 21:2958–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske, C.H., and Y. Subbarow. 1925. The colorimetric determination of phosphorus. J. Biol. Chem. 66:375–400. [Google Scholar]
- Flynn, G.C., T.G. Chappell, and J.E. Rothman. 1989. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 245:385–390. [DOI] [PubMed] [Google Scholar]
- Gaut, J.R., and L.M. Hendershot. 1993. Mutations within the nucleotide binding site of immunoglobulin-binding protein inhibit ATPase activity and interfere with release of immunoglobulin heavy chain. J. Biol. Chem. 268:7248–7255. [PubMed] [Google Scholar]
- Greene, M.K., K. Maskos, and S.L. Landry. 1998. Role of the J-domain in the cooperation of Hsp40 with Hsp70. Proc. Natl. Acad. Sci. USA. 95:6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh, N.G., and A.E. Johnson. 2002. A new role for BiP: closing the aqueous translocon pore during protein integration into the ER membrane. J. Cell Biol. 156:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman, B.D., J.-C. Chen, E.E. Johnson, and A.E. Johnson. 1997. The aqueous pore through the translocon has a diameter of 40-60 Å during cotranslational protein translocation at the ER membrane. Cell. 89:535–544. [DOI] [PubMed] [Google Scholar]
- Hamman, B.D., L.M. Hendershot, and A.E. Johnson. 1998. BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell. 92:747–758. [DOI] [PubMed] [Google Scholar]
- Hanein, D., K.E. Matlack, B. Jungnickel, K. Plath, K.U. Kalies, K.R. Miller, T.A. Rapoport, and C.W. Akey. 1996. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 87:721–732. [DOI] [PubMed] [Google Scholar]
- Hendershot, L., J. Wei, J. Gaut, J. Melnick, S. Aviel, and Y. Argon. 1996. Inhibition of immunoglobulin folding and secretion by dominant negative BiP ATPase mutants. Proc. Natl. Acad. Sci. USA. 93:5269–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld, J., Y. Minami, and F.U. Hartl. 1995. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 83:589–598. [DOI] [PubMed] [Google Scholar]
- Johnson, A.E., and M.A. van Waes. 1999. The translocon: a dynamic gateway at the ER membrane. Annu. Rev. Cell Dev. Biol. 15:799–842. [DOI] [PubMed] [Google Scholar]
- Kabani, M., S.S. Kelley, M.W. Morrow, D.L. Montgomery, R. Sivendran, M.D. Rose, L.M. Gierasch, and J.L. Brodsky. 2003. Dependence of endoplasmic reticulum-associated degradation on the peptide binding domain and concentration of BiP. Mol. Biol. Cell. 14:3437–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata, Y., Y.I. Kimata, Y. Shimizu, H. Abe, I.C. Farcasanu, M. Takeuchi, M.D. Rose, and K. Kohno. 2003. Genetic evidence for a role of BiP/Kar2 that regulates Ire1 in response to accumulation of unfolded proteins. Mol. Biol. Cell. 14:2559–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladokhin, A.S., M.E. Selsted, and S.H. White. 1997. Sizing membrane pores in lipid vesicles by leakage of co-encapsulated markers: pore formation by melittin. Biophys. J. 72:1762–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, R., M. Wiedmann, and G. Kreibich. 2001. In vitro binding of ribosomes to the β subunit of the Sec61p protein translocation complex. J. Biol. Chem. 276:2340–2346. [DOI] [PubMed] [Google Scholar]
- Liao, S., J. Lin, H. Do, and A.E. Johnson. 1997. Both lumenal and cytosolic gating of the aqueous ER translocon pore is regulated from inside the ribosome during membrane protein integration. Cell. 90:31–41. [DOI] [PubMed] [Google Scholar]
- Ma, K., K.M. Vattem, and R.C. Wek. 2002. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J. Biol. Chem. 277:18728–18735. [DOI] [PubMed] [Google Scholar]
- Matlack, K.E., B. Misselwitz, K. Plath, and T.A. Rapoport. 1999. BiP acts as a molecular ratchet during posttranslational transport of prepro-α factor across the ER membrane. Cell. 97:553–564. [DOI] [PubMed] [Google Scholar]
- Mayer, M., J. Reinstein, and J. Buchner. 2003. Modulation of the ATPase cycle of BiP by peptides and proteins. J. Mol. Biol. 330:137–144. [DOI] [PubMed] [Google Scholar]
- McClellan, A.J., J.B. Endres, J.P. Vogel, D. Palazzi, M.D. Rose, and J.L. Brodsky. 1998. Specific molecular chaperone interactions and an ATP-dependent conformational change are required during posttranslational protein translocation into the yeast ER. Mol. Biol. Cell. 9:3533–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, H.A., H. Grau, R. Kraft, S. Kostka, S. Prehn, K.U. Kalies, and E. Hartmann. 2000. Mammalian Sec61 is associated with Sec62 and Sec63. J. Biol. Chem. 275:14550–14557. [DOI] [PubMed] [Google Scholar]
- Miller, M., A.J. Jermy, G.J. Steel, H.J. Garside, S. Carter, and C.J. Stirling. 2003. An in vitro assay using overexpressed yeast SRP demonstrates that cotranslational translocation is dependent upon the J-domain of Sec63p. Biochemistry. 42:7171–7177. [DOI] [PubMed] [Google Scholar]
- Misselwitz, B., O. Staeck, and T.A. Rapoport. 1998. J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences. Mol. Cell. 2:593–603. [DOI] [PubMed] [Google Scholar]
- Misselwitz, B., O. Staeck, K.E. Matlack, and T.A. Rapoport. 1999. Interaction of BiP with the J-domain of the Sec61p component of the endoplasmic reticulum protein translocation complex. J. Biol. Chem. 274:20110–20115. [DOI] [PubMed] [Google Scholar]
- Nicchitta, C.V., and G. Blobel. 1993. Lumenal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell. 73:989–998. [DOI] [PubMed] [Google Scholar]
- Raden, D., W. Song, and R. Gilmore. 2000. Role of the cytoplasmic segments of Sed61a in the ribosome-binding and translocation-promoting activities of the Sec61 complex. J. Cell Biol. 150:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J., X. Chen, L.M. Hendershot, and R. Prywes. 2002. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell. 3:99–111. [DOI] [PubMed] [Google Scholar]
- Shen, Y., L. Meunier, and L.M. Hendershot. 2002. Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue, which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J. Biol. Chem. 277:15947–15956. [DOI] [PubMed] [Google Scholar]
- Simon, S.M., and G. Blobel. 1991. A protein-conducting channel in the endoplasmic reticulum. Cell. 65:371–380. [DOI] [PubMed] [Google Scholar]
- Suh, W.-C., W.F. Burkholder, C.Z. Lu, X. Zhao, M.E. Gottesman, and C.A. Gross. 1998. Interaction of the Hsp70 molecular chaperone, DnaK, with its cochaperone DnaJ. Proc. Natl. Acad. Sci. USA. 95:15223–15228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theyssen, H., H.P. Schuster, L. Packschies, B. Bukau, and J. Reinstein. 1996. The second step of ATP binding induces peptide release. J. Mol. Biol. 263:657–670. [DOI] [PubMed] [Google Scholar]
- Tyedmers, J., M. Lerner, C. Bies, J. Dudek, M.H. Skowronek, I.G. Haas, N. Heim, W. Nastainczyk, J. Volkmer, and R. Zimmermann. 2000. Homologs of the yeast Sec complex subunits Sec62p and Sec63p are abundant proteins in dog pancreas microsomes. Proc. Natl. Acad. Sci. USA. 97:7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhove, M., Y.-K. Usherwood, and L.M. Hendershot. 2001. Unassembled Ig heavy chains do not cycle from BiP in vivo, but require light chains to trigger their release. Immunity. 15:105–114. [DOI] [PubMed] [Google Scholar]
- Walter, P., and G. Blobel. 1983. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 96:84–93. [DOI] [PubMed] [Google Scholar]
- Wei, J., J.R. Gaut, and L.M. Hendershot. 1995. In vitro dissociation of BiP-peptide complexes requires a conformational change in BiP after ATP binding but does not require ATP hydrolysis. J. Biol. Chem. 270:26677–26682. [DOI] [PubMed] [Google Scholar]
- Wirth, A., M. Jung, C. Bies, M. Frien, J. Tyedmers, R. Zimmermann, and R. Wagner. 2003. The Sec61p complex is a dynamic precursor activated channel. Mol. Cell. 12:261–268. [DOI] [PubMed] [Google Scholar]
- Woolhead, C., P.J. McCormick, and A.E. Johnson. 2004. Nascent membrane and secretory proteins differ in their FRET-detected folding far inside the ribosome and in their exposure to ribosomal proteins. Cell. 116:725–736. [DOI] [PubMed] [Google Scholar]
- Young, B.P., R.A. Craven, P.J. Reid, M. Willer, and C.J. Stirling. 2001. Sec63p and Kar2p are required for the translocation of SRP-dependent precursors into the yeast endoplasmic reticulum in vivo. EMBO J. 20:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, M., R.H.A. Haslam, and D.B. Haslam. 2000. HEDJ, an Hsp40 co-chaperone localized to the endoplasmic reticulum of human cells. J. Biol. Chem. 275:24984–24992. [DOI] [PubMed] [Google Scholar]
- Zhu, X., X. Zhao, W.F. Burkholder, A. Gragerov, C.M. Ogata, M.E. Gottesman, and W.A. Hendrickson. 1996. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 272:1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]