Abstract
Myosin VI plays a role in the maintenance of Golgi morphology and in exocytosis. In a yeast 2-hybrid screen we identified optineurin as a binding partner for myosin VI at the Golgi complex and confirmed this interaction in a range of protein interaction studies. Both proteins colocalize at the Golgi complex and in vesicles at the plasma membrane. When optineurin is depleted from cells using RNA interference, myosin VI is lost from the Golgi complex, the Golgi is fragmented and exocytosis of vesicular stomatitis virus G-protein to the plasma membrane is dramatically reduced. Two further binding partners for optineurin have been identified: huntingtin and Rab8. We show that myosin VI and Rab8 colocalize around the Golgi complex and in vesicles at the plasma membrane and overexpression of constitutively active Rab8-Q67L recruits myosin VI onto Rab8-positive structures. These results show that optineurin links myosin VI to the Golgi complex and plays a central role in Golgi ribbon formation and exocytosis.
Introduction
In membrane trafficking pathways, motor proteins moving along cytoskeletal tracks play a major role in transporting vesicles between donor and acceptor compartments and may also be involved in processes such as cargo sorting, vesicle formation, and steady-state localization of organelles. Short-range movement of cargo or vesicles along actin filaments, around internal organelles, or within the cortical regions of the cell is powered by members of the myosin superfamily, which is comprised of at least 18 different classes (Hodge and Cope, 2000; Berg et al., 2001). Although in recent years the localization and functions of a few of these myosins have been identified, there is still limited information regarding the molecular mechanism linking myosin function and cargo attachment. For example, how does a myosin recognize its cargo; how is the interaction regulated and what influence does cargo binding have on motor activity?
Myosin VI is a multifunctional motor protein found in a number of different intracellular compartments including endocytic vesicles (Buss et al., 2001b; Aschenbrenner et al., 2003), membrane ruffles (Buss et al., 1998), the Golgi complex, and secretory vesicles (Buss et al., 1998; Warner et al., 2003). Unlike all the other myosins that have been studied so far that move toward the plus end of actin filaments, myosin VI moves toward the minus end of actin (Wells et al., 1999). Functional studies have indicated that myosin VI plays a major role in endocytic and secretory membrane traffic pathways (Buss et al., 2001b; Warner et al., 2003) and it has been postulated that the diverse functions of myosin VI are mediated by interaction with a number of different binding partners (Buss et al., 2004). Recently, three binding partners of myosin VI were identified, Dab2, GIPC, and SAP97, all of which target myosin VI to vesicular compartments (Bunn et al., 1999; Morris et al., 2002; Wu et al., 2002). So far, the best-characterized myosin VI–binding partner is Dab2; its interaction with myosin VI has been shown to form a dynamic link between cell surface receptors, clathrin-mediated endocytosis, and the actin cytoskeleton (Morris and Cooper, 2001; Morris et al., 2002). In contrast, no binding partners have been identified that targets myosin VI to the Golgi complex and the secretory pathway.
In this paper, we have identified and characterized optineurin, a novel myosin VI–binding partner, which is found at the Golgi complex. Optineurin was first discovered as a binding partner of the adenoviral protein E3-14.7K (14.7K-interacting protein-2 and therefore named FIP-2) and was shown to protect infected cells from TNF-α–induced cytolysis (Li et al., 1998b). It is a conserved 67-kD protein with multiple leucine zipper domains and a putative zinc finger domain at the COOH terminus. Optineurin shows strong homology (53% identity) with NF-κB essential modulator and was therefore also called NEMO-related protein (Schwamborn et al., 2000). Mutations in the human optineurin gene are associated with adult-onset open angle glaucoma (hence it was named “optic neuropathy inducing” protein optineurin; Rezaie et al., 2002).
Although optineurin was previously localized to the Golgi complex (Schwamborn et al., 2000; Stroissnigg et al., 2002) its functions at this organelle have not yet been established. However, two binding partners for optineurin have been identified which link it to membrane trafficking events. One is huntingtin, the protein mutated in the neurodegenerative disorder Huntington's disease (Faber et al., 1998), and the other is the small GTPase Rab8 (Hattula and Peranen, 2000). Although the precise cellular functions of the wild-type huntingtin protein are not known, its intracellular localization to the Golgi complex and to endocytic and exocytic vesicles (DiFiglia et al., 1995; Velier et al., 1998), as well as the identity of its binding partners (Harjes and Wanker, 2003), suggests a role in membrane trafficking pathways (Gauthier et al., 2004). The other optineurin binding partner Rab8 belongs to a large family of small GTPases that participate in and regulates intracellular membrane trafficking pathways (Zerial and McBride, 2001). In their active GTP-bound state different Rab proteins bind to different membrane compartments and recruit specific effector proteins, which are not only involved in docking and fusion with the target membrane but also in the formation of transport vesicles and in binding motor proteins for vesicle transport (Zerial and McBride, 2001; Hammer and Wu, 2002; Seabra and Coudrier, 2004). Several examples of motor protein–Rab complexes are known; for example those involving microtubule motors such as the kinesin-like protein (Rabkinesin-6) and Rab6 as well as actin-based motor complexes such as myosin Va and Rab27a and myosin Vb and Rab11 (Hammer and Wu, 2002).
Rab8 interacts specifically with the NH2 terminus of optineurin (Hattula and Peranen, 2000). Rab8 has been localized to the Golgi region, to TGN-derived transport vesicles and to the plasma membrane (Huber et al., 1993b) and has been shown to regulate biosynthetic trafficking pathways from the TGN to the cell surface (Huber et al., 1993b; Ang et al., 2003).
In our present study, we have identified an RRL sequence in the tail domain of myosin VI that binds to a site in the COOH-terminal region of optineurin. Both proteins colocalize at the Golgi complex and in vesicular structures close to the plasma membrane. The depletion of optineurin using small interfering RNA (siRNA) techniques drastically alters the morphology of the Golgi complex and reduces significantly transport of vesicular stomatitis virus G-protein (VSV-G) to the cell surface indicating that optineurin plays an important role in Golgi ribbon formation and in exocytosis. In addition, depletion of optineurin causes a marked reduction in the amount of myosin VI associated with the Golgi complex suggesting that optineurin is the anchor for myosin VI at this organelle. We further show that myosin VI colocalizes with Rab8 at the Golgi complex and at the plasma membrane, which is interesting, because Rab8 is a regulator of post-Golgi membrane traffic from the TGN to the plasma membrane.
Results
Optineurin binds to the COOH-terminal tail of myosin VI
The COOH-terminal tail domain of myosin VI is required for targeting to intracellular locations such as clathrin-coated vesicles (Buss et al., 2001a) and the Golgi complex (Warner et al., 2003). To identify binding partners for myosin VI in the Golgi complex we used the whole tail of myosin VI as a bait in a yeast two-hybrid screen of a human umbilical vein epithelial cell cDNA library (Morris et al., 2002). A 375–amino acid COOH-terminal fragment of optineurin (residues 198–577) was identified in the screen as a myosin VI–binding partner (Fig. 1 A). To confirm this interaction both myosin VI and optineurin were overexpressed in CHO cells and the binding was measured in a mammalian two-hybrid assay (Fig. 1 B). Deletion mutants of optineurin revealed that the myosin VI–binding site on optineurin is between amino acids 412 and 520 (not depicted). To determine the site on myosin VI that binds to optineurin a combination of deletion mutants followed by site-directed mutagenesis was used (Fig. 1 B). The results indicate that the globular tail (GT; aa 1034–1276) contains the binding site, because the NH2-terminal helical part of the tail (HT; aa 845–1033; Fig. 1 B, columns 1 and 2) did not bind. Furthermore, myosin VI interaction with optineurin does not require the large insert (LI) found in some isoforms (Fig. 1 B, columns 3 and 4); this part of the tail has previously been shown to be involved in targeting myosin VI to clathrin-coated vesicles at the apical domain of polarized cells (Buss et al., 2001b). Because deletion of the COOH-terminal 159 aa of the myosin VI tail did not abolish binding (Fig. 1 B, column 5), we used site-directed mutagenesis to change three adjacent residues at a time to alanine in order to narrow down the binding site in the remainder of the GT region, i.e., between aa 1060 and 1117. Fig. 1 B shows that changing WKS to AAA, KVY to AAA, and REE to AAA did not reduce binding (Fig. 1, columns 6, 7, and 9), however mutating RRL to AAA abolished binding of the myosin VI tail to optineurin (column 8), clearly showing that these residues constitute the binding site for optineurin.
Figure 1.
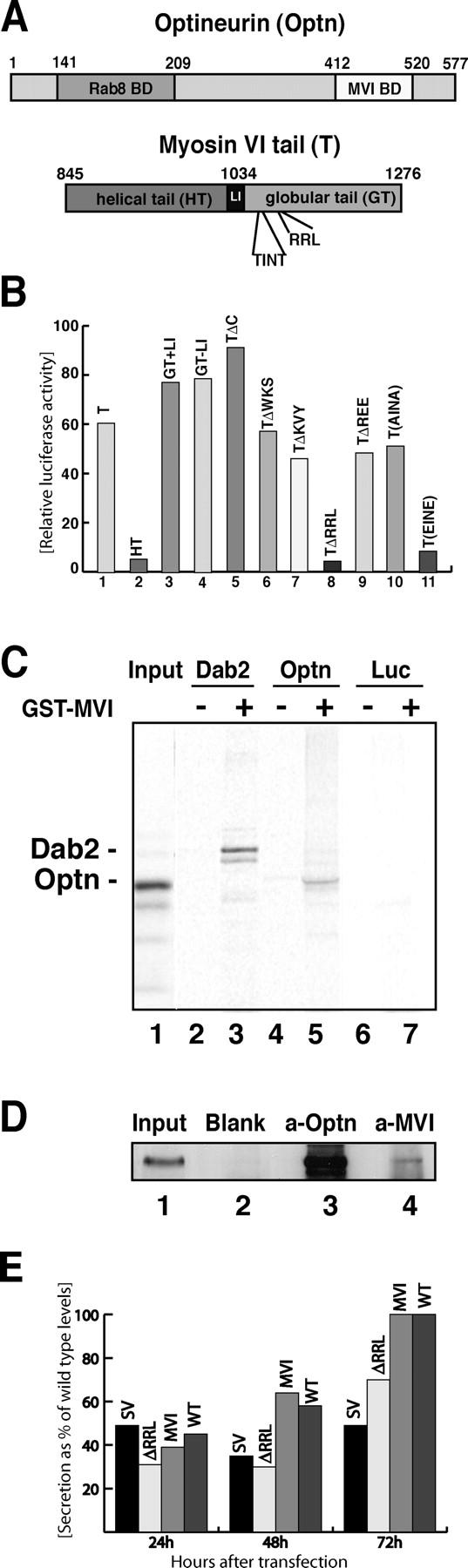
Optineurin is a myosin VI–binding partner. (A) A carton showing the major regions in optineurin and myosin VI tail. Optineurin contains an NH2-terminal Rab8-binding domain (aa 141–209; Hattula and Peranen, 2000) and a COOH-terminal myosin VI–binding domain (aa 412–520). Myosin VI tail has a proposed helical tail domain (HT) and a globular tail (GT), which contains the optineurin-binding site RRL in position 1107–1109. The putative phosphorylation site TINT is at position 1088–1091. (B) Using the ProQuest yeast two-hybrid screen optineurin was identified as a myosin interacting protein. The interaction was verified using the mammalian two-hybrid system. To narrow down the optineurin-binding site on the myosin VI tail the whole tail (T; column 1), the helical tail (HT; column 2) or the globular tail (GT) with or without the large insert (GT+LI or GT-LI; columns 3 and 4) were fused to the Gal4 DNA-binding domain and coexpressed with full-length optineurin fused to the VP16 activating domain in CHO cells. Luciferase activity is shown relative to control cells containing only the myosin VI construct with the empty prey vector. Deletion of myosin VI tail at residue 1117 (TΔC; column 5) did not abolish binding. Mutating three adjacent amino acids each (WKS, KVY, RRL, and REE to AAA) revealed RRL as the optineurin-binding site (columns 6–9). Two threonine residues in positions 1088 and 1091 (TINT) were mutated to either alanine (AINA) or glutamate (EINE; columns 10 and 11). (C) Optineurin binds to purified myosin VI tail. A pull down was performed using in vitro–translated optineurin and GST-myosin VI tail. 35S-labeled in vitro–translated full-length optineurin (lanes 4 and 5), Dab2 as a positive control (lanes 2 and 3) or luciferase as a negative control (lanes 6 and 7) were incubated with either 5 μg GST alone (lanes 2, 4, and 6) or with 5 μg GST-myosin VI tail (lanes 3, 5, and 7). Lane 1 shows 50% of the input used for the pulldown with [35S]optineurin in lane 5. The band below Dab2 is possibly caused by degradation (lane 3). (D) Co-immunoprecipitation of endogenous myosin VI and optineurin from the cytosol of A431 cells. Immunoprecipitation was performed under native conditions using no antibody, only protein A beads as a control (lane 2, blank), an antibody to full-length optineurin (lane 3, a-Optn) or a pAb to the tail of myosin VI (lane 4, a-MVI). Lane 1 is equivalent to 5% of the input used for each immunoprecipitation. Immunoprecipitates were run out on a 10% PAGE gel, blotted, and analyzed using an antibody to optineurin. (E) Constitutive secretion in Snell's waltzer fibroblasts cannot be rescued by overexpression of the mutant myosin VI lacking the optineurin-binding site (ΔRRL). Rescue experiments were performed by generating Snell's waltzer cell lines stably expressing only GFP (SV), the mutant myosin VI without the optineurin-binding site (ΔRRL), or wild-type whole myosin VI (MVI). Levels of secretion were compared with wild-type cells stably expressing only GFP (WT). To measure constitutive secretion, a construct, which expresses a SEAP was transfected into the various Snell's waltzer cell lines and the wild-type fibroblasts. The amount of alkaline phosphatase secreted into the media was measured 24, 48, and 72 h after transfection and plotted as a percentage of total maximal secretion in wild-type cells after 72 h. Transfection efficiency was normalized by cotransfection of a control plasmid expressing a red fluorescent protein. The data from two separate experiments run in triplicate are shown.
In vitro phosphorylation studies using recombinant p21–activated kinase (PAK; Buss et al., 1998) and the tail of myosin VI expressed in E. coli indicated that threonine 1088 and threonine 1091 (the sequence TINT; Fig. 1 A) are potential phosphorylation sites (unpublished data). These threonines are also phosphorylated in vivo, because when full-length myosin VI was isolated from A431 cells by immunoprecipitation and subjected to MALDI mass spectrometric analysis a peptide containing this phosphorylated TINT motif was detected. This peptide was dephosphorylated after alkaline phosphatase treatment (unpublished data). We mutated these two threonines to either alanines or glutamates to mimic the nonphosphorylated and phosphorylated states respectively in order to test their effects on the binding of myosin VI to optineurin in the mammalian two-hybrid assay. Whereas no effect was seen with the alanine-mutated construct AINA (Fig. 1 B, column 10), TINT mutated to EINE abolished optineurin binding to myosin VI (column 11) but did not abolish myosin VI binding to Dab2, which was used as a control. Because the EINE mutation potentially mimics the phosphorylated form, this data suggests that phosphorylation regulates the binding of optineurin to myosin VI.
A GST pull-down assay gave further confirmation that optineurin binds directly to the tail of myosin VI. In vitro–translated [35S]methionine labeled optineurin or Dab2 as a positive control (Fig. 1 C, lanes 2 and 3) were incubated with either GST or GST myosin VI tail. The autoradiogram in Fig. 1 C shows that optineurin binds to the myosin VI tail (Fig. 1 C, lane 5), but not to GST alone (lane 4) or to luciferase as a negative control protein (lanes 6 and 7). To confirm that myosin VI binds to optineurin in vivo, we immunoprecipitated endogenous myosin VI and endogenous optineurin from the cytosol of A431 cells. When myosin VI was immunoprecipitated with myosin VI tail antibodies optineurin is coimmunoprecipitated (Fig. 1 D, lane 4), indicating that optineurin and myosin VI exist in a protein complex in vivo.
Mutant myosin VI (ΔRRL) does not rescue secretion in Snell's waltzer fibroblasts
In fibroblastic cell lines derived from Snell's waltzer mice (sv/sv) the absence of myosin VI causes a reduction in constitutive secretion. As shown previously this phenotype can be restored by overexpression of a fully functional myosin VI (Warner et al., 2003). To test whether binding of optineurin is essential for myosin VI function at the Golgi complex in vivo, we performed similar rescue experiments in sv/sv fibroblasts. Immortal fibroblastic sv/sv or wild-type cell lines were stably transfected with GFP, GFP-tagged whole wild-type myosin VI or myosin VI with mutations in the optineurin-binding site (ΔRRL). Constitutive secretion in these stable cell lines was measured using a soluble secreted form of the alkaline phosphatase (SEAP) assay as previously described (Warner et al., 2003). We observed that only fully functional myosin VI is able to restore wild-type levels of secretion, whereas cells expressing myosin VI with the mutant optineurin-binding site are unable to increase secretion levels back to those of the wild type (Fig. 1 E).
Myosin VI and optineurin colocalize at the Golgi complex and in vesicles underneath the plasma membrane
To test the interaction between myosin VI and optineurin in vivo, we investigated their intracellular localization in normal rat kidney (NRK) cells. Previously, both proteins had been localized independently to the Golgi complex (Buss et al., 1998; Hattula and Peranen, 2000; Schwamborn et al., 2000; Stroissnigg et al., 2002; Warner et al., 2003). Using an affinity-purified pAb to optineurin and the TGN marker, TGN38, we confirm that optineurin is localized at the Golgi complex (Fig. 2, a–c). Double labeling experiments using optineurin antibodies and an mAb to myosin VI (Warner et al., 2003) showed considerable colocalization of both endogenous proteins at the Golgi complex (Fig. 2, d–f). In addition, both proteins were found in vesicular structures in the cell periphery close to the plasma membrane (Fig. 2, g–i and g′–i′). We transiently overexpressed optineurin or fragments of optineurin tagged with either GFP, Flag, or myc at their COOH or NH2 termini in a number of different cell types for colocalization studies with the aim of expressing optineurin mutants that might function as dominant negative. However, none of the transiently expressed optineurin constructs gave the same intracellular localization as endogenous optineurin (unpublished data); the overexpressed optineurin was not observed at the Golgi complex but showed an overall vesicular distribution and formed aggregates of various sizes in the cytoplasm. It would appear that the addition of the GFP, Flag, or myc tags to the NH2 or COOH terminus of optineurin or even to its fragments alters the protein conformation in some way and masks domains important for intracellular targeting to the Golgi complex. Interestingly, endogenous myosin VI was recruited from its cytosolic pool into these optineurin aggregates (Fig. 2, j–l), indicating that the myosin VI–binding site is maintained and accessible. NH2-terminal optineurin constructs without the myosin VI–binding domain (aa 412–520) were unable to recruit endogenous myosin VI into these aggregates (not depicted). These results strongly indicate that myosin VI binds to and forms a protein complex with optineurin in vivo.
Figure 2.
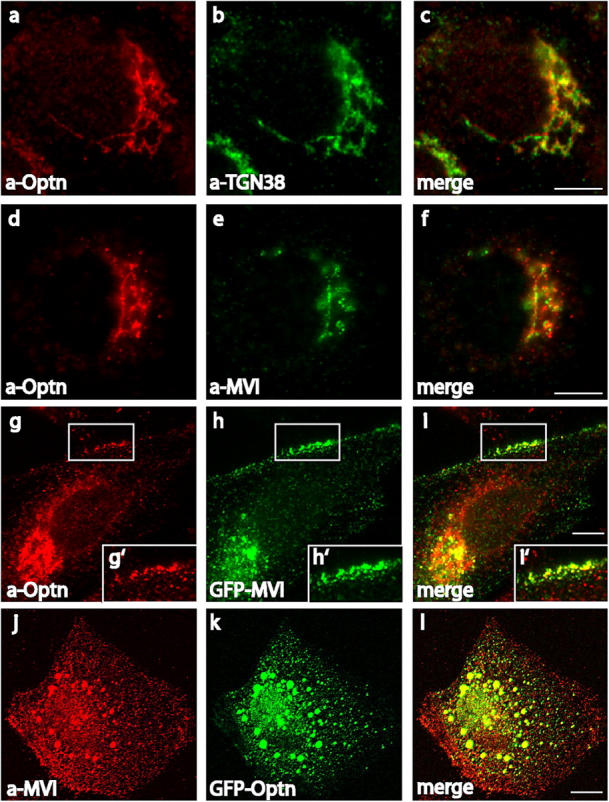
Optineurin and myosin VI colocalize in NRK cells. Endogenous optineurin localizes to the Golgi complex using a pAb to optineurin (a) and an mAb to TGN38 (b). Optineurin and myosin VI are localized at the Golgi complex using a pAb to optineurin (d) and an mAb to myosin VI (e). Colocalization in vesicular structures close to the plasma membrane was demonstrated with the pAb to optineurin (g) and by transiently overexpressing myosin VI tagged with GFP (h). White boxes indicate areas enlarged in the pictures below (g′, h′, and i′). Endogenous myosin VI, visualized using the pAb against the whole tail, is recruited into aggregates containing overexpressed GFP-optineurin (j–k). The merged images are shown in c, f, i, i′, and l. Bars, 10 μm.
Depletion of optineurin by siRNA causes loss of myosin VI from the Golgi complex
Does optineurin play a role in linking myosin VI to the Golgi complex? To address this question we used siRNA to reduce cellular expression levels of optineurin (Elbashir et al., 2001). For the knockdown experiments two siRNA duplexes, specific for the nucleotide sequence of optineurin, were tested in NRK and HeLa cells (see Materials and methods) and the best results were achieved after repeated transfection over a period of 4 d. In these cells optineurin was depleted to <10% of its original level as shown by Western blotting of equal amounts of mock and siRNA-treated cells 48 h after the second transfection (Fig. 3 A, a and B, a). In the same cells expression levels of myosin VI and actin were unaffected (Fig. 3 A, a and B, a). In the knockdown cells immunofluorescence microscopy showed that very little optineurin was present in the Golgi complex (Fig. 3 A, d and B, d), although in some cells residual cytosolic labeling was apparent (Fig. 3 A, d). To find out what happens to the localization of myosin VI in optineurin knockdown cells, we double labeled siRNA and mock-transfected NRK cells with a myosin VI COOH-terminal globular tail antibody and with antibodies to GM130 and TGN38. In optineurin-depleted cells there was a marked reduction in the amount of myosin VI associated with the Golgi complex (Fig. 4, c and g) as compared with mock-transfected cells (Fig. 4, a and e) indicating that optineurin plays a role in anchoring myosin VI to the Golgi complex.
Figure 3.
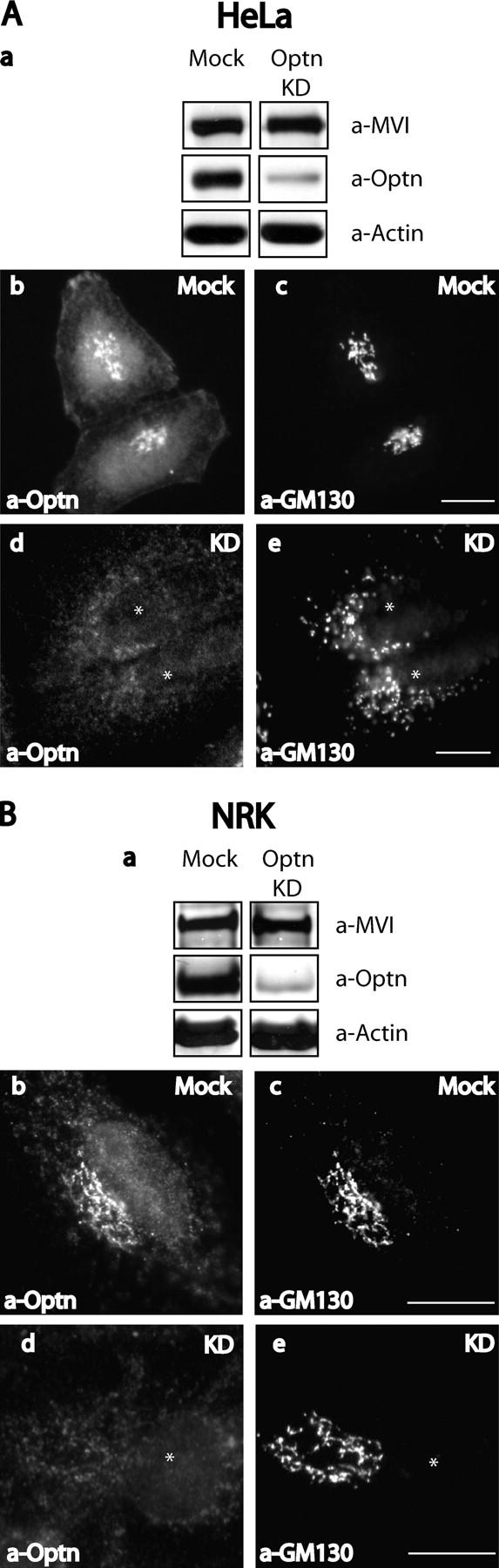
Optineurin is depleted from NRK and HeLa cells using siRNA. HeLa (A) or NRK (B) cells were either mock transfected with water or transfected twice at 48-h intervals with siRNA specific to optineurin. After 4 d cells were blotted and probed with antibodies to myosin VI, optineurin, or actin (A, a and B, a). In a parallel experiment mock-transfected, siRNA-transfected NRK, or HeLa cells were used for immunofluorescence and double labeled with antibodies to optineurin (A, b and d; B, b and d) and GM130 (A, c and e; B, c and e). Bars, 10 μm.
Figure 4.
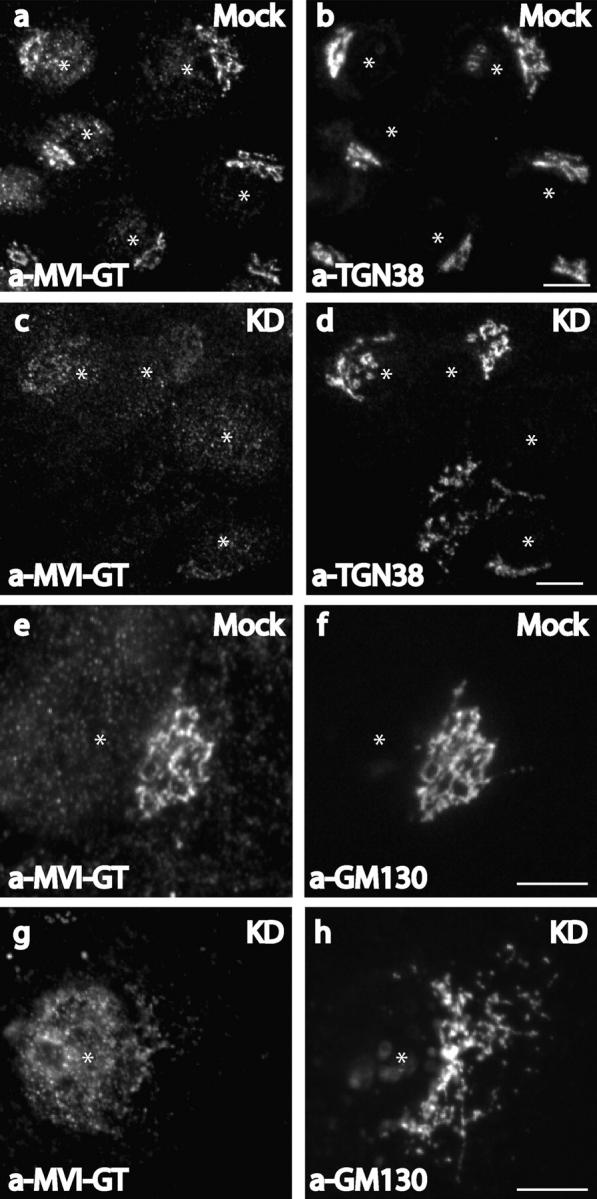
Knockdown of optineurin in NRK cells causes loss of myosin VI from the Golgi complex. Mock-transfected (a, b, e, and f) or siRNA-transfected (c, d, g, and h) NRK cells were used for immunofluorescence and were double labeled with antibodies to the globular tail of myosin VI (a, c, e, and g) and antibodies to TGN38 (b and d) or GM130 (f and h). Asterisks mark the position of the nucleus. Bars, 10 μm.
Loss of optineurin disrupts the Golgi ribbon structure
In mammalian cells the Golgi complex is formed by stacks of flattened membrane cisternae that are interconnected by tubular membranes into a ribbon like structure (Rambourg and Clermont, 1990). After depolymerization of microtubules using drugs such as nocodazole, these tubular connections are lost and the Golgi stacks are disconnected and dispersed throughout the cells, indicating that the cytoskeleton plays a major role in maintaining this perinuclear organization of the Golgi complex (Rogalski and Singer, 1984).
When optineurin was depleted by siRNA transfection in NRK cells (Fig. 4, d and h) or especially in HeLa cells (Fig. 5, d–f) there was a dramatic effect on the structure of the Golgi complex. In the optineurin-depleted HeLa cells the Golgi complex was fragmented into vesicular and sometimes short tubular structures dispersed throughout the whole cytoplasm. These Golgi fragments not only contained the Golgi matrix (GM130) and TGN (TGN46) markers (Fig. 5, d–f) but also markers for the cis- and medial-Golgi stacks (not depicted). Although the Golgi matrix and TGN markers were present in a single structure, they do not completely colocalize, indicating that some compartmentalization and organization of the Golgi was still preserved in these fragments (Fig. 5, d′–f′). The Golgi structure was not disrupted due to a breakdown of the microtubular network, because this network was still intact in optineurin-depleted cells (unpublished data).
Figure 5.
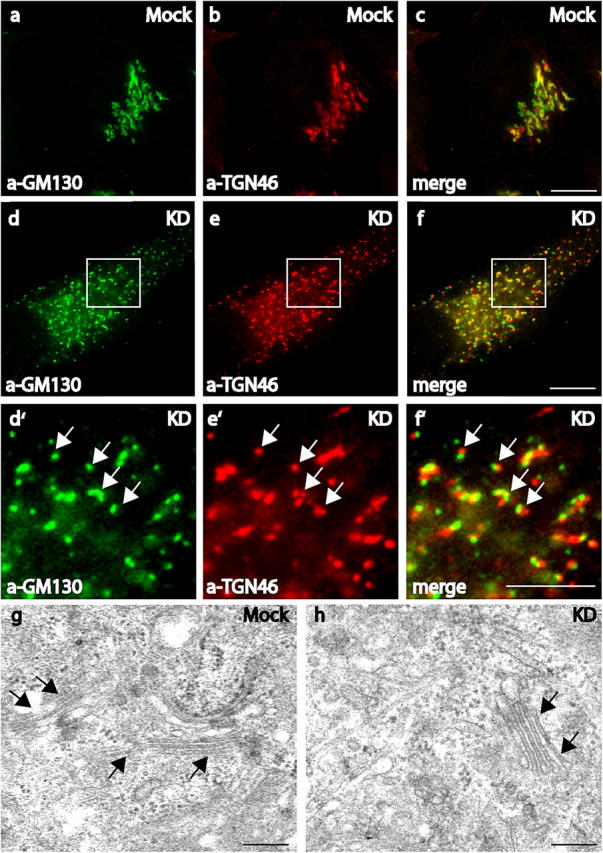
The loss of optineurin results in Golgi fragmentation. Mock (a–c) or optineurin siRNA-treated HeLa cells (d–f and d′–f′) were double labeled in immunofluorescence experiments with TGN46 (b, e, and e′) and GM130 (a, d, and d′). White boxes indicate areas enlarged in the pictures below (d′, e′, and f′). The Golgi fragments contain both marker proteins as indicated by the yellow color in the merged images (c, f, and f′). The arrows highlight the overlap between the marker proteins in (d′–f′). Bars, 10 μm. TEM analysis of mock (g) or siRNA-transfected (h) HeLa cells. Arrows indicate the position of Golgi stacks that can be found in both mock-transfected and siRNA-transfected cells. Bars, 200 nm.
To characterize the phenotype of optineurin-depleted cells at the ultrastructural level, electron microscopy was performed on mock and siRNA-treated HeLa cells. In optineurin knockdown cells mini-stacks of Golgi membranes similar to those in control cells were still observed (Fig. 5, g and h). This indicates that although the loss of optineurin caused a breakdown of the Golgi ribbon structure, the overall morphology of individual Golgi stacks was maintained and no large-scale vesiculation was present.
Colocalization of myosin VI and Rab8 is optineurin dependent
Optineurin has been shown to form a link between huntingtin and Rab8 (Hattula and Peranen, 2000) and optineurin and huntingtin can be colocalized at the Golgi complex (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200501162/DC1). These proteins are both found at the TGN, in transport vesicles throughout the cytoplasm and at the plasma membrane (Huber et al., 1993b; Velier et al., 1998). Optineurin specifically interacts with wild-type Rab8 and with the constitutively active GTP-bound mutant Rab8-Q67L, but not with the dominant-negative GDP-bound mutant Rab8-T22N in vitro (Hattula and Peranen, 2000; Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200501162/DC1). By immunofluorescence optineurin was shown to colocalize with wild-type Rab8 and the constitutively active Rab8-Q67L at the Golgi complex and in vesicular and tubular structures in the cytoplasm (Fig. S2 B).
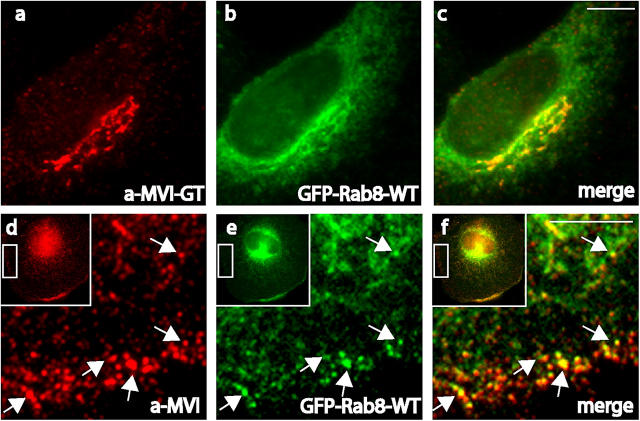
We suggest that optineurin may link myosin VI to Rab8, as it has been suggested that it links huntingtin to Rab8 (Hattula and Peranen, 2000). To check this idea we determined the intracellular distribution of myosin VI and Rab8 in NRK cells. Both proteins showed good colocalization around the Golgi complex (Fig. 6, a–c), and also in peripheral vesicles close to the plasma membrane (Fig. 6, d–f). Overexpression of the constitutively active form of Rab8-Q67L colocalized with myosin VI at the Golgi complex (Fig. 7, a and b) but also resulted in the formation of long tubular structures emerging from the Golgi complex (Fig. 7, f and f′). These long tubular carriers containing Rab8-Q67L were able to recruit endogenous myosin VI onto their membranes and led to a dramatic redistribution of myosin VI (Fig. 7, e and e′). The recruitment of myosin VI to these Rab8-positive structures requires optineurin, because in optineurin knockdown cells myosin VI is no longer present at the fragmenting Golgi complex (Fig. 7, c and d) or in the tubular structures (Fig. 7, g, h, g′, and h′).
Figure 6.
Myosin VI colocalizes with Rab8 at the Golgi complex and the plasma membrane. In NRK cells transiently overexpressing Rab8 tagged with GFP (b and e) myosin VI was detected with an antibody to the globular tail (a) or to the whole tail (d). Myosin VI and Rab8 are found in the same labeled structures/vesicles at the Golgi complex (c) and at the plasma membrane (f). Arrows indicate vesicles containing both proteins. Bars, 10 μm.
Figure 7.
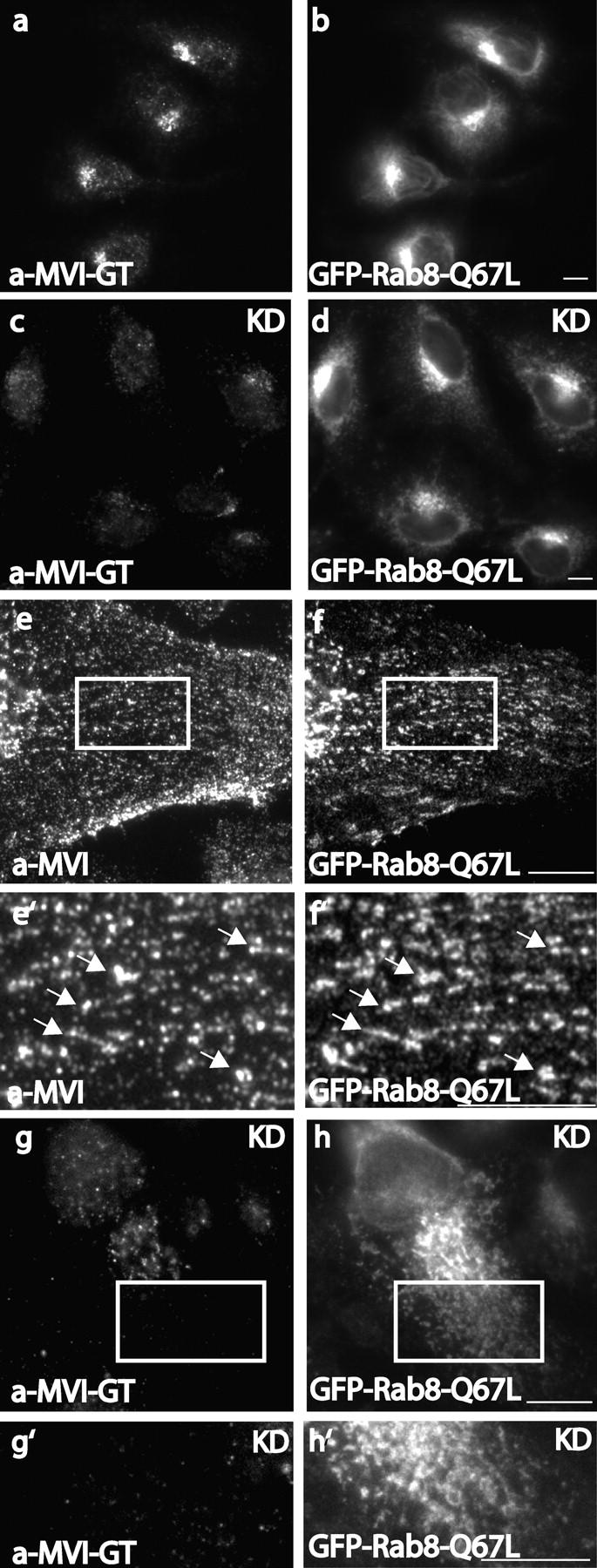
The recruitment of myosin VI to tubular vesicular structures formed after overexpression of the constitutively active form of Rab8 is lost in optineurin-depleted cells. The constitutively active form of Rab8 (GFP-Rab8-Q67L) overexpressed in NRK cells localizes with myosin VI at the Golgi complex (a and b) and recruits myosin VI onto tubular and vesicular structures emerging from the Golgi complex (e and f). In optineurin-depleted NRK cells, which are overexpressing GFP-Rab8-Q67L myosin VI no longer colocalizes with Rab8-Q67L at the Golgi complex (c and d) and is not recruited to tubular and vesicular structures positive for Rab8-Q67L (g and h). White boxes highlight the areas of the cells enlarged in the pictures below (e′,f′, g′, and h′). Endogenous myosin VI was detected with either a pAb to the globular tail (a, c, g, and g′) or to the whole tail (e and e′). Bars, 10 μm.
Transport of VSV-G from the Golgi complex to the plasma membrane is reduced in optineurin knockdown cells
Myosin VI has been shown to be important for constitutive secretion in fibroblasts (Warner et al., 2003). To test whether optineurin as a myosin VI–binding partner present at the Golgi complex, plays a role in post-Golgi membrane traffic to the cell surface, we used a thermoreversible folding mutant of the VSV-G fused to GFP at its cytoplasmic tail (ts045 VSV-G-GFP) as a reporter molecule for membrane transport in the secretory pathway (Wehland et al., 1982; Kreis and Lodish, 1986; Balch et al., 1994). This membrane protein misfolds and is retained in the ER at 39.5°C, but upon a temperature shift to 32°C, it can fold and move to the Golgi complex and subsequently to the plasma membrane. We measured the amount of VSV-G at the cell surface relative to the total amount of VSV-G expressed in the cell using an antibody specific to the luminal domain of this protein (Pepperkok et al., 1993; Seemann et al., 2000).
The loss of optineurin has a dramatic effect on transport of VSV-G from the Golgi complex to the cell surface. After 40 min in mock-transfected cells VSV-G could be detected at the cell surface (Fig. 8 A, b), whereas in optineurin knockdown cells it is still found in the fragmented Golgi (Fig. 8 A, c) and not at the cell surface (Fig. 8 A, d). After 100 min very little VSV-G still remained in the Golgi complex in the mock-transfected cells and most was on the cell surface (Fig. 8 A, e and f), however, in the optineurin-depleted cells VSV-G was still retained in the Golgi complex with only a small proportion of it present on the cell surface (Fig. 8 A, g and h). To quantify these observations ∼30 cells per time point were measured in two independent experiments. At every time point less VSV-G was detected at the plasma membrane of siRNA-treated cells compared with mock-transfected cells (Fig. 8 B). Thus, the transport of VSV-G from the Golgi complex to the cell surface was dramatically delayed in the optineurin-depleted cells and only at later time points did it reach 50% of the control levels. These results clearly indicate that optineurin plays an important role in post-Golgi membrane trafficking of VSV-G from the Golgi complex to the cell surface.
Figure 8.
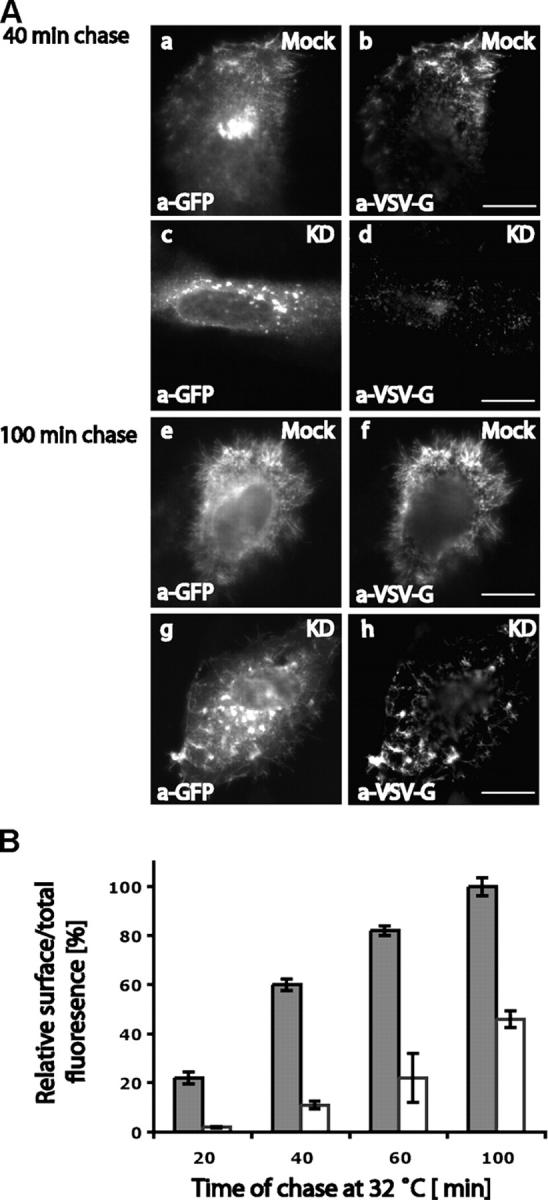
The transport of VSV-G to the cell surface is dramatically reduced in optineurin-depleted HeLa cells. Mock-treated or siRNA-treated HeLa cells were transfected with ts045-VSV-G-GFP to measure the rate of exocytosis . VSV-G at the cell surface was detected in indirect immunofluorescence using an mAb to the luminal domain of VSV-G and total VSV-G expressed was detected using a pAb to GFP. (A) Representative cells for the 40- and 100-min time points are shown to compare the amount of VSV-G on the cell surface in mock- and optineurin-depleted cells. (B) The ratio of cell surface over total VSV-G fluorescence was measured as described in Materials and methods to determine the rate of transport from the Golgi complex to the cell surface. Error bars: ±SEM. Bars, 10 μm.
Discussion
We have previously shown that myosin VI is present at the Golgi complex (Buss et al., 1998) and is involved not only in the steady-state organization of the Golgi complex but also in post-Golgi membrane traffic (Warner et al., 2003). To investigate the precise roles that myosin VI plays in these processes we identified optineurin, a peripheral Golgi protein as a binding partner for myosin VI. This interaction found in a yeast two-hybrid screen was confirmed using a range of different protein–protein interaction studies. Myosin VI and optineurin colocalize at the Golgi complex and in vesicular structures close to the plasma membrane. The loss of optineurin not only leads to Golgi fragmentation and a reduction in exocytosis, but we also show that optineurin is essential for targeting myosin VI to the Golgi complex. Most interestingly optineurin plays a role in colocalizing myosin VI and Rab8, a member of the Rab family of G-proteins, which are important regulators of membrane traffic events.
Optineurin binds directly to the sequence RRL (residues 1107–1109) in the globular tail of myosin VI. This binding site is distinct from the Dab2-binding site and is independent of the large insert. The binding of optineurin to myosin VI is regulated by phosphorylation at the TINT (1088–1091) site in the globular tail region. Our results suggest that a PAK phosphorylates myosin VI not only in the motor domain (Buss et al., 1998) but also in the globular tail domain at threonine 1088 and 1091, thus coordinating the regulation of motor function with cargo binding in the tail domain.
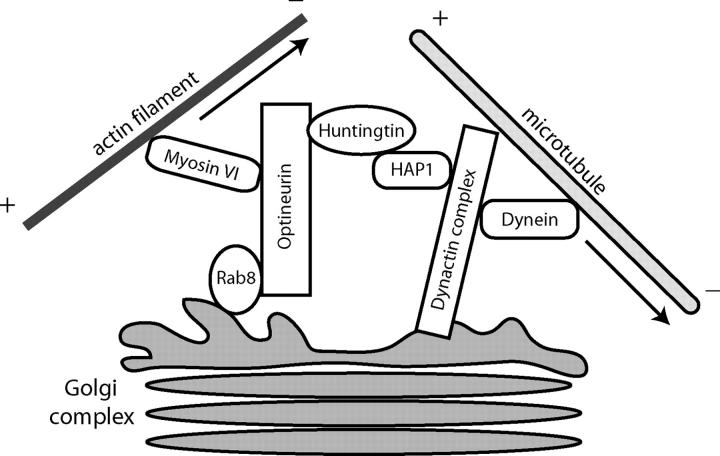
In the absence of optineurin the Golgi complex is fragmented. The ribbon structure of interconnected stacks of membrane cisternae is broken up and the disconnected Golgi stacks are dispersed throughout the cytoplasm. The overall appearance of the fragmented Golgi stacks, however, is similar to the appearance of the Golgi in control cells and no gross vesiculation was observed at the EM level. Golgi resident proteins such as TGN46 and GM130 were still present in the Golgi fragments. These morphological changes are very similar to those observed when microtubules are disrupted by drugs such as nocodazole (Rogalski and Singer, 1984), suggesting that optineurin might be involved in linking Golgi membranes directly or indirectly to microtubules around the microtubule organizing center (MTOC). One of the optineurin-binding partners is huntingtin, known to interact with HAP1 (Huntingtin-associated protein; Li et al., 1995), which binds directly to the dynactin subunit p150glued (Engelender et al., 1997; Li et al., 1998a). The dynactin complex is involved in linking the minus end directed microtubule motor protein dynein to membrane vesicles (Gill et al., 1991). It has been proposed that huntingtin and HAP1 act as scaffolding proteins regulating the interaction between the dynein–dynactin complex and cargo such as Golgi membranes (Harjes and Wanker, 2003). The absence or inactivation of cytoplasmic dynein leads to fragmentation and spreading of the Golgi complex into the cytoplasm (a phenotype similar to that observed after the loss of optineurin; Harada et al., 1998); thus it is possible that optineurin via the huntingtin–HAP1 complex may be involved in targeting dynein to Golgi membranes (Fig. 9). Recent results (Gauthier et al., 2004) indicate a further role for the huntingtin–HAP1 complex in the axonal transport of vesicles containing brain-derived neurotrophic factor along microtubules and highlight the importance of huntingtin-binding partners in the progression of the disease.
Figure 9.
A cartoon illustrating the possible interaction of optineurin with motor protein complexes at the Golgi complex. Optineurin may play a central role in coordinating actin-based and microtubule-based motor function for maintaining Golgi morphology. The optineurin-binding partner huntingtin has been shown to interact with HAP1, which in turn was reported to form a complex with the dynactin subunit p150glued and modulate/regulate the dynein–dynactin complex. Loss of minus end–directed dynein motor activity causes in fragmentation of the Golgi ribbon structure. On the other hand loss of myosin VI results in a reduction in the size of the Golgi complex, because now the dynein complex is still active and able to retract the Golgi complex toward the MTOC. However, if optineurin is depleted, both motor complexes are nonfunctional and the Golgi complex is fragmented.
The loss of optineurin not only disrupts the structure of the Golgi complex, but also dramatically reduces secretion of VSV-G to the plasma membrane. Reduced transport is not due to Golgi fragmentation because Golgi stacks dispersed by depolymerization of microtubules remain fully functional with only a slight reduction in protein secretion (Van De Moortele et al., 1993). Reduced exocytosis of VSV-G in the optineurin knockdown cells is a very similar phenotype to that observed in the myosin VI knockout mouse, where secretion of alkaline phosphatase is also significantly reduced (Warner et al., 2003). Secretion in these cells lacking myosin VI is not restored by mutant myosin VI, which is unable to bind to optineurin, indicating that a functional complex between myosin VI and optineurin is required for constitutive exocytosis.
Rab8 binds to optineurin and plays an important role in polarized membrane transport both from the Golgi complex to the basolateral membrane in polarized epithelial cells and to dendrites in neurons (Huber et al., 1993a,b). Rab8 is present in the same intracellular compartments as myosin VI and in this paper we show that myosin VI and Rab8 colocalize in the perinuclear region around the Golgi complex and both are present on the same vesicles underneath the plasma membrane. In addition Rab8 has been localized to the recycling endosome, a compartment shown to be involved in post-Golgi membrane trafficking to the plasma membrane (Ang et al., 2003, 2004). However, at present we don't know whether some myosin VI is also associated with the fraction of Rab8 present in the recycling endosome. On the other hand because Rab8 may interact via optineurin with myosin VI it suggests a possible pathway whereby TGN-derived vesicles are transported short distances along actin filaments by myosin VI, before a kinesin motor picks up the cargo for long distance transport along microtubules. Similarly, close to the plasma membrane the Rab8–optineurin–myosin VI complex might be involved in presenting the secretory vesicle to the docking site, thus tethering the vesicle close to the plasma membrane before fusion. Overexpression of Rab8-Q67L leads to the formation of long tubular extensions from the Golgi complex and it recruits endogenous myosin VI onto these tubular structures. These results indicate that Rab8 serves as a membrane receptor for myosin VI, recruiting this myosin motor onto a specific intracellular membrane compartment via optineurin. Similarly, it was shown recently that Rab27a recruits myosin Va onto melanosomes via the linker molecule melanophillin (Seabra and Coudrier, 2004).
Optineurin is the first myosin VI–binding partner identified at the Golgi complex and may play an adaptor or linker role. The recent demonstration that myosin VI can exist as a nonprocessive monomer (Lister et al., 2004) and also possibly as a processive dimer (Rock et al., 2001; Nishikawa et al., 2002) may provide a mechanism for the distinct dual roles for myosin VI in structural maintenance and vesicle transport action away from the Golgi complex. We therefore suggest that optineurin may serve two major “linker” functions for myosin VI in the Golgi complex (Fig. 9): First, optineurin together with huntingtin links via the minus end directed motor dynein to the microtubule network and also by directly interacting with myosin VI, is linked to the actin cytoskeleton, thus it may play a role in coordinating microtubule-based and actin-based motor activity around the Golgi complex. Support for such a role is provided by our previous observation that in Snell's waltzer mice the absence of myosin VI causes a reduction in the size of the Golgi complex (Warner et al., 2003), which would be due to the dynein motor complex pulling the Golgi membranes in toward the MTOC resulting in a smaller more compact Golgi complex. Therefore, in the absence of myosin VI the balance of motor proteins is disturbed and dynein wins the “tug of war”. Second, optineurin might link myosin VI to Rab8, a regulatory protein known to be involved in sorting molecules in the exocytic pathway at the TGN and in membrane fusion at the plasma membrane. The linker function of optineurin may be temporally regulated, for example by phosphorylation of individual proteins, including myosin VI and optineurin (Schwamborn et al., 2000).
In addition, because optineurin is obviously a dimer with multiple leucine zippers it may induce monomeric myosin VI to dimerize for processive movement of vesicles from the TGN to the plasma membrane.
Our work provides new information on the intracellular functions of optineurin, which will help elucidate how mutations in the optineurin gene lead to primary open angle glaucoma (Rezaie et al., 2002). Previous reports, that reduced secretion of the glycoprotein myocilin leads to primary open angle glaucoma (Joe et al., 2003), support our results that optineurin plays a major role in Golgi morphology and exocytosis.
Materials and methods
Antibodies
The following antibodies were used: affinity-purified rabbit pAb to human full-length optineurin (α-Optn; GenBank/EMBLDDBJ accession no. AF061034); monoclonal α-VSV-G to the luminal domain (a gift from R. Pepperkok and J. Simpson, EMBL, Heidelberg, Germany); polyclonal α-optineurin (Cayman); monoclonal α-GM130 (BD Transduction Laboratories); monoclonal α-TGN38 (Luzio et al., 1990); monoclonal α-GFP (Qbiogene); polyclonal α-GFP (Molecular Probes); monoclonal, polyclonal α-TGN46 (Serotec); and monoclonal and pAbs to the whole tail (α-MVI) and globular tail of myosin VI (α-MVI-GT; Buss et al., 1998; Warner et al., 2003).
Yeast two-hybrid screen
Myosin VI interacting proteins were identified in a ProQuest Two-Hybrid System (Invitrogen) using the tail domain of chicken intestinal brush border myosin VI (amino acids 846–1277) containing the large insert to screen a human umbilical vein epithelial cell cDNA library (provided by M. McCormack and T. Rabbits, MRC-LMB, Cambridge, UK) as described previously (Morris et al., 2002). 100 mM 3-amino-1,2,4-TRIzol was used to overcome the observed self-activation by the myosin VI tail domain. The potential myosin VI tail domain interacting proteins were identified in the yeast two-hybrid screen by selecting transformants that were able to grow on plates lacking leucine, tryptophan, and histidine in the presence of 100 mM 3-amino-1,2,4-TRIzol. Colonies containing candidate cDNAs for interacting proteins were isolated and replated on selection agar to test for activation of the three reporter genes HIS3, URA3, and lacZ. Colonies positive for all three reporter genes were confirmed in the mammalian two-hybrid assay.
Cloning of optineurin and construction of pEGFP-Rab8-Q67L
The full-length version of human optineurin cDNA was generated by PCR from Caco-2 cDNA and subcloned into pEGFP (Clontech) or pRSET (Invitrogen) for pAb production. pEGFP-Rab8 WT, containing the human wild-type Rab8 cDNA sequence (GenBank/EMBL/DDBJ accession no. 498943) was a gift from P. Row (University of Liverpool, Liverpool, UK) and H. Davidson (University of Colorado, Denver, CO). The constitutively active form of Rab8, Rab8-Q67L, was generated by QuikChange Site-Directed Mutagenesis (Stratagene) according to the manufacturer's instructions.
Mammalian two-hybrid assay
Chicken myosin VI tail fragments HT (aa 845–1033) and TΔC (aa 845–1117) were generated by PCR and inserted into the pM “bait” vector (CLONTECH Laboratories, Inc.). The following alanine mutations were generated using QuikChange Site-Directed Mutagenesis (Stratagene): WKS, KVY, RRL, and REE to AAA at aa positions 1115–1117, 1110–1112, 1107–1109, and 1102–1104, respectively, (named TΔWKS, etc.) along with T1088A/T1091A and T1088E/T1091E double mutants. T (chicken Myosin VI whole tail with the large insert (LI)), CC (aa 846–1034), GT+LI (aa 1035–1277), and GT-LI (aa 1061–1277) were constructed previously (Morris et al., 2002). The mammalian two-hybrid assay was performed as previously described (Morris et al., 2002).
Cell culture and transfection
NRK cells, CHO cells, and HeLa cells were cultured as described before (Buss et al., 1998). For transient transfection experiments cells were transfected either with 2 μg of pEGFP-optineurin, pEGFP-MyosinVI, pEGFP-Rab8-WT, or pEGFP-Rab8-Q67L for 16–18 h using FuGENE (Roche Diagnostics) according to the manufacturer's instructions. Snell's waltzer mouse immortal cell lines (Warner et al., 2003) were transfected using the MEF-1 Nucleofector kit (Amaxa Biosystems) according to the manufacturer's instructions with chicken full-length myosin VI, the mutant myosin VI (ΔRRL), or only GFP cloned into ΔpMEP (Buss et al., 2001a). The cells were grown and selection performed as previously described (Warner et al., 2003).
SEAP expression and assay
Snell's waltzer fibroblasts (SV), wild-type fibroblasts (WT; Warner et al., 2003) and Snell's waltzer expressing either full-length wild-type myosin VI-GFP (MVI) or the mutant myosin VI (ΔRRL) were transiently transfected with the pSEAP2-control mammalian expression plasmid (CLONTECH Laboratories, Inc.) containing SEAP cDNA using the MEF-1 Nucleofector kit (Amaxa Biosystems) according to the manufacturer's instructions. The SEAP assay was performed as previously described (Warner et al., 2003).
Knockdown of optineurin by siRNA
HeLa and NRK cells were transfected with siRNA duplex (20 μM; Dharmacon Research) using OligofectAMINE (Invitrogen) according to the manufacturer's manual. For efficient knockdown of optineurin cells were transfected twice with siRNA duplex on day 1 and day 3. On day 5 cells were processed for indirect immunofluoresence and the efficiency of knockdown was assayed by Western blotting. In the mock experiment the siRNA duplex was substituted with H2O. Human and rat optineurin were targeted with two independent siRNAs: 5′-CCACCAGCTGAAAGAAGCC-3′ and 5′-CTGCAGCTCAAGCTGAACT-3′. Both siRNAs gave the same level of knockdown and phenotypes in optineurin-depleted cells.
Indirect immunofluorescence microscopy
Indirect immunofluorescence staining was performed as described in Buss et al. (1998). To visualize optineurin at the Golgi complex, cells were pre-permeabilized with 0.05% saponin in cytosol buffer (Morris and Cooper, 2001) for 30 s. Cells were analyzed and photographed using 63× or 100× objectives of an Axiophot microscope (Carl Zeiss MicroImaging, Inc.) equipped with a CCD camera. Images were further processed in Adobe Photoshop.
VSV-G-GFP transport assay
4-d mock- or siRNA-treated HeLa cells were transfected with ts045G VSV-G-GFP (a gift from R. Duden, University of London, London, UK) using FuGENE as described above. Cells were first incubated for 2 h at 37°C and then for 15 h at 39.5°C. Before a temperature shift from 39.5°C to 32°C, 100 μg/ml cycloheximide (Sigma-Aldrich) was added to each dish and cells were incubated at 4°C for 30 min. After 20, 40, 60, and 100 min at 32°C cells were fixed with 4% PFA and VSV-G present on the cell surface detected with an mAb to the luminal domain of VSV-G. GFP fluorescence gave the total amount of VSV-G expressed in the cell. The ratio of cell surface over total fluorescence was calculated as described previously (Pepperkok et al., 1993; Seemann et al., 2000) using IP lab software.
Immunoprecipitation and immunoblotting
For immunoprecipitation A431 cells were lysed and sonicated in extraction buffer containing PBS, 1 mM EDTA, 5 mM ATP, 5 mM MgCl2, 0.5% IGEPAL CA-630 (Sigma-Aldrich) and protease inhibitor cocktail (Complete; Roche) and processed for immunoprecipitation followed by SDS PAGE and immunoblotting as described previously (Buss et al., 1998).
GST pull-down assay
The chicken brush border myosin VI tail residues 840–1277 tagged with GST was expressed in E. coli BL21 (DE3) cells and purified as described previously (Buss et al., 1998). Full-length optineurin and Dab2 were cloned into pcDNA3 (Invitrogen) and in vitro translated using the TNT-coupled Reticulocyte Lysate System (Promega). The [35S]methionine in vitro–translated protein was incubated for 2 h at 4°C with 5 μg of GST or GST myosin VI tail coupled to glutathione beads in 10 mM Hepes, pH 7.4, 150 mM NaCl, 1% Triton X-100. After extensive washing the protein complexes bound to glutathione beads were separated by SDS PAGE and analyzed by autoradiography.
Transmission electron microscopy
Cells were grown in 6-cm tissue cultures dishes and fixed with 2.5% glutaraldehyde/2% PFA in 0.1 M Na cacodylate buffer, pH 7.2, at 37°C. After washing with 0.1 M Na cacodylate buffer, pH 7.2, cells were fixed after in 1% osmium tetroxide in 0.1 M Na cacodylate buffer, pH 7.2, for 1 h and washed with 0.05 M Na maleate buffer, pH 5.2. The cells were then en bloc stained with 0.5% uranyl acetate in 0.05 M Na maleate buffer, pH 5.2, dehydrated in ethanol, exchanged into 1,2-epoxy propane, and embedded in Araldite CY212 epoxy resin (Agar Scientific). Ultrathin sections were collected on EM grids and stained with uranyl acetate and Reynolds lead citrate. The sections were observed in a transmission electron microscope (model CM100; Philips Electron Optics) at an operating voltage of 80 kV.
Online supplemental material
Fig. S1 shows colocalization of optineurin with wild-type huntingtin at the Golgi complex in HeLa cells. Fig. S2 A demonstrates that optineurin binds to wild-type Rab8 and the Rab8 Q67L mutant but not the Rab8 T22N mutant in vitro. Fig. S2 B shows colocalization of endogenous optineuin with wild-type GFP-Rab8 and the GFP-Rab8 Q67L mutant at the Golgi complex and in vesicular structures throughout the cytosol. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200501162/DC1.
Acknowledgments
We thank Drs R. Duden, R. Pepperkok, J. Simpson, and H. Davidson for generous gifts of antibodies and reagents; and Drs. M. Chibalina and P. Pryor for help and advice.
This work was funded by a Wellcome Trust Senior Fellowship (F. Buss), a Wellcome Trust studentship (D.A. Sahlender) and a Royal Society USA postdoctoral fellowship (G. Spudich) and supported by the Medical Research Council. Cambridge Institute for Medical Research is in receipt of a strategic award from the Wellcome Trust.
D.A. Sahlender and R.C. Roberts contributed equally to this work.
Abbreviations used in this paper: MTOC, microtubule organizing center; NRK, normal rat kidney; SEAP, soluble secreted form of the alkaline phosphatase; siRNA, small interfering RNA; VSV-G, vesicular stomatitis virus G-protein.
References
- Ang, A.L., H. Folsch, U.M. Koivisto, M. Pypaert, and I. Mellman. 2003. The Rab8 GTPase selectively regulates AP-1B–dependent basolateral transport in polarized Madin-Darby canine kidney cells. J. Cell Biol. 163:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang, A.L., T. Taguchi, S. Francis, H. Folsch, L.J. Murrells, M. Pypaert, G. Warren, and I. Mellman. 2004. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 167:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner, L., T. Lee, and T. Hasson. 2003. Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol. Biol. Cell. 14:2728–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch, W.E., J.M. McCaffery, H. Plutner, and M.G. Farquhar. 1994. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 76:841–852. [DOI] [PubMed] [Google Scholar]
- Berg, J.S., B.C. Powell, and R.E. Cheney. 2001. A millennial myosin census. Mol. Biol. Cell. 12:780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn, R.C., M.A. Jensen, and B.C. Reed. 1999. Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol. Biol. Cell. 10:819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, F., J. Kendrick-Jones, C. Lionne, A.E. Knight, G.P. Cote, and J. Paul Luzio. 1998. The localization of myosin VI at the Golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J. Cell Biol. 143:1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, F., S.D. Arden, M. Lindsay, J.P. Luzio, and J. Kendrick-Jones. 2001. a. Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J. 20:3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, F., J.P. Luzio, and J. Kendrick-Jones. 2001. b. Myosin VI, a new force in clathrin mediated endocytosis. FEBS Lett. 508:295–299. [DOI] [PubMed] [Google Scholar]
- Buss, F., G. Spudich, and J. Kendrick-Jones. 2004. Myosin VI: cellular fuctions and motor properties. Annu. Rev. Cell Dev. Biol. 20:649–676. [DOI] [PubMed] [Google Scholar]
- DiFiglia, M., E. Sapp, K. Chase, C. Schwarz, A. Meloni, C. Young, E. Martin, J.P. Vonsattel, R. Carraway, S.A. Reeves, et al. 1995. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 14:1075–1081. [DOI] [PubMed] [Google Scholar]
- Elbashir, S.M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498. [DOI] [PubMed] [Google Scholar]
- Engelender, S., A.H. Sharp, V. Colomer, M.K. Tokito, A. Lanahan, P. Worley, E.L. Holzbaur, and C.A. Ross. 1997. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum. Mol. Genet. 6:2205–2212. [DOI] [PubMed] [Google Scholar]
- Faber, P.W., G.T. Barnes, J. Srinidhi, J. Chen, J.F. Gusella, and M.E. MacDonald. 1998. Huntingtin interacts with a family of WW domain proteins. Hum. Mol. Genet. 7:1463–1474. [DOI] [PubMed] [Google Scholar]
- Gauthier, L.R., B.C. Charrin, M. Borrell-Pages, J.P. Dompierre, H. Rangone, F.P. Cordelieres, J. De Mey, M.E. MacDonald, V. Lessmann, S. Humbert, and F. Saudou. 2004. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 118:127–138. [DOI] [PubMed] [Google Scholar]
- Gill, S.R., T.A. Schroer, I. Szilak, E.R. Steuer, M.P. Sheetz, and D.W. Cleveland. 1991. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 115:1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer, J.A., III, and X.S. Wu. 2002. Rabs grab motors: defining the connections between Rab GTPases and motor proteins. Curr. Opin. Cell Biol. 14:69–75. [DOI] [PubMed] [Google Scholar]
- Harada, A., Y. Takei, Y. Kanai, Y. Tanaka, S. Nonaka, and N. Hirokawa. 1998. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J. Cell Biol. 141:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjes, P., and E.E. Wanker. 2003. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem. Sci. 28:425–433. [DOI] [PubMed] [Google Scholar]
- Hattula, K., and J. Peranen. 2000. FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr. Biol. 10:1603–1606. [DOI] [PubMed] [Google Scholar]
- Hodge, T., and M.J. Cope. 2000. A myosin family tree. J. Cell Sci. 113:3353–3354. [DOI] [PubMed] [Google Scholar]
- Huber, L.A., M.J. de Hoop, P. Dupree, M. Zerial, K. Simons, and C. Dotti. 1993. a. Protein transport to the dendritic plasma membrane of cultured neurons is regulated by rab8p. J. Cell Biol. 123:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, L.A., S. Pimplikar, R.G. Parton, H. Virta, M. Zerial, and K. Simons. 1993. b. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J. Cell Biol. 123:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe, M.K., S. Sohn, W. Hur, Y. Moon, Y.R. Choi, and C. Kee. 2003. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem. Biophys. Res. Commun. 312:592–600. [DOI] [PubMed] [Google Scholar]
- Kreis, T.E., and H.F. Lodish. 1986. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 46:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S.H., C.A. Gutekunst, S.M. Hersch, and X.J. Li. 1998. a. Interaction of huntingtin-associated protein with dynactin P150Glued. J. Neurosci. 18:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.J., S.H. Li, A.H. Sharp, F.C. Nucifora Jr., G. Schilling, A. Lanahan, P. Worley, S.H. Snyder, and C.A. Ross. 1995. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 378:398–402. [DOI] [PubMed] [Google Scholar]
- Li, Y., J. Kang, and M.S. Horwitz. 1998. b. Interaction of an adenovirus E3 14.7-kilodalton protein with a novel tumor necrosis factor alpha-inducible cellular protein containing leucine zipper domains. Mol. Cell. Biol. 18:1601–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister, I., S. Schmitz, M. Walker, J. Trinick, F. Buss, C. Veigel, and J. Kendrick-Jones. 2004. A monomeric myosin VI with a large working stroke. EMBO J. 23:1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio, J.P., B. Brake, G. Banting, K.E. Howell, P. Braghetta, and K.K. Stanley. 1990. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38). Biochem. J. 270:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, S.M., and J.A. Cooper. 2001. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2:111–123. [DOI] [PubMed] [Google Scholar]
- Morris, S.M., S.D. Arden, R.C. Roberts, J. Kendrick-Jones, J.A. Cooper, J.P. Luzio, and F. Buss. 2002. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 3:331–341. [DOI] [PubMed] [Google Scholar]
- Nishikawa, S., K. Homma, Y. Komori, M. Iwaki, T. Wazawa, A. Hikikoshi Iwane, J. Saito, R. Ikebe, E. Katayama, T. Yanagida, and M. Ikebe. 2002. Class VI myosin moves processively along actin filaments backward with large steps. Biochem. Biophys. Res. Commun. 290:311–317. [DOI] [PubMed] [Google Scholar]
- Pepperkok, R., J. Scheel, H. Horstmann, H.P. Hauri, G. Griffiths, and T.E. Kreis. 1993. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 74:71–82. [DOI] [PubMed] [Google Scholar]
- Rambourg, A., and Y. Clermont. 1990. Three-dimensional electron microscopy: structure of the Golgi apparatus. Eur. J. Cell Biol. 51:189–200. [PubMed] [Google Scholar]
- Rezaie, T., A. Child, R. Hitchings, G. Brice, L. Miller, M. Coca-Prados, E. Heon, T. Krupin, R. Ritch, D. Kreutzer, et al. 2002. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 295:1077–1079. [DOI] [PubMed] [Google Scholar]
- Rock, R.S., S.E. Rice, A.L. Wells, T.J. Purcell, J.A. Spudich, and H.L. Sweeney. 2001. Myosin VI is a processive motor with a large step size. Proc. Natl. Acad. Sci. USA. 98:13655–13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski, A.A., and S.J. Singer. 1984. Associations of elements of the Golgi apparatus with microtubules. J. Cell Biol. 99:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamborn, K., R. Weil, G. Courtois, S.T. Whiteside, and A. Israel. 2000. Phorbol esters and cytokines regulate the expression of the NEMO-related protein, a molecule involved in a NF-kappa B-independent pathway. J. Biol. Chem. 275:22780–22789. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C., and E. Coudrier. 2004. Rab GTPases and myosin motors in organelle motility. Traffic. 5:393–399. [DOI] [PubMed] [Google Scholar]
- Seemann, J., E.J. Jokitalo, and G. Warren. 2000. The role of the tethering proteins p115 and GM130 in transport through the Golgi apparatus in vivo. Mol. Biol. Cell. 11:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroissnigg, H., M. Repitz, A. Miloloza, I. Linhartova, H. Beug, G. Wiche, and F. Propst. 2002. FIP-2, an IkappaB-kinase-gamma-related protein, is associated with the Golgi apparatus and translocates to the marginal band during chicken erythroblast differentiation. Exp. Cell Res. 278:133–145. [DOI] [PubMed] [Google Scholar]
- Van De Moortele, S., R. Picart, A. Tixier-Vidal, and C. Tougard. 1993. Nocodazole and taxol affect subcellular compartments but not secretory activity of GH3B6 prolactin cells. Eur. J. Cell Biol. 60:217–227. [PubMed] [Google Scholar]
- Velier, J., M. Kim, C. Schwarz, T.W. Kim, E. Sapp, K. Chase, N. Aronin, and M. DiFiglia. 1998. Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp. Neurol. 152:34–40. [DOI] [PubMed] [Google Scholar]
- Warner, C.L., A. Stewart, J.P. Luzio, K.P. Steel, R.T. Libby, J. Kendrick-Jones, and F. Buss. 2003. Loss of myosin VI reduces secretion and the size of the Golgi in fibroblasts from Snell's waltzer mice. EMBO J. 22:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland, J., M.C. Willingham, M.G. Gallo, and I. Pastan. 1982. The morphologic pathway of exocytosis of the vesicular stomatitis virus G protein in cultured fibroblasts. Cell. 28:831–841. [DOI] [PubMed] [Google Scholar]
- Wells, A.L., A.W. Lin, L.Q. Chen, D. Safer, S.M. Cain, T. Hasson, B.O. Carragher, R.A. Milligan, and H.L. Sweeney. 1999. Myosin VI is an actin-based motor that moves backwards. Nature. 401:505–508. [DOI] [PubMed] [Google Scholar]
- Wu, H., J.E. Nash, P. Zamorano, and C.C. Garner. 2002. Interaction of SAP97 with minus-end-directed actin motor myosin VI. Implications for AMPA receptor trafficking. J. Biol. Chem. 277:30928–30934. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117. [DOI] [PubMed] [Google Scholar]