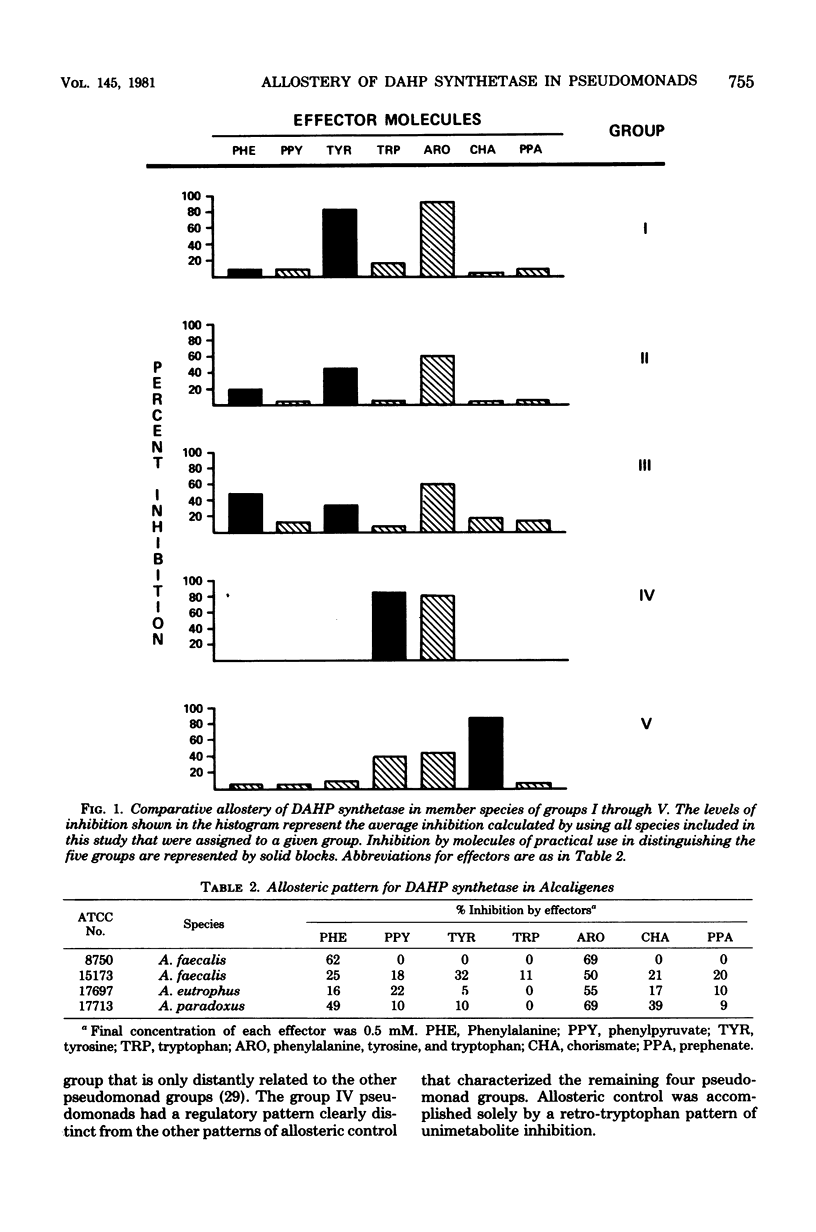
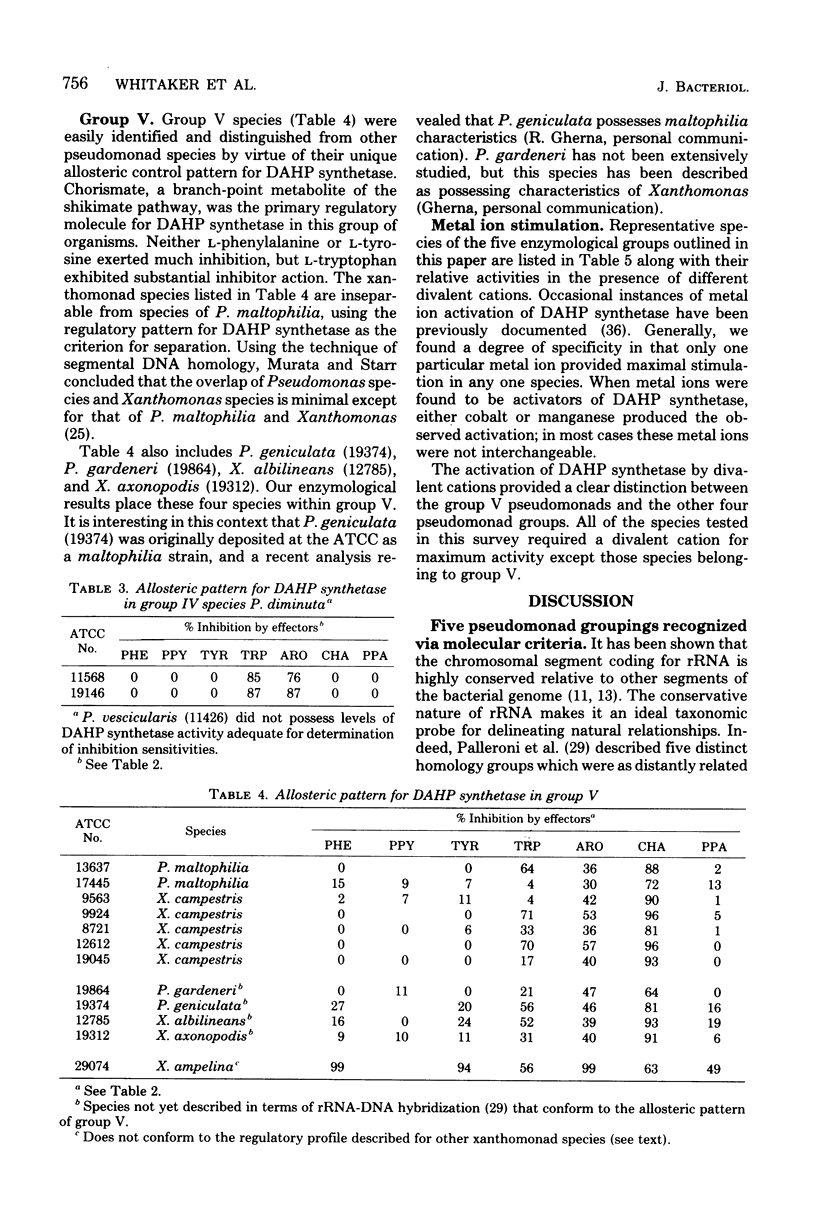
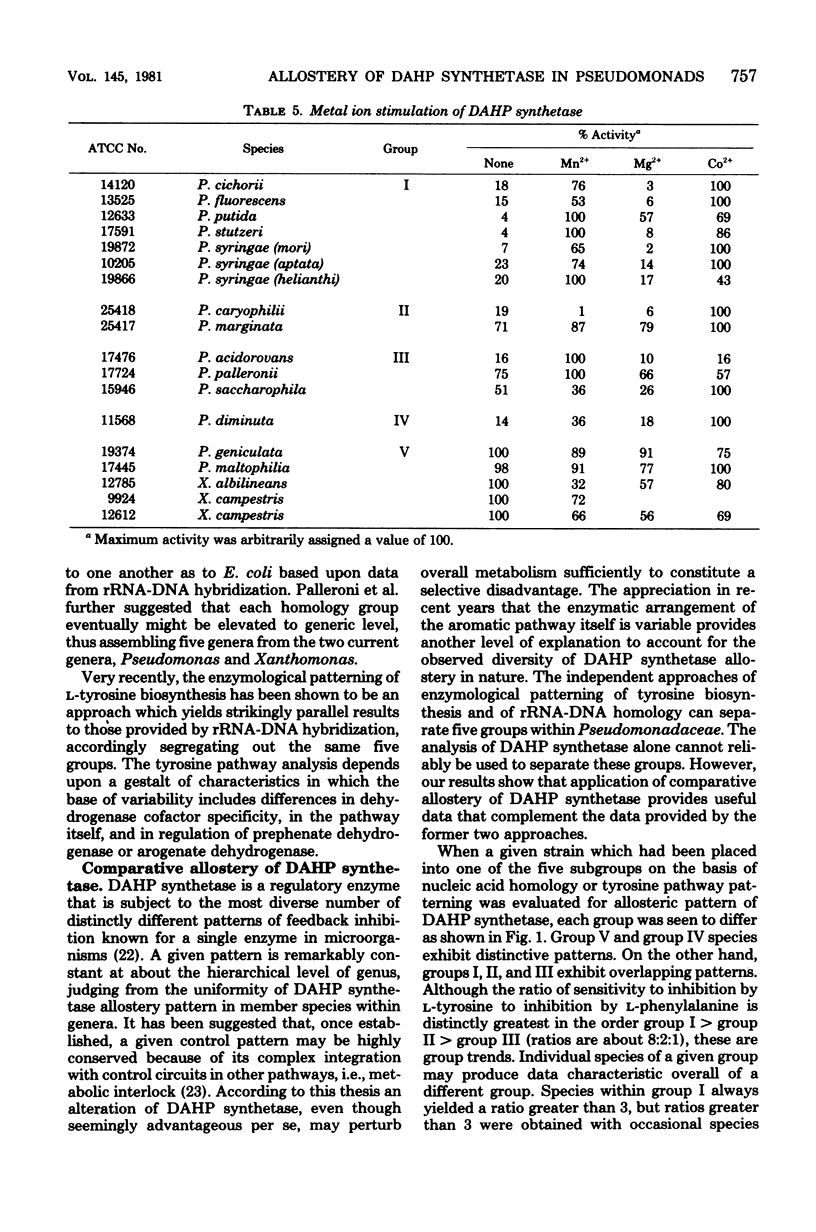
Abstract
Recently, an analysis of the enzymological patterning of L-tyrosine biosynthesis was shown to distinguish five taxonomic groupings among species currently named Pseudomonas, Xanthomonas, or Alcaligenes (Byng et al., J. Bacteriol. 144:247--257, 1980). These groupings paralleled with striking consistency those previously defined by ribosomal ribonucleic acid-deoxyribonucleic acid homology relationships. The comparative allostery of 3-deoxy-D-arabino-heptulosonate 7-phosphate (DAHP) synthetase has previously been shown to be a useful indicator of taxonomic relationship at about the level of genus. The comparative allostery of DAHP synthetase was evaluated in relationship to data available from the same pseudomonad species previously studied. Species of Xanthomonas and some named species of Pseudomonas, e.g., P. maltophilia, were unmistakably recognized as belonging to group V, having a DAHP synthetase sensitive to sequential feedback inhibition by chorismate. This control pattern is thus far unique to group V pseudomonads among microorganisms. Group V organisms were also unique in their possession of DAHP synthetase enzymes that were unstimulated by divalent cations. Group IV pseudomonads (P. diminuta) were readily distinguished by the retro-tryptophan pattern of control for DAHP synthetase. Activity for DAHP synthetase was not always recovered in group IV species, e.g., P. vesicularis. The remaining three groups exhibited overlapping patterns of DAHP synthetase sensitivity to both L-phenylalanine and L-tyrosine. Individual species cannot be reliably keyed to group I. II, or III without other data. However, each group overall exhibited a different trend of relative sensitivity to L-tyrosine and L-phenylalanine. Thus, although enzymological patterning of L-tyrosine biosynthesis alone can be used to separate the five pseudomonad groups, the independent assay of DAHP synthetase control pattern can be used to confirm assignments. The latter approach is, in fact, the easiest and most definitive method for recognition of group V (and often of group IV) species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard R. W., Doudoroff M., Stanier R. Y., Mandel M. Taxonomy of the aerobic psuedomonads: Pseudomonas diminuta and P. vesiculare. J Gen Microbiol. 1968 Oct;53(3):349–361. doi: 10.1099/00221287-53-3-349. [DOI] [PubMed] [Google Scholar]
- Ballard R. W., Palleroni N. J., Doudoroff M., Stanier R. Y., Mandel M. Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola and P. caryophylli. J Gen Microbiol. 1970 Feb;60(2):199–214. doi: 10.1099/00221287-60-2-199. [DOI] [PubMed] [Google Scholar]
- Baumann L., Baumann P. Studies of relationship among terrestrial Pseudomonas, Alcaligenes, and enterobacteria by an immunological comparison of glutamine synthetase. Arch Microbiol. 1978 Oct 4;119(1):25–30. doi: 10.1007/BF00407923. [DOI] [PubMed] [Google Scholar]
- Byng G. S., Whitaker R. J., Gherna R. L., Jensen R. A. Variable enzymological patterning in tyrosine biosynthesis as a means of determining natural relatedness among the Pseudomonadaceae. J Bacteriol. 1980 Oct;144(1):247–257. doi: 10.1128/jb.144.1.247-257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. Channel-shuttle mechanism for the regulation of phenylalanine and tyrosine synthesis at a metabolic branch point in Pseudomonas aeruginosa. J Bacteriol. 1973 Jan;113(1):241–251. doi: 10.1128/jb.113.1.241-251.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- DOI R. H., IGARASHI R. T. CONSERVATION OF RIBOSOMAL AND MESSENGER RIBONUCLEIC ACID CISTRONS IN BACILLUS SPECIES. J Bacteriol. 1965 Aug;90:384–390. doi: 10.1128/jb.90.2.384-390.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Giovanelli J., Mudd S. H. Homocysteine biosynthesis in green plants. O-Phosphorylhomoserine as the physiological substrate for cystathionine gamma-synthase. J Biol Chem. 1974 Feb 25;249(4):1139–1155. [PubMed] [Google Scholar]
- Dubnau D., Smith I., Morell P., Marmur J. Gene conservation in Bacillus species. I. Conserved genetic and nucleic acid base sequence homologies. Proc Natl Acad Sci U S A. 1965 Aug;54(2):491–498. doi: 10.1073/pnas.54.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. Y., Sprinson D. B. Properties of tyrosine-inhibitable 3-deoxy-d-arabinoheptulosonic acid-7-phosphate synthase from Salmonella. J Bacteriol. 1977 Jan;129(1):177–183. doi: 10.1128/jb.129.1.177-183.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Montoya A. L., Nester E. W. Characterization of the functional activities of the subunits of 3-deoxy-D-arabinoheptulosonate 7-phosphate synthetase-chorismate mutase from Bacillus subtilis 168. J Biol Chem. 1974 Jul 25;249(14):4473–4470. [PubMed] [Google Scholar]
- Jensen R. A., Calhoun D. H., Stenmark S. L. Allosteric inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase by tyrosine, tryptophan and phenylpyruvate in Pseudomonas aeruginosa. Biochim Biophys Acta. 1973 Jan 12;293(1):256–268. doi: 10.1016/0005-2744(73)90398-7. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nester E. W. Regulatory enzymes of aromatic amino acid biosynthesis in Bacillus subtilis. II. The enzymology of feedback inhibition of 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. J Biol Chem. 1966 Jul 25;241(14):3373–3380. [PubMed] [Google Scholar]
- Jensen R. A., Pierson D. L. Evolutionary implications of different types of microbial enzymology for L-tyrosine biosynthesis. Nature. 1975 Apr 24;254(5502):667–671. doi: 10.1038/254667a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Stenmark S. L. Comparative allostery of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthetase as a molecular basis for classification. J Bacteriol. 1970 Mar;101(3):763–769. doi: 10.1128/jb.101.3.763-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano H., Zalkin H. Tyrosine-inhibited 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase. Properties of the partially purified enzyme from Salmonella typhimurium. Arch Biochem Biophys. 1970 May;138(1):58–65. doi: 10.1016/0003-9861(70)90284-5. [DOI] [PubMed] [Google Scholar]
- Palleroni N. J., Ballard R. W., Ralston E., Doudoroff M. Deoxyribonucleic acid homologies among some Pseudomonas species. J Bacteriol. 1972 Apr;110(1):1–11. doi: 10.1128/jb.110.1.1-11.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleroni N. J., Doudoroff M. Phenotypic characterization and deoxyribonucleic acid homologies of Pseudomonas solanacearum. J Bacteriol. 1971 Sep;107(3):690–696. doi: 10.1128/jb.107.3.690-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Ralston E., Palleroni N. J., Doudoroff M. Deoxyribonucleic acid homologies of some so-called "Hydrogenomonas" species. J Bacteriol. 1972 Jan;109(1):465–466. doi: 10.1128/jb.109.1.465-466.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoner R., Herrmann K. M. 3-Deoxy-D-arabino-heptulosonate 7-phosphate synthase. Purification, properties, and kinetics of the tyrosine-sensitive isoenzyme from Escherichia coli. J Biol Chem. 1976 Sep 25;251(18):5440–5447. [PubMed] [Google Scholar]
- Staub M., Dénes G. Purification and properties of the 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (phenylalanine sensitive) of Escherichia coli K12. II. Inhibition of activity of the enzyme with phenylalanine and functional group-specific reagents. Biochim Biophys Acta. 1969 May 27;178(3):599–608. doi: 10.1016/0005-2744(69)90228-9. [DOI] [PubMed] [Google Scholar]
- Udaka S. Pathway-specific pattern of control of arginine biosynthesis in bacteria. J Bacteriol. 1966 Feb;91(2):617–621. doi: 10.1128/jb.91.2.617-621.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



