Abstract
Canonical Wnt signaling instructively promotes sensory neurogenesis in early neural crest stem cells (eNCSCs) (Lee, H.Y., M. Kléber, L. Hari, V. Brault, U. Suter, M.M. Taketo, R. Kemler, and L. Sommer. 2004. Science. 303:1020–1023). However, during normal development Wnt signaling induces a sensory fate only in a subpopulation of eNCSCs while other cells maintain their stem cell features, despite the presence of Wnt activity. Hence, factors counteracting Wnt signaling must exist. Here, we show that bone morphogenic protein (BMP) signaling antagonizes the sensory fate-inducing activity of Wnt/β-catenin. Intriguingly, Wnt and BMP act synergistically to suppress differentiation and to maintain NCSC marker expression and multipotency. Similar to NCSCs in vivo, NCSCs maintained in culture alter their responsiveness to instructive growth factors with time. Thus, stem cell development is regulated by combinatorial growth factor activities that interact with changing cell-intrinsic cues.
Introduction
Because of its broad developmental potential, the neural crest represents an ideal model system to study stem cell biology. Neural crest cells delaminate from the developing dorsal neural tube, migrate to various locations within the embryo, and generate multiple neural cell types of the vertebrate peripheral nervous system (PNS) as well as several nonneural tissues (Le Douarin and Dupin, 2003). At least some neural crest cells are multipotent, giving rise to neuronal, glial, and nonneural derivatives, as has been shown by clonal analysis in culture and in vivo. Moreover, some crest cells have features of stem cells with the capacity to self-renew. Both in migrating neural crest and in crest-derived tissues, such multipotent stem and progenitor cells coexist with cells that display more restricted developmental potential.
Recently we have described an early population of neural crest stem cells (eNCSCs) that is highly homogeneous with respect to marker expression and the potential to generate autonomic and sensory neurons, glia, and smooth muscle cells (Lee et al., 2004). Clonal cell culture experiments revealed the responsiveness of these cells to several instructive growth factors that promote the generation of specific cell fates at the expense of other possible fates. These factors include members of the TGFβ factor family that promote autonomic neurogenesis and the development of smooth muscle–like cells, neuregulin1 (NRG1) isoforms that induce the generation of peripheral glia, and Wnt signaling that promotes sensory neurogenesis in a β-catenin–dependent manner (Le Douarin and Dupin, 2003; Kléber and Sommer, 2004). In vivo and in cell culture, eNCSCs that lack the Wnt signaling component β-catenin fail to generate sensory neurons (Hari et al., 2002). Complementary to this, embryos that express a constitutively active form of β-catenin specifically in NCSCs generate almost exclusively sensory neural cells at the expense of other possible neural crest–derived lineages (Lee et al., 2004). Thus, unlike in other stem cell types, canonical Wnt signaling on its own does not support stem cell expansion in NCSCs, but rather promotes sensory neurogenesis. So far, the signals supporting NCSC maintenance and self-renewal have not been identified, although it has been possible to passage both migratory and postmigratory NCSCs for some generations in a nondefined medium (Stemple and Anderson, 1992; Bixby et al., 2002).
Given the expression of Wnts in the dorsal neural tube at the time of neural crest emigration and the sensitivity of almost all eNCSCs to Wnt signaling (Parr et al., 1993; Lee et al., 2004), the question arises as to why during normal development only some cells adopt a sensory neural fate as they emigrate while other neural crest cells remain multipotent and can contribute to multiple nonsensory neural crest derivatives (Kléber and Sommer, 2004). Similarly, it remains to be shown why bone morphogenic proteins (BMPs) that are able to induce autonomic neurogenesis (Reissmann et al., 1996; Shah et al., 1996) and that are secreted by the ectoderm and the dorsal neural tube (Liem et al., 1995) do not lead to the generation of autonomic neurons in neural crest cells immediately after their delamination from the neural tube. Convergence of BMP and canonical Wnt signaling has been reported in several studies (Nelson and Nusse, 2004). We therefore investigated whether BMP and Wnt might act in a mutually antagonistic fashion to suppress neurogenesis in NCSCs. We find that combinatorial Wnt/BMP signaling not only suppresses differentiation of NCSCs, but also acts synergistically to maintain stem cell multipotency.
Results
BMP signaling suppresses sensory neurogenesis in early neural crest stem cells
Previously, conditions have been established that are permissive for the generation of sensory neurons from neural crest explant cultures (Greenwood et al., 1999). At early stages, a high percentage of all neural crest cells in these cultures are early neural crest stem cells (eNCSCs) that in clonal cell cultures can be challenged by various instructive growth factors to give rise to sensory or autonomic neurons, or nonneural smooth muscle–like cells (Lee et al., 2004). These data indicated that with respect to their potentials, explants of eNCSCs represent a highly homogeneous cell population. Consistent with these findings, the cells in such explants expressed the NCSC markers p75 and Sox10 after 20 h in culture (Stemple and Anderson, 1992; Paratore et al., 2001), whereas no sensory neuronal precursors marked by Brn-3A (Fedtsova and Turner, 1995) or neurofilament (NF) 160 were detectable at this stage (Fig. 1, A–D) (Lee et al., 2004). However, upon incubation for an additional 24 h, 9.4 ± 1.6% of all cells per explant expressed Brn-3A (Fig. 1, E, F, and K), as described previously (Greenwood et al., 1999). Based on our previous clonal analyses (Lee et al., 2004), these Brn-3A–positive cells likely arose from multipotent eNCSCs. Alternatively, they might have been generated from lineage-restricted sensory neuron precursors that cannot be respecified by factors promoting alternative fates (Greenwood et al., 1999). To distinguish between these possibilities, we challenged neural crest explant cultures with BMP2, a factor inducing autonomic neurogenesis in NCSCs at the expense of other lineages (Shah et al., 1996). BMP2-treated cultures gave rise to only 0.1 ± 0.1% Brn-3A–positive sensory neurons, whereas the generation of autonomic progenitors expressing the transcription factor Mash-1 was promoted (Fig. 1, G–K). In the presence of BMP2, sensory neurogenesis was fully suppressed rather than simply delayed, as even after prolonged incubation no sensory neurons formed (0.5 ± 0.1% and 29.7 ± 6.3% Brn-3A–positive cells in BMP2-treated vs. control cultures) (Fig. 2, A and B). In contrast, the formation of autonomic neurons that expressed NF but not Brn-3A was induced by BMP2. Thus, BMP signaling prevents sensory neurogenesis in early neural crest explant cultures, demonstrating the absence of committed sensory progenitors in these cultures.
Figure 1.

BMP suppresses sensory neurogenesis in eNCSCs. eNCSCs were allowed to emigrate from neural tubes in conditions permissive for sensory neurogenesis. After 20 h in culture, all eNCSCs express the stem cell markers Sox10 (green) and p75 (red) (B), but not the sensory marker Brn-3A or NF (D). Neural crest explants that were challenged with BMP2 for an additional 24 h fail to generate Brn-3A–positive sensory neurons (H) and instead express the autonomic marker Mash-1 (J, arrows). In the absence of BMP2, Brn-3A–positive sensory neurons are generated (F, arrowhead). (K) Quantification of the average percentage of Brn-3A–positive cells per explant 24 h after growth in “No add” or upon addition of BMP2. The bars represent the numbers (mean ± SD) from three independent experiments (*, P < 0.05, t test). Three explants per experiment were analyzed, scoring 1,500–3,000 cells per explant. (A, C, E, G, and I), corresponding phase-contrast pictures. Bar, 10 μm.
Figure 2.
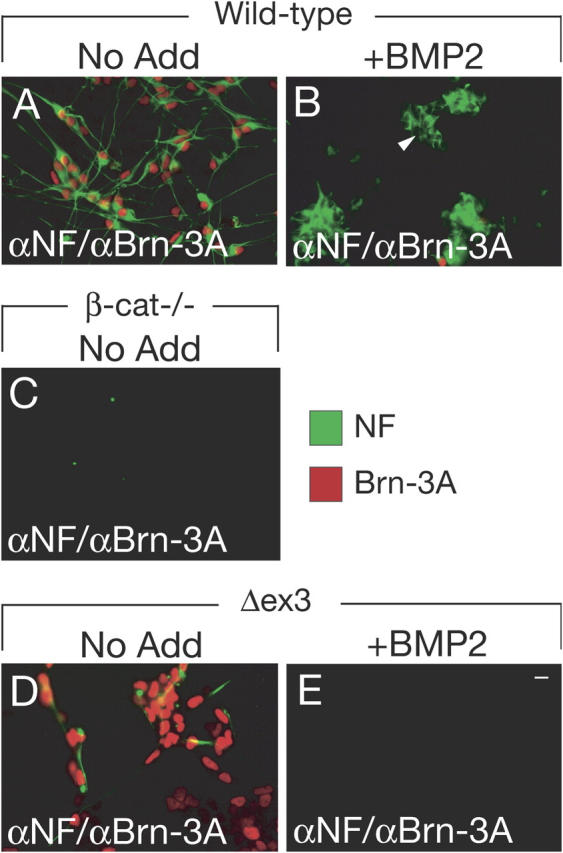
BMP signaling counteracts Wnt/β-catenin–dependent sensory neurogenesis. Wild-type eNCSCs cultured in conditions permissive for sensory neurogenesis for 60 h generate differentiated sensory neurons (A) expressing Brn-3A and NF, whereas eNCSCs challenged with BMP2 fail to generate sensory neurons and instead produce Brn-3A–negative/NF-positive autonomic neurons (B, arrowhead). β-Catenin–deficient NCSCs (β-cat−/−) are unable to generate Brn-3A–positive/NF-positive sensory neurons (C). Stabilized β-catenin (Δex3) promotes sensory neurogenesis (D), whereas neural crest cells expressing stabilized β-catenin fail to generate both sensory and autonomic neuronal cells after BMP2 treatment (E). Bar, 10 μm.
BMP signaling counteracts Wnt/β-catenin-dependent sensory neurogenesis
Canonical Wnt signaling is both required and sufficient for sensory neuronal fate specification in eNCSCs (Hari et al., 2002; Lee et al., 2004). Accordingly, as in BMP2-treated cultures, the formation of Brn-3A–positive sensory neurons was abolished in explants of β-catenin–deficient eNCSCs (0 ± 0% Brn-3A–positive cells; Fig. 2 C) (Hari et al., 2002). In neural crest explants, sensory neurogenesis is presumably promoted by Wnts expressed in the neural tube that is present in these cultures (Parr et al., 1993). The restricted expression of Wnts and their short-range activity likely explains the relatively low percentage of sensory neurons found in these explants and their generation in close proximity to the neural tube (Fig. 1 K) (Greenwood et al., 1999). Therefore, the suppression of sensory neurogenesis in neural crest explants by BMP2 conceivably reflects BMP activity counteracting neural tube-derived Wnt signaling. To address whether BMP2 would also counteract more pronounced Wnt/β-catenin activity, neural crest cells expressing a constitutively active form of β-catenin (Lee et al., 2004) were exposed to BMP2. In the absence of BMP2, sustained β-catenin activity promoted sensory neurogenesis in eNCSCs (82.2 ± 7.8% Brn-3A–positive cells) (Fig. 2 D), as described previously (Lee et al., 2004). In the presence of BMP2, sensory neurogenesis was completely inhibited, even upon constitutive β-catenin signal activation in eNCSCs (0 ± 0% Brn-3A–positive cells). However, unlike in cultures of wild-type neural crest cells (Fig. 2 B), BMP2 was unable to promote the formation of autonomic neurons (Fig. 2 E). Thus, persistent β-catenin signaling appears to suppress BMP2-induced autonomic neurogenesis, whereas BMP2 prevents sensory neurogenesis promoted by β-catenin signal activation. We conclude that Wnt and BMP signaling are possibly involved in a cross-talk to regulate neurogenesis in neural crest cells.
Maintenance of NCSC markers and suppression of differentiation by combined Wnt and BMP signaling
To further address the interaction between Wnt and BMP signaling, we exposed explant cultures of wild-type neural crest cells to either Wnt1, BMP2, or both of these factors. While BMP2 alone induced the generation of ganglia-like structures consisting of NF+/Brn-3A− autonomic neurons (Fig. 3, A, D, and G), exposure of neural crest explants to Wnt1 led to the formation of ganglia containing NF+/Brn-3A+ sensory neurons (Fig. 3, B, E, and H). Wnt1-induced sensory neurons were located close to the neural tube (mean distance to neural tube: 58.7 ± 14.7 μm), whereas BMP2-induced autonomic neurons developed distal to the neural tube (mean distance to neural tube: 287.0 ± 58.7 μm), reflecting the localization of sensory and autonomic ganglia in vivo. Intriguingly, the simultaneous application of BMP2 and Wnt1 to neural crest cells prevented the development of NF-expressing neurons, and no ganglia-like structures were present in these cultures (Fig. 3, C and F). This was not simply due to the loss of neural crest cells or impaired migration because many Sox10-positive neural crest cells were detectable in Wnt1/BMP2-treated explant cultures (Fig. 3, F, I, K, and L). These data indicate that the combined activity of Wnt1 and BMP2 suppresses both autonomic and sensory neuron formation. Moreover, Wnt1/BMP2 actively supported maintenance of Sox10-positive cells because in the absence of both growth factors neural crest cells either die or differentiate within a few days in the chosen culture conditions (not depicted; Lee et al., 2004).
Figure 3.
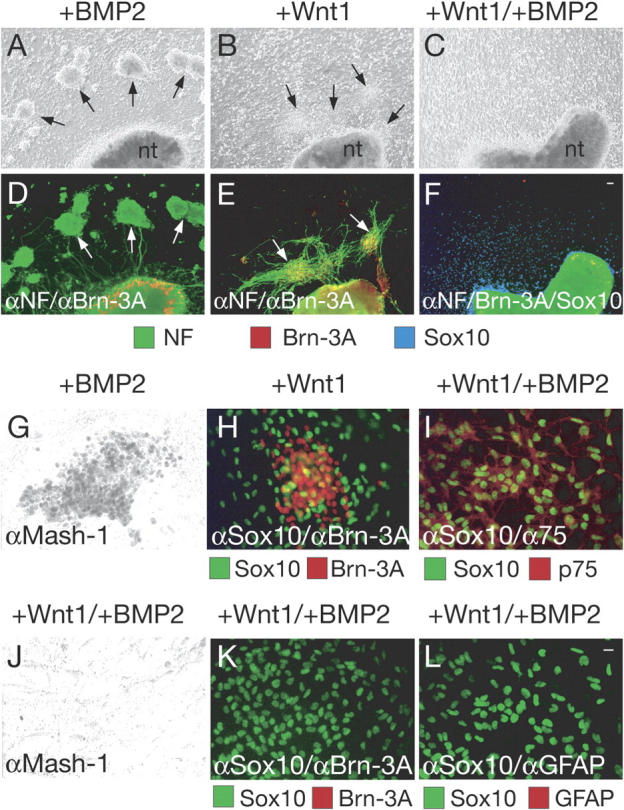
Maintenance of NCSC marker expression and suppression of differentiation by combined Wnt and BMP signaling. Wild-type eNCSCs exposed to monolayers supplemented with BMP2 generate autonomic ganglion-like structures (A, arrows) expressing NF (D, white arrows). eNCSCs exposed to Wnt1 monolayers form sensory ganglion-like cell aggregates (B, arrows), expressing Brn-3A and NF (E, white arrows). In the presence of Wnt1 and BMP2, eNCSCs fail to generate autonomic and sensory ganglia (C) and instead remain Sox10 positive (F). The Sox10 staining was artificially colored in blue. nt, neural tube. eNCSCs exposed to BMP2 generate Mash-1–positive autonomic progenitor cells (G) but no Brn-3A–positive cells (not depicted), whereas eNCSCs exposed to Wnt1 generate sensory ganglia expressing Brn-3A (H) but not Mash-1 (not depicted). eNCSCs exposed to both Wnt1 and BMP2 maintain the NCSC markers Sox10 and p75 (I). Moreover, they do not express the autonomic marker Mash-1 (J), the sensory marker Brn-3A (K), or the glial markers GFAP (L) and S100 (not depicted). Bars, 10 μm.
Early stages of neurogenesis were also suppressed in Wnt1/BMP2-treated neural crest explant cultures. Although BMP2 alone induced Mash1 expression (Fig. 3 G), which labels autonomic progenitor cells, combined Wnt1 plus BMP2 did not allow the appearance of Mash-1–positive cells in neural crest explants (Fig. 3 J). Likewise, Wnt1 (which prevents the generation of Mash-1–expressing cells; Lee et al., 2004) promoted formation of Brn-3A–positive sensory precursor cells only in the absence of BMP2 (Fig. 3, E, H, and K). Instead virtually all cells in Wnt1/BMP2-treated neural crest explants expressed p75 and Sox10 (Fig. 3 I). Both of these markers are found in NCSCs, but are also expressed during gliogenesis (Stemple and Anderson, 1992; Paratore et al., 2001). To address whether combinatorial Wnt and BMP signaling would suppress neurogenesis by promoting gliogenesis, we stained neural crest cultures with the glial differentiation markers glial fibrillary acidic protein (GFAP) and S100. However, GFAP and S100 expression was not detectable in cultures simultaneously exposed to Wnt1 and BMP2, even after prolonged incubation (Fig. 3 L; not depicted). Thus, Wnt1/BMP2-treated neural crest explants consisted of many cells expressing the NCSC-markers p75 and Sox10, while neither neuronal nor glial differentiation was observed.
Combined Wnt and BMP signaling instructively promotes NCSC maintenance and factor responsiveness
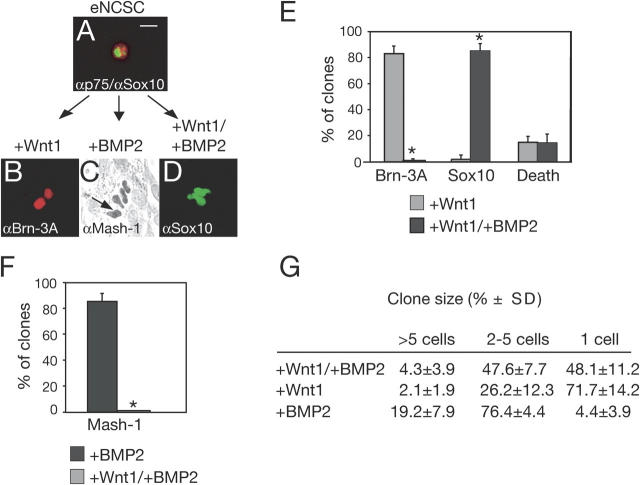
The lack of apparent neurogenesis and gliogenesis upon treatment of neural crest cells with Wnt1 plus BMP2 could indicate a selective elimination of neural crest cells as they undergo differentiation. Alternatively, our data could reflect an instructive role of combined Wnt and BMP signaling on individual eNCSCs to maintain expression of stem cell markers while preventing their differentiation. To distinguish between these possibilities, explants of neural crest cells were replated at clonal density, individual eNCSCs were prospectively identified by virtue of their p75 expression (Stemple and Anderson, 1992), and their developmental potential was assessed by challenging them with either Wnt1, BMP2, or a combination of both factors. On sister dishes, 92.1 ± 1.9% of all p75-positive cells also expressed Sox10 (Fig. 4 A). In agreement with our previous findings (Lee et al., 2004), exposure of p75-labeled cells to Wnt1 alone induced the formation of clones containing Brn-3A–positive sensory neurons in 83.1 ± 5.8% of all eNCSCs (Fig. 4, B and E), whereas upon BMP2 treatment 85.4 ± 5.7% of all eNCSCs adopted an autonomic fate marked by Mash-1 (Fig. 4, C and F). In contrast, combined application of Wnt1 and BMP2 suppressed both sensory and autonomic neurogenesis in eNCSCs, so that only very few or no sensory and autonomic cells were produced in these conditions (0.8 ± 1.4% and 0 ± 0%, respectively). Instead, 84.9 ± 5.9% of all eNCSCs challenged with Wnt1 plus BMP2 continued to express Sox10 (Fig. 4, D and E). Furthermore, cell death was minimal in all clonal experiments, excluding selective effects of Wnt and BMP signaling. Thus, the combined activities of Wnt1 and BMP2 do not selectively eliminate neuronal progenitors, but rather instructively promote maintenance of the NCSC marker Sox10 in the vast majority of all NCSCs. Moreover, although cells proliferated less than in the presence of BMP2 alone, 51.9 ± 11.2% of the Wnt1/BMP2-treated cells divided once or more times (Fig. 4 G), indicating that combined Wnt and BMP signaling also supports self-renewal of Sox10-positive NCSCs.
Figure 4.
Wnt and BMP instructively promote stem cell features in eNCSCs. Clonal analysis demonstrates responsiveness of eNCSCs to the instructive growth factors Wnt1 and BMP2 and the combination of both. p75-positive (red) founder cells coexpress Sox10 (green) (A). Founder cells exposed to Wnt1 generate clones of Brn-3A–positive sensory neurons (B). BMP2 instructs eNCSCs to generate Mash-1–expressing autonomic cells (C, arrow). In the presence of both Wnt1 and BMP2, founder cells generate clones of Sox10-positive cells (D). Bar, 10 μm. (E and F) Quantification of clone composition. The data are expressed as the mean ± SD of three independent experiments. *, P < 0.001. 50–80 clones were analyzed per experiment, scoring a clone when at least one cell within the clone expressed the marker indicated. The cell number within individual Wnt1-, BMP2-, and Wnt1/BMP2-treated clones was analyzed (G). Numbers (percentage of all clones per condition) are shown as the mean ± SD of three independent experiments, scoring 50–80 clones per experiment.
Sox10 has been implicated in maintaining multipotency of NCSCs both in culture and in the enteric nervous system in vivo (Paratore et al., 2002; Kim et al., 2003). Therefore, we asked whether sustained Sox10 expression in Wnt1/BMP2-treated eNCSCs also reflects maintenance of NCSC multipotency. In a first experiment, explants of eNCSCs (Fig. 5 A) were incubated for 5 d with Wnt1 plus BMP2. Thereafter, some dishes were fixed and analyzed for p75/Sox10 expression, while sets of sister dishes received one of the following treatments (Fig. 5): (1) some dishes were kept in Wnt1/BMP2 to assess whether maintenance of NCSC marker expression can occur over prolonged time periods and to exclude differentiation events arising by default over time; (2) to investigate whether the cells were still responsive to the sensory fate-inducing activity of Wnt1, Wnt1 treatment was continued while BMP activity was suppressed by the addition of the BMP-inhibitor noggin; (3) to assess the potential to generate nonneural smooth muscle–like cells, the sensory neuron–inducing activity of Wnt1 was suppressed by the addition of chicken embryo extract (which inhibits Wnt/β-catenin–dependent sensory neurogenesis; not shown) and TGFβ was applied to the cultures; (4) to test the gliogenic potential of the cells, Wnt activity was suppressed and NRG1 was added; and (5) to monitor autonomic neurogenesis, Wnt signaling was suppressed and BMP2 was continuously administered. Although the uninterrupted exposure of NCSCs to both Wnt1 and BMP2 sustained expression of Sox10 and p75 in all cells without apparent differentiation (Fig. 5 C), TGFβ-, NRG1-, and BMP2 treatment of cells that had been maintained in Wnt1/BMP2 for 5 d promoted the efficient generation of nonneural smooth muscle actin (SMA)–positive cells, glia, and autonomic progenitors, respectively (Fig. 5, F–H). However, when neural crest cells that had been maintained in culture for 5 d were treated with Wnt1, only relatively few sensory neurons were formed and Wnt1 appeared to have no effect on most of the cells (Fig. 5, D and E). In all conditions, cell death was minimal (unpublished data). Thus, the combined application of Wnt1 and BMP2 not only allows maintenance of Sox10/p75 expression in neural crest cell populations, but also preserves their responsiveness to various growth factors including TGFβ, NRG1, and BMP2. In contrast, these neural crest cells appear to be less sensitive to the sensory neuron–inducing activity of Wnt.
Figure 5.
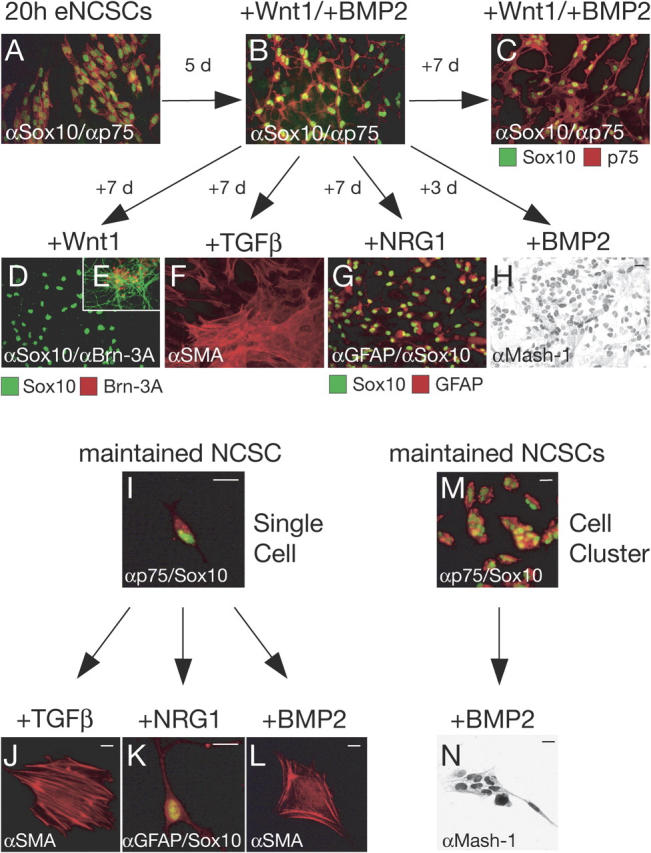
Maintenance of multipotency in NCSCs by combinatorial Wnt and BMP signaling. After emigration, eNCSCs (A) were exposed to Wnt1 and BMP2 and maintained for 5 d (B). Some cultures were incubated for an additional 7 d in the presence of Wnt1/BMP2 and continued to express the NCSC markers Sox10 and p75 (C). On sister plates, in the presence of Wnt1 alone, most of the cells maintain Sox10 expression (D) while sensory neurons expressing Brn-3A and NF are rarely found (E). However, NCSCs after prolonged exposure to Wnt1/BMP2 efficiently generate SMA-positive smooth muscle–like cells in the presence of TGFβ (F) and Sox10/GFAP-positive glia in the presence of NRG1 (G). In the absence of Wnt1, NCSCs express the autonomic marker Mash-1 in the presence of BMP2 (H). To challenge individual cells with instructive growth factors, NCSCs that have been maintained in the presence of Wnt1/BMP2 for 11 d were replated at clonal density (I) (Table I) or as cell clusters (M). In the presence of TGFβ, founder cells generate clones of smooth muscle–like cells (J), whereas NRG1 promotes gliogenesis (K). BMP2 induces a smooth muscle–like fate at clonal density (L) and autonomic neurogenesis in cell communities (N). Bars, 10 μm.
To address whether maintained responsiveness to instructive growth factors is a feature of the vast majority of Wnt1/BMP2-treated neural crest cells or only of subpopulations of the cells, NCSCs that had been maintained with Wnt1/BMP2 for prolonged time periods were replated at clonal density and prospectively identified by virtue of their p75 expression. 96.4 ± 1.9% of all p75-positive cells also expressed Sox10 (Fig. 5 I). Subsequently, p75-positive cells at clonal density were challenged with either TGFβ, NRG1, or BMP2 (Fig. 5, J–L). Consistent with the data obtained in mass cultures, the majority of all NCSCs that had been maintained in culture were responsive to these instructive growth factors (Table I). However, although TGFβ promoted the generation of smooth muscle–like cells and NRG1 the formation of glia, as expected, BMP2-treated founder cells predominantly gave rise to clones of smooth muscle–like cells and only a minority of the clones contained autonomic neurons (Table I). Thus, similar to eNCSCs present in the emigrating neural crest (Fig. 4), NCSCs maintained in culture were sensitive to various instructive growth factors, but the quality of their response changed with time (Fig. 5). This suggests that during culture intrinsic properties had changed in NCSCs, similar to the intrinsic changes observed in NCSCs isolated at different developmental stages in vivo (White et al., 2001; Kruger et al., 2002; Kubu et al., 2002).
Table 1. Clonal analysis of p75-positive NCSCs that have been maintained in culture by Wnt1 and BMP2.
| Phenotype of progeny (% ± SD)
|
||||||
|---|---|---|---|---|---|---|
| Condition | Death | Undiff. | sN | G | aN | Non-neural |
| TGFβ | 26.4 ± 3.7 | 12.4 ± 3.6 | 0 | 0 | 1.7 ± 1.6 | 59.5 ± 4.4 |
| NRG1 | 17.7 ± 5.6 | 0 | 0 | 82.3 ± 5.6 | 0 | 0 |
| BMP2 | 27.4 ± 2.9 | 8.4 ± 1.6 | 0 | 0 | 9.0 ± 3.2 | 57.9 ± 4.2 |
| Wnt1 | 15.4 ± 4.0 | 71.1 ± 3.4 | 13.5 ± 3.7 | 0 | 0 | 0 |
After 11 d in culture in the presence of Wnt1 and BMP2, NCSCs were replated at clonal density, prospectively identified, and exposed to different instructive growth factors. The plating efficiency was 65–70%. The progeny of single p75-positive cells was characterized by immunocytochemistry. The numbers indicate the phenotype of the progeny in percentage of all prospectively identified cells (mean ± SD of three experiments, scoring 40–80 clones per experiment). “Death” designates lost clones. A clone was labelled “Undiff.” (undifferentiated) when it contained Sox10-positive cells but no cells expressing lineage differentiation markers; “sN” (sensory neuron) indicates clones with at least one Brn-3A–positive cell; “G” (glia), clones with at least one GFAP-positive cell; “aN” (autonomic neuron), clones with at least one cell expressing NF but not Brn-3A; and “non-neural”, clones with at least one SMA cell. The low numbers of lost clones demonstrate that BMP2, NRG1, and TGFβ are not selectively eliminating certain cell lineages, but are acting instructively on maintained NCSCs. Note that after expansion in culture most NCSCs have lost their responsiveness to Wnt1, as only few clones contained sensory neurons.
Previously, community effects provided by short-range cell–cell interactions have been shown to influence fate decisions in NCSCs (Hagedorn et al., 1999, 2000). In particular, community effects in cell clusters modulate signaling of TGFβ family members to promote autonomic neurogenesis rather than smooth muscle cell formation in NCSCs. Such context-dependent signal modulation might explain the discrepancy between the strong neurogenic effect of BMP2 seen in mass cultures (Fig. 5 H) and the predominant smooth muscle–inducing activity of BMP2 in clonal cell cultures (Fig. 5 L). To test this idea, NCSCs that had been maintained in the presence of Wnt1/BMP2 were replated as cell clusters (Fig. 5 M), allowing short-range cell–cell interactions to occur. Subsequent treatment of BMP2 induced autonomic neurogenesis in most cells within NCSC clusters (Fig. 5 N). In fact, 98.6 ± 1.3 of all cells within BMP2-treated NCSC clusters expressed the autonomic marker Mash1 while cell death was minimal (1.4 ± 1.3% of all cells). Thus, depending on the context, BMP2 could efficiently instruct NCSCs after prolonged Wnt1/BMP2 exposure to become autonomic neurons. In sum, the combined activities of Wnt1 plus BMP2 support maintenance of multipotency and responsiveness to TGFβ, NRG1, and BMP2 in NCSCs.
Loss of Wnt responsiveness in NCSCs over time
Although NCSCs that had been maintained in culture were sensitive to various instructive growth factors, their response to the sensory neuron–inducing activity of Wnt1 was clearly reduced when compared with the response of eNCSCs emigrating from the neural tube. Possibly, maintenance of NCSCs in culture leads to loss of their Wnt responsiveness with time. To address this issue, Wnt1/BMP2-treated NCSCs were replated at clonal density, as described in the previous paragraph, and exposed to Wnt1 alone. In contrast to single eNCSCs (Fig. 4), only 13.5 ± 3.7% of all maintained NCSCs generated sensory neuron–containing clones while the remaining cells displayed no obvious response to Wnt1 (Fig. 6, A and B; Table I). This finding might represent a cell culture artifact with no relevance to neural crest development in vivo. Alternatively, the change in Wnt1 responsiveness of NCSCs might reflect processes also occurring in vivo. Cells with NCSC features persist during development in various postmigratory targets of the neural crest, including the sciatic nerve and the dorsal root ganglia (DRG) (Hagedorn et al., 1999; Bixby et al., 2002). To assess whether Wnt1 could induce sensory neurogenesis in postmigratory NCSCs present at later developmental stages, NCSCs were isolated either from the sciatic nerve or from the DRG at embryonic day 12 (E12), plated at clonal density, prospectively identified by p75 labeling, and incubated with or without Wnt1. Previous studies have shown that 70–90% of the undifferentiated p75-positive neural crest cells present in the sciatic nerve and in the DRG at these developmental stages are sensitive to the instructive activities of TGFβ, NRG1, and BMP2 (not depicted; Hagedorn et al., 1999; Bixby et al., 2002). However, in contrast to migratory eNCSCs, neither sciatic nerve– nor DRG-derived NCSCs were able to undergo increased sensory neurogenesis in response to Wnt1 (Fig. 6, C and D), indicating changes in Wnt signal interpretation that have been acquired over time in vivo.
Figure 6.
Loss of Wnt activity and Wnt responsiveness during neural crest development. NCSCs that had been maintained for prolonged time with Wnt1/BMP2 were replated at clonal density (A) and exposed to Wnt1 alone. In contrast to eNCSCs (Fig. 4), NCSCs expanded in culture lose responsiveness to Wnt1 and mostly remain Sox10 positive without expressing sensory markers (B, Table I). Bars, 10 μm. Clonal analysis revealed that postmigratory NCSCs isolated from sciatic nerve do not generate sensory neurons in response to Wnt1, as no Brn-3A–expressing clone was generated from p75-positive founder cells, from which 81.4 ± 4.0% coexpressed Sox10 (C). Likewise, postmigratory NCSCs isolated from DRG are not Wnt responsive; no significant difference in clone composition between Wnt1-treated and control (co) cultures was observed (D). Of the p75-positive founder cells, 65 ± 5.9% coexpressed Sox10. (E–L) Whole-mount and section analysis of mice harbouring TOPGAL, in which a β-galactosidase gene is under the control of a TCF/Lef and β-catenin inducible promoter. At E9.5 (E–G), Wnt/β-catenin activity is observed in the dorsal neural tube and in regions of emigrating neural crest (F, arrow). At E10.5 (H–J), Wnt/β-catenin activity decreases at sites of postmigratory NCSCs (I, arrow). At E12.5 (K), there is no Wnt/β-catenin activity at sites of postmigratory NCSCs (L, open arrow). Enlarged areas (F, I, and L) are marked by boxes in E, H, and K, respectively.
These data suggest that canonical Wnt signaling might play a role predominantly at early stages of neural crest development. To further address this issue, we investigated whether Wnt/β-catenin activity persists at sites of postmigratory NCSCs. To this end, we analyzed embryos harbouring TOPGAL, in which a β-galactosidase gene is under the control of a TCF/Lef and β-catenin inducible promoter (DasGupta and Fuchs, 1999). Although canonical Wnt activity was observed in neural crest cells emigrating from the neural tube at E9.5 (Fig. 6, E–G), Wnt/β-catenin activity decreased over time and at E12.5 was no longer detectable at sites of postmigratory neural crest cells including DRG and sympathetic ganglia (Fig. 6, K and L). These findings suggest that Wnt/β-catenin is not directly involved in the induction of late sensory neurogenesis in the DRG, nor is it likely to modulate BMP-induced autonomic neurogenesis in the sympathetic ganglia or to regulate persistence of NCSCs in postmigratory neural crest target tissues. However, the data are consistent with an involvement of canonical Wnt signaling in maintenance and early sensory lineage segregation in emigrating eNCSCs.
Discussion
Factors supporting NCSC maintenance and self-renewal have not been described so far. Here, we show that the synergistic activity of Wnt and BMP signaling regulate NCSC maintenance, whereas the individual factors alone promote lineage decisions in these stem cells. In the CNS, expansion of neural stem cells has been achieved before in neurosphere and in adhesive cultures (Alvarez-Buylla et al., 2001). However, the cellular composition in these culture systems is heterogeneous, with the actual stem cells representing only a minor fraction of all cells. In contrast, combined Wnt/BMP activity allows the expansion of an almost pure population of NCSCs while suppressing differentiation. Stem cell maintenance is not only reflected by persistent expression of the NCSC markers p75 and Sox10 in virtually all cells, but also by the maintained potential of these cells to generate neurons, glia, and nonneural cell types. Although NCSCs proliferate in the presence of Wnt/BMP, cell cycle progression is slower than in lineage-specified progenitors. This is comparable to the reduced proliferation rate of stem cells as opposed to transient amplifying progenitors in the CNS (Alvarez-Buylla et al., 2001). Similar to NCSCs isolated at different time points in vivo, NCSCs change certain intrinsic properties over time and acquire differential responsiveness to instructive growth factors.
Stem cell maintenance and early sensory lineage segregation in emigrating neural crest cells
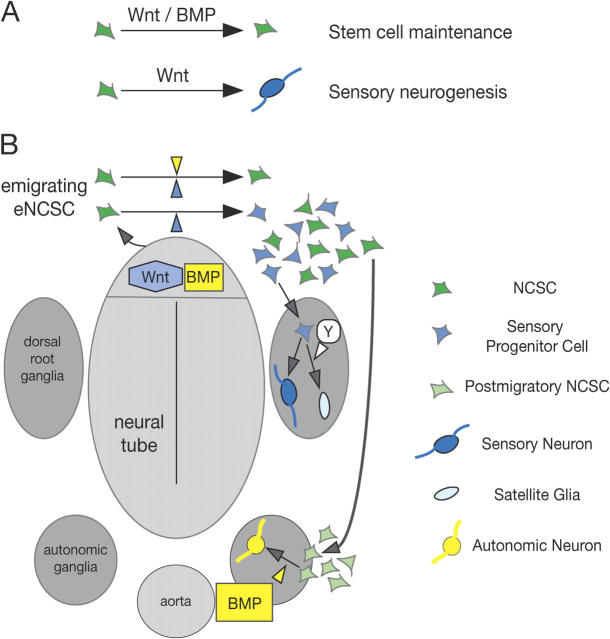
Although in culture emigrating eNCSCs initially represent a homogeneous cell population with respect to their potentials (Lee et al., 2004), the cells quickly undergo lineage segregation (Henion and Weston, 1997; Greenwood et al., 1999; Luo et al., 2003). This is likely due to the limited signaling range and activity of growth factors such as neural tube–derived Wnt that in explant cultures induces sensory neurogenesis in a subset of all neural crest cells and that can be modulated by BMP signaling (Figs. 1 and 2). Likewise, in vivo the migratory neural crest is heterogeneous as has been shown by single-cell tracing experiments (Fraser and Bronner-Fraser, 1991). Although some of the labeled cells in these experiments contributed to both sensory and autonomic ganglia, others were restricted and generated either only sensory or only sympathetic cells. Moreover, Ngn2-positive cells fated predominantly for sensory ganglia are found in neural crest cells as they emigrate from the neural tube, intermingled with multifated Sox10-positive cells (Zirlinger et al., 2002). Thus, maintenance vs. sensory lineage segregation is regulated very early in neural crest development. Based on our data, we propose (Fig. 7) that neural crest cells emerge from the neural tube with equal competence; combinatorial Wnt and BMP signaling maintains multipotency in some emigrating NCSCs and suppresses their neuronal specification, whereas other emigrating neural crest cells are not (or at least not continuously) exposed to the synergistic activity of Wnt plus BMP and adopt a Wnt-dependent sensory fate. How this cellular heterogeneity is established remains elusive. Possibly, restricted signal availability or factors locally inhibiting BMP activity might be involved. Moreover, it is presently not clear whether Wnt/BMP signaling maintains NCSCs with potentials exceeding smooth muscle formation, neurogenesis, and gliogenesis. In particular, recent evidence indicates that the melanocyte lineage segregates from other neural crest lineages already in the neural tube, before neural crest emigration (Wilson et al., 2004). In any case, however, our finding that BMP signaling can counteract the sensory neuron–inducing activity of canonical Wnt signaling provides one conceivable solution to the paradox that in normal neural crest development not all emigrating neural crest cells adopt a sensory fate, despite the general responsiveness of eNCSCs to Wnt signaling and the expression of Wnts at the site of neural crest delamination. Similarly, the inhibition of BMP-dependent autonomic neurogenesis by Wnt signaling also offers an explanation for why the generation of autonomic neurons is prevented in emigrating neural crest cells in spite of the presence of BMPs in the ectoderm and the dorsal neural tube.
Figure 7.
Model of combinatorial Wnt/BMP signaling regulating NCSC development. Wnt in synergy with BMP signaling promotes NCSC maintenance while on its own, canonical Wnt signaling induces sensory fate acquisition (A). Based on these findings we propose (B) that Wnt proteins expressed in the dorsal neural tube induce the formation of sensory neural cells (blue) from eNCSCs (green) that emigrate from the neural tube. Sensory progenitor cells predominantly populate the DRG (Zirlinger et al., 2002), where an unknown factor Y prevents some cells from differentiating into sensory neurons, allowing the formation of satellite glia. BMPs (yellow) that are also expressed in the dorsal midline region at the time point of neural crest emigration suppress Wnt-dependent sensory neurogenesis in some eNCSCs and, together with Wnt, instructively promote maintenance of NCSC features. With time, NCSCs change intrinsic properties and, as postmigratory NCSCs (light green), have lost their responsiveness to Wnt but not to other instructive growth factors. For simplicity, of all possible nonsensory neural crest–derived structures only autonomic ganglia are shown, in which dorsal aorta–derived BMP induces autonomic neurogenesis in postmigratory NCSCs.
Consistent with a role of Wnt signaling in NCSC expansion in vivo, ablation of wnt1/wnt3a in mice results in the reduction of most neural crest lineages (Ikeya et al., 1997), and Wnt signal inhibition at the onset of neural crest emigration in zebrafish leads to reduced expression of several neural crest markers, including Sox10 (Lewis et al., 2004). However, numbers of Sox10-positive cells appear normal and neural crest cells still contribute to multiple lineages after neural crest–specific deletion of β-catenin and of BMP receptor IA (Hari et al., 2002; Stottmann et al., 2004). This could point to stage- or signaling component–specific requirements for Wnt/BMP signaling in stem cell maintenance, which has to be addressed in future studies.
Although Wnt in combination with BMP signaling maintains multipotency of NCSCs and supports cell division in many of these cells, their responsiveness to instructive growth factors changes with time. Most intriguingly, expanded NCSCs lose their sensitivity to the sensory neuron–inducing activity of canonical Wnt signaling while remaining responsive to other instructive growth factors including BMP2, NRG1, and TGFβ. The loss of Wnt responsiveness cannot be explained by the selective elimination of cells with sensory potential, as demonstrated by clonal analysis of cells that have been maintained in the presence of Wnt1 and BMP2 (Figs. 5 and 6). Rather, during maintenance in culture individual NCSCs have acquired intrinsic differences as compared with eNCSCs emigrating from the neural tube. Strikingly, these changes correspond to processes occurring in vivo: Although in the case of sciatic nerve cells increased cell death might have masked an effect of Wnt on sensory neurogenesis, postmigratory NCSCs present in both the sciatic nerve and the DRG displayed an altered Wnt response as compared with migratory NCSCs and failed to generate sensory neurons (Fig. 6). Similar changes also occur in response to other growth factors, both in NCSCs isolated at different time points and in postmigratory NCSCs derived from different PNS regions (Bixby et al., 2002; Kruger et al., 2002). Thereby, changes in cell-intrinsic determinants influence cell fate decisions by changing the sensitivity of neural crest cells to specific extracellular signals (White et al., 2001; Kubu et al., 2002). For instance, the level of the transcription factor Sox10 determines how neural crest cells interpret their environment and which fate they adopt (Paratore et al., 2001). Such changes acquired over time might also explain the various functions attributed to Wnts and BMPs during neural crest development, ranging from neural crest induction, delamination, and NCSC expansion to melanocyte formation and neurogenesis (Ikeya et al., 1997; Dorsky et al., 1998; Garcia-Castro et al., 2002; Burstyn-Cohen et al., 2004; Lee et al., 2004). Indeed, blocking Wnt signaling at various time points in zebrafish embryogenesis indicated its reiterated but distinct roles in neural crest development (Lewis et al., 2004).
Neural stem cells from the CNS also undergo intrinsic changes during development, biasing a cell to self-renew, to generate either neurons or glia, or to produce specific neuronal cell types (Alvarez-Buylla et al., 2001). In particular, canonical Wnt signaling promotes stem cell expansion at early stages of cortical development, whereas it induces neuronal lineage commitment at later stages (Hirabayashi et al., 2004). Thus, as is the case with NCSCs found at different stages of PNS development, the proposed transition from neural stem cells present in the embryo to adult neural stem cells is accompanied by alterations in the stem cell's genetic program (Alvarez-Buylla et al., 2001). It follows that continuous self-renewal used as a key feature of stem cells might not apply in its strictest sense to neural (and conceivably other) stem cells during development. Rather, although maintaining their multipotency, stem cells adapt to signals present in their extracellular environment.
The environment, however, also changes with time. In the PNS, canonical Wnt activity is observed only at early development stages, when neural crest cells emigrate from the neural tube (Fig. 6). At later stages, Wnt/β-catenin activity was no longer detectable in neural crest–derived tissues, such as DRG, peripheral nerves, and sympathetic ganglia. Thus, not only do NCSCs lose their Wnt responsiveness with time, but also postmigratory neural crest cells are not exposed to canonical Wnt signaling any more. We propose that this dual regulation of Wnt signaling ensures the tight control, both spatially and temporally, of NCSC maintenance by Wnt/BMP and sensory lineage segregation by Wnt signaling alone. Consequently, late sensory neurogenesis in the forming DRG may occur in a Wnt-independent manner. Likewise, maintenance of NCSCs at later stages may be regulated by cues not involving canonical Wnt signaling. Alternatively, transient priming of NCSCs by Wnt plus BMP at early stages may be sufficient to maintain NCSCs also at later stages until they are exposed to new instructive cues, such as BMPs that are expressed in the dorsal aorta and induce autonomic neurogenesis (Reissmann et al., 1996; Shah et al., 1996). In sum, NCSC maintenance and lineage decisions appear to be regulated by the dynamic interaction between cell-intrinsic properties and cell-extrinsic cues, including Wnt signaling, that change with time and location.
Combinatorial Wnt and BMP signaling
Our study provides multiple lines of evidence for a cross-talk between Wnt and BMP signaling in eNCSCs. Wnt1 treatment as well as sustained β-catenin activity interferes with the autonomic neuron–inducing activity of BMP2 (Figs. 2 and 3). Likewise, BMP signaling suppresses β-catenin–dependent sensory neurogenesis from eNCSCs (Fig. 2) and prevents sensory neuron formation in eNCSCs treated with exogenous Wnt1 (Fig. 3). The fact that BMP2 also blocks sensory neurogenesis in eNCSCs expressing a constitutively active form of β-catenin indicates that BMP signal transduction interacts with canonical Wnt signaling at the level of or downstream of β-catenin. Strikingly, the cross-talk between Wnt and BMP signaling is not just mutually antagonistic but synergistic, leading to a new fate not seen with the individual signals alone. Indeed, combined Wnt and BMP signaling suppresses also glial and nonneural cell differentiation, apart from sensory and autonomic neurogenesis, while maintaining stem cell features in neural crest cells.
Wnt/β-catenin signaling interacts with several other signal transduction pathways, which influences its signaling output (Nelson and Nusse, 2004). In particular, Wnt and BMP signaling cooperate in various species in either a synergistic or antagonistic manner to regulate cell lineage decisions, patterning, or tumorigenesis. Thereby, the β-catenin/Lef/TCF transcription factor complex acts as signal coordinator that physically interacts with the BMP signaling components SMAD4 or SMAD1 to induce specific target genes. Most recently, BMP has been shown to antagonize Wnt/β-catenin in intestinal stem cells by signaling through the tumor suppressor PTEN and Akt kinase (He et al., 2004). Intriguingly, in these stem cells Wnt promotes self-renewal and Wnt/BMP promotes cell fate decisions, unlike in NCSCs, indicating that the effects of Wnt signaling and Wnt signal modulation by BMP are dependent on the stem cell type (Kléber and Sommer, 2004).
The signaling components involved and the target genes of Wnt/BMP signal convergence in NCSCs remain to be identified. The homeodomain factor Msx2 might possibly be implicated, at least in the cranial crest, because Msx2 expression is regulated by cooperative Wnt/BMP signaling in embryonic stem cells (Hussein et al., 2003) and suppresses chondrogenic differentiation in migratory cranial neural crest cells (Takahashi et al., 2001). Another candidate transcription factor is Sox10, which is persistently expressed in NCSCs maintained by Wnt1 plus BMP2 (Figs. 3–5). Similar to the combinatorial activity of Wnt and BMP, Sox10 promotes maintenance of multipotency and growth factor responsiveness in cultured NCSCs (Kim et al., 2003), and is required for maintenance of enteric NCSCs in vivo (Paratore et al., 2002). Although there is precedence for Wnt/β-catenin signaling regulating expression of Sox genes (Blache et al., 2004), a functional interplay between Sox10 and the canonical Wnt signaling pathway might take place at the protein level, as several Sox proteins physically interact with β-catenin (Zorn et al., 1999; Akiyama et al., 2004; Sinner et al., 2004). It is open whether such interactions occur in NCSCs, but they might play a part in the modulation of the signaling network provided by Wnt and BMP that controls NCSC maintenance and cell fate decisions.
Materials and methods
Mating scheme, genotyping, and in vivo fate mapping
Control and mutant embryos were generated and genotyped as described previously (Hari et al., 2002; Lee et al., 2004). Wnt/β-catenin activity was assessed by analyzing mice harboring TOPGAL, in which a β-galactosidase gene is under the control of a TCF/Lef and β-catenin inducible promoter (DasGupta and Fuchs, 1999). X-gal staining was performed as described previously (Paratore et al., 2002).
Neural crest explant cultures
Mouse neural crest explant cultures under conditions permissive for sensory neurogenesis were performed as reported previously (Hari et al., 2002; Lee et al., 2004). To inhibit sensory neurogenesis, early neural crest explants were supplemented with 50 ng/ml BMP2 (R&D Systems). Exposure of neural crest cells to Wnt1-expressing and control fibroblasts was done as described previously (Lee et al., 2004). In some experiments, neural crest cells on fibroblast monolayers were treated with BMP2 20 h after emigration and cultured for an additional 5–12 d. To challenge Wnt1/BMP2-treated NCSCs after 5 d in explant culture, cells were washed three times in Dulbecco's minimum essential medium and cultured for an additional 7 d either in defined medium (Greenwood et al., 1999) supplemented with 125 ng/ml noggin (R&D Systems) or in medium containing chicken embryo extract (Stemple and Anderson, 1992) supplemented with 20 ng/ml IGF-1 (R&D Systems) and either 50 ng/ml BMP2, 0.1 ng/ml TGFβ1 (R&D Systems), or 1 nM NRG1 (R&D Systems). The number of sensory neurons (mean ± SD) was determined for three different explants, counting at least 2,000 cells per explant. Five ganglia per explant in at least three different explants were assayed to quantify the mean distances between ganglia and neural tube, using NIH Image 1.62 software.
Clonal analysis of NCSCs
Rat eNCSCs and postmigratory NCSCs from rat DRG and sciatic nerve were prepared as reported previously (Hagedorn et al., 1999; Leimeroth et al., 2002; Lee et al., 2004). Clonal analysis of prospectively identified NCSCs plated onto Wnt1-expressing or control fibroblast monolayers was done as in Lee et al. (2004). The plating efficiency was ∼60–70%. Some cultures on fibroblast monolayers were supplemented with 50 ng/ml BMP2 (R&D Systems) 2 d after plating. To evaluate maintenance of multipotency, rat NCSCs were incubated on Wnt1-expressing monolayers in the presence of BMP2 for 11 d. After 11 d, the remaining fibroblasts and neural tubes were removed and the explants were replated at clonal density onto freshly prepared Wnt1-expressing or control feeder monolayers, or onto pdL/fibronectin-coated plates. Clone founder cells were identified and mapped as explained above. Cells exposed to Wnt1 were cultured in medium permissive for sensory neurogenesis (Lee et al., 2004). Cultures in the absence of Wnt1 were treated with 50 ng/ml BMP2 (R&D Systems), 0.1 ng/ml TGFβ (R&D Systems), or 1 nM NRG1 (R&D Systems) for 4, 5, and 7 d, respectively, in medium containing chicken embryo extract (Stemple and Anderson, 1992).
Immunocytochemistry
Anti-p75, anti-Sox10, anti-Brn-3A, and anti-NF160 antibody stainings were done as in Hari et al. (2002). Anti-GFAP and anti-S100 stainings were performed using pAbs (each at 1:200 dilution; DakoCytomation). SMA was stained with an mAb (1:400 dilution; Sigma-Aldrich); for Mash-1 staining, cells were incubated with an mAb (1:100 dilution; BD Biosciences) overnight at 4°C, followed by incubation with a HRP-coupled anti–mouse IgG antibody (1:200 dilution; DakoCytomation) and by HRP development using DAB as substrate. Immunofluorescence was analyzed using an Axiovert 100 microscope and AxioVision 4.1 software (Carl Zeiss MicroImaging, Inc.).
Acknowledgments
We thank Ned Mantei and Joao Relvas for critical reading of the manuscript; Rolf Kemler (Max-Planck Institute of Immunology, Freiburg, Germany) and Makoto M. Taketo (Kyoto University, Kyoto, Japan) for providing transgenic animals; and Michael Wegner (University of Erlangen, Erlangen, Germany) and Eric Turner (University of California, San Diego, San Diego, CA) for antibodies.
Supported by grants of the Swiss National Science Foundation, the National Center of Competence in Research “Neural Plasticity and Repair,” and the Swiss Federal Institute of Technology.
Abbreviations used in this paper: BMP, bone morphogenic protein; DRG, dorsal root ganglia; eNCSC, early neural crest stem cell; GFAP, glial fibrillary acidic protein; NCSC, neural crest stem cell; NF, neurofilament; NRG, neuregulin; PNS, peripheral nervous system; SMA, smooth muscle actin.
References
- Akiyama, H., J.P. Lyons, Y. Mori-Akiyama, X. Yang, R. Zhang, Z. Zhang, J.M. Deng, M.M. Taketo, T. Nakamura, R.R. Behringer, et al. 2004. Interactions between Sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 18:1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla, A., J.M. Garcia-Verdugo, and A.D. Tramontin. 2001. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2:287–293. [DOI] [PubMed] [Google Scholar]
- Bixby, S., G.M. Kruger, J.T. Mosher, N.M. Joseph, and S.J. Morrison. 2002. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 35:643–656. [DOI] [PubMed] [Google Scholar]
- Blache, P., M. van de Wetering, I. Duluc, C. Domon, P. Berta, J.N. Freund, H. Clevers, and P. Jay. 2004. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J. Cell Biol. 166:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen, T., J. Stanleigh, D. Sela-Donenfeld, and C. Kalcheim. 2004. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development. 131:5327–5339. [DOI] [PubMed] [Google Scholar]
- DasGupta, R., and E. Fuchs. 1999. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 126:4557–4568. [DOI] [PubMed] [Google Scholar]
- Dorsky, R.I., R.T. Moon, and D.W. Raible. 1998. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 396:370–373. [DOI] [PubMed] [Google Scholar]
- Fedtsova, N.G., and E.E. Turner. 1995. Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech. Dev. 53:291–304. [DOI] [PubMed] [Google Scholar]
- Fraser, S.E., and M.E. Bronner-Fraser. 1991. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development. 112:913–920. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro, M.I., C. Marcelle, and M. Bronner-Fraser. 2002. Ectodermal Wnt function as a neural crest inducer. Science. 297:848–851. [DOI] [PubMed] [Google Scholar]
- Greenwood, A.L., E.E. Turner, and D.J. Anderson. 1999. Identification of dividing, determined sensory neuron precursors in the mammalian neural crest. Development. 126:3545–3559. [DOI] [PubMed] [Google Scholar]
- Hagedorn, L., U. Suter, and L. Sommer. 1999. P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-β family factors. Development. 126:3781–3794. [DOI] [PubMed] [Google Scholar]
- Hagedorn, L., J. Floris, U. Suter, and L. Sommer. 2000. Autonomic neurogenesis and apoptosis are alternative fates of progenitor cell communities induced by TGFβ. Dev. Biol. 228:57–72. [DOI] [PubMed] [Google Scholar]
- Hari, L., V. Brault, M. Kléber, H.Y. Lee, F. Ille, R. Leimeroth, C. Paratore, U. Suter, R. Kemler, and L. Sommer. 2002. Lineage-specific requirements of β-catenin in neural crest development. J. Cell Biol. 159:867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X.C., J. Zhang, W.G. Tong, O. Tawfik, J. Ross, D.H. Scoville, Q. Tian, X. Zeng, X. He, L.M. Wiedemann, et al. 2004. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-β-catenin signaling. Nat. Genet. 36:1117–1121. [DOI] [PubMed] [Google Scholar]
- Henion, P.D., and J.A. Weston. 1997. Timing and pattern of cell fate restrictions in the neural crest lineage. Development. 124:4351–4359. [DOI] [PubMed] [Google Scholar]
- Hirabayashi, Y., Y. Itoh, H. Tabata, K. Nakajima, T. Akiyama, N. Masuyama, and Y. Gotoh. 2004. The Wnt/β-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 131:2791–2801. [DOI] [PubMed] [Google Scholar]
- Hussein, S.M., E.K. Duff, and C. Sirard. 2003. Smad4 and β-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. J. Biol. Chem. 278:48805–48814. [DOI] [PubMed] [Google Scholar]
- Ikeya, M., S.M. Lee, J.E. Johnson, A.P. McMahon, and S. Takada. 1997. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 389:966–970. [DOI] [PubMed] [Google Scholar]
- Kim, J., L. Lo, E. Dormand, and D.J. Anderson. 2003. SOX10 Maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 38:17–31. [DOI] [PubMed] [Google Scholar]
- Kléber, M., and L. Sommer. 2004. Wnt signaling and the regulation of stem cell function. Curr. Opin. Cell Biol. 16:681–687. [DOI] [PubMed] [Google Scholar]
- Kruger, G.M., J.T. Mosher, S. Bixby, N. Joseph, T. Iwashita, and S.J. Morrison. 2002. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 35:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubu, C.J., K. Orimoto, S.J. Morrison, G. Weinmaster, D.J. Anderson, and J.M. Verdi. 2002. Developmental changes in Notch1 and numb expression mediated by local cell-cell interactions underlie progressively increasing delta sensitivity in neural crest stem cells. Dev. Biol. 244:199–214. [DOI] [PubMed] [Google Scholar]
- Le Douarin, N.M., and E. Dupin. 2003. Multipotentiality of the neural crest. Curr. Opin. Genet. Dev. 13:529–536. [DOI] [PubMed] [Google Scholar]
- Lee, H.Y., M. Kléber, L. Hari, V. Brault, U. Suter, M.M. Taketo, R. Kemler, and L. Sommer. 2004. Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science. 303:1020–1023. [DOI] [PubMed] [Google Scholar]
- Leimeroth, R., C. Lobsiger, A. Lussi, V. Taylor, U. Suter, and L. Sommer. 2002. Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent progenitor cells. Dev. Biol. 246:245–258. [DOI] [PubMed] [Google Scholar]
- Lewis, J.L., J. Bonner, M. Modrell, J.W. Ragland, R.T. Moon, R.I. Dorsky, and D.W. Raible. 2004. Reiterated Wnt signaling during zebrafish neural crest development. Development. 131:1299–1308. [DOI] [PubMed] [Google Scholar]
- Liem, K.F., G. Tremml, H. Roelink, and T.M. Jessell. 1995. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 82:969–979. [DOI] [PubMed] [Google Scholar]
- Luo, R., J. Gao, B. Wehrle-Haller, and P.D. Henion. 2003. Molecular identification of distinct neurogenic and melanogenic neural crest sublineages. Development. 130:321–330. [DOI] [PubMed] [Google Scholar]
- Nelson, W.J., and R. Nusse. 2004. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 303:1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratore, C., D.E. Goerich, U. Suter, M. Wegner, and L. Sommer. 2001. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 128:3949–3961. [DOI] [PubMed] [Google Scholar]
- Paratore, C., C. Eichenberger, U. Suter, and L. Sommer. 2002. Sox10 haploinsufficiency affects maintenance of progenitor cells in a mouse model of Hirschsprung disease. Hum. Mol. Genet. 11:3075–3085. [DOI] [PubMed] [Google Scholar]
- Parr, B.A., M.J. Shea, G. Vassileva, and A.P. McMahon. 1993. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 119:247–261. [DOI] [PubMed] [Google Scholar]
- Reissmann, E., U. Ernsberger, P.H. Francis-West, D. Rueger, P.M. Brickell, and H. Rohrer. 1996. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 122:2079–2088. [DOI] [PubMed] [Google Scholar]
- Shah, N.M, A.K. Groves, and D.J. Anderson. 1996. Alternative neural crest cell fates are instructively promoted by TGFβ superfamily members. Cell. 85:331–343. [DOI] [PubMed] [Google Scholar]
- Sinner, D., S. Rankin, M. Lee, and A.M. Zorn. 2004. Sox17 and β-catenin cooperate to regulate the transcription of endodermal genes. Development. 131:3069–3080. [DOI] [PubMed] [Google Scholar]
- Stemple, D.L., and D.J. Anderson. 1992. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 71:973–985. [DOI] [PubMed] [Google Scholar]
- Stottmann, R.W., M. Choi, Y. Mishina, E.N. Meyers, and J. Klingensmith. 2004. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 131:2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., G.H. Nuckolls, I. Takahashi, K. Nonaka, M. Nagata, T. Ikura, H.C. Slavkin, and L. Shum. 2001. Msx2 is a repressor of chondrogenic differentiation in migratory cranial neural crest cells. Dev. Dyn. 222:252–262. [DOI] [PubMed] [Google Scholar]
- White, P.M., S.J. Morrison, K. Orimoto, C.J. Kubu, J.M. Verdi, and D.J. Anderson. 2001. Neural crest stem cells undergo cell-intrinsic developmental changes in sensitivity to instructive differentiation signals. Neuron. 29:57–71. [DOI] [PubMed] [Google Scholar]
- Wilson, Y.M., K.L. Richards, M.L. Ford-Perriss, J.J. Panthier, and M. Murphy. 2004. Neural crest cell lineage segregation in the mouse neural tube. Development. 131:6153–6162. [DOI] [PubMed] [Google Scholar]
- Zirlinger, M., L. Lo, J. McMahon, A.P. McMahon, and D.J. Anderson. 2002. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc. Natl. Acad. Sci. USA. 99:8084–8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn, A.M., G.D. Barish, B.O. Williams, P. Lavender, M.W. Klymkowsky, and H.E. Varmus. 1999. Regulation of Wnt signaling by Sox proteins: XSox17 α/β and XSox3 physically interact with β-catenin. Mol. Cell. 4:487–498. [DOI] [PubMed] [Google Scholar]