Abstract
We have identified an unusual potential dual Akt/protein kinase B consensus phosphorylation motif in the protein Synip (RxKxRS97xS99). Surprisingly, serine 97 is not appreciably phosphorylated, whereas serine 99 is only a specific substrate for Akt2 but not Akt1 or Akt3. Although wild-type Synip (WT-Synip) undergoes an insulin-stimulated dissociation from Syntaxin4, the Synip serine 99 to phenylalanine mutant (S99F-Synip) is resistant to Akt2 phosphorylation and fails to display insulin-stimulated Syntaxin4 dissociation. Furthermore, overexpression of WT-Synip in 3T3L1 adipocytes had no effect on insulin-stimulated recruitment of glucose transporter 4 (GLUT4) to the plasma membrane, whereas overexpression of S99F-Synip functioned in a dominant-interfering manner by preventing insulin-stimulated GLUT4 recruitment and plasma membrane fusion. These data demonstrate that insulin activation of Akt2 specifically regulates the docking/fusion step of GLUT4-containing vesicles at the plasma membrane through the regulation of Synip phosphorylation and Synip–Syntaxin4 interaction.
Introduction
Akt, also known as protein kinase B (PKB), is an important regulator of several cellular processes, including proliferation, metabolism, and programmed cell death (Lawlor and Alessi, 2001; Whiteman et al., 2002). Akt has three isoforms, Akt1 (PKBα), Akt2 (PKBβ), and Akt3 (PKBγ), each with overlapping but distinct cellular function. In particular, recent studies have demonstrated that Akt1 plays an important role on growth and antiapoptosis, whereas Akt2 functions primarily as a regulator of glucose metabolism (Cho et al., 2001b; Bae et al., 2003). For example, Akt1−/− mice have reduced body size but relatively normal glucose homeostasis, whereas Akt2−/− mice displayed insulin-resistant glucose metabolism in liver and muscle (Cho et al., 2001a). However, the insulin resistance is relatively mild and becomes significantly more pronounced in conjunction with the loss of Akt1 (Jiang et al., 2003). Consistent with a primary role for Akt2, a family with severe insulin resistance and overt diabetes was mapped to a single point mutation in Akt2 (George et al., 2004). Thus, there seems to exist intracellular signal specificity and some compensation mechanism for the regulation of glucose metabolism between Akt1 and Akt2.
It is well documented that Akt is involved in the regulation of glucose metabolism by inhibiting glycogen synthesis through the inhibition of glycogen synthesis kinase 3 (GSK3) activity (Cross et al., 1995; Coghlan et al., 2000; Doble and Woodgett, 2003). However, how Akt regulates glucose uptake (muscle and adipose tissue), its association with peripheral insulin resistance, and the molecular basis for the apparent Akt2 specificity is still unknown.
Peptide substrate mapping studies have identified the preferred Akt1 phosphorylation consensus site as RxRxxS/T (Alessi et al., 1996). Currently, over 20 substrates for Akt have been identified; however, none of these substrates has been reported to exhibit Akt isoform selectivity. Thus, at present, the molecular basis for the physiologic specificities of Akt isoform function remains a fundamental issue that has yet to be resolved. In this regard, we previously identified Synip as a Syntaxin4 interacting protein (Min et al., 1999). Under the basal conditions, Synip was constitutively bound to Syntaxin4 and prevented the interaction of Syntaxin4 with both SNAP23 (synaptosome-associated proteins of 23 kD) and VAMP2 (vesicle-associated membrane protein 2; Min et al., 1999). Insulin treatment resulted in a dissociation of the Synip–Syntaxin4 complex allowing for the assembly of a fusogenic Syntaxin4–SNAP23–VAMP2 complex necessary for glucose transporter 4 (GLUT4) translocation (Min et al., 1999).
In this paper, we now demonstrate that Synip is a preferred Akt2-specific substrate with an unusual dual consensus phosphorylation site. The specific Akt2-dependent phosphorylation of serine 99 is essential for the insulin-stimulated dissociation of Synip from Syntaxin4, translocation, and plasma membrane fusion of GLUT4-containing vesicles.
Results
Analysis of insulin signal-regulating Synip–Syntaxin4 interaction
Insulin stimulates the translocation of GLUT4 proteins from intracellular storage sites to the plasma membrane. To date, two major insulin-mediated signal transduction pathways have been implicated in the regulation of this process (Saltiel and Pessin, 2003). The insulin activation and/or targeting of the type 1A phosphatidylinositol 3 (PI3) kinase generate PI3, 4, 5P3 in the plasma membrane (Okada et al., 1994). PI3, 4, 5P3 recruits and/or activates phosphoinositide-dependent kinase 1 that serves as an immediate upstream kinase for Akt and the atypical protein kinase C isoforms λ and ζ (Bellacosa et al., 1991; Belham et al., 1999). The plasma membrane translocation of GLUT4 requires the specific interaction of the plasma membrane t-SNARE proteins Syntaxin4 and SNAP23 with the v-SNARE protein VAMP2 in GLUT4-containing cargo vesicles (Pessin et al., 1999).
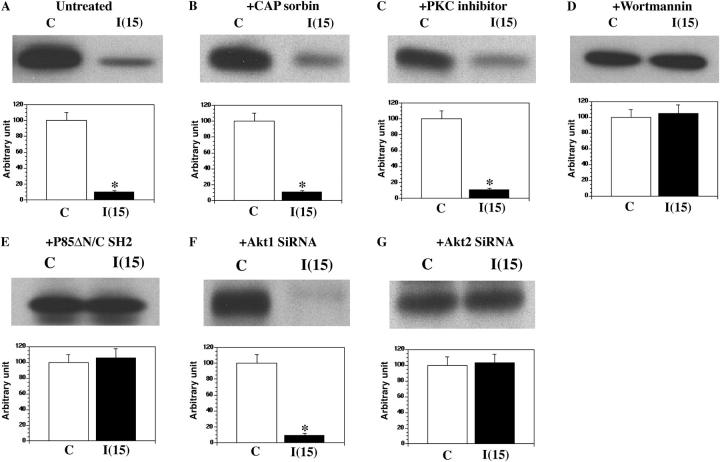
To determine the specific insulin signaling pathway responsible for the dissociation of Synip from Syntaxin4, we treated cells with various pharmacological agents and small interfering RNA (siRNA; Fig. 1). As previously observed, after insulin stimulation there was an ∼90% decrease in the amount of Synip protein coimmunoprecipitated with Syntaxin4 (Fig. 1 A). Several studies have suggested that in addition to the PI3 kinase pathway a second PI3 kinase–independent insulin signal pathway is necessary for the efficient translocation of GLUT4 in adipocytes (Watson et al., 2004). This latter pathway involves the function of the Cbl adaptor protein CAP (Cbl-associated protein) as expression of a dominant-interfering CAP blocks insulin-stimulated GLUT4 translocation without significant effect on insulin activation of PI3 kinase signaling and Akt activation (Baumann et al., 2000). However, expression of a dominant-interfering CAP mutant (CAP sorbin) had no significant effect on the insulin-stimulated dissociation of Synip from Syntaxin4 with also an ∼90% reduction in coimmunoprecipitation (Fig. 1 B). Similarly, treatment with a high concentration (10 μM) of PKC inhibitor GF109203X, which inhibits atypical PKCs (Li et al., 1999), resulted in an ∼85% decrease of Synip protein coimmunoprecipitated with Syntaxin4 after insulin stimulation (Fig. 1 C).
Figure 1.
Analysis of insulin signal-regulating Synip–Syntaxin4 interaction. FLAG-WT-Synip is expressed in CHOIR (CHO cell with overexpressed human insulin receptor) by electroporation. After 48 h of recovery, 6 h of serum starvation followed. 100 nM insulin was added for 15 min to see Synip–Syntaxin4 interaction. After 4 mg of whole cell lysates were made with NP-40 lysis buffer, 2 μg of Syntaxin4 antibody was added to precipitate Syntaxin4. Coimmunoprecipitated FLAG-WT-Synip was detected by FLAG immunoblotting. In each experiment it was confirmed that same amount of Syntaxin4 was immunoprecipitated by Syntaxin4 immunoblotting, and FLAG-WT-Synip expression was identical by FLAG immunoblotting in each sample (not depicted). Data show representative experiments independently performed and each experiment was repeated three to four times. (A) A typical result of Synip–Syntaxin4 interaction before and after insulin stimulation. Insulin causes Synip dissociation from Syntaxin4 after insulin stimulation, which is recognized as weaker band intensity compared with basal condition. It was statistically significant (*, P < 0.01). (B) The effect of the disruption of CAP/TC10 signal (PI3 kinase–independent pathway) by the design of sorbin overexpression on Synip–Syntaxin4 interaction. After insulin stimulation, Synip dissociation was still observed. The difference in band intensity was statistically significant (*, P < 0.01). (C) The effect of a pharmacological PKC inhibitor such as GF 109203X. Synip dissociation could be still observed. The difference in band intensity was statistically significant (*, P < 0.01). (D) The effect of pharmacological PI3 kinase inhibitor, such as Wortmannin, pretreatment on Synip–Syntaxin4 interaction. Synip dissociation disappeared. (E) The effect of PI3 kinase negative-dominant overexpression. Synip dissociation also disappeared. (F) The effect of siRNA of Akt1. Synip dissociation could be observed. The difference in band intensity was statistically significant (*, P < 0.01). (G) The effect of siRNA of Akt2. Synip dissociation could not be observed.
In contrast, inhibition of PI3 kinase with Wortmannin or by expression of a dominant-interfering p85 regulatory subunit mutant prevented the insulin-stimulated Synip dissociation (Fig. 1, D and E). Furthermore, reduction of Akt2 protein levels with a specific Akt2 siRNA blocked the insulin-stimulated dissociation of Synip from Syntaxin4, whereas reduction of Akt1 protein levels by a specific Akt1 siRNA resulted in a 94% insulin-stimulated decrease of Synip binding (Fig. 1, F and G). Under these siRNA conditions, the protein levels of Akt1 and Akt2 were reduced by more than 90% compared with untreated cells or incubated with a random siRNA (unpublished data). Together, these data strongly suggested that the PI3 kinase–dependent pathway leading specifically to Akt2 activation is responsible for the insulin-stimulated dissociation of Synip–Syntaxin4 complex. Furthermore, these results indicate that neither the CAP nor atypical protein kinase C pathways are involved in this process.
Akt2 but not Akt1 or Akt3 phosphorylates Synip
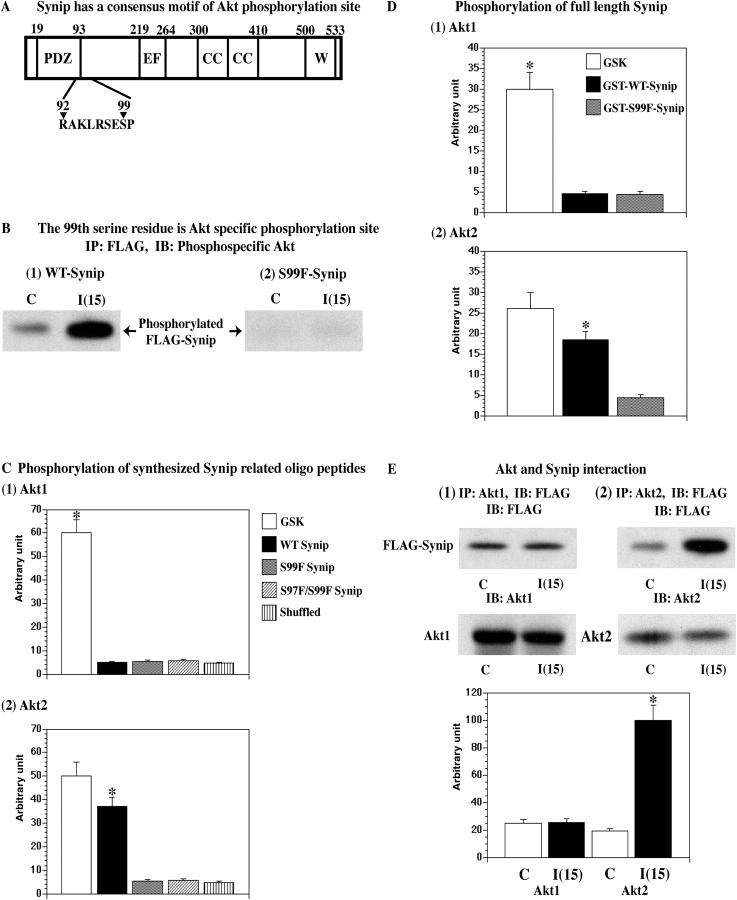
Next, we determined whether or not Synip phosphorylation by Akt2 was responsible for the regulation of the Synip–Syntaxin4 interaction. Inspection of the Synip amino acid sequence revealed the presence of an overlapping dual Akt consensus phosphorylation site at serine 97 and 99 (RAKLRSESP) (Fig. 2 A). Because arginine at position −5 is preferred for Akt1 (Alessi et al., 1996; Obata et al., 2000), this suggests that serine 97 would be a preferred Akt1 phosphorylation site. However, to date no Akt2-specific consensus motif has been reported.
Figure 2.
Synip phosphorylation by Akt2. (A) Candidate Akt phosphorylation consensus motif in Synip is presented. (B) Either FLAG-WT-Synip or FLAG-S99F-Synip was expressed in CHOIR cells by electroporation. After 15 min of insulin stimulation, Synip was immunoprecipitated by FLAG antibody and samples were separated on SDS-PAGE. Phosphorylated Synip signal was analyzed by Akt phosphospecific substrate antibody immunoblotting. These are representative experiments independently performed three times. (C) Synthesized oligo peptides were phosphorylated as described in Materials and methods. These were repeated four to five times. Recombinant Akt2 was capable of phosphorylating the Synip peptide compared with Akt1 (*, P < 0.01). A scramble peptide with the same composition as well as mutation of the equivalent position of serine 99 to phenylalanine markedly reduced Akt2-dependent phosphorylation (*, P < 0.01). (D) GST-Synip fusion proteins were phosphorylated as described in Materials and methods. These were repeated five times. Recombinant Akt2 was capable of phosphorylating GST-WT-Synip (*, P < 0.05). However, Akt2 did not phosphorylate GST-S99F-Synip. GST alone showed negligible count. (E) Akt and Synip interaction was estimated by the design of coimmunoprecipitation experiments. FLAG-Synip was expressed in CHO/IR cells by electroporation and either Akt1 or Akt2 was immunoprecipitated by specific antibody. Synip amount associated with Akt was estimated by FLAG immunoblot. These were repeated five times. Synip significantly associates with not Akt1 but Akt2 after insulin stimulation (*, P < 0.01).
Therefore, we performed the following series of experiments. First, we assessed the Akt-dependent phosphorylation of Synip in cells using an Akt phosphospecific substrate antibody. Wild-type Synip (WT-Synip) or Synip serine 99 to phenylalanine mutant (S99F-Synip) was expressed in CHOIR cells and stimulated with insulin for 15 min. Synip was then immunoprecipitated, and phosphorylated signal was detected by Western blotting with Akt phosphospecific substrate antibody. As shown in Fig. 2 B, WT-Synip was clearly phosphorylated after insulin stimulation. In contrast, S99F-Synip was unable to undergo insulin-stimulated phosphorylation (Fig. 2 B). To assess whether or not serine 99 is a specific Akt2 phosphorylation site, we examined the in vitro phosphorylation of synthetic peptides corresponding to this region of Synip (Fig. 2 A). We were unable to detect any significant Akt1-dependent phosphorylation of the WT-Synip peptide, the S99F mutant, the S97F/S99F double mutant, or a scramble peptide. In contrast, the WT-Synip peptide was an effective substrate for recombinant Akt2 (Fig. 2 C). As controls, both a scramble peptide with the same composition as well as mutation of the equivalent position of serine 99 to phenylalanine markedly reduced Akt2-dependent phosphorylation (Fig. 2 C). In addition, Akt2 did not phosphorylate the peptide, which has mutation of the equivalent position of serine 97 and 99 to phenylalanine. Similarly, the activated form of purified recombinant Akt3 did not phosphorylate the Synip peptide (unpublished data). Confirming the functional activity of the recombinant Akt1, Akt2, and Akt3, all three isoforms were fully capable of phosphorylating a GSK3 control peptide (Fig. 2 C and not depicted).
Because these data demonstrate that the RAKLRSESP Synip peptide sequence displays Akt2-specific substrate specificity, we examined if serine 99 is the critical Akt2 substrate site in the full-length Synip protein. GSK3 control peptide and the full-length Synip proteins expressed as GST-fusions were phosphorylated in vitro. As expected, GSK3 control peptide was an effective substrate for both Akt1 and Akt2, whereas the full-length Synip protein was only a functional substrate for Akt2 (Fig. 2 D). Consistent with a required role for S99, the Synip S99F mutant was not phosphorylated by either Akt1 or Akt2.
Although the mechanism of Akt substrate recognition is not known, if it is through the active site then an increased binding of Synip to Akt2 compared with Akt1 would be expected. To test this possibility, we examined the coimmunoprecipitation of Synip with Akt1 and Akt2 (Fig. 2 E). The immunoprecipitation of Akt1 resulted in the coimmunoprecipitation of Synip but this interaction was unaffected by insulin stimulation. In contrast, insulin stimulation resulted in a marked increase in the amount of Synip that was coimmunoprecipitated with Akt2. Together, these data provide compelling evidence that the Synip serine 99 residue is a selective Akt2 phosphorylation site and that the RAKLRSESP sequence context might provide an Akt2-specific consensus motif.
Phosphorylation of serine 99 is required for reduced Synip–Synatxin4 binding
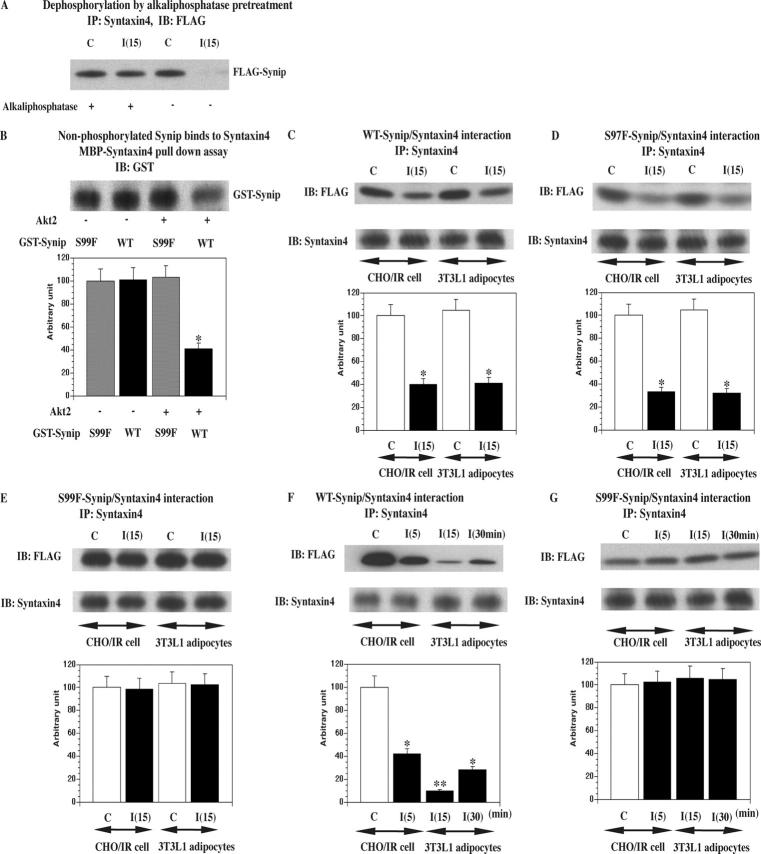
To determine the functional consequence of this phosphorylation event, we nonspecifically dephosphorylated the Synip protein with alkaline phosphatase before coimmunoprecipitation (Fig. 3 A). Under these conditions, Synip failed to display an insulin-stimulated dissociation from Syntaxin4, suggesting nonphosphorylated Synip preferentially binds to Syntaxin4. Consistent with a complete Synip dephosphorylation, we did not detect any significant difference of Synip–Syntaxin4 complex amount between unstimulated and insulin-stimulated samples. To directly assess the effect of Synip phosphorylation, we examined the in vitro binding of GST-Synip with MBP (maltose binding protein)-Syntaxin4 after Akt2 phosphorylation (Fig. 3 B). In the absence of Akt2, both WT-Synip and S99F displayed identical extents of binding. In contrast, Akt2-dependent phosphorylation of WT-Synip resulted in a 70% reduction in Syntaxin4 binding, whereas there was no effect on the S99F mutant.
Figure 3.
Synip phosphorylation is required for Synip dissociation from Syntaxin4. (A) In this experiment whole cell lysates were pretreated with alkaline phosphatase first as described previously (Zhao et al., 1998) to dephosphorylate all the phosphorylated molecules. Thereafter, an ordinal coimmunoprecipitation experiment was conducted to see Synip–Syntaxin4 interaction. These are representative experiments independently performed two times. (B) Either GST-WT-Synip or GST-S99F-Synip was phosphorylated by Akt2 first and then incubated with MBP-Syntaxin4. Samples were separated on SDS-PAGE and GST-Synip associated with MBP-Syntaxin4 was estimated by GST immunoblot (*, P < 0.01). These were repeated five times. In addition, we stripped the GST antibody from filters and reprobed them with phosphospecific Akt substrate antibody. We confirmed that there was no signal (not depicted) and that nonphosphorylated Synip binds to Syntaxin4. (C–E) FLAG-WT-Synip or FLAG-S99F-Synip or FLAG-S97F-Synip was electroporated to CHOIR cells and 3T3L1 adipocytes and the cells stimulated with or without insulin for 15 min. In these experiments, the amount of expressed Synip protein was adjusted to match the expression levels in 3T3L1 adipocytes that have a lower level of transfection efficiency and protein expression than CHOIR cells (Min et al., 1999). In comparison to Fig. 1, 8 mg of detergent whole cell lysates were immunoprecipitated with 4 μg of Syntaxin4 antibody. Coimmunoprecipitated Synip amount was estimated by FLAG immunoblotting. FLAG-WT-Synip and FLAG-S97F-Synip dissociation could be observed in CHOIR cells and 3T3L1 adipocytes. The difference in band intensity was statistically significant (*, P < 0.05). These are representative experiments independently performed four times. (F and G) FLAG-WT-Synip or FLAG-S99F-Synip was electroporated to CHOIR. After Syntaxin4 was immunoprecipitated, coimmunoprecipitated Synip amount was estimated by FLAG immunoblotting. FLAG-WT-Synip dissociation appeared 5 min after insulin stimulation and continued up to 30 min. The difference in band intensity was statistically significant (*, P < 0.05; **, P < 0.01). These are representative experiments independently performed three times. In this design, insulin stimulation was done at 5, 15, and 30 min.
As expected for site-specific phosphorylation, both the wild type and a serine 97 to phenylalanine (S97F) mutant displayed the typical insulin-stimulated dissociation from Syntaxin4 in both CHO/IR and 3T3L1 adipocytes (Fig. 3, C and D). In contrast, the serine 99 to phenylalanine (S99F) mutant was refractory to insulin-stimulated dissociation (Fig. 3 E). In fact, whereas 5 min of insulin stimulation was sufficient to induce WT-Synip dissociation from Syntaxin4 (Fig. 3 F), S99F-Synip was persistently associated at least over the 30 min time course examined (Fig. 3 G). Insulin-stimulated WT-Synip dissociation continued up to 30 min after insulin stimulation (Fig. 3 F). These data are consistent with phosphorylation of serine 99 but not serine 97 as a necessary step in the insulin-stimulated dissociation of the Synip–Syntaxin4 complex. Together these data provide compelling evidence that the RxKxRSxS motif is essential for Synip phosphorylation and dissociation from Syntaxin4 by Akt2.
Synip regulates the docking/fusion steps of GLUT4-containing vesicles by Akt2 phosphorylation
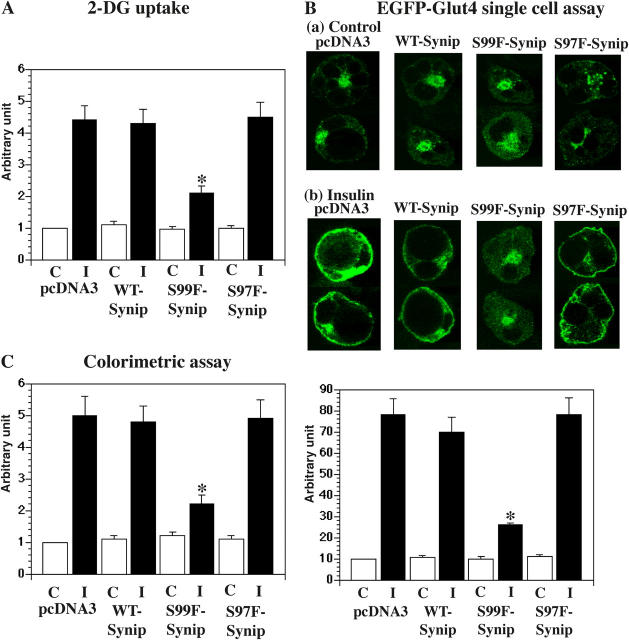
Having established a biochemical consequence of Synip phosphorylation, we next examined the physiological requirement of this event. Expression of WT-Synip or S97F-Synip in 3T3L1 adipocytes had no significant effect on insulin-stimulated glucose uptake compared with empty vector-transfected cells (Fig. 4 A). However, expression of S99F-Synip significantly suppressed insulin-stimulated glucose uptake. To determine if the reduction of insulin-stimulated glucose uptake was due to an inhibition of GLUT4 translocation, we compared the cell surface localization of GLUT4 by confocal fluorescent microscopy (Fig. 4 B). Expression of S99F-Synip but not WT-Synip or S97F-Synip reduced insulin-stimulated GLUT4 translocation compared with control cells (Fig. 4 B). Finally, a quantitative colorimetric assay for the translocation of a myc epitope-tagged GLUT4 (Konrad et al., 2002) demonstrated that expression of S99F-Synip reduced insulin-stimulated GLUT4 translocation by 52% compared with control, WT-Synip, and S97F-Synip–expressing cells (Fig. 4 C). In this particular assay, because the myc epitope tag is located on the extracellular loop between transmembrane domains 1 and 2, only the GLUT4 proteins that have undergone plasma membrane fusion are detected.
Figure 4.
Synip phosphorylation is required for glucose uptake and GLUT4 translocation. (A) In 3T3L1 adipocytes, pcDNA3, FLAG-WT-Synip, FLAG-S99F-Synip, or FLAG-S97F-Synip was introduced by electroporation (Min et al., 1999). After 48 h of recovery, serum was removed for 6 h. Cells were stimulated by insulin for 30 min. 3H-labeled 2-deoxyglucose uptake was measured as described in Materials and methods. FLAG-S99F-Synip significantly inhibited 3H-labeled 2-deoxyglucose uptake (*, P < 0.05). (B) In 3T3L1 adipocytes, eGFP-GLUT4 and either pcDNA3, FLAG-WT-Synip, FLAG-S99F-Synip, or FLAG-S97F-Synip were introduced by electroporation (Min et al., 1999). After 48 h of recovery, serum was removed for 6 h. Then, cells were stimulated by insulin for 30 min. EGFP signal was detected by confocal microscopy, and rim formation was considered as completely translocated eGFP-GLUT4 in this assay. FLAG-S99F-Synip significantly inhibited GLUT4 translocation (*, P < 0.05). Experiments were repeated four times. (C) In 3T3L1 adipocytes, eGFP-myc-GLUT4 and either pcDNA3, FLAG-WT-Synip, FLAG-S99F-Synip, or FLAG-S97F-Synip were introduced by electroporation (Min et al., 1999). Myc was inserted in the second loop of GLUT4 to expose to the outside of the plasma membrane after insulin stimulation. When myc signal was detected, docking/fusion step was completed and GLUT4 translocation was accomplished. Myc signal was estimated as described in Materials and methods. FLAG-S99F-Synip significantly inhibited GLUT4 translocation (*, P < 0.05). Experiments were repeated eight times.
Discussion
It is well recognized that the interaction of Syntaxin4 as t-SNARE and VAMP2 as v-SNARE is necessary for insulin-stimulated GLUT4 translocation in adipose tissue and skeletal muscle (Foster and Klip, 2000). For example, overexpression of the Syntaxin4 cytoplasmic domain inhibits insulin-stimulated GLUT4 translocation (Olson et al., 1997). This inhibition was specific for the VAMP2 binding domain within Syntaxin4 because deletion of this region did not result in inhibition of insulin-stimulated GLUT4 translocation (Olson et al., 1997). Thus, Syntaxin4 and VAMP2 binding is one of the necessary steps for GLUT4 translocation. However, it is still unknown about precise mechanism how insulin controls Syntaxin4–VAMP2 interaction directly. In this regard, Synip is a Syntaxin4 specific binding protein that regulates the interaction between Syntaxin4 and VAMP2 in an insulin-dependent manner (Min et al., 1999). Insulin causes Synip dissociation from Syntaxin4, and this dissociation allows VAMP2 to bind with Syntaxin4 because Synip and VAMP2 use the same binding site on Syntaxin4 (Min et al., 1999). However, it has not yet been determined how insulin causes Synip dissociation from Syntaxin4. Therefore, we attempted to identify the Synip dissociation mechanism to understand how insulin regulates the Syntaxin4–VAMP2 interaction and docking/fusion step of GLUT4-containing vesicle.
PI3 kinase–independent pathway regulated by CAP/Cbl/TC10 molecules is recently reported to regulate the final steps of GLUT4 exocytosis by recruiting Exo70 to the plasma membrane (Inoue et al., 2003). However, as shown in Fig. 1 B, CAP-sorbin (Baumann et al., 2000), which has a dominant-negative effect for the CAP/Cbl/TC10 pathway, did not affect Synip dissociation from Syntaxin4. Furthermore, our data strongly suggested that the PI3 kinase–Akt pathway specifically regulates Synip–Syntaxin4 interaction and that the PI3 kinase–atypical PKC pathway is not likely involved (Fig. 1, C–G). Furthermore, our siRNA experiments strongly suggested that Akt2 but not Akt1 regulates Synip–Syntaxin4 interaction (Fig. 1, F and G).
The next question is why Akt2 alone is involved in the regulation of Synip–Syntaxin4 interaction? What kind of mechanism does determine the Akt specificity in this pathway? As shown in Fig. 2 A, Synip seemed to have a consensus motif including the 99th serine residue as Akt phosphorylation site. Therefore, we tested the possibility of whether or not Akt phosphorylates the 99th serine of Synip. We have observed that Akt2, but not Akt1, specifically phosphorylates Synip at serine 99 residue after insulin stimulation (Fig. 2 B). However, because it was formally possible that this was an indirect phosphorylation event, we synthesized the WT-Synip peptide, the S99F Synip mutant, the S97F/S99F Synip double mutant, or a scramble peptide including the serine 99 and performed in vitro phosphorylation experiments with recombinant activated forms of Akt1, Akt2, and Akt3. Surprisingly, only Akt2 was capable of directly phosphorylating the WT-Synip peptide (Fig. 2 C). Although serine 97 is also in an appropriate context as an Akt1 substrate, neither Akt1, Akt2, nor Akt3 was capable of phosphorylating the S99F Synip mutant, the S97F/S99F Synip double mutant, or a scramble peptide. Moreover, the similar substrate selectivity was observed in the case of in vitro phosphorylation using full-length WT-Synip and S99F-Synip (Fig. 2 D). Currently, there are more than 20 reported substrates for Akt, yet none of them have been shown to display Akt isoform specificity. It is generally assumed that either Akt and/or substrate spatial compartmentalization is the primary determinant of substrate selectivity. Although our data do not exclude this mechanism, our data indicate the presence of at least one unusual consensus site that displays relative Akt2 specificity in a physiologically regulated manner.
As Synip is a Syntaxin4 binding protein, its functional activity is assumed to exist at the plasma membrane. Although insulin-stimulated Akt2 translocates to the plasma membrane (Hanada et al., 2004), Akt1 was reported to undergo nuclear localization after growth factor stimulation (Pekarsky et al., 2000). As shown in Fig. 2 E, insulin stimulation resulted in a specific increase in Akt2 but not Akt1 association with Synip. Whether or not the Synip–Akt2 interaction occurs through direct or indirect interaction, these results are consistent with the specificity and subcellular compartmentalization of Akt1 and Akt2.
Our data also provide a physiological consequence for Akt2-specific Synip phosphorylation. As shown in Fig. 3 A, the dephosphorylated Synip binds Syntaxin4, whereas Akt2 phosphorylated Synip displayed significantly less binding compared with nonphosphorylated Synip (Fig. 3 B). Furthermore, S99F-Synip binding to Syntaxin4 was refractory to insulin stimulation and was unaffected by the presence or absence of active Akt2 (Fig. 3 B). Consistent with these results, only Synip, which has an Akt2 phosphorylation site, was permissive for insulin-stimulated glucose uptake and GLUT4 translocation, whereas the nonphosphorylatable mutant S99F was inhibitory. Thus, our data demonstrate that insulin-stimulated Akt2-dependent phosphorylation of Synip on serine residue 99 results in reduced binding interactions between Synip and Syntaxin4. These data are consistent with the requirement of Akt2 for insulin-stimulated glucose uptake and insulin sensitivity in culture cells, mice, and humans (Cho et al., 2001a; Bae et al., 2003; George et al., 2004).
In summary, we have identified Synip as the first specific Akt2 substrate. Akt2-dependent phosphorylation at serine 99 directly modulates the interaction of Synip with Syntaxin4, suggesting a direct mechanism linking Akt2 function with the t-SNARE–mediated docking/fusion of GLUT4 cargo vesicles.
Materials and methods
Materials
The FLAG M2 mAb was obtained from Sigma-Aldrich, and Syntaxin4 sheep polyclonal and Synip rabbit polyclonal antibodies were prepared and affinity purified as described previously (Olson et al., 1997; Min et al., 1999). Phosphospecific Akt substrate antibody was purchased from Cell Signaling Technology, and Akt1- and Akt2-specific antibodies and siRNA for Akt1 and Akt2 were obtained from Upstate Cell Signaling Solutions. Although the Akt substrate phosphospecific antibody can recognize the RxRxxS/T motif, it apparently has a sufficient cross reactivity to the KxRxxS sequence present in Synip. GST protein expression system including GST antibody was obtained from Amersham Biosciences. MBP expression system was obtained from New England Biolabs, Inc. ECL+plus Western Blotting Detection System and 3H-2-deoxyglucose were obtained from Amersham Biosciences. The anti–sheep and anti–rabbit IgG-HRP were obtained from Pierce Chemical Co. The VAMP2 antibody was purchased from Calbiochem, and cell culture media and reagents were purchased from Life Technologies. Synthesis of oligo-peptides was done by Shimazu Corporation. All of other chemicals used in this study were purchased from Sigma-Aldrich.
Cell culture
CHO cells expressing the human insulin receptor (CHO/IR) were obtained as described previously (Waters et al., 1995). These cells were maintained in minimal Eagle's medium containing 10% FBS at 37°C with 5% CO2. 3T3L1 preadipocytes were cultured in DME containing 25 mM glucose, 10% calf serum at 37°C with 8% CO2. Confluent cultures were induced to differentiate into adipocytes as described previously (Min et al., 1999).
Immunoprecipitation
Scraped frozen cells were rocked for 10 min at 4°C with NP-40 lysis buffer (25 mM Hepes, pH 7.4, 10% glycerol, 50 mM sodium fluoride, 10 mM sodium phosphate, 137 mM sodium chloride, 1 mM sodium orthovanadate, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/ml pepstatin, and 5 μg/ml leupeptin). Insoluble material was separated from the soluble extract by centrifugation for 10 min at 4°C, and the total protein amount in the supernatant was determined by BCA method. After the addition of 4.5 μg of antibody to the whole cell lysates, samples (typically 2–3 mg of lysates) were incubated for 2 h at 4°C. 50 μl of protein A–agarose (Santa Cruz Biotechnology, Inc.) was added and samples were consequently rocked during the next 1 h at 4°C. After the incubation, samples were extensively washed three times with the NP-40 lysis buffer. The washed samples were resuspended in SDS sample buffer (125 mM Tris-HCl, pH 6.8, 20% [vol/vol] glycerol, 4% [wt/vol] SDS, 100 mM DTT, and 0.1% [wt/vol] Bromophenol blue) and heated at 100°C for 5 min.
Immunoblotting
Samples were separated by SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes. The samples were immunoblotted with monoclonal or polyclonal specific antibody as indicated in the figures and legends.
To assess Synip–Syntaxin4 interaction, 90% of samples were applied on SDS-PAGE and subjected to FLAG immunoblotting to detect FLAG-Synip signal. For the estimation of immunoprecipitated Syntaxin4 amount, the rest of 10% immunoprecipitated samples were applied on a different SDS-PAGE and subjected to Syntaxin4 immunoblotting. Although figures were not presented, FLAG-Synip and Syntaxin4 signal from whole cell lysate samples were checked and it was confirmed that electroporation efficiency was the same among each sample and Syntaxin4 expression was not affected by sample treatment in each experiment. The primary monoclonal and polyclonal antibodies for immunoblottings were detected with HRP-conjugated anti–mouse or anti–rabbit IgG antibodies (Pierce Chemical Co.). Specific signals were visualized by ECL+plus Western Blotting Detection System (Amersham Biosciences). Each band was developed to X-ray film from Amersham Biosciences and scanned by UMAX MagicScan 4.3 system.
Transfection of CHO/IR and 3T3L1 adipocytes
CHO/IR cells were quantitatively transfected by electroporation as previously described (Waters et al., 1995). 3T3L1 adipocytes were put into suspension by mild trypsinization and electroporated with a total of 600 μg of plasmid under low-voltage condition (0.16 kV, 950 μF). The cells were allowed to adhere to collagen-coated tissue culture dishes for 30–48 h, and the adipocytes were then serum starved for 2 h before incubation in the absence or presence of 100 nM insulin for 15 min at 37°C. In the case of cotransfection of siRNA and plasmids, 2 nmol siRNA was used.
In vitro Synip phosphorylation
Akt substrate phosphorylation assay kit was purchased from Upstate Cell Signal Solutions and we followed the manufacture's procedure. 200 mU of recombinant Akts were incubated with 2.5 mM of synthesized oligo-Synip peptides and 4.3 μM of GST-Synip fusion proteins with supplied kinase reaction buffer. The kinase reaction was stopped by the addition of ice cold TCA and applied on phosphocellulose paper. After extensive washing of phosphocellulose paper, scintillation cocktail was added to the phosphocellulose paper and the radiation activity was measured.
EGFP-GLUT4 translocation assay
50 μg of eGFP-GLUT4 plasmid with 550 μg of interesting plasmids was electroporated to 3T3L1 adipocytes. The cells were allowed to adhere to collagen-coated tissue culture dishes for 30–48 h, and the adipocytes were serum starved for 2 h before incubation in the absence or presence of 100 nM insulin for 15 min at 37°C. Transfected adipocytes were washed in PBS and fixed for 10 min in PBS containing 4% PFA and 0.2% Triton X-100. The samples were mounted on glass slides with Fluorescent Mounting Medium (DakoCytomation). Cells were imaged using a confocal fluorescence microscope (model MRC-1024; Bio-Rad Laboratories).
Colorimetric assay and 2-deoxyglucose uptake
In the case of colorimetric assay, 50 μg of eGFP-cMyc-GLUT4 with 550 μg of interesting plasmids was electroporated to 3T3L1 adipocytes. 2-Deoxyglucose uptake and colorimetric assay were performed as described previously (Olson et al., 1997; Konrad et al., 2002; Brozinick et al., 2003).
Statistical analysis
Each band of Western blots was scanned and analyzed by Molecular Imager FX (Bio-Rad Laboratories). We set and used the same size rectangle box to surround each band and analyzed the intensity of each band alone by the program in Molecular Imager FX. Before we calculated the band intensity, we subtracted the background intensity, which is estimated from the band-free area by using the same rectangle box.
All values are expressed as mean ± SEM. Data were evaluated for statistical significance by analysis of variance and t test with InStat 2 program.
Acknowledgments
We would like to thank Dr. Jeffrey E. Pessin (State University of New York at Stony Brook, Stony Brook, NY) for critical suggestions on our manuscript.
Abbreviations used in this paper: GLUT4, glucose transporter 4; GSK3, glycogen synthesis kinase 3; PI3, phosphatidylinositol 3; siRNA, small interfering RNA; WT, wild-type.
References
- Alessi, D.R., F.B. Caudwell, M. Andjelkovic, B.A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333–338. [DOI] [PubMed] [Google Scholar]
- Bae, S.S., H. Cho, J. Mu, and M.J. Birnbaum. 2003. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J. Biol. Chem. 278:49530–49536. [DOI] [PubMed] [Google Scholar]
- Baumann, C.A., V. Ribon, M. Kanzaki, D.C. Thurmond, S. Mora, S. Shigematsu, P.E. Bickel, J.E. Pessin, and A.R. Saltiel. 2000. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 407:202–207. [DOI] [PubMed] [Google Scholar]
- Belham, C., S. Wu, and J. Avruch. 1999. Intracellular signalling: PDK1—a kinase at the hub of things. Curr. Biol. 9:R93–R96. [DOI] [PubMed] [Google Scholar]
- Bellacosa, A., J.R. Testa, S.P. Staal, and P.N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 254:274–277. [DOI] [PubMed] [Google Scholar]
- Brozinick, J.T., Jr., B.R. Roberts, and G.L. Dohm. 2003. Defective signaling through Akt-2 and -3 but not Akt-1 in insulin-resistant human skeletal muscle: potential role in insulin resistance. Diabetes. 52:935–941. [DOI] [PubMed] [Google Scholar]
- Cho, H., J. Mu, J.K. Kim, J.L. Thorvaldsen, Q. Chu, E.B. Crenshaw III, K.H. Kaestner, M.S. Bartolomei, G.I. Shulman, and M.J. Birnbaum. 2001. a. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science. 292:1728–1731. [DOI] [PubMed] [Google Scholar]
- Cho, H., J.L. Thorvaldsen, Q. Chu, F. Feng, and M.J. Birnbaum. 2001. b. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349–38352. [DOI] [PubMed] [Google Scholar]
- Coghlan, M.P., A.A. Culbert, D.A. Cross, S.L. Corcoran, J.W. Yates, N.J. Pearce, O.L. Rausch, G.J. Murphy, P.S. Carter, L.R. Cox, et al. 2000. Selective small molecule inhibitors of GSK-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 7:793–803. [DOI] [PubMed] [Google Scholar]
- Cross, D.A., D.R. Alessi, P. Cohen, M. Andjelkovich, and B.A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 378:785–789. [DOI] [PubMed] [Google Scholar]
- Doble, B.W., and J.R. Woodgett. 2003. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, L.J., and A. Klip. 2000. Mechanism and regulation of GLUT-4 vesicle fusion in muscle and fat cells. Am. J. Physiol. Cell Physiol. 279:C877–C890. [DOI] [PubMed] [Google Scholar]
- George, S., J.J. Rochford, C. Wolfrum, S.L. Gray, S. Schinner, J.C. Wilson, M.A. Soos, P.R. Murgatroyd, R.M. Williams, C.L. Acerini, et al. 2004. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 304:1325–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada, M., J. Feng, and B.A. Hemmings. 2004. Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim. Biophys. Acta. 1697:3–16. [DOI] [PubMed] [Google Scholar]
- Inoue, M., L. Chang, J. Wang, S.H. Chiang, A.R. Saltiel. 2003. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 422:629–633. [DOI] [PubMed] [Google Scholar]
- Jiang, Z.Y., Q.L. Zhou, K.A. Coleman, M. Chouinard, Q. Boese, and M.P. Czech. 2003. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA. 100:7569–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad, D., P.J. Bilan, Z. Nawaz, G. Sweeney, W. Niu, Z. Liu, C.N. Antonescu, A. Rudich, and A. Klip. 2002. Need for GLUT4 activation to reach maximum effect of insulin-mediated glucose uptake in brown adipocytes isolated from GLUT4myc-expressing mice. Diabetes. 51:2719–2726. [DOI] [PubMed] [Google Scholar]
- Lawlor, M.A., and D.R. Alessi. 2001. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114:2903–2910. [DOI] [PubMed] [Google Scholar]
- Li, D., G. Sweeney, Q. Wang, and A. Klip. 1999. Participation of PI3K and atypical PKC in Na+-K+-pump stimulation by IGF-I in VSMC. Am. J. Physiol. 276:H2109–H2116. [DOI] [PubMed] [Google Scholar]
- Min, J., S. Okada, M. Kanzaki, J.S. Elmendorf, K.J. Coker, B.P. Ceresa, L.J. Syu, Y. Noda, A.R. Saltiel, and J.E. Pessin. 1999. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol. Cell. 3:751–760. [DOI] [PubMed] [Google Scholar]
- Obata, T., M.B. Yaffe, G.G. Leparc, E.T. Piro, H. Maegawa, A. Kashiwagi, R. Kikkawa, and L.C. Cantley. 2000. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J. Biol. Chem. 275:36108–36115. [DOI] [PubMed] [Google Scholar]
- Okada, T., Y. Kawano, T. Sakakibara, O. Hazeki, and M. Ui. 1994. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J. Biol. Chem. 269:3568–3573. [PubMed] [Google Scholar]
- Olson, A.L., J.B. Knight, and J.E. Pessin. 1997. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 17:2425–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky, Y.Y., A. Koval, C. Hallas, R. Bichi, M. Tresini, S. Malstrom, G. Russo, P. Tsichlis, and C.M. Croce. 2000. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc. Natl. Acad. Sci. USA. 97:3028–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin, J.E., D.C. Thurmond, J.S. Elmendorf, K.J. Coker, and S. Okada. 1999. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J. Biol. Chem. 274:2593–2596. [DOI] [PubMed] [Google Scholar]
- Saltiel, A.R., and J.E. Pessin. 2003. Insulin signaling in microdomains of the plasma membrane. Traffic. 4:711–716. [DOI] [PubMed] [Google Scholar]
- Waters, S.B., K. Yamauchi, and J.E. Pessin. 1995. Insulin-stimulated disassociation of the SOS-Grb2 complex. Mol. Cell. Biol. 15:2791–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, R.T., M. Kanzaki, and J.E. Pessin. 2004. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 25:177–204. [DOI] [PubMed] [Google Scholar]
- Whiteman, E.L., H. Cho, and M.J. Birnbaum. 2002. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13:444–451. [DOI] [PubMed] [Google Scholar]
- Zhao, H., S. Okada, J.E. Pessin, G.A. Koretzky. 1998. Insulin receptor-mediated dissociation of Grb2 from Sos involves phosphorylation of Sos by kinase(s) other than extracellular signal-regulated kinase. J. Biol. Chem. 273:12061–12067. [DOI] [PubMed] [Google Scholar]