Abstract
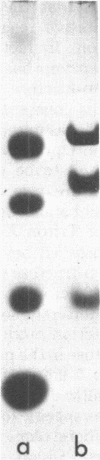
D-Fructose dehydrogenase was solubilized and purified from the membrane fraction of glycerol-grown Gluconobacter industrius IFO 3260 by a procedure involving solubilization of the enzyme with Triton X-100 and subsequent fractionation on diethylaminoethyl-cellulose and hydroxylapatite columns. The purified enzyme was tightly bound to a c-type cytochrome and another peptide existing as a dehydrogenase-cytochrome complex. The purified enzyme was deemed pure by analytical ultracentrifugation as well as by gel filtration on a Sephadex G-200 column. The molecular weight of the enzyme complex was determined to be about 140,000, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed the presence of three components having molecular weights of 67,000 (dehydrogenase), 50,800 (cytochrome c), and 19,700 (unknown function). Only D-fructose was readily oxidized by the enzyme in the presence of dyes such as ferricyanide, 2,6-dichlorophenolindophenol, or phenazine methosulfate. Nicotinamide adenine dinucleotide, nicotinamide adenine dinucleotide phosphate, and oxygen did not function as electron acceptors. The optimum pH of D-fructose oxidation was 4.0. The enzyme was stable at pH 4.5 to 6.0 Stability of the purified enzyme was much enhanced by the presence of detergent in the enzyme solution. Removal of detergent from the enzyme solution facilitated the aggregation of the enzyme and caused its inactivation. An apparent Michaelis constant for D-fructose was observed to be 10(-2) M with the purified enzyme. D-Fructose dehydrogenase was shown to be a satisfactory reagent for microdetermination of D-fructose.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAD G., ENGLARD S. 5-KETO-D-FRUCTOSE. I. CHEMICAL CHARACTERIZATION AND ANALYTICAL DETERMINATION OF THE DICARBONYLHEXOSE PRODUCED BY GLUCONOBACTER CERINUS. J Biol Chem. 1965 Jun;240:2290–2296. [PubMed] [Google Scholar]
- Avigad G., Englard S., Pifko S. 5-Keto-D-fructose. IV. A specific reduced nicotinamide adenine dinucleotide phosphate-linked reductase from Gluconobacter cerinus. J Biol Chem. 1966 Jan 25;241(2):373–378. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- ENGLARD S., AVIGAD G. 5-KETO-D-FRUCTOSE. II. PATTERNS OF FORMATION AND OF ASSOCIATED DEHYDROGENASE ACTIVITIES IN GLUCONOBACTER CERINUS. J Biol Chem. 1965 Jun;240:2297–2310. [PubMed] [Google Scholar]
- ENGLARD S., AVIGAD G., PROSKY L. 5-KETO-D-FRUCTOSE. 3. PROOF OF STRUCTURE BASED ON STEREOSPECIFIC PATTERNS OF ENZYMATIC REDUCTION. J Biol Chem. 1965 Jun;240:2302–2307. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsushita K., Shinagawa E., Adachi O., Ameyama M. Membrane-bound D-gluconate dehydrogenase from Pseudomonas aeruginosa. Purification and structure of cytochrome-binding form. J Biochem. 1979 May;85(5):1173–1181. [PubMed] [Google Scholar]
- Mowshowitz S., Avigad G., Englard S. 5-Keto-D-fructose: formation and utilization in the course of D-fructose as similation by Gluconabacter cerinus. J Bacteriol. 1974 Jun;118(3):1051–1058. doi: 10.1128/jb.118.3.1051-1058.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowshowitz S., Englard S., Avigad G. Metabolic consequences of a block in the synthesis of 5-keto-D-fructose in a mutant of Gluconobacter cerinus. J Bacteriol. 1974 Aug;119(2):363–370. doi: 10.1128/jb.119.2.363-370.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Yamada Y., Aida K., Uemura T. Enzymatic studies on the oxidation of sugar and sugar alcohol. 8. Particle-bound L-sorbose dehydrogenase from Gluconobacter suboxydans. J Biochem. 1969 Oct;66(4):521–527. doi: 10.1093/oxfordjournals.jbchem.a129177. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamada Y., Aida K., Uemura T. Enzymatic studies on the oxidation of sugar and sugar alcohol. I. Purification and properties of particle-bound fructose dehydrogenase. J Biochem. 1967 May;61(5):636–646. doi: 10.1093/oxfordjournals.jbchem.a128594. [DOI] [PubMed] [Google Scholar]