Abstract
One of the characteristics of the mammalian Golgi is its position adjacent to the nucleus. This characteristic is maintained through the action of the microtubule (MT) minus end–directed motor dynein and MT-associated proteins (MAPs). Recent findings suggest that GMAP-210, a member of the golgin family of proteins, may help to link Golgi membranes and vesicles with the MT cytoskeleton. However, there are good grounds to doubt that either GMAP-210 or its yeast homologue Rud3p is a MAP. Instead, they appear to function in vesicle trafficking events at the Golgi together with the GTPase ARF1 and a small membrane protein, Erv14. As such, the interesting question of how the Golgi interacts with MTs may well remain open to further investigation.
Golgi position and the polarity of protein transport
In mammalian cells the position of the Golgi correlates with that of the microtubule (MT)-organizing center or centrosome (Kupfer et al., 1982). Disruption of normal MT cytoskeleton dynamics with antibodies or agents such as colchicine and nocodazole that cause MT depolymerization, or paclitaxel, which stabilizes MTs, results in dispersal of the Golgi into smaller units or “mini-stacks” spread throughout the cell (Robbins and Gonatas, 1964; Wehland et al., 1983; Rogalski and Singer, 1984). Exactly how these structures arise is the subject of some controversy; in brief, two models have been proposed. Either Golgi proteins and membranes directly fragment into smaller units or they are recycled via the ER and reform at the so-called ER exit sites. This topic has been extensively reviewed elsewhere (Glick, 2002). Remarkably, these smaller units still retain the classical stacked cisternal organization of the Golgi (Rogalski et al., 1984). Therefore, MTs are important for the formation of the so-called “ribbon” of Golgi stacks and positioning this adjacent to the centrosome and nucleus. However, despite many years of study, there is still no satisfactory answer to the question of what role MT-dependent Golgi positioning and MTs play in protein secretion.
Early studies suggested that MT-dependent spatial organization of the ER and Golgi might facilitate ER to Golgi traffic in polarized cells of isolated lacrimal glands (Busson-Mabillot et al., 1982). Several more recent observations could explain how this is achieved. First, MT minus end–directed movement of COPII vesicles from ER exit sites toward the Golgi involves dynein (Presley et al., 1997). Second, dynactin, a multisubunit complex that increases the processivity of the dynein motor (King and Schroer, 2000), can bind to components of the COPII vesicle coat. The p150glued subunit of the dynactin complex directly interacts with the COPII coat subunits Sec23A and Sec24D, and expression of the COPII-binding domain of p150glued causes a delay in the formation of ER–Golgi vesicular tubular transport intermediates from COPII vesicles (Watson et al., 2004). However, this is not the whole story because studies using fibroblast cell cultures indicate that the transport kinetics of a model glycoprotein, the vesicular stomatitis virus G protein, between the ER and Golgi or through the Golgi are not significantly altered in cells treated with paclitaxel or nocodazole (Rogalski et al., 1984). Rather, the only aspect of vesicular stomatitis virus G protein transport found to be altered concerned the delivery to the plasma membrane. This occurred adjacent to the ribbonlike Golgi in the control cells, but uniformly over the entire plasma membrane in cells where the Golgi was dispersed by nocodazole treatment (Rogalski et al., 1984). A role for Golgi position in directing secretion has also been suggested by observations that the Golgi polarizes together with the centrosome in migrating fibroblasts, and that this appears to be of significance for the establishment and maintenance of cell polarity (Kupfer et al., 1982; Bergmann et al., 1983; Preisinger et al., 2004). The kinetics of both constitutive and regulated vesicle transport from the TGN to the plasma membrane are reduced in the absence of MTs (Tooze et al., 1991), suggesting that transport from the TGN to the cell surface may therefore be more sensitive to MTs than ER to Golgi and intra-Golgi trafficking. Consistent with this idea, post-TGN transport intermediates have been observed to move along MTs (Toomre et al., 1999).
A more complex situation is found in polarized epithelial cells where MTs are important for cell polarization in addition to membrane trafficking events. It is difficult to make a general case for the role of MT-dependent transport in polarized epithelial cells because they do not all show the same type of MT organization. To give two examples, kidney-derived epithelial cells have vertical MT arrays (Bacallao et al., 1989), whereas polarized hepatocytes have horizontal MT arrays (Meads and Schroer, 1995), which probably reflects the different types of epithelial organization seen in tissues such as the kidney and liver (Cohen et al., 2004). One heavily studied cell type is the kidney-derived MDCK cell, which forms columnar epithelial monolayers, with an apical or lumenal surface separated by tight junctions from the basal and lateral (basolateral) surfaces. These cells have vertical MT arrays, with the MT minus end facing the apical surface, whereas the Golgi and centrosome are positioned above the nucleus below the apical membrane (Bacallao et al., 1989). MT depolymerizing drugs cause a reduction in the rate of transport to the apical but not the basolateral surface and cause some of the apical cargo to be mis-sorted to the basolateral surface (Rindler et al., 1987; Breitfeld et al., 1990; Grindstaff et al., 1998). Consistent with the MT polarity in these cells, vesicle delivery to the apical surface involves the MT minus end–directed motor dynein, and potentially additional motor proteins of the kinesin family (Lafont et al., 1994). Thus, MTs are likely to be important for organizing the secretory pathway and directing vesicles to specific destinations within the cell or subdomains of the plasma membrane in both polarized and fibroblast-like cells.
Positioning the Golgi
The first clues to the mechanism underlying MT-dependent Golgi positioning in the perinuclear region came from experiments using an in vitro cell assay, where the ability of permeabilized cells to capture exogenous Golgi membranes was followed (Corthesy-Theulaz et al., 1992). This assay was dependent on cytosolic factors and could be blocked by the depletion of dynein or the addition of antibodies inhibitory to dynein function (Corthesy-Theulaz et al., 1992). Some years later it was shown that expression of the p50 subunit of dynactin results in dispersal of the Golgi from the perinuclear region (Burkhardt et al., 1997). Finally, cells lacking dynein are unable to concentrate organelles such as the Golgi and lysosomes in the perinuclear region, although, interestingly, the Golgi fragments are still associated with MTs (Harada et al., 1998). These and other observations have led to a model in which dynactin together with additional accessory factors regulates dynein-dependent vesicle and organelle movement at the level of cargo binding (Allan et al., 2002).
How do these components actually result in positioning of the Golgi? Cellular MTs are in a constant state of flux termed dynamic instability, where they switch from growth to collapse, and thus over time the volume of the cytoplasm is probed by MTs. Dynactin is concentrated at MT plus ends and can transiently capture Golgi membranes that are then transported toward the minus ends by dynein (Vaughan et al., 2002). Golgi membranes are thus concentrated in the perinuclear region by a MT search, dynein–dynactin capture process. It is important to note that this does not suggest that the dynein–dynactin complex is the only factor mediating attachment of the Golgi to MTs.
Identifying Golgi MT-associated proteins (MAPs}
Several groups have attempted to purify factors linking the Golgi with MTs using sophisticated biochemical assays (Bashour and Bloom, 1998; Hennig et al., 1998); however, this approach has not proven to be as successful as originally hoped for. Using such assays, two groups identified the same Golgi-associated factor, the enzyme formiminotransferase cyclodeaminase (FTCD). However, this is a liver-specific enzyme and unlikely to act as a general MT linker at the Golgi (Bashour and Bloom, 1998; Hennig et al., 1998). Tubulin is typically purified from brain for such assays, and with hindsight this turns out to be problematic. Bovine brain tubulin is heavily modified by the addition of polyglutamates, and it is suspected that FTCD interacts with MTs via these because it does not bind to MTs assembled from liver tubulin that essentially lacks this modification (Bashour and Bloom, 1998). Although in some cases posttranslational modification of tubulin may play an important role in modulating the interaction of motor proteins and MAPs with the MT surface (Rosenbaum, 2000), it is unlikely that binding of FTCD to polyglutamylated MTs is of significance for Golgi positioning. Moreover, the specificity of the MT interaction has to be questioned because FTCD has also been found associated with the vimentin intermediate filament network (Gao and Sztul, 2001). Therefore, the search for proteins linking the Golgi to MTs has continued and has recently become focused on the role of the golgin family of proteins as potential Golgi MAPs.
A confusing MAP of the Golgi
GMAP-210 was originally identified as a cis-Golgi localized autoantigen of 210 kD using the serum from a patient with an autoimmune condition known as Sjögrens syndrome (Rios et al., 1994). It was subsequently characterized at the molecular level and found to have extensive regions of predicted coiled-coil (Infante et al., 1999), justifying its classification as a member of the golgin family of proteins (Barr and Short, 2003; Gillingham and Munro, 2003). Golgins have diverse functions at the Golgi, but it is believed that together they form a Golgi matrix giving identity, shape, and stacked organization to Golgi cisternae (Barr and Short, 2003; Gillingham and Munro, 2003). Therefore, the finding that GMAP-210 interacts with MTs was significant (Infante et al., 1999) because it provided a link between Golgi structure and positioning. When the MTs were sheared, increasing the number of free ends without changing the concentration of MTs themselves, an increase in bound GMAP-210 was seen hinting that it is a MT end binding protein. This interaction was mapped to amino acids 1597 to 1979 at the COOH terminus of GMAP-210, whereas the Golgi-targeting signal was found in the first 375 amino acids. Both of these domains, when expressed at high level, caused perturbations in Golgi structure. Thus, GMAP-210 was proposed to bind a subpopulation of MT ends through its COOH terminus and to the Golgi via its NH2 terminus (Infante et al., 1999). An interesting aspect of this work was the finding that the GMAP-210 COOH-terminal domain accumulated at the centrosome when expressed as a GFP fusion rather than decorating MTs and could displace a marker for the pericentriolar material defined using the CTR453 monoclonal antibody (Infante et al., 1999). One of the key centrosomal components required for initiating MT assembly in vivo is the γ-tubulin ring complex (γ-TURC; for review see Schiebel, 2000). Recently, the same authors have shown that GMAP-210 could be immunoprecipitated with components of the γ-TURC under conditions where α-tubulin was not (Rios et al., 2004). This finding might explain the increased binding of GMAP-210 to sheared MTs, as γ-TURC interacts with MT minus ends. Therefore, displacement of centrosomal proteins caused by the GMAP-210 COOH terminus may be due to sequestration of the γ-TURC. Because the γ-TURC plays a key role in MT nucleation and attachment at the centrosome, Rios et al. (2004) reasoned that GMAP-210 could nucleate and/or attach MTs at the Golgi (Chabin-Brion et al., 2001). Consistent with the hypothesis, they found that cells expressing high levels of GMAP-210 show some recruitment of γ-tubulin to Golgi structures, indicating that GMAP-210 may act as a targeting factor for γ-TURC. They also found that a polyclonal antibody to γ-tubulin stained both the centrosomes and the Golgi, although this is a little surprising because other antibodies to γ-tubulin and GFP-tagged γ-tubulin have not indicated a Golgi pool of this protein (Murphy et al., 1998; Khodjakov and Rieder, 1999).
GMAP-210 is not the only protein reported to interact with both MT ends and the Golgi. CLASPs (CLIP-associating proteins) are a family of proteins important for MT organization at the leading edge of motile fibroblasts that bind to MT plus ends and localize to both the cell cortex and the Golgi apparatus (Akhmanova et al., 2001; Mimori-Kiyosue et al., 2005). Together with the MT end binding factor, EB1, their main activity is to promote MT stability and interaction of MT plus ends with the cell cortex (Mimori-Kiyosue et al., 2005). What role CLASPs play at the Golgi is less clear, but because cells depleted of CLASPs using RNA interference have a less dense disorganized MT network and a pool of CLASP1 and CLASP2 is present at the Golgi (Mimori-Kiyosue et al., 2005) it is possible that this also alters MT organization around the Golgi and, as a consequence, Golgi positioning.
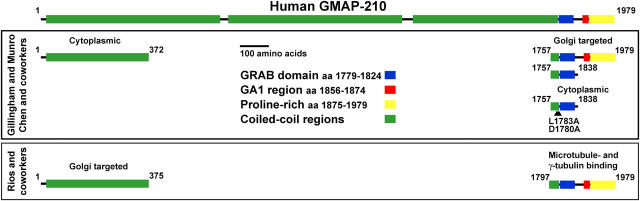
These findings have been brought together in a model where GMAP-210 captures short MT seeds formed at the centrosome by their minus ends and, together with the Golgi-localized pool of the CLASPs that attach to and stabilize the MT plus ends, generates a meshwork of short MTs associated with adjacent Golgi stacks and links them to form a ribbon (Rios et al., 2004). By interacting with MTs emanating from the centrosome, this meshwork would then keep the Golgi in the pericentriolar region. However, there are some problems with this model concerning the function of GMAP-210 and how it targets to the Golgi (summarized in Fig. 1).
Figure 1.
GMAP-210 targeting to the Golgi. A schematic depiction of human GMAP-210 showing the predicted segments of coiled-coil (green), the GRAB domain (blue) and GRAB-associated region (GA1; red), and the proline-rich hydrophobic sequence (yellow). Nomenclature is that which has been used previously (Gillingham et al., 2004). The data of Gillingham and Munro (2003) and Chen et al. (1999) are summarized in the top panel. They identified a minimal Golgi-targeting region between amino acids 1757 and 1838, the GRAB domain. Deletion of 1754–1866 aa, removing the GRAB domain, or mutation of the conserved aspartate 1780 and leucine 1783 to alanine abolishes Golgi targeting of GMAP-210 (Chen et al., 1999; Gillingham et al., 2004). Neither group found a role for the NH2 terminus in Golgi targeting. The data of Rios et al. (2004) and Infante et al. (1999) are summarized in the bottom panel. They described 1–375 aa as a Golgi-targeting region and 1797–1979 aa as a MT- and γ-tubulin complex–binding domain.
Rud3p is a putative yeast golgin associated with early Golgi compartments, originally identified as a suppressor of mutants in ER to Golgi vesicle traffic (Kim et al., 1999; VanRheenen et al., 1999). Rud3p is not an essential gene in budding yeast but its deletion results in glycosylation defects, suggesting it is important for Golgi function, perhaps in recycling glycosyltransferases (Kim, 2003). Initially, Rud3p was proposed to be the yeast homologue of golgin-160; however, a recent paper shows that it is in fact related to GMAP-210 (Gillingham et al., 2004). When analyzing the mechanism by which Rud3p targets to the budding yeast Golgi, Gillingham and Munro (2003) identified a COOH-terminal region of 126 amino acids responsible for Golgi localization. This region shows some similarities with the GRIP domain found in a family of other golgins (Barr and Short, 2003; Gillingham and Munro, 2003). Because the GRIP domain functions by binding a Golgi-localized GTPase of the ARF-like (ARL) family, they reasoned that the Golgi-targeting region of Rud3p might also bind a GTPase. In vitro binding assays revealed that the Golgi-targeting region of Rud3p bound to two ARF family GTPases, Arf1p and Arf3p, and that mutations in a key leucine residue abolished Golgi targeting and Arf1p binding. Therefore, this region was termed the GRIP-related ARF-binding (GRAB) domain. Sequence analysis showed that the GRAB domain is also found in GMAP-210 and a variety of related coiled-coiled proteins from a wide range of eukaryotes. One aspect of these proteins that is not conserved is the NH2-terminal region proposed to be responsible for Golgi targeting of GMAP-210 (Rios et al., 2004). Gillingham and Munro (2003) then analyzed the targeting of GMAP-210 and found that like Rud3p the GRAB domain–containing COOH terminus localized to the Golgi, whereas the NH2-terminal 372 amino acids did not. Furthermore, neither full-length GMAP-210 nor the GRAB domain were found at centrosomes, and expression of the GMAP-210 COOH-terminal domain also failed to recruit γ-tubulin to the Golgi (Gillingham et al., 2004). A previous paper on GMAP-210, where the authors believed that it was a potential transcriptional coactivator they called Trip230, also found that the region containing GRAB domain targets to the Golgi (Chen et al., 1999). This finding raises two apparent contradictions. First, the GRAB domain falls within the region previously shown by Rios et al. (2004) to bind to the γ-TURC and hence MTs, and also to localize to the centrosome (Infante et al., 1999). Second, the GMAP-210 NH2 terminus and not the COOH terminus was claimed to localize to the Golgi (Infante et al., 1999). One potential explanation for these differences is that the different constructs and epitope tags used give confusing results. However, our unpublished observations using constructs designed to match those of Rios et al. (2004) with a variety of epitope tags fused at either the NH2 or COOH terminus fail to confirm their findings, but are in agreement with Chen et al. (1999) and Gillingham et al. (2004). We have also raised specific antibodies to detect endogenous human GMAP-210, and these stain the Golgi but not centrosomes. Thus, the COOH-terminal GRAB domain of GMAP-210 contains Golgi rather than centrosomal targeting information. Whether or not it also binds MTs is still open to debate, but this dual function seems unlikely because mutations that abolish ARF binding are diffuse in the cytoplasm and do not obviously target to centrosomes or MT structures (Gillingham et al., 2004). It is of course possible that the GRAB domain can also bind to the γ-TURC, but one would then have to assume that the mutations inactivating ARF binding also abolish an interaction with γ-TURC. Such a model would then only be true for GMAP-210 in mammalian cells because budding yeast Golgi is not reported to show any interactions with MTs. One outstanding issue is that a direct interaction between human ARF1 and GMAP-210 has not been demonstrated to date; thus, further investigation of GMAP-210 will be needed to clarify both its Golgi targeting and putative MT binding properties.
What exactly is the primary function of the GRAB domain–containing proteins if not in MT binding? Synthetic lethality is a powerful tool of the yeast genetic toolbox that allows insight into the function of cellular pathways that are redundant or not required for growth under laboratory conditions but are likely to be important under physiological growth conditions. In simple terms, one asks which genes, when deleted or mutated in combination with the gene of interest, cause a lethal growth defect. Further investigation of the RUD3 gene revealed that it is synthetically lethal with RIC1, the gene encoding the exchange factor for budding yeast Ypt6p (Rab6 in mammals; Gillingham et al., 2004). A previous paper had defined a list of genes synthetically lethal with the RIC1 deletion, and strains deleted for these genes were then tested for the ability to target a GFP-Rud3p fusion protein to the Golgi. A single gene encoding a small membrane protein Erv14p was identified by this approach (Gillingham et al., 2004). Erv14p recycles between the ER and Golgi and is required for the selection of a subset of cargo molecules into COPII vesicles (Powers and Barlowe, 1998). Exactly how Erv14p contributes to Rud3p targeting requires further investigation because it does not appear to directly interact with Rud3p, but it is a highly conserved molecule and thus likely to be important for Golgi targeting of GRAB domain proteins in other organisms. It is difficult to assemble all these findings into a compelling model, but they suggest that Rud3p, and by extension other GRAB domain proteins including GMAP-210, cooperates with both the Ypt6/Rab6-dependent transport pathway and Erv14p-dependent ER to Golgi transport to maintain normal Golgi function. Consistent with this idea, overexpression of GMAP-210 blocks recycling between the ER and Golgi (Pernet-Gallay et al., 2002).
Rab6 and the MT cytoskeleton
If GMAP-210 is not a Golgi MAP, then what other factors are important for MT-dependent Golgi positioning? Two candidates can be found in the literature, both putative binding partners for the Rab family GTPase Rab6. The first of these is rabkinesin-6, identified in a yeast two-hybrid screen for interaction partners of mammalian Rab6a and initially proposed to function in a MT-dependent transport step from the Golgi to the ER (Echard et al., 1998; White et al., 1999). Subsequently, it was found that Rabkinesin-6 is a cell cycle–regulated protein absent from interphase cells and enriched during mitosis where it plays an essential role in cytokinesis (Hill et al., 2000; Fontijn et al., 2001). Rather than carrying vesicle membranes, it seems that its key function is to transport two mitotic kinases, Plk1 and Aurora B (Neef et al., 2003; Gruneberg et al., 2004).
The second candidate is Bicaudal-D, a Golgi-localized binding partner for the dynein–dynactin complex (Hoogenraad et al., 2001). Bicaudal-D targets to membranes through an interaction with Rab6, and thus recruits the dynein–dynactin complex to Golgi membranes (Matanis et al., 2002; Short et al., 2002). The main function of this interaction appears to be in a MT-dependent recycling pathway from the TGN to the ER (Young et al., 2005). Therefore, it is unlikely that either of these factors plays a significant role in maintenance of Golgi positioning in interphase cells.
Understanding MTs and Golgi function
Although MTs are important for Golgi positioning in mammalian cells, this is not true in all organisms. In, Drosophila melanogaster for example, the Golgi does not form a ribbon and is found as discrete exocytic units evenly dispersed throughout the cytoplasm (Stanley et al., 1997). Nevertheless, there is order to Golgi positioning in D. melanogaster, and Golgi stacks are associated with ER exit sites (Kondylis and Rabouille, 2003). Like mammalian cells, D. melanogaster cells are capable of polarized delivery of cargo to the plasma membrane. The question is how is this achieved? Rather than MT-dependent positioning, D. melanogaster can restrict specific mRNAs to subregions of the ER such that specific exocytic units (Golgi stacks) deliver this cargo to a local region of the cell surface (Herpers and Rabouille, 2004). Similar considerations apply to plants, which also lack a ribbonlike Golgi and organize many small Golgi stacks at ER exit sites (daSilva et al., 2004). Again ER to Golgi transport is not disrupted by nocodazole (Brandizzi et al., 2002), suggesting that the MT cytoskeleton is not required for Golgi positioning or secretion in plants.
Specializations of the secretory pathway have clearly arisen throughout evolution, for example, the regulated secretory pathway important for release of neurotransmitters, stored peptide hormones, and digestive enzymes, and it is important to understand these. The role of MTs in vesicle transport and Golgi positioning is probably best viewed as just such a specialization. This problem, like many other aspects of Golgi form and function, seems to have generated more arguments than answers (Farquhar and Palade, 1998). Maybe this reflects the problems associated with understanding the complex problem of how organelles work and our willingness to oversimplify complex problems. As we have discussed here, there are still many open questions about how the mammalian Golgi interacts with MTs. That is surely a good reason to continue studying the problem.
Acknowledgments
The authors wish to thank Martina Casenghi, Alison Gillingham, and Sean Munro for helpful discussion and comments; Dr. Anna Akhmanova for invaluable advice on the function of CLASPs; and members of the Barr group for allowing discussion of their unpublished data and for reading the manuscript.
The Max-Planck Society supports research in the laboratory of F.A. Barr.
Abbreviations used in this paper: FTCD, formiminotransferase cyclodeaminase; GRAB, GRIP-related ARF binding; MAP, MT-associated protein; MT, microtubule.
References
- Akhmanova, A., C.C. Hoogenraad, K. Drabek, T. Stepanova, B. Dortland, T. Verkerk, W. Vermeulen, B.M. Burgering, C.I. De Zeeuw, F. Grosveld, and N. Galjart. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 104:923–935. [DOI] [PubMed] [Google Scholar]
- Allan, V.J., H.M. Thompson, and M.A. McNiven. 2002. Motoring around the Golgi. Nat. Cell Biol. 4:E236–E242. [DOI] [PubMed] [Google Scholar]
- Bacallao, R., C. Antony, C. Dotti, E. Karsenti, E.H. Stelzer, and K. Simons. 1989. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J. Cell Biol. 109:2817–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, F.A., and B. Short. 2003. Golgins in the structure and dynamics of the Golgi apparatus. Curr. Opin. Cell Biol. 15:405–413. [DOI] [PubMed] [Google Scholar]
- Bashour, A.M., and G.S. Bloom. 1998. 58K, a microtubule-binding Golgi protein, is a formiminotransferase cyclodeaminase. J. Biol. Chem. 273:19612–19617. [DOI] [PubMed] [Google Scholar]
- Bergmann, J.E., A. Kupfer, and S.J. Singer. 1983. Membrane insertion at the leading edge of motile fibroblasts. Proc. Natl. Acad. Sci. USA. 80:1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi, F., E.L. Snapp, A.G. Roberts, J. Lippincott-Schwartz, and C. Hawes. 2002. Membrane protein transport between the endoplasmic reticulum and the Golgi in tobacco leaves is energy dependent but cytoskeleton independent: evidence from selective photobleaching. Plant Cell. 14:1293–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld, P.P., W.C. McKinnon, and K.E. Mostov. 1990. Effect of nocodazole on vesicular traffic to the apical and basolateral surfaces of polarized MDCK cells. J. Cell Biol. 111:2365–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt, J.K., C.J. Echeverri, T. Nilsson, and R.B. Vallee. 1997. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139:469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson-Mabillot, S., A.M. Chambaut-Guerin, L. Ovtracht, P. Muller, and B. Rossignol. 1982. Microtubules and protein secretion in rat lacrimal glands: localization of short-term effects of colchicine on the secretory process. J. Cell Biol. 95:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabin-Brion, K., J. Marceiller, F. Perez, C. Settegrana, A. Drechou, G. Durand, and C. Pous. 2001. The Golgi complex is a microtubule-organizing organelle. Mol. Biol. Cell. 12:2047–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., P.L. Chen, C.F. Chen, Z.D. Sharp, and W.H. Lee. 1999. Thyroid hormone, T3-dependent phosphorylation and translocation of Trip230 from the Golgi complex to the nucleus. Proc. Natl. Acad. Sci. USA. 96:4443–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, D., P.J. Brennwald, E. Rodriguez-Boulan, and A. Musch. 2004. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol. 164:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy-Theulaz, I., A. Pauloin, and S.R. Pfeffer. 1992. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J. Cell Biol. 118:1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva, L.L., E.L. Snapp, J. Denecke, J. Lippincott-Schwartz, C. Hawes, and F. Brandizzi. 2004. Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell. 16:1753–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echard, A., F. Jollivet, O. Martinez, J.J. Lacapere, A. Rousselet, I. Janoueix-Lerosey, and B. Goud. 1998. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 279:580–585. [DOI] [PubMed] [Google Scholar]
- Farquhar, M.G., and G.E. Palade. 1998. The Golgi apparatus: 100 years of progress and controversy. Trends Cell Biol. 8:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontijn, R.D., B. Goud, A. Echard, F. Jollivet, J. van Marle, H. Pannekoek, and A.J. Horrevoets. 2001. The human kinesin-like protein RB6K is under tight cell cycle control and is essential for cytokinesis. Mol. Cell. Biol. 21:2944–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y., and E. Sztul. 2001. A novel interaction of the Golgi complex with the vimentin intermediate filament cytoskeleton. J. Cell Biol. 152:877–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham, A.K., and S. Munro. 2003. Long coiled-coil proteins and membrane traffic. Biochim. Biophys. Acta. 1641:71–85. [DOI] [PubMed] [Google Scholar]
- Gillingham, A.K., A.H. Tong, C. Boone, and S. Munro. 2004. The GTPase Arf1p and the ER to Golgi cargo receptor Erv14p cooperate to recruit the golgin Rud3p to the cis-Golgi. J. Cell Biol. 167:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick, B.S. 2002. Can the Golgi form de novo? Nat. Rev. Mol. Cell Biol. 3:615–619. [DOI] [PubMed] [Google Scholar]
- Grindstaff, K.K., R.L. Bacallao, and W.J. Nelson. 1998. Apiconuclear organization of microtubules does not specify protein delivery from the trans-Golgi network to different membrane domains in polarized epithelial cells. Mol. Biol. Cell. 9:685–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg, U., R. Neef, R. Honda, E.A. Nigg, and F.A. Barr. 2004. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, A., Y. Takei, Y. Kanai, Y. Tanaka, S. Nonaka, and N. Hirokawa. 1998. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J. Cell Biol. 141:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig, D., S.J. Scales, A. Moreau, L.L. Murley, J. De Mey, and T.E. Kreis. 1998. A formiminotransferase cyclodeaminase isoform is localized to the Golgi complex and can mediate interaction of trans-Golgi network-derived vesicles with microtubules. J. Biol. Chem. 273:19602–19611. [DOI] [PubMed] [Google Scholar]
- Herpers, B., and C. Rabouille. 2004. mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes. Mol. Biol. Cell. 15:5306–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, E., M. Clarke, and F.A. Barr. 2000. The Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO J. 19:5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad, C.C., A. Akhmanova, S.A. Howell, B.R. Dortland, C.I. De Zeeuw, R. Willemsen, P. Visser, F. Grosveld, and N. Galjart. 2001. Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J. 20:4041–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante, C., F. Ramos-Morales, C. Fedriani, M. Bornens, and R.M. Rios. 1999. GMAP-210, a cis-Golgi network-associated protein, is a minus end microtubule-binding protein. J. Cell Biol. 145:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov, A., and C.L. Rieder. 1999. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.W. 2003. Characterization of Grp1p, a novel cis-Golgi matrix protein. Biochem. Biophys. Res. Commun. 303:370–378. [DOI] [PubMed] [Google Scholar]
- Kim, D.W., M. Sacher, A. Scarpa, A.M. Quinn, and S. Ferro-Novick. 1999. High-copy suppressor analysis reveals a physical interaction between Sec34p and Sec35p, a protein implicated in vesicle docking. Mol. Biol. Cell. 10:3317–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S.J., and T.A. Schroer. 2000. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat. Cell Biol. 2:20–24. [DOI] [PubMed] [Google Scholar]
- Kondylis, V., and C. Rabouille. 2003. A novel role for dp115 in the organization of tER sites in Drosophila. J. Cell Biol. 162:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer, A., D. Louvard, and S.J. Singer. 1982. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc. Natl. Acad. Sci. USA. 79:2603–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont, F., J.K. Burkhardt, and K. Simons. 1994. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature. 372:801–803. [DOI] [PubMed] [Google Scholar]
- Matanis, T., A. Akhmanova, P. Wulf, E. Del Nery, T. Weide, T. Stepanova, N. Galjart, F. Grosveld, B. Goud, C.I. De Zeeuw, et al. 2002. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat. Cell Biol. 4:986–992. [DOI] [PubMed] [Google Scholar]
- Meads, T., and T.A. Schroer. 1995. Polarity and nucleation of microtubules in polarized epithelial cells. Cell Motil. Cytoskeleton. 32:273–288. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue, Y., I. Grigoriev, G. Lansbergen, H. Sasaki, C. Matsui, F. Severin, N. Galjart, F. Grosveld, I. Vorobjev, S. Tsukita, and A. Akhmanova. 2005. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 168:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, S.M., L. Urbani, and T. Stearns. 1998. The mammalian γ-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p. J. Cell Biol. 141:663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef, R., C. Preisinger, J. Sutcliffe, R. Kopajtich, E.A. Nigg, T.U. Mayer, and F.A. Barr. 2003. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet-Gallay, K., C. Antony, L. Johannes, M. Bornens, B. Goud, and R.M. Rios. 2002. The overexpression of GMAP-210 blocks anterograde and retrograde transport between the ER and the Golgi apparatus. Traffic. 3:822–832. [DOI] [PubMed] [Google Scholar]
- Powers, J., and C. Barlowe. 1998. Transport of axl2p depends on erv14p, an ER-vesicle protein related to the Drosophila cornichon gene product. J. Cell Biol. 142:1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger, C., B. Short, V. De Corte, E. Bruyneel, A. Haas, R. Kopajtich, J. Gettemans, and F.A. Barr. 2004. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3ζ. J. Cell Biol. 164:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley, J.F., N.B. Cole, T.A. Schroer, K. Hirschberg, K.J. Zaal, and J. Lippincott-Schwartz. 1997. ER-to-Golgi transport visualized in living cells. Nature. 389:81–85. [DOI] [PubMed] [Google Scholar]
- Rindler, M.J., I.E. Ivanov, and D.D. Sabatini. 1987. Microtubule-acting drugs lead to the nonpolarized delivery of the influenza hemagglutinin to the cell surface of polarized Madin-Darby canine kidney cells. J. Cell Biol. 104:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, R.M., A.M. Tassin, C. Celati, C. Antony, M.C. Boissier, J.C. Homberg, and M. Bornens. 1994. A peripheral protein associated with the cis-Golgi network redistributes in the intermediate compartment upon brefeldin A treatment. J. Cell Biol. 125:997–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, R.M., A. Sanchis, A.M. Tassin, C. Fedriani, and M. Bornens. 2004. GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell. 118:323–335. [DOI] [PubMed] [Google Scholar]
- Robbins, E., and N.K. Gonatas. 1964. Histochemical and ultrastructural studies on Hela cell cultures exposed to spindle inhibitors with special reference to the interphase cell. J. Histochem. Cytochem. 12:704–711. [DOI] [PubMed] [Google Scholar]
- Rogalski, A.A., and S.J. Singer. 1984. Associations of elements of the Golgi apparatus with microtubules. J. Cell Biol. 99:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski, A.A., J.E. Bergmann, and S.J. Singer. 1984. Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J. Cell Biol. 99:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, J. 2000. Cytoskeleton: functions for tubulin modifications at last. Curr. Biol. 10:R801–R803. [DOI] [PubMed] [Google Scholar]
- Schiebel, E. 2000. Gamma-tubulin complexes: binding to the centrosome, regulation and microtubule nucleation. Curr. Opin. Cell Biol. 12:113–118. [DOI] [PubMed] [Google Scholar]
- Short, B., C. Preisinger, J. Schaletzky, R. Kopajtich, and F.A. Barr. 2002. The Rab6 GTPase regulates recruitment of the dynactin complex to Golgi membranes. Curr. Biol. 12:1792–1795. [DOI] [PubMed] [Google Scholar]
- Stanley, H., J. Botas, and V. Malhotra. 1997. The mechanism of Golgi segregation during mitosis is cell type-specific. Proc. Natl. Acad. Sci. USA. 94:14467–14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomre, D., P. Keller, J. White, J.C. Olivo, and K. Simons. 1999. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J. Cell Sci. 112:21–33. [DOI] [PubMed] [Google Scholar]
- Tooze, S.A., T. Flatmark, J. Tooze, and W.B. Huttner. 1991. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J. Cell Biol. 115:1491–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen, S.M., X. Cao, S.K. Sapperstein, E.C. Chiang, V.V. Lupashin, C. Barlowe, and M.G. Waters. 1999. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J. Cell Biol. 147:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, P.S., P. Miura, M. Henderson, B. Byrne, and K.T. Vaughan. 2002. A role for regulated binding of p150Glued to microtubule plus ends in organelle transport. J. Cell Biol. 158:305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, P., R. Forster, K.J. Palmer, R. Pepperkok, and D.J. Stephens. 2004. Coupling of ER exit to microtubules through direct interaction of COPII with dynactin. Nat Cell Biol. 7:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland, J., M. Henkart, R. Klausner, and I.V. Sandoval. 1983. Role of microtubules in the distribution of the Golgi apparatus: effect of taxol and microinjected anti-alpha-tubulin antibodies. Proc. Natl. Acad. Sci. USA. 80:4286–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J., L. Johannes, F. Mallard, A. Girod, S. Grill, S. Reinsch, P. Keller, B. Tzschaschel, A. Echard, B. Goud, and E.H. Stelzer. 1999. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 147:743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J., T. Stauber, E. Del Nery, I. Vernos, R. Pepperkok, and T. Nilsson. 2005. Regulation of microtubule-dependent recycling at the trans-Golgi network by Rab6A and Rab6A'. Mol. Biol. Cell. 16:162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]