Abstract
In contrast to its well-established actions as an organizer of synaptic differentiation at the neuromuscular junction, the proteoglycan agrin is still in search of a function in the nervous system. Here, we report an entirely unanticipated role for agrin in the dual modulation of electrical and chemical intercellular communication that occurs during the critical period of synapse formation. When applied at the developing splanchnic nerve–chromaffin cell cholinergic synapse in rat adrenal acute slices, agrin rapidly modified cell-to-cell communication mechanisms. Specifically, it led to decreased gap junction–mediated electrical coupling that preceded an increase in nicotinic synaptic transmission. This developmental switch from predominantly electrical to chemical communication was fully operational within one hour and depended on the activation of Src family–related tyrosine kinases. Hence, agrin may play a pivotal role in synaptogenesis in promoting a rapid switch between electrical coupling and synaptic neurotransmission.
Introduction
The heparan sulfate proteoglycan agrin is a nerve-derived signaling molecule that has been originally isolated from the electric organ of Torpedo californica (Nitkin et al., 1987). Agrin has been first identified from its ability to cause acetylcholine receptors to aggregate in cultured myotubes (McMahan, 1990). It has since been described to play a crucial role during neuromuscular junction (NMJ) formation in vivo (Gautam et al., 1996). At the NMJ, agrin is responsible for the assembly of the postsynaptic apparatus of the myofiber by inducing clustering of the neurotransmitter receptors and their associated signaling components (Wallace, 1989).
Particularly pertinent is the observation that agrin is not exclusively expressed at the NMJ. Its broad expression pattern in numerous neuronal and nonneuronal tissues suggests that it may play additional roles (Smith and Hilgenberg, 2002; Bezakova and Ruegg, 2003). Until now, the functional role of agrin in the central nervous system (CNS) has remained elusive. In the CNS, agrin is expressed at early developmental stage (Stone and Nikolics, 1995) in areas involved in synaptogenesis (Cohen et al., 1997). This prompted us to hypothesize that agrin may participate in formation and/or maturation of functional synapses between neuronal cells, as it does at the NMJ. However, it is worth noting that in contrast to the NMJ, the synaptogenesis in the developing CNS is associated with a finely tuned coordination between two communication modes, i.e., the gap junction–mediated electrical coupling and synaptic neurotransmission (Kandler, 1997; Kandler and Katz, 1998; Szabo et al., 2004). During development, gap junction–mediated electrotonic coupling is widespread among neurons (Kandler and Katz, 1995; Naus and Bani-Yaghoub, 1998) and gap junction–coupled neuronal assemblies often precede the formation of synaptically connected neuronal network (Kandler, 1997). In addition, the number of gap junctions gradually decreases during postnatal development, coinciding with the establishment of functional chemical synapses (Personius and Balice-Gordon, 2001; Mentis et al., 2002). As a common finding, the critical phase of synapse formation is accompanied by a switch from a predominant gap junction–mediated electrotonic coupling to a fully mature synaptic neurotransmission. At present, the cellular mechanisms underlying this developmental switch remain unknown. We therefore investigated the effects of agrin on both synaptic neurotransmission and gap junctional coupling during synaptogenesis. We addressed this issue at the developing splanchnic nerve–chromaffin cell cholinergic synapse in newborn rat adrenal acute slices. At birth, the innervation of rat chromaffin cells by the splanchnic nerve terminals is not fully competent but completely matures during the first postnatal week (Slotkin, 1986). In addition, neonate chromaffin cells displayed a high level of electrical coupling (Martin et al., 2003). The neonate adrenal medulla therefore represents a suitable model to examine how the two major modes of intercellular communication that take place in the nervous system are regulated during synaptogenesis. The present study reports the discovery of an entirely unexpected role for agrin in the dual regulation of both electrical and chemical synapses. We described agrin as a crucial developmental factor able to reduce gap junction–driven electrical coupling between neonate chromaffin cells within a few minutes and to increase nicotinic synaptic transmission within 1 h. Lastly, we showed that both effects of agrin depended on the activation of the same intracellular transduction pathway involving the Src family–related tyrosine kinase–mediated signaling. Thus, in addition to its well-established function in the postsynaptic assembly of the NMJ (Ferns et al., 1992), we propose that agrin may play a pivotal role essential in synaptogenesis and the maturation of stimulus-secretion mechanisms by promoting a rapid switch from electrical coupling to synaptic transmission.
Results
Postnatal up-regulation of agrin expression in the adrenal medulla
Agrin was detected by Western blot from whole adrenal glands of adults and neonates. Total agrin was recognized as a single band of ∼200 kD (Fig. 1 A), as reported previously (Rupp et al., 1991). Kidney protein extracts were used as positive controls (Groffen et al., 1998). The expression level of total agrin and its Z+ variant, which has been reported as the most potent isoform in clustering nicotinic acetylcholine receptors (nAChRs) at the NMJ (Ferns et al., 1992), underwent up-regulation during adrenal gland postnatal development, as indicated by a weaker expression in neonates when compared with adults (Fig. 1 B). To characterize the tissular localization of agrin, a double-immunofluorescent detection of total agrin and the Z+ insert-containing isoform was combined with tyrosine hydroxylase (TH) staining to visualize chromaffin cells. In adults, intense staining for both total agrin and the Z+ variant was detected throughout the medulla outlining chromaffin cell clusters (Fig. 1 C, a). By contrast, neonate medulla displayed faint labeling for agrin (Fig. 1 C, b). Both agrin isoforms were likely apposed to the ECM, as evidenced by colocalization with laminin (unpublished data). In adults, the Z+ variant was preferentially expressed at cholinergic synaptic contacts, which were stained by a vesicular acetylcholine transporter (VAChT)–specific antibody (Fig. 1 D), suggesting that agrin likely originated from cholinergic neurons synapsing onto chromaffin cells. Agrin was never detected in chromaffin cells, ruling out a possible autocrine/paracrine mechanism of action.
Figure 1.
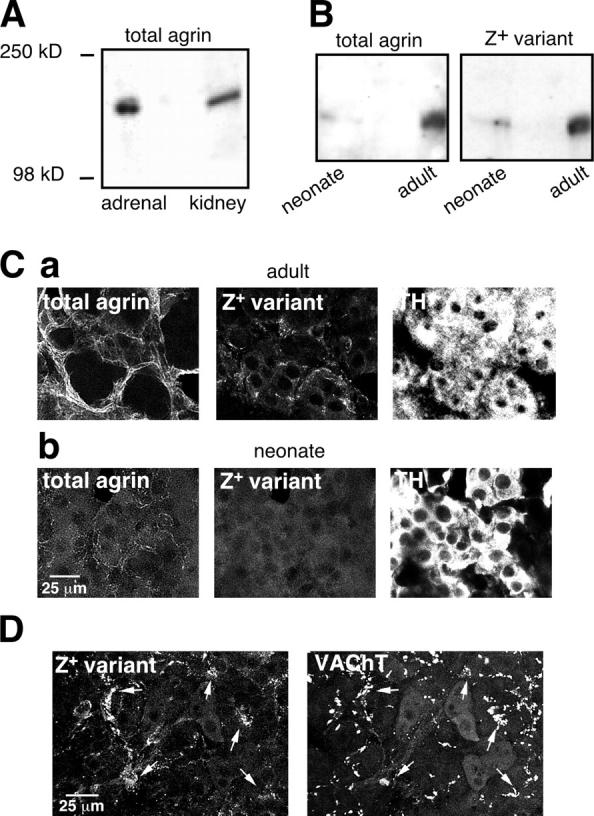
Agrin expression in adult and newborn rat adrenal glands. (A and B) Western blots showing that total agrin or the Z+ variant is highly expressed in adults (3 glands) and more weakly detected in neonates (6 glands). (C) Double immunofluorescent detection of total agrin and its Z+ isoform combined with TH staining to visualize chromaffin cells in both adult (a) and neonatal (b) adrenal medulla. (D) Preferential expression of the Z+ variant at cholinergic synaptic contacts stained by the VAChT (white arrows).
Agrin induces postsynaptic changes in nicotinic synaptic transmission in neonates: effects on both amplitude and pharmacological profile of spontaneous excitatory synaptic currents
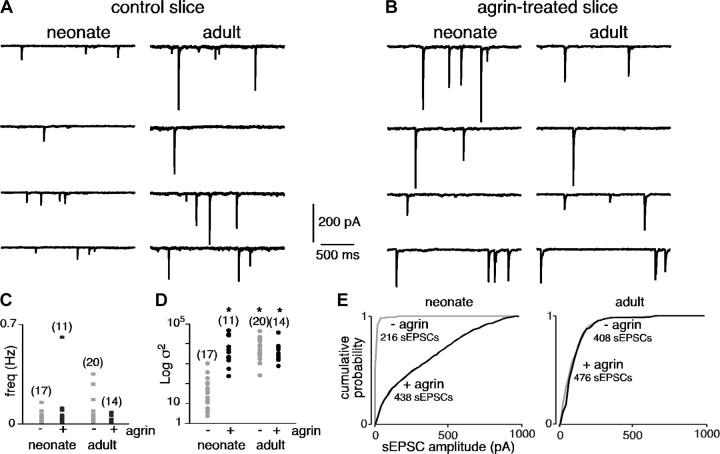
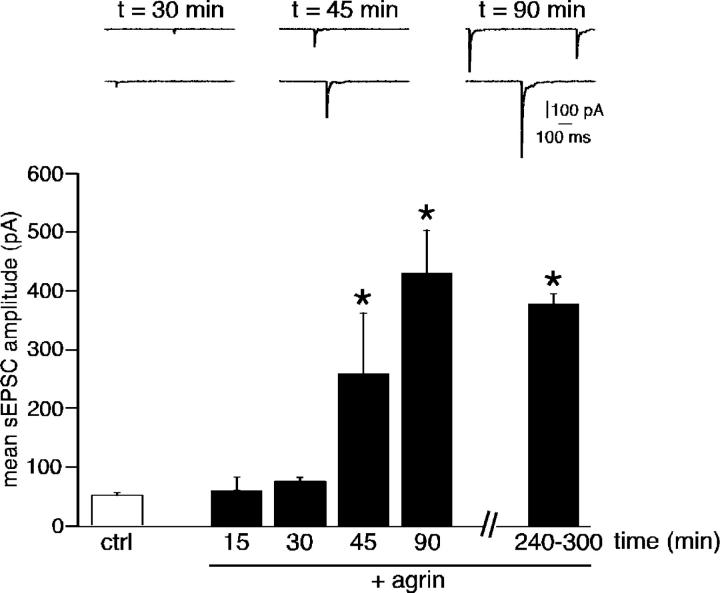
Excitatory synaptic transmission between splanchnic nerve endings and chromaffin cells is mainly mediated by neuronal-type nAChRs (Barbara and Takeda, 1996). We recorded spontaneous excitatory postsynaptic currents (sEPSCs) in whole-cell voltage-clamped chromaffin cells in acute adrenal slices from adults and neonates (Fig. 2). About 20% of cells recorded in both adults and neonates exhibited sEPSCs. The mean sEPSC amplitude (but not the frequency) was significantly lower in neonates than in adults (51.8 ± 2.6 pA, n = 216 sEPSCs, 17 cells in neonates vs. 126.3 ± 6.6 pA, n = 408 sEPSCs, 20 cells in adults, P < 0.01). Because neonate chromaffin cells are smaller in size than adult cells, the corresponding mean current density was calculated. Given a mean membrane capacitance of 7.50 ± 0.55 pF (n = 16) in neonates and 11.64 pF in adults (Martin et al., 2001), the mean sEPSC amplitude corresponded to a mean current density of 6.91 ± 0.51 pA/pF in neonates and 10.85 ± 0.37 pA/pF in adults (P < 0.01). Prolonged exposure to recombinant rat COOH-terminal agrin (4–5 h, 50 ng/ml) induced a dramatic increase in sEPSC amplitude in neonates (Fig. 2 B; 51.8 ± 2.6 pA, n = 17 cells in control slices vs. 380.4 ± 13.4 pA, n = 438 sEPSCs, 11 cells in agrin-treated slices, P < 0.01) by inducing the appearance of high amplitude events. Consistent with this finding, sEPSC amplitude variance (σ2) displayed a wide distribution range in agrin-treated slices—a change that was statistically significant compared with neonate control slices (Fig. 2 D, P < 0.01). The plots illustrating the cumulative probability of sEPSC amplitude clearly indicate that agrin treatment modified sEPSC amplitude in neonates (Fig. 2 E). sEPSC frequency was not modified by agrin treatment (Fig. 2 C, P > 0.01). Increased synaptic transmission induced by agrin was fully prevented in the presence of an anti–rat agrin-neutralizing pAb (R&D Systems; 100 ng/ml, 4–5 h, mean sEPSC amplitude 61.8 ± 8.4 pA, n = 8 cells, P > 0.01, as compared with untreated neonate slices; unpublished data). In adults, agrin did not have any detectable effects on either sEPSC frequency or σ2 (Fig. 2, B–D; P > 0.01, n = 476 sEPSCs, 14 cells). To ascertain that agrin likely acted postsynaptically, we investigated the effects of agrin on nicotine-triggered inward currents in neonates (100-ms puff, 100 μM). This resulted in a statistically significant increase in the mean current amplitude (68.4 ± 19.1 pA, n = 18 in control slices vs. 175 ± 33 pA, n = 17 in agrin-treated slices, P < 0.01; unpublished data). In addition, the number of cells in which nicotine evoked an inward current was significantly higher after agrin exposure (94.4 vs. 62.1% in untreated slices, P < 0.01).
Figure 2.
Effect of prolonged agrin treatment on spontaneous excitatory synaptic activity recorded in adult and neonate chromaffin cells (holding potential −80 mV). (A and B) Chart recordings illustrating sEPSCs in control and agrin-treated slices (4–5 h before recordings). (C and D) Summarized effects of agrin on sEPSC frequency and amplitude variance. *, P < 0.01 compared with untreated slices. (E) Plots showing the cumulative probability of sEPSC amplitude.
Because a number of different spliced variants of agrin exist (Tsim et al., 1992), we next investigated the effects of agrin lacking the insert at site Z (agrin0,0; donated by Dr. C. Fuhrer, Brain Research Institute, Zurich, Switzerland). Prolonged exposure to agrin0,0 (4–5 h, 50 ng/ml) had no effect on synaptic transmission (mean sEPSC amplitude 55.6 ± 9.7 pA, n = 6 cells, P > 0.01, as compared with untreated slices; unpublished data).
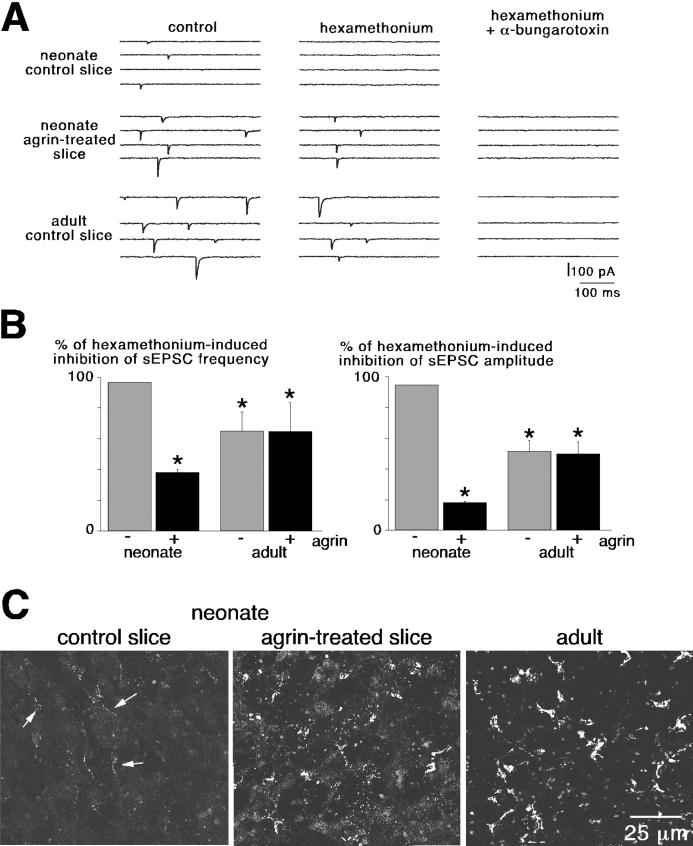
As recently described (Martin et al., 2003), sEPSCs recorded in adults are mediated by the coactivation of α3 and α7 nAChR subtypes that are antagonized by hexamethonium and α-bungarotoxin (α-Bgt), respectively. In neonate control slices, bath-applied hexamethonium (200 μM) fully inhibited sEPSCs (Fig. 3, A and B), pointing to a major contribution of the α3 subunit–containing nAChRs. In agrin-treated slices, hexamethonium only partly reduced sEPSCs, and as found in adults the remaining synaptic activity was blocked by subsequent application of α-Bgt (10 μg/ml). These results indicate that a change in the nAChR subtype distribution takes place during postnatal development at the splanchnic nerve–chromaffin cell cholinergic synapse. To test this hypothesis, α7 nAChRs were labeled in living slices using Alexa 488–conjugated α-Bgt. As illustrated in Fig. 3 C, prolonged agrin exposure (4–5 h) modified the expression pattern of fluorescent α7 nAChRs by promoting the formation of numerous nAChR aggregates, thus resembling the distribution found in adults.
Figure 3.
Agrin-induced recruitment of α-Bgt–sensitive nAChRs in neonates. (A) Effect of 200 μM hexamethonium and 10 μg/ml α-Bgt on sEPSCs in neonates and adults. In neonates, sEPSCs were fully blocked by hexamethonium. After exposure to agrin, the complete inhibition of sEPSCs required the additional application of α-Bgt, as in adults. (B) Pooled data summarizing the effects of hexamethonium on sEPSC frequency and amplitude. *, P < 0.01 when compared with untreated slices. (C) Effect of agrin treatment on the expression pattern of α7 nAChRs stained with Alexa 488–labeled α-Bgt.
Agrin reduces gap junction–mediated electrical cell-to-cell communication between neonate chromaffin cells: an entirely unexpected function for agrin
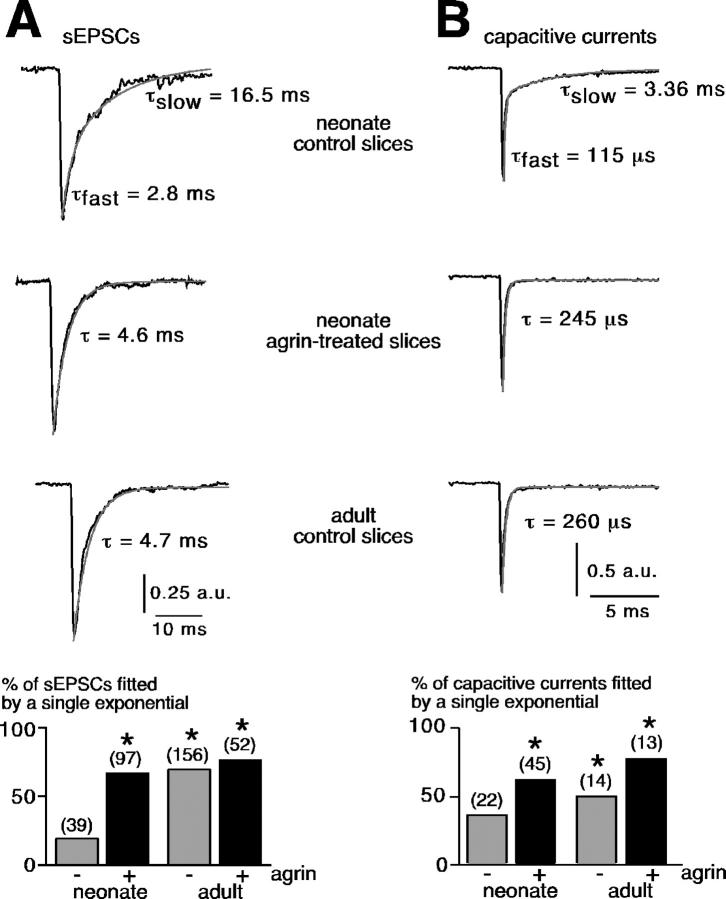
In addition to the effect on the sEPSC variance amplitude, we found that agrin also altered the sEPSC kinetics by modifying the decay phase (Fig. 4 A). No effect was observed on the activation phase (unpublished data). In adults, most of sEPSCs were well fitted by a single exponential curve (71% of sEPSCs, n = 156), as previously reported (Barbara and Takeda, 1996). Conversely, in neonates the decay phase preferentially displayed bi-exponential kinetics (80% of sEPSCs, n = 39). Nevertheless, after agrin treatment, 69% sEPSCs (n = 97) in neonates exhibited a single exponential decay phase, equivalent to the adult curve (P > 0.01). In adults, agrin treatment remained without significant effect on sEPSC kinetics (71% monoexponential sEPSCs in control vs. 78% in agrin-treated slices, P > 0.01). Similar findings were obtained for capacitive currents. In neonates, 63.6% capacitive currents generated by square voltage pulses at −90 mV (100-ms duration) displayed a bi-exponential decay phase (n = 22). After prolonged agrin exposure, the percentage dropped to 37.8% (Fig. 4 B; n = 45, P < 0.01). Because gap junctional coupling modifies decay kinetics of capacitive currents, i.e., uncoupled cells exhibit single-exponential capacitive current decays while the decays are fitted with a double-exponential in coupled cells (Moser, 1998; Postma et al., 1998), we hypothesized that agrin decreased gap junctional coupling between neonate chromaffin cells. sEPSCs were then recorded in the presence of the decoupling agent carbenoxolone (Ishimatsu and Williams, 1996). Carbenoxolone (100 μM, 10 min before recordings) mimicked, although to a lesser extent, the effect of agrin on sEPSC amplitude (unpublished data). The average amplitude significantly increased (70.9 ± 4.5 pA, n = 89 sEPSCs in carbenoxolone-treated slices vs. 51.8 ± 2.6 pA, n = 47 sEPSCs in control slices, P < 0.01). Similarly, the mean current amplitude evoked by a brief application of nicotine (100 μM, 100 ms) significantly increased in the presence of the gap junction blocker (116.9 ± 33.4 pA, n = 6 in carbenoxolone-treated slices vs. 68.4 ± 19.1 pA, n = 18 in control slices, P < 0.01; unpublished data). In addition, in the presence of carbenoxolone the percentage of sESPCs in which the decay phase could be fitted by a single exponential significantly increased (unpublished data), approaching values found in agrin-treated slices. In adults, 86.3% sEPSC decays were fitted by a single exponential in the presence of carbenoxolone (63 out of 73 sEPSCs, 8 cells) versus 71.2% in untreated slices, consistent with the uncoupling effect of carbenoxolone on gap junctional coupling in adult chromaffin cells (Martin et al., 2001). Together, these findings confirmed that a double-exponential fit reflects the presence of electrical coupling between the recorded cell and nearby cells. In this case the slow time constant τslow likely corresponded to the electrotonic spread of current from coupled cells.
Figure 4.
Agrin-induced changes in sEPSC and capacitive current kinetics. (A) Effect of agrin on the sEPSC decay phase. Typical examples of a double-exponential sEPSC recorded in neonate and a single-exponential sEPSC recorded in adult and in neonate after agrin treatment. Pooled data in the histogram show the percentage of sEPSCs that could be fitted by a single exponential. *, P < 0.01 when compared with control slices in neonates. (B) Similar effect of agrin on the decay phase of capacitive currents evoked by square voltage pulses from −80 to −90 mV (100-ms duration).
Studies were next undertaken to examine the effect of agrin on both gap junction–mediated electrical and metabolic coupling between neonate chromaffin cells. Metabolic coupling was assessed using Lucifer yellow (LY) to label coupled cells, whereas electrical coupling was evidenced by dual whole-cell patch-clamp recording of intercellular macroscopic junctional currents (Ij). The probability of LY spreading between chromaffin cells was significantly reduced in agrin-treated slices compared with untreated slices (Fig. 5 A; 31 vs. 57%, P < 0.01). In the presence of the anti–rat agrin-neutralizing pAb, agrin failed to decrease LY diffusion between neonate chromaffin cells (58.3%, n = 24; unpublished data). Similarly, the dye coupling was not modified by inactive agrin0,0 (61.5%, n = 13; unpublished data). In adults, agrin did not change the incidence of LY diffusion (37.9% in treated-slices, n = 29 vs. 44% under control conditions, P > 0.01; unpublished data). Agrin decreased the percentage of electrically coupled cell pairs (Fig. 5 A; 53.8%, n = 26 pairs in control slices vs. 22.2%, n = 18 pairs in agrin-treated slices, P < 0.01). In addition, agrin reduced the incidence of robustly coupled cells (Fig. 5 B). The voltage changes in response to depolarizing current injected into the stimulated cell were robustly reflected in the unstepped cell in 64.3% (n = 9/14 cell pairs) and 50% (n = 4 cell pairs) in control and agrin-treated slices, respectively (P < 0.01). The resulting coupling ratios exhibited a wide distribution range from 0.01 to 0.97 (Fig. 5 C). This strongly suggests the presence of two chromaffin cell populations—a weakly coupled cell population and a highly coupled cell population. Recordings of Ij in chromaffin cell pairs voltage clamped at −60 mV confirmed these findings (unpublished data). Delivering voltage steps with command pulses of both polarities triggered Ij in the unstepped cell that exhibited variable degrees of attenuation. In control slices, 81.8% recorded pairs (n = 11), Ij amplitude could reach 1–2 nA, whereas in the remaining pairs Ij did not exceed several picoamperes. In agrin-treated slices, the percentage of coupled cell pairs exhibiting a high Ij amplitude dropped to 50%. When plotted as a function of the transjunctional potential, the I/V curve of Ij displayed a linear relationship within the membrane potential range of −60 to +120 mV. The curve used to fit the data was derived from a linear regression, given a macroscopic junctional conductance (Gj) of 43 and 60 pS for the weak coupling and 12 and 13 nS for the robust coupling (Fig. 5 C, insets).
Figure 5.
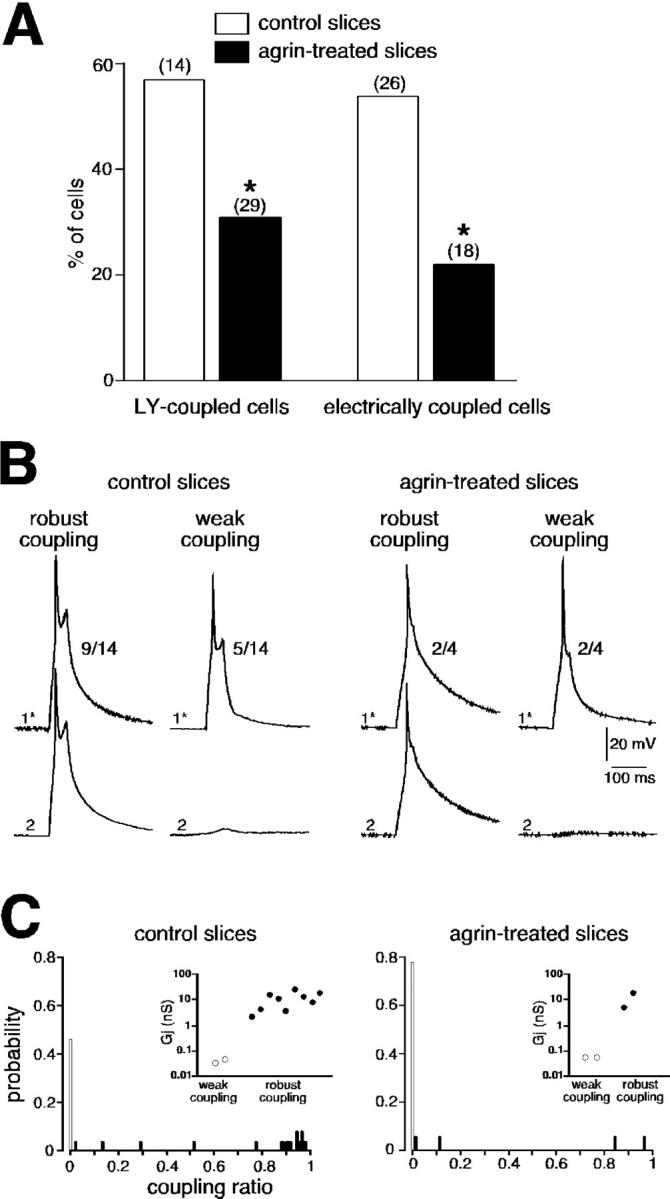
Agrin-mediated decrease in gap junction–dependent intercellular communication between chromaffin cells in neonates. (A) Reduced number of electrically and LY-coupled chromaffin cells in neonatal agrin-treated slices. *, P < 0.01 when compared with control slices. (B) Monitoring of electrical coupling between chromaffin cell pairs in control and agrin-containing saline showing examples of robust and weak coupling. Cells were current-clamped at −65 mV. A robust coupling was less frequently observed in agrin-treated slices. (C) Histograms illustrating the wide distribution range of the coupling ratio calculated in 26 control and 18 agrin-treated cells from voltage-clamp measurements of Ij (holding potential −60 mV, transjunctional potential from −120 to +60 mV, 150 ms duration). Insets: pooled data of Gj calculated in 11 control cell pairs and 4 agrin-treated cell pairs.
All these results are in agreement with an agrin-mediated reduction of electrical junctional communication between neonate chromaffin cells, and emphasize an entirely novel and not previously anticipated role for the protein agrin.
The agrin-mediated switch from electrical coupling to synaptic transmission is fully operational within 1 h
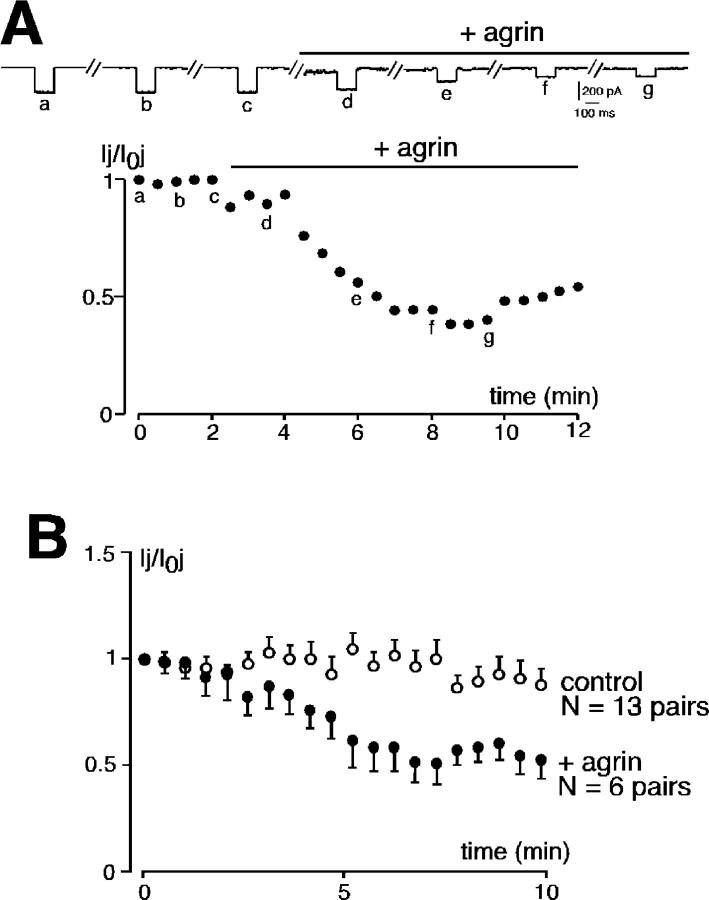
As a first step toward the characterization of mechanisms involved in agrin-induced changes in cell-to-cell communication, we investigated the time course of agrin effects on both electrical coupling and synaptic nicotinic transmission. Ij was continuously monitored before and during 10 min bath-applied agrin (50 ng/ml). As illustrated in Fig. 6 A, Ij amplitude rapidly and dramatically decreased upon agrin application, with a maximal effect observed within 5–6 min (n = 6/6 cell pairs). Pooled data show that a 10-min exposure to agrin reduced Ij amplitude by ∼50% (range from 15 to 63%), whereas it remained unchanged in control saline (Fig. 6 B, n = 13 cell pairs). Regarding synaptic transmission, a statistically significant increase in sEPSC amplitude was observed after 45–60 min of continuously applied agrin (Fig. 7). These results clearly indicate that (1) the effect of agrin on the two intercellular communication modes is sequential, i.e., the reduction of gap junctional communication precedes the increase of synaptic neurotransmission; and (2) the switch between electrical and chemical coupling occurs rapidly and is fully operational within 1 h.
Figure 6.
Agrin-evoked rapid decrease in Ij. (A) Ij was continuously monitored in a neonatal chromaffin cell pair voltage- clamped at −60 mV. A voltage step (60 mV, 150 ms) was elicited every 30 s. Bath-applied agrin (50 ng/ml, 10 min) reduced Ij amplitude by ∼60% within a few minutes. (B) Pooled data showing that a 10-min agrin exposure irreversibly reduced Ij amplitude by 50%, with a maximal effect within 6 min.
Figure 7.
Time course of agrin-mediated increase in synaptic currents in neonates. sEPSCs were recorded every 15 min after the onset of agrin application. 2–4 cells were tested at each time point. A statistically significant increase in synaptic activity was observed after 45 min. *, P < 0.01 when compared with untreated slices.
Are these two agrin-mediated effects on intercellular communication independent of each other? We addressed this issue by first examining the spreading of LY between neonatal chromaffin cells in agrin-treated slices in the presence of hexamethonium and α-Bgt. The complete blockade of nicotinic synaptic transmission did not prevent agrin-induced reduction of dye coupling (23.8% of dye-coupled cells in nAChR blocker–containing saline, n = 21 vs. 31.0% without antagonists, n = 29, P > 0.01; unpublished data). These results strengthen the fact that agrin reduces junctional coupling independently of its effect on synaptic transmission. Reciprocally, pretreating neonatal slices with carbenoxolone (100 μM) did not prevent subsequent agrin effects on synaptic transmission. Large sEPSC amplitude (>100 pA) occurred in 55% cells (n = 20) in presence of carbenoxolone versus 63.6% in carbenoxolone-free saline (n = 11 cells, P > 0.01; unpublished data).
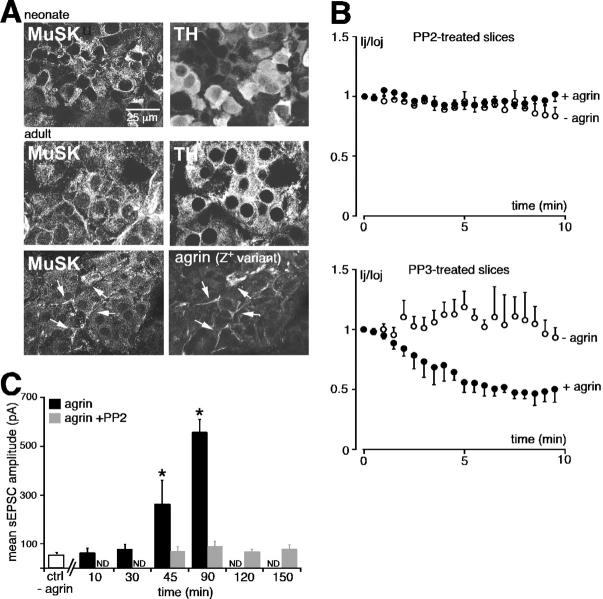
Agrin-mediated effects on both junctional current and nicotinic transmission depend on activation of Src family tyrosine kinases
As shown in Figs. 6 and 7, agrin acted sequentially on both electrical coupling and synaptic transmission. Do these effects require activation of a unique or different intracellular signaling pathways? To address this issue we first looked for the expression of the muscle-specific kinase (MuSK) in both adult and neonate adrenal medulla. This question was prompted by the finding that at the NMJ agrin activates a multicomponent receptor complex including MuSK (Glass et al., 1996). As illustrated in Fig. 8 A, MuSK is highly expressed in neonate and adult chromaffin cells. The subsequent colocalization with the Z+ agrin variant in adults strongly supports the hypothesis that agrin might activate MuSK in the adrenal gland. Additional experiments were then performed to determine whether agrin effects depended on the activation of Src family–related kinases, as reported at the NMJ (Mittaud et al., 2001). This was achieved by incubating adrenal neonatal slices with the specific Src family tyrosine kinase inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) (5 μM, 20 min; Hanke et al., 1996) before agrin addition. As illustrated in Fig. 8 B, agrin failed to reduce Ij in the presence of PP2 (n = 8 cell pairs). By contrast, the use of the inactive structural analogue 4-amino-7-phenylpyrazolo[3,4-d]pyrimidine (PP3) (5 μM, 20 min) did not prevent agrin-mediated Ij decrease (n = 3 cell pairs). Similarly, PP2 pretreatment impaired the agrin-mediated increase in sEPSC amplitude (Fig. 8 C). Even after 150 min of agrin exposure, sEPSC amplitude did not significantly differ from that found in control slices (78.6 ± 18.3 pA, n = 5 cells in PP2-treated slices vs. 51.8 ± 2.6 pA, n = 17 cells in control, P > 0.01). Together, these results indicate that both agrin-induced changes in cell–cell communication depend on activation of Src family tyrosine kinase–mediated intracellular pathway.
Figure 8.
Activation of Src family–related tyrosine kinases is required for agrin effects on both gap junctional coupling and synaptic transmission. (A) Double-immunofluorescent staining showing that MuSK is constitutively expressed in both adult and neonatal chromaffin cells, and colocalized with the Z+ agrin variant in adults. Arrows indicate the sites of colocalized MuSK with the agrin Z+ variant. (B) PP2, a specific Src family tyrosine kinase inhibitor (5 μM, 20 min before agrin) fully blocked the effect of agrin on electrical coupling. By contrast, PP3 pretreatment (5 μM, 20 min before agrin), the inactive structural analogue of PP2, did not antagonize the agrin-mediated decrease in Ij. (C) Blockade of the agrin-mediated increase in sEPSC amplitude in PP2-containing saline. *, P < 0.01 when compared with untreated slices.
Discussion
We discovered an unanticipated function for agrin as the first identified factor to dualistically modulate both electrical and chemical synapses. Because it operates a rapid switch from gap junction–mediated electrical communication to synaptic transmission, we propose that agrin acts as a developmental switch essential in synaptogenesis and the maturation of stimulus-secretion mechanisms in the adrenal medulla. At birth, the innervation of chromaffin cells is not fully competent (Slotkin, 1986) and the postnatal synaptic regulation of catecholamine secretion is marked by a switch from nonneurogenic to neurogenic control. We show that agrin promotes the acquisition of the neurogenic control of the stimulus-secretion coupling by rendering neonate chromaffin cells readily responsive to synaptically released neurotransmitters, while foregoing reducing information transfer through gap junctions.
What mechanisms could account for agrin-mediated developmental switch from electrical to chemical synapses?
The more striking and unexpected effect of agrin reported here was its rapid and persistent inhibitory effect on gap junction–mediated communication between neonate chromaffin cells. As evidenced by the continuous monitoring of Ij amplitude, agrin rapidly reduced electrotonic coupling (within a few minutes). Injection of LY up to several hours after agrin treatment revealed a persistent reduced dye spreading, thus indicating an agrin-mediated long-lasting effect. The blocking effect of the tyrosine kinase inhibitor PP2 on agrin-induced decreased gap junctional communication further indicates that Src family tyrosine kinases are involved in agrin-stimulated intracellular signaling pathway, as shown at the NMJ (Mittaud et al., 2001; Mohamed et al., 2001). Our result is consistent with the abundant expression of Src family kinases in neural tissue (Ross et al., 1988) from which chromaffin cells originated. Compelling evidence argues that connexin function can be regulated by activated tyrosine protein kinases. In particular, phosphorylation of connexin-43 (Cx43) by c-Src and v-Src kinases has been reported to disrupt gap junctional communication (Azarnia et al., 1988; Postma et al., 1998; Giepmans et al., 2001; Lin et al., 2001). Because rat adrenal chromaffin cells are coupled by Cx43-built gap junctions (Martin et al., 2001), one could reasonably propose that activation of Src class kinases by agrin may cause tyrosine phosphorylation of Cx43, thus leading to a decrease in junctional communication. A reduced Ij may have resulted from effects on the mean open probability of unitary gap junction channels. This hypothesis is prompted by a recent finding showing the ability of v-Src to reduce open probability of Cx43-built channels (Cottrell et al., 2003). Alternatively, the unitary conductance and/or the number of open channels anchored at the plasma membrane might also be affected by agrin treatment. Recordings of electrical activity from unitary gap junction channels would be required to definitively address this issue.
What mechanisms could account for both initiation and maintenance of agrin effect on cell-to-cell communication? The regulation of gap junctional communication can potentially occur at multiple levels from transcription of connexin gene to degradation of the connexin proteins and with a wide spectrum of time courses (from millisecond to hours). The rapid agrin-induced Ij decrease strongly suggests that the regulation of gap junctions by agrin may reside at the level of channel gating. Translational and post-translational modification of connexins by phosphorylation act as a gating mechanism (Lampe and Lau, 2000) and therefore could account for agrin-mediated Ij decrease. The persistent effect of agrin on cell-to-cell communication within a few hours could involve any of the steps in connexin biosynthesis. Changes in connexin mRNA transcription rate and mRNA stability could be affected by activation of Src signaling cascade, as reported for cAMP-activated intracellular pathways (Bennett et al., 1991). Because of the rapid turnover of gap junctions (Laird, 1996), assembly of connexons at the plasma membrane may be subject to regulation at the level of connexin turnover (Musil et al., 2000). Alternatively, agrin may down-regulate gap junction assembly in the membrane by modifying the connexin/connexon trafficking (Govindarajan et al., 2002) or scaffolding protein or connexon trafficking–associated regulatory proteins (Giepmans and Moolenaar, 1998; Toyofuku et al., 2001; Li et al., 2004). The assembly process of gap junction channel at the plasma membrane is dynamically regulated by connexin phosphorylation (Cooper and Lampe, 2002), and therefore may be affected by agrin signaling.
Agrin-induced increase in synaptic transmission at nicotinic synapses
In neonates, after prolonged agrin exposure, excitatory nicotinic synaptic transmission undergoes two main postsynaptic changes. First, sEPSC amplitude variance is dramatically increased, and second, an additional pharmacological sensitivity to α7 antagonists is acquired. Increased sEPSC amplitude can be explained either by an increase in the number of acetylcholine-containing presynaptic vesicles released by the splanchnic nerve terminals or by an increase in the number of postsynaptic functional nAChRs in chromaffin cells. We favor the latter hypothesis, as agrin did not change sEPSC frequency, consistent with a postsynaptic action. Based on the principal known postsynaptic effect of agrin at the NMJ (Nitkin et al., 1987; Ferns et al., 1992), one can reasonably assume that agrin induces a clustering of nAChRs in the adrenal medulla. One main argument for this assertion comes from an examination of quantal release at this synapse. Assuming a quantal size of 20 pA (Barbara and Takeda, 1996), the maximum sEPSC amplitude in neonates corresponds to the synchronous release of ∼10 quanta, whereas in agrin-treated slices or in adults, the maximum sEPSC amplitude reflects the presynaptic release of ∼90–100 quanta. In addition, our finding that a significant increase in sEPSC amplitude was detected within 45–60 min after agrin treatment is consistent with previous data indicating that agrin-induced increase in tyrosine phosphorylation of the β subunit of nAChRs occurs within 30 min of adding agrin (Wallace et al., 1991). The appearance of large amplitude events in agrin-treated neonate when compared with adults suggests that neonate chromaffin cells displayed an oversensitivity to agrin. This hypothesis can be supported by electron microscopic data indeed indicating that the surface of the apposed pre- and postsynaptic membranes corresponding to the putative vesicle release area is more extensive in neonates compared with adults (Tomlinson and Coupland, 1990). Assuming that the whole postsynaptic surface would be responsive to agrin, a subsequent exposure to exogenous agrin would lead to an overrecruitment of nAChRs compared with that observed in adults, and would therefore ultimately increase sEPSC amplitude.
An intriguing question is raised by our pharmacological experiments showing that agrin recruits α-Bgt–sensitive α7 nAChRs. The clustering effect of agrin depends on the phosphorylation of the nAChR β subunit (Borges and Ferns, 2001). Because the α7-containing nAChRs are homopentamers that do not express a β subunit (Chen and Patrick, 1997), α7 nAChRs would therefore be unlikely directly clustered by agrin. Nevertheless, α7 nAChRs could be phosphorylated by agrin (Wallace et al., 1991). Although a double labeling with synaptic markers was not performed, the staining of α7 nAChRs using fluorescent α-Bgt strongly suggests that they could be subsequently recruited by agrin either directly at the synapse or nearby in a perisynaptic region (Temburni et al., 2000). One possible explanation would be that agrin-triggered α3 nAChR clustering could thereafter activate an intracellular signaling cascade that in turn would regulate α7 nAChRs (Brumwell et al., 2002).
Because agrin-mediated increased synaptic activity was prevented by PP2 pretreatment, one could reasonably propose that agrin acts on nAChRs by activating Src family kinases. This is in agreement with a recent study reporting that α3 nAChRs are tyrosine phosphorylated by Src family kinases and that PP2 treatment reduces the peak amplitude of nicotine-activated currents in bovine chromaffin cells (Wang et al., 2004).
Constitutive expression of MuSK in neonatal and adult chromaffin cells
To date, two distinct agrin receptors have been described; namely the well-documented MuSK–MASC multicomponent complex expressed at the NMJ (Glass et al., 1996) and the more recently identified neuronal form expressed in CNS neurons (Hoover et al., 2003). Our data showing the presence of MuSK in neonatal and adult chromaffin cells strongly suggest that MuSK might be involved in agrin-induced changes in cell-to-cell coupling. The fast effects of agrin on junctional currents (i.e., within a few minutes) are consistent with previous data showing that agrin-induced Src family kinase activation occurs as early as 5 min after addition of agrin and is highly correlated with the activation of MuSK (Fuhrer et al., 1999). Similarly, the activation of MuSK may account for the delayed action of agrin on synaptic transmission. As recently reported, a single 5-min pulse of agrin is efficient enough to trigger long-lasting MuSK and nAChR phosphorylation and subsequent clustering (Mittaud et al., 2004).
Agrin as a molecular switch from electrical to chemical communication operating at developing neuron–neuron synapses?
There is growing evidence of a mutual inhibitory action between gap junction– and synapse-mediated cell-to-cell communication during synaptogenesis (Martin et al., 2003; Pastor et al., 2003; Szabo et al., 2004). Understanding how nervous system shifts from electrical coupling to synaptic transmission is crucial to better understanding the subsequent formation of synaptically connected neural networks. The sequential and opposite action of agrin on gap junctional communication and synaptic activity reported in this study assigns to agrin a potential role in this developmental switch. Although it is reasonably speculated that agrin may have an organizing role in the synapse formation (Hoch et al., 1993; Li et al., 1997, 1999; Böse et al., 2000; Burgess et al., 2000; Lesuisse et al., 2000; Gingras and Ferns, 2001; Gingras et al., 2002; Hoover et al., 2003), this protein is still in search of a function in the CNS (Smith and Hilgenberg, 2002; Bezakova and Ruegg, 2003). Our findings provide evidence that agrin would exert a crucial role in synaptogenesis by operating a rapid switch from gap junctional coupling to synaptic neurotransmission that occurs during synapse formation. This is consistent with the expression of agrin at early developmental stage (Stone and Nikolics, 1995) in areas involved in synaptogenesis (Cohen et al., 1997). Because agrin expression is not restricted to cholinergic regions (O'Connor et al., 1994), we therefore propose that the unanticipated role of agrin reported here might be extended to numerous developing neuron–neuron synapses.
Materials and methods
Acute adrenal slice preparation
Adrenal slices were prepared from neonatal rats and from 12–16-wk-old Wistar female rats as reported previously (Martin et al., 2003). All experimental procedures were done in accordance with the Society for Neuroscience guidelines and were approved by our institutional animal care and use committee. After removal, the glands were kept in ice-cold saline for 2 min. A gland was next glued onto an agarose cube and transferred to the stage of a vibratome (DSK, DTK-1000; Dosaka EM Co., LTD). Slices of 150- and 250-μm thickness (for neonates and adults, respectively) were then cut with a razor blade and transferred to a storage chamber maintained at 32°C containing Ringer's saline ([mM]: 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 12 glucose) and buffered to pH 7.4. The saline was continuously bubbled with carbogen (95% O2/5% CO2).
Electrophysiology
All experiments were performed in the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981). Patch pipettes were pulled to a resistance of 5–8 MΩ from borosilicate glass and filled with the following internal solution (mM): 140 potassium-gluconate, 2 MgCl2, 1.1 EGTA, and 5 Hepes, that was titrated to pH 7.2 with KOH. sEPSCs were acquired with an EPC-9 patch-clamp amplifier (HEKA Electronik) in chromaffin cells voltage-clamped at −80 mV and were filtered at 1 kHz. Membrane potentials of chromaffin cell pairs were recorded under current-clamp conditions using an EPC-9 dual patch-clamp amplifier and filtered at 3 kHz. Ij was monitored under dual voltage-clamp conditions (Neyton and Trautmann, 1985). To calculate Gj, the I/V curve in which Ij amplitude was plotted as a function of the transjunctional voltage Vj was fitted by a computed linear regression y = ax + b (where y corresponds to Ij and x to Vj). Gj was then given by the slope of the linear regression. Signals were analyzed with Axograph 4.0 (Axon instruments, Inc.). Normalized sEPSC decays were fitted by a single or double exponential using the Simplex fit algorithm based on the sum of squared errors (SSE). Only fits with SSE <0.001 were taken into consideration.
LY diffusion
The fluorescent dye LY (1 mM; Sigma-Aldrich) was introduced into chromaffin cells using patch pipettes. Dye transfer between gap junction–coupled cells was visualized with confocal microscopy using the 488-nm–centered wavelength of the laser beam. The extent of LY diffusion between chromaffin cells was estimated by counting the number of labeled cells. The probability of LY diffusion was expressed as a ratio corresponding to the number of injected cells that show dye transfer to adjacent cells over the total number of injected cells.
Western blot
Extracts from adrenal glands and kidney were homogenized in a buffer containing 50 mM Tris, 1 mM PMSF, and 10 μg/ml leupeptin and were centrifuged for 30 min at 13,000 rpm. Supernatants were collected and 50% glycerol was added. 100 ng of each sample was deposited, subjected to 10% SDS-PAGE, and electrophoretically transferred onto nitrocellulose membranes. Nonspecific binding sites were blocked by incubation with TBS (50 mM Tris and 150 mM NaCl, pH 7.4) containing 8% powdered milk for 1 h at RT. Membranes were then incubated overnight at 4°C with a pAb raised against the conserved NH2-terminal part of the protein (R&D Systems) for total agrin detection, or with an mAb directed against the COOH-terminal end of agrin (StressGen Biotechnologies) for the Z+ variant detection. After washing in TBS containing 0.1% Tween 20, membranes were incubated with secondary antibodies (peroxidase-linked IgGs, 1:4,000; Amersham Biosciences) for 1 h at RT. Immunoreactive bands were visualized with a chemiluminescence detection kit (Boehringer).
Immunostaining
To process for immunolabeling, the adrenal glands were rapidly removed and fixed by immersion in 4% PFA in 0.1 M phosphate buffer (overnight at 4°C). They were then cut using a vibratome (VT1000S; Leica) into 40-μm-thick sections. Sections were incubated for 48 h at 4°C with different primary antibodies including the two antibodies raised against agrin (total agrin, 1:200; Z+ variant, 1:1,000) mentioned above, a rabbit IgG antibody against TH (1:5,000; Jacques Boy Laboratories), a goat IgG antibody against VAChT (1:1,000; CHEMICON International, Euromedex), and a goat IgG antibody against MuSK (1:100; Santa Cruz Biotechnology, Inc.). Sections were then incubated for 4 h at RT with appropriate secondary antibodies conjugated to Alexa 488 (1:2,000; Molecular Probes, Inc.) or Cy3 or Cy5 (1:2,000; Jackson ImmunoResearch Laboratories). Primary and secondary antibodies were diluted in PBS containing 2% BSA and 0.1% Triton X-100. Stained sections were imaged with a confocal laser scanning microscope (MRC 1024; Bio-Rad Laboratories) with a 20× objective, 0.75 NA (Nikon), equipped with a krypton–argon mixed gas laser emitting at 488, 568, and 645 nm. The specificity of the commercial antibodies has been assessed by absorption tests. Negative controls were performed by omitting primary antibodies.
Staining of α7 nAChRs
Tissular distribution of α7-built nAChRs in living slices from neonates and adults was visualized using Alexa 488–conjugated α-Bgt (Molecular Probes, Inc.). Acute slices (150-μm thickness) were incubated for 2 h at 37°C in the presence of fluorescent α-Bgt (dilution 1:500). The distribution of fluorescent nAChRs was imaged by collecting 10 consecutive confocal images through the slice and by projecting them onto the same plane.
Solutions and chemicals
The effects of agrin were studied in acute slices from neonates and adults that were treated for 4–5 h before recordings with a recombinant rat COOH-terminal agrin (50 ng/ml; R&D Systems). To investigate the effects of agrin on gap junction–mediated electrical coupling, agrin was acutely bath-applied for 10 min. Hexamethonium, α-Bgt, and carbenoxolone (Sigma-Aldrich), were bath-applied 5–10 min before testing their effects on sEPSCs. For Src family kinase inhibition studies, slices were incubated either with the Src family tyrosine kinase inhibitor PP2 or the inactive structural analogue PP3 (Calbiochem) at 5 μM for at least 20 min before and during Ij recording.
Statistics
Numerical data are expressed as the mean ± SEM. Differences between groups were assessed by using the nonparametric Mann-Whitney U test. Unpaired t test was used to compare means. Statistical comparisons between groups were made by using one-way ANOVA and Fisher PLSD as post-tests when appropriate. Percentages were compared using a contingency table and the chi-square test. Differences with P < 0.01 were considered significant.
Acknowledgments
We thank Dr. C. Fuhrer for the gift of inactive agrin0,0; Drs. U. Gerber, C. Legraverend, and P. Mollard for critical reading; and M. Passama, D. Haddou, and E. Galibert for technical assistance.
Supported by INSERM and grants from European Union (QLGA-CT-2001-51890), Région Languedoc-Roussillon (994195), and Fondation pour la Recherche Médicale (1000322/5-E).
A.O. Martin's present address is Division of Molecular Neurobiology, National Institute for Medical Research, the Ridgeway, Mill Hill, London NW7 1AA, England, UK.
Abbreviations used in this paper: α-Bgt, α-bungarotoxin; CNS, central nervous system; Cx43, connexin-43; Gj, macroscopic junctional conductance; Ij, intercellular macroscopic junctional current; LY, Lucifer yellow; MuSK, muscle-specific kinase; NMJ, neuromuscular junction; nAChR, nicotinic acetylcholine receptor; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; PP3, 4-amino-7-phenylpyrazolo[3,4-d]pyrimidine; sEPSC, spontaneous excitatory postsynaptic current; TH, tyrosine hydroxylase; VAChT, vesicular acetylcholine transporter.
References
- Azarnia, R., S. Reddy, T.E. Kmiecik, D. Shalloway, and W.R. Loewenstein. 1988. The cellular src gene product regulates junctional cell-to-cell communication. Science. 239:398–401. [DOI] [PubMed] [Google Scholar]
- Barbara, J.-G., and K. Takeda. 1996. Quantal release at a neuronal nicotinic synapse from rat adrenal gland. Proc. Natl. Acad. Sci. USA. 93:9905–9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M.V.L., L.C. Barrio, T.A. Bargiello, D.C. Spray, E. Hertzberg, and J.C. Sáez. 1991. Gap junctions: new tools, new answers, new questions. Neuron. 6:305–320. [DOI] [PubMed] [Google Scholar]
- Bezakova, G., and M.A. Ruegg. 2003. New insights into the roles of agrin. Nat. Rev. Mol. Cell Biol. 4:295–308. [DOI] [PubMed] [Google Scholar]
- Borges, L.S., and M. Ferns. 2001. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J. Cell Biol. 153:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böse, C.M., D. Qiu, A. Bergamaschi, B. Gravante, M. Bossi, A. Villa, F. Rupp, and A. Malgaroli. 2000. Agrin controls synaptic differentiation in hippocampal neurons. J. Neurosci. 20:9086–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumwell, C.L., J.L. Johnson, and M.H. Jacob. 2002. Extrasynaptic α7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity. J. Neurosci. 22:8101–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, R.W., W.C. Skarnes, and J.R. Sanes. 2000. Agrin isoforms with distinct amino termini: differential expression, localization, and function. J. Cell Biol. 151:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., and J.W. Patrick. 1997. The α-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the α7 subunit. J. Biol. Chem. 272:24024–24029. [DOI] [PubMed] [Google Scholar]
- Cohen, N.A., W.E. Kaufmann, P.F. Worley, and F. Rupp. 1997. Expression of agrin in the developing and adult rat brain. Neuroscience. 76:581–596. [DOI] [PubMed] [Google Scholar]
- Cooper, C.D., and P.D. Lampe. 2002. Casein kinase 1 regulates connexin-43 gap junction assembly. J. Biol. Chem. 277:44962–44968. [DOI] [PubMed] [Google Scholar]
- Cottrell, G.T., R. Lin, B.J. Warn-Cramer, A.F. Lau, and J.M. Burt. 2003. Mechanism of v-Src- and mitogen-activated protein kinase-induced reduction of gap junction communication. Am. J. Physiol. Cell Physiol. 284:C511–C520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns, M.J., W. Hoch, J.T. Campanelli, F. Rupp, and Z.W. Hall. 1992. RNA splicing regulates agrin-mediated acetylcholine receptor clustering activity on cultured myotubes. Neuron. 8:1079–1086. [DOI] [PubMed] [Google Scholar]
- Fuhrer, C., M. Gautam, J.E. Sugiyama, and Z.W. Hall. 1999. Roles of rapsyn and agrin in interaction of postsynaptic proteins with acetylcholine receptors. J. Neurosci. 19:6405–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam, M., P.G. Noakes, L. Moscoso, F. Rupp, R.H. Scheller, J.P. Merlie, and J.R. Sanes. 1996. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 85:525–535. [DOI] [PubMed] [Google Scholar]
- Giepmans, B.N., and W.H. Moolenaar. 1998. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 8:931–934. [DOI] [PubMed] [Google Scholar]
- Giepmans, B.N., T. Hengeveld, F.R. Postma, and W.H. Moolenaar. 2001. Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J. Biol. Chem. 276:8544–8549. [DOI] [PubMed] [Google Scholar]
- Gingras, J., and M. Ferns. 2001. Expression and localization of agrin during sympathetic synapse formation in vitro. J. Neurobiol. 48:228–242. [DOI] [PubMed] [Google Scholar]
- Gingras, J., S. Rassadi, E. Cooper, and M. Ferns. 2002. Agrin plays an organizing role in the formation of sympathetic synapses. J. Cell Biol. 158:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, D.J., D.C. Bowen, T.N. Stitt, C. Radziejewski, J. Bruno, T.E. Ryan, D.R. Gies, S. Shah, K. Mattsson, S.J. Burden, et al. 1996. Agrin acts via a MuSK receptor complex. Cell. 85:513–523. [DOI] [PubMed] [Google Scholar]
- Govindarajan, R., S. Zhao, X.-H. Song, R.-J. Guo, M. Wheeloock, K.R. Johnson, and P.P. Mehta. 2002. Impaired trafficking of connexins in androgen-independent human prostate cancer cell lines and its mitigation by α-catenin. J. Biol. Chem. 277:50087–50097. [DOI] [PubMed] [Google Scholar]
- Groffen, A., M.A. Ruegg, H. Dijkman, T.J. van de Velden, C.A. Buskens, J. van der Born, K.J. Assmann, L.A. Monnens, J.H. Veerkamp, and L.P. van den Heuvel. 1998. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J. Histochem. Cytochem. 46:19–27. [DOI] [PubMed] [Google Scholar]
- Hamill, O.P., A. Marty, E. Neher, B. Sakmann, and F.J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- Hanke, J.H., J.P. Gardner, R.L. Dow, P.S. Changelian, W.H. Brissette, E.J. Weringer, B.A. Pollok, and P.A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. J. Biol. Chem. 271:695–701. [DOI] [PubMed] [Google Scholar]
- Hoch, W., M. Ferns, J.T. Campanelli, Z.W. Hall, and R.H. Scheller. 1993. Developmental regulation of highly active alternatively spliced forms of agrin. Neuron. 11:479–490. [DOI] [PubMed] [Google Scholar]
- Hoover, C.L., L.G.W. Hildenberg, and M.A. Smith. 2003. The COOH-terminal domain of agrin signals via a synaptic receptor in central nervous system neurons. J. Cell Biol. 161:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimatsu, M., and J.T. Williams. 1996. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J. Neurosci. 16:5196–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler, K. 1997. Coordination of neuronal activity by gap junctions in the developing neocortex. Semin. Cell Dev. Biol. 8:43–51. [DOI] [PubMed] [Google Scholar]
- Kandler, K., and L.C. Katz. 1995. Neuronal coupling and uncoupling in the developing nervous system. Curr. Opin. Neurobiol. 5:98–105. [DOI] [PubMed] [Google Scholar]
- Kandler, K., and L.C. Katz. 1998. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J. Neurosci. 18:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird, D.W. 1996. The life cycle of a connexin: gap junction formation, removal, and degradation. J. Bioenerg. Biomembr. 28:311–318. [DOI] [PubMed] [Google Scholar]
- Lampe, P.D., and A.F. Lau. 2000. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 384:205–215. [DOI] [PubMed] [Google Scholar]
- Lesuisse, C., D. Qiu, C.M. Böse, K. Nakaso, and F. Rupp. 2000. Regulation of agrin expression in hippocampal neurons by cell contact and electrical activity. Brain Res. Mol. Brain Res. 81:92–100. [DOI] [PubMed] [Google Scholar]
- Li, X., C. Olson, S. Lu, N. Kamasawa, T. Yasumura, J.E. Rash, and J.I. Nagy. 2004. Neuronal connexin36 association with zonula occludens-1 protein (ZO-1) in mouse brain and interaction with the first PDZ domain of ZO-1. Eur. J. Neurosci. 19:2132–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., J.L. Massengill, D.K. O'Dowd, and M.A. Smith. 1997. Agrin gene expression in mouse somatosensory cortical neurons during development in vivo and in cell culture. Neuroscience. 79:191–201. [DOI] [PubMed] [Google Scholar]
- Li, Z., L.G. Hilgenberg, D.K. O'Dowd, and M.A. Smith. 1999. Formation of functional synaptic connections between cultured cortical neurons from agrin-deficient mice. J. Neurobiol. 39:547–557. [DOI] [PubMed] [Google Scholar]
- Lin, R., B.J. Warn-Cramer, W.E. Kurata, and A.F. Lau. 2001. V-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J. Cell Biol. 154:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A.O., M.-N. Mathieu, C. Chevillard, and N.C. Guérineau. 2001. Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: A role in catecholamine release. J. Neurosci. 21:5397–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A.O., M.-N. Mathieu, and N.C. Guérineau. 2003. Evidence for long-lasting cholinergic control of gap junctional communication between adrenal chromaffin cells. J. Neurosci. 23:3669–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan, U.J. 1990. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 55:407–418. [DOI] [PubMed] [Google Scholar]
- Mentis, G.Z., E. Diaz, L.B. Moran, and R. Navarrete. 2002. Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J. Physiol. 544:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittaud, P., P.A. Marangi, S. Erb-Vögtli, and C. Fuhrer. 2001. Agrin-induced activation of acetylcholine receptor-bound Src family kinases requires rapsyn and correlates with acetylcholine receptor clustering. J. Biol. Chem. 276:14505–14513. [DOI] [PubMed] [Google Scholar]
- Mittaud, P., A.A. Camilleri, R. Willmann, S. Erb-Vögtli, S.J. Burden, and C. Fuhrer. 2004. A single pulse of agrin triggers a pathway that acts to cluster acetylcholine receptors. Mol. Cell. Biol. 24:7841–7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, A.S., K.A. Rivas-Plata, J.R. Kraas, S.M. Saleh, and S.L. Swope. 2001. Src-class kinases act within the agrin/MuSK pathway to regulate acetylcholine receptor phosphorylation, cytoskeletal anchoring, and clustering. J. Neurosci. 21:3806–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, T. 1998. Low-conductance intercellular coupling between mouse chromaffin cells in situ. J. Physiol. 506:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil, L.S., C.A.N. Le, J.K. VanSlyke, and L.M. Roberts. 2000. Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J. Biol. Chem. 275:25207–25215. [DOI] [PubMed] [Google Scholar]
- Naus, C.C.G., and M. Bani-Yaghoub. 1998. Gap junctional communication in the developing central nervous system. Cell Biol. Int. 22:751–763. [DOI] [PubMed] [Google Scholar]
- Neyton, J., and A. Trautmann. 1985. Single-channel currents on an intercellular junction. Nature. 317:331–335. [DOI] [PubMed] [Google Scholar]
- Nitkin, R.M., M.A. Smith, C. Magill, J.R. Fallon, Y.-M.M. Yao, B.G. Wallace, and U.J. McMahan. 1987. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J. Cell Biol. 105:2471–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, L.T., J.C. Lauterborn, C.M. Gall, and M.A. Smith. 1994. Localization and alternative splicing of agrin mRNA in adult rat brain: transcripts encoding isoforms that aggregate acetylcholine receptors are not restricted to cholinergic regions. J. Neurosci. 14:1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor, A.M., G.Z. Mentis, R.R. De La Cruz, E. Diaz, and R. Navarrete. 2003. Increased electrotonic coupling in spinal motoneurons after transient botulinum neurotoxin paralysis in the neonatal rat. J. Neurophysiol. 89:793–805. [DOI] [PubMed] [Google Scholar]
- Personius, K.E., and R.J. Balice-Gordon. 2001. Loss of correlated motor neuron activity during synaptic competition at developing neuromuscular synapses. Neuron. 31:395–408. [DOI] [PubMed] [Google Scholar]
- Postma, F.R., T. Hengeveld, J. Alblas, B.N.G. Giepmans, G.C.M. Zondag, K. Jalink, and W.H. Moolenaar. 1998. Acute loss of cell-cell communication caused by G protein-coupled receptors: a critical role for c-Src. J. Cell Biol. 140:1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, C.A., G.E. Wright, M.D. Resh, R.C. Pearson, and S.H. Snyder. 1988. Brain-specific src oncogene mRNA mapped in rat brain by in situ hybridization. Proc. Natl. Acad. Sci. USA. 85:9831–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp, F., D.G. Payan, C. Magill-Solc, D.M. Cowan, and R.H. Scheller. 1991. Structure and expression of a rat agrin. Neuron. 6:811–823. [DOI] [PubMed] [Google Scholar]
- Slotkin, T.A. 1986. Development of the sympathoadrenal axis. In Developmental Neurobiology of the Autonomic Nervous System. P.M. Gootman, editor. Humana Press, Totowa, NJ. 69–96.
- Smith, M.A., and L.G.W. Hilgenberg. 2002. Agrin in the CNS: a protein in search of a function. Neuroreport. 13:1485–1495. [DOI] [PubMed] [Google Scholar]
- Stone, D.M., and K. Nikolics. 1995. Tissue- and age-specific expression patterns of alternatively spliced agrin mRNA transcripts in embryonic rat suggest novel developmental roles. J. Neurosci. 15:6767–6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, T.M., D.S. Faber, and M.J. Zoran. 2004. Transient electrical coupling delays the onset of chemical neurotransmission at developing synapses. J. Neurosci. 24:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temburni, M.K., R.C. Blitzblau, and M.H. Jacob. 2000. Receptor targeting and heterogeneity at interneuronal nicotinic cholinergic synapses in vivo. J. Physiol. 525:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson, A., and R.E. Coupland. 1990. The innervation of the adrenal gland. IV. Innervation of the rat adrenal medulla from birth to old age. A description and quantitative morphometric biochemical study of the innervation of the chromaffin cells and adrenal medullary neurons in Wistar rats. J. Anat. 169:209–236. [PMC free article] [PubMed] [Google Scholar]
- Toyofuku, T., Y. Akamatsu, H. Zhang, T. Kuzuya, M. Tada, and M. Hori. 2001. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J. Biol. Chem. 276:1780–1788. [DOI] [PubMed] [Google Scholar]
- Tsim, K.W., M.A. Ruegg, G. Escher, S. Kroger, and U.J. McMahan. 1992. cDNA that encodes active agrin. Neuron. 8:677–689. [DOI] [PubMed] [Google Scholar]
- Wallace, B.G. 1989. Agrin-induced specializations contain cytoplasmic, membrane, and extracellular matrix-associated components of the postsynaptic apparatus. J. Neurosci. 9:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, B.G., Z. Qu, and R.L. Huganir. 1991. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron. 6:869–878. [DOI] [PubMed] [Google Scholar]
- Wang, K., J.T. Hackett, M.E. Cox, M. van Hoek, J.M. Lindstrom, and S.J. Parsons. 2004. Regulation of the neuronal nicotinic acetylcholine receptor by Src family tyrosine kinases. J. Biol. Chem. 279:8779–8786. [DOI] [PubMed] [Google Scholar]